Atherosclerosis is characterized by the gradual deposition of fat and cholesterol in the arterial wall, which over time can progress to yield inflammation and extracellular matrix-rich lesions that occlude vessels and restrict blood flow. During the formation of atherosclerotic lesions, monocytes are recruited through a mechanism that involves upregulation of the monocyte chemoattractant protein (MCP)-1/chemokine receptor CCR2 signaling pathway [1]. In addition to uptake of lipids, macrophages secrete high concentrations of inflammatory mediators and proteinases such as matrix metalloproteinases (MMPs), which contribute to atherosclerotic plaque progression [2]. Collagen, a key component in the fibrous cap of atherosclerotic plaque, helps sustain the plaque tension and stability. MMPs degrade collagen and lead to increased plaque instability [3]. As such, inflammation and MMPs are implicated in all phases of atherosclerosis, from disease initiation through progression and eventually to the onset of symptoms.
In this issue of Atherosclerosis, Tan and colleagues investigated the connection between MMP-9 and MCP-1 concentrations with the severity of carotid atherosclerosis in patients [4]. They measured serum concentrations of MMP-9 and MCP-1, and evaluated plaque score, plaque stability (assessed by surface characteristics, echogenicity, and texture of atherosclerotic plaque), and intima-media thickness (IMT). Using multinomial logistic regression model, the authors showed that MMP-9 was highly associated with plaque score and instability in a concentration-dependent manner; while MCP-1 correlated with IMT but not plaque score or stability. The authors concluded that MMP-9 and MCP-1 may be useful biomarkers to distinguish stable and unstable plaques and predict future cardiovascular events.
MMP-9 proteolytically processes all major extracellular matrix (ECM) components of the atherosclerotic plaque, including collagen, elastin, and proteoglycans, and as such is a critical modulator of plaque stability [5, 6]. Increased MMP-9 was shown in advanced atherosclerotic lesions in the Ldl4−/−Apob100/100 mouse model [7, 8]. Gough and colleagues revealed that macrophage expression of active, but not pro-, MMP-9 induced plaque disruption [9]. MMP-9 is overexpressed in progressive atherosclerotic plaques obtained from humans undergoing endarterectomy and is especially prevalent in the cap regions where macrophages accumulate, implicating macrophages and MMP-9 in plaque rupture [10]. The current study provides translational support by demonstrating an increased risk of severe atherosclerosis and unstable plaques in patients with higher MMP-9 levels.
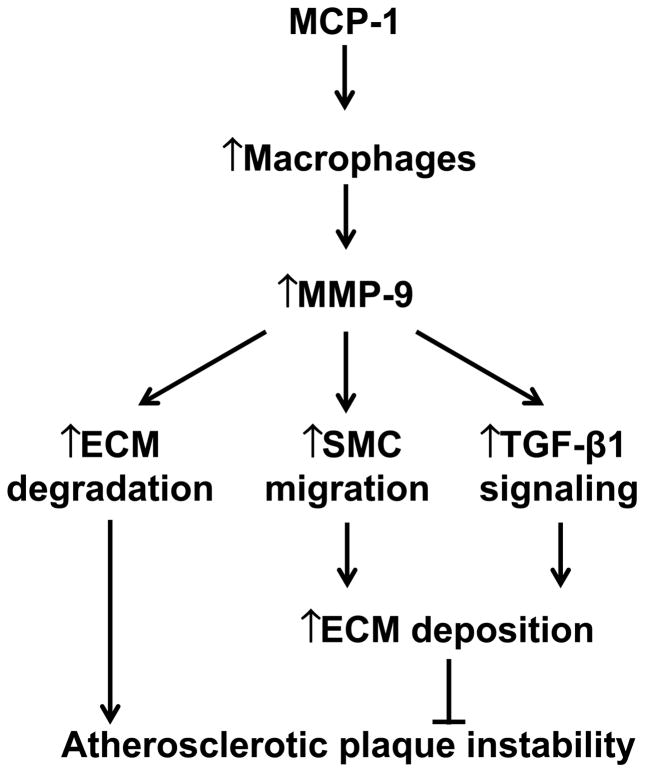
In addition to ECM substrates, MMP-9 also proteolytically cleaves a wide array of inflammatory mediators and growth factors, which implicate MMP-9 in plaque growth. Mice expressing human MMP-9 at pathological levels in macrophages show increased collagen deposition in atherosclerotic lesions, which occurs through increased MMP-9- mediated activation of transforming growth factor-β, suggesting pro-fibrotic and protective roles of MMP-9 in atherosclerosis [5]. Targeted disruption of the MMP-9 gene impairs smooth muscle cell migration and limits arterial remodeling, suggesting a growth role of MMP-9 in atherosclerosis [11]. Our lab and others have revealed dual roles of MMP-9 in left ventricular remodeling following myocardial infarction. Both MMP-9 deletion and macrophage MMP-9 overexpression attenuate cardiac remodeling in a mouse model of myocardial infarction [12, 13]. MMP-9 roles depend on the cellular source, time course, and surrounding microenvironment. Fig. 1 summarizes how MMP-9 modulates atherosclerosis by mediating both plaque growth and instability through ECM regulation.
Fig. 1.
A diagram of the mechanisms by which MCP-1 and MMP-9 regulate the development and progression of atherosclerosis. MCP-1 recruits monocytes to the site of atherosclerotic lesion, where they mature into macrophages. MMP-9, derived mainly from macrophages, exerts dual roles in regulating atherosclerosis. On the one hand, MMP-9 cleaves ECM substrates (especially collagen) in the fibrous cap to increase plaque vulnerability. On the other hand, MMP-9 stimulates smooth muscle cell (SMC) migration and transforming growth factor (TGF)-β1 signaling to facilitate ECM deposition. To what extent both effects occur in vivo remain to be elucidated.
MCP-1, a member of the CC chemokine family, is a potent monocyte attractant upregulated by oxidized lipids [14]. MCP-1 deletion prevents macrophage recruitment and the development of atherosclerotic lesions in Apob overexpressing mice, suggesting an early pro-atherosclerotic role for MCP-1 [15]. The lower expression of MCP-1 in carotid artery may account for its relative resistance to atherosclerosis compared to the more atherosclerosis-prone aorta [16]. In patients, plasma MCP-1 levels significantly correlated with peripheral artery disease, independent of traditional risk factors for coronary artery disease [17].
IMT, a measure of the arterial intima and media thickness, is used to monitor the extent of atherosclerosis in humans and experimental animal models. The MCP-1 gene A2518G polymorphism correlates with IMT in patients with type 2 diabetes, linking MCP-1 with increased smooth muscle proliferation [18]. Tan and colleagues determined that plasma MCP-1 levels were significantly associated with IMT, suggesting that mechanisms in addition to its regulation of macrophage recruitment may be important (Fig. 1).
The findings of this study are encouraging, but several aspects need to be taken into account when interpreting and translating these results. First, large-scale prospective trials are warranted to confirm the findings above. Whether MMP-9 and MCP-1 also predict future cardiovascular events (e.g. myocardial infarction or stroke) has not been unraveled.
Second, the molecular mechanisms whereby MMP-9 mediates atherosclerotic progression to instability and rupture are not totally understood. Emerging evidence has shown that ECM or non-ECM MMP-9 substrates modulate tissue remodeling by regulating inflammatory and fibrotic responses [19, 20]. Hence, a better understanding of the biological activity of MMP-9 proteolysis may provide new intervention opportunities to slow, delay, or even reverse the development and rupture of an unstable atherosclerotic lesion. A proteomics approach that targets inflammation and ECM would help identify the missing pieces of the puzzle by providing a more thorough and high throughput identification of novel ECM and non-ECM substrates [21, 22].
Third, atherosclerosis is pathologically complicated and no single biomarker will be the perfect indicator. It will likely be necessary to utilize several biomarkers (e.g. C-reactive protein, MMP-9, MCP-1, and uric acid) in combination to diagnose and monitor atherosclerosis progression or treatment efficacy. In order for the optimal biomarkers to be defined, a computational approach will likely be needed to refine the list of most informative indicators [23, 24].
In summary, inflammation plays a key role in the initiation and progression of atherosclerosis, and Tan and colleagues have identified MCP-1 and MMP-9 as key mediators. Identification and stabilization of vulnerable plaques is highly important for clinicians, as these plaques are the ones that cause acute syndromes (e.g. myocardial infarction and stroke). Therefore, a strategy evaluating these biomarkers (specifically MMP-9) seems appealing for helping identify which patients may benefit from more aggressive medical therapies (e.g. statins, antiplatelet agents, etc) to prevent these acute unstable plaque ruptures and subsequent complications.
Acknowledgments
We acknowledge support from the NIH/NHLBI HHSN 268201000036C (N01-HV-00244) for the San Antonio Cardiovascular Proteomics Center, HL051971, and R01 HL075360, and from the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Award 5I01BX000505.
Footnotes
Conflict of interest
All authors declare that there is no conflict of interest associated with this manuscript.
References
- 1.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sukhova GK, Schonbeck U, Rabkin E, Schoen FJ, Poole AR, Billinghurst RC, et al. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation. 1999;99:2503–2509. doi: 10.1161/01.cir.99.19.2503. [DOI] [PubMed] [Google Scholar]
- 4.Tan C, Liu Y, Li W, Deng F, Liu X, Wang X, et al. Associations of matrix metalloproteinase-9 and monocyte chemoattractant protein-1 concentrations with carotid atherosclerosis, based on measurements of plaque and intima–media thickness. Atherosclerosis. doi: 10.1016/j.atherosclerosis.2013.11.040. In press. [DOI] [PubMed] [Google Scholar]
- 5.Lemaitre V, Kim HE, Forney-Prescott M, Okada Y, D’Armiento J. Transgenic expression of matrix metalloproteinase-9 modulates collagen deposition in a mouse model of atherosclerosis. Atherosclerosis. 2009;205:107–112. doi: 10.1016/j.atherosclerosis.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Halade GV, Jin YF, Lindsey ML. Matrix metalloproteinase (MMP)-9: a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol Ther. 2013;139:32–40. doi: 10.1016/j.pharmthera.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagsater D, Zhu C, Bjorkegren J, Skogsberg J, Eriksson P. MMP-2 and MMP-9 are prominent matrix metalloproteinases during atherosclerosis development in the Ldlr(−/−)Apob(100/100) mouse. Int J Mol Med. 2011;28:247–253. doi: 10.3892/ijmm.2011.693. [DOI] [PubMed] [Google Scholar]
- 8.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85:1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 9.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stintzing S, Heuschmann P, Barbera L, Ocker M, Jung A, Kirchner T, et al. Overexpression of MMP9 and tissue factor in unstable carotid plaques associated with Chlamydia pneumoniae, inflammation, and apoptosis. Ann Vasc Surg. 2005;19:310–319. doi: 10.1007/s10016-005-0003-7. [DOI] [PubMed] [Google Scholar]
- 11.Galis ZS, Johnson C, Godin D, Magid R, Shipley JM, Senior RM, et al. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ Res. 2002;91:852–859. doi: 10.1161/01.res.0000041036.86977.14. [DOI] [PubMed] [Google Scholar]
- 12.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamilpa R, Ibarra J, de Castro Bras LE, Ramirez TA, Nguyen N, Halade GV, et al. Transgenic overexpression of matrix metalloproteinase-9 in macrophages attenuates the inflammatory response and improves left ventricular function post-myocardial infarction. J Mol Cell Cardiol. 2012;53:599–608. doi: 10.1016/j.yjmcc.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M, Parhami F, et al. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, et al. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka E, Shimokawa H, Kamiuneten H, Eto Y, Matsumoto Y, Morishige K, et al. Disparity of MCP-1 mRNA and protein expressions between the carotid artery and the aorta in WHHL rabbits: one aspect involved in the regional difference in atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:244–250. doi: 10.1161/01.atv.0000051876.26766.fd. [DOI] [PubMed] [Google Scholar]
- 17.Hoogeveen RC, Morrison A, Boerwinkle E, Miles JS, Rhodes CE, Sharrett AR, et al. Plasma MCP-1 level and risk for peripheral arterial disease and incident coronary heart disease: Atherosclerosis Risk in Communities study. Atherosclerosis. 2005;183:301–307. doi: 10.1016/j.atherosclerosis.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Yuasa S, Maruyama T, Yamamoto Y, Hirose H, Kawai T, Matsunaga-Irie S, et al. MCP-1 gene A-2518G polymorphism and carotid artery atherosclerosis in patients with type 2 diabetes. Diabetes Res Clin Pract. 2009;86:193–198. doi: 10.1016/j.diabres.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, et al. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell. 2003;3:589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trial J, Rossen RD, Rubio J, Knowlton AA. Inflammation and ischemia: macrophages activated by fibronectin fragments enhance the survival of injured cardiac myocytes. Exp Biol Med (Maywood) 2004;229:538–545. doi: 10.1177/153537020422900612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yabluchanskiy A, Li Y, de Castro Bras LE, Hakala K, Weintraub ST, Lindsey ML. Proteomic Analysis of the Left Ventricle Post-myocardial Infarction to Identify In Vivo Candidate Matrix Metalloproteinase Substrates. Methods Mol Biol. 2013;1066:185–199. doi: 10.1007/978-1-62703-604-7_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Castro Bras LE, Ramirez TA, DeLeon-Pennell KY, Chiao YA, Ma Y, Dai Q, et al. Texas 3-step decellularization protocol: looking at the cardiac extracellular matrix. J Proteomics. 2013;86:43–52. doi: 10.1016/j.jprot.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Yang T, Ma Y, Halade GV, Zhang J, Lindsey ML, et al. Mathematical modeling and stability analysis of macrophage activation in left ventricular remodeling post-myocardial infarction. BMC Genomics. 2012;13 (Suppl 6):S21. doi: 10.1186/1471-2164-13-S6-S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang T, Chiao YA, Wang Y, Voorhees A, Han HC, Lindsey ML, et al. Mathematical modeling of left ventricular dimensional changes in mice during aging. BMC Syst Biol. 2012;6 (Suppl 3):S10. doi: 10.1186/1752-0509-6-S3-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]