Abstract
In 2012, we conducted a retrospective cross-sectional study to assess the number of people living with HIV linked to care and, among these, the number of people on antiretroviral therapy. The health authority in each of the 20 Italian Regions provided the list of Public Infectious Diseases Clinics providing antiretroviral therapy and monitoring people with HIV infection. We asked every Public Infectious Diseases Clinic to report the number of HIV-positive people diagnosed and linked to care and the number of those on antiretroviral therapy during 2012. In 2012, 94,146 people diagnosed with HIV and linked to care were reported. The majority were males (70.1%), Italians (84.4%), and aged between 25 and 49 years (63.4%); the probable route of transmission was heterosexual contact in 37.5% of cases, injecting drug use in 28.1%, and male-to-male contact in 27.9%. Among people in care, 20.1% had less than 350 CD4 cells/μl, 87.6% received antiretroviral therapy, and among these, 62.4% had a CD4 cell count higher than 350 cells/μl. The overall estimated prevalence of individuals diagnosed and linked to care in 2012 in Italy was 0.16 per 100 residents (all ages). Adding the estimated proportion of undiagnosed people, the estimated HIV prevalence would range between 0.19 and 0.26 per 100 residents. In Italy, the majority of people diagnosed and linked to care receive antiretroviral therapy. A higher prevalence of individuals diagnosed and linked to care was observed in Northern Italy and among males. More information for developing the HIV care continuum is necessary to improve the entire engagement in care, focusing on test-and-treat strategies to substantially reduce the proportion of people still undiagnosed or with a detectable viral load.
Introduction
Aknowledge of the number of people living with HIV (PLHIV) is essential to address the requirements of the national health system, plan the allocation of economic funds, and implement prevention campaigns.
After the introduction of the antiretroviral therapy (ART) in the mid-1990s, the number of PLHIV has notably increased due to the effect of treatment that improves the survival of HIV-infected people and decreases the number of deaths correlated with AIDS, thus transforming HIV infection into a chronic life-long infection.1 ART has improved both the length and the quality of life of HIV-positive people maintained on treatment. On the other hand, HIV-positive people who are not diagnosed or engaged in the care system cannot take advantage of these benefits, suggesting damage not only at the individual level in terms of a faster progression to AIDS and death, but also at the public health level in determining the potential for ongoing transmission in a community.2
Specifically, in recent years, gaps in the detection of HIV-positive people among IDUs and migrants have been reported in Italy, in terms of both a low access to HIV testing and a high percentage of late diagnosis.3,4
The distribution of PLHIV at various stages of the engagement-in-care process has been synthesized in the “HIV care continuum” that spans from HIV acquisition to viral suppression providing a simple quantitative depiction of every step along the HIV care continuum2,5–8; the results of the cascade can be used to assess the effectiveness of interventions and improve engagement in the care of HIV-positive individuals. The steps of the HIV care continuum start with the estimated number of HIV-infected individuals, followed by the number of those who are diagnosed, which includes those linked to HIV care and those retained in care, which includes in turn those who need ART and those who are on ART; the cascade ends with the number of those with undetectable HIV viral load.6
In Italy, national data on the number of HIV-diagnosed individuals and their characteristics (clinical, immunological, behavioral, and treatment) are lacking, and so are the other figures needed to describe the HIV care continuum. Therefore, we conducted a cross-sectional study in 2012 to assess the number of PLHIV linked to care and, among these, the number of people on ART. These data were used also to estimate the number of HIV-infected individuals in Italy.
Materials and Methods
In Italy, Public Infectious Diseases Clinics (PIDC) offer monitoring and health management free of charge to all HIV-positive individuals, including non-nationals and undocumented migrants. The majority of individuals who test HIV positive are diagnosed in PIDC and those who are tested in other health facilities are then addressed to a PIDC for confirmatory testing and diagnosis. Therefore, the probability for an individual who tests HIV positive to escape the PIDC network is extremely low.
A law issued in 19909 in Italy established that only PIDC are allowed to provide ART to HIV-positive individuals. ART is offered free of charge regardless of nationality, residence, and (for non-Italians) legal status. For these reasons, in 2012 we decided to conduct a retrospective cross-sectional study with the collaboration of PIDC located throughout Italy.
We contacted the regional health authority located in each of the 20 Italian Regions and requested the list of all PIDC providing ART and monitoring people with HIV infection in the region. Then, every PIDC was contacted by e-mail and asked to report (1) the number of HIV-positive people diagnosed and linked to care, defined as individuals who had at least one medical visit at any time after HIV diagnosis between January 1 and December 31, 2012, and (2) the number of HIV-positive people on ART, defined as individuals linked to care who received and took at least one ART regimen between January 1 and December 31, 2012.
Each of these two groups was stratified by gender (male, female), age (≤24 years, 25–49 years, 50–59 years, ≥60 years), probable route of transmission [these groups are mutually exclusive: heterosexuals (HET), men having sex with men (MSM), injecting drug user (IDU), other modes (i.e., mother-to-child transmission or blood transfusion)], last CD4 measurement (CD4 <200 cells/μl, CD4 200–349 cells/μl, CD4 ≥350 cells/μl), and U.S. Centers for Disease Control and Prevention (CDC) HIV clinical stage (A, B, C).
An aggregate data collection form was sent to all PIDC by e-mail; all data were requested as aggregate numbers or percentages of the total number of people linked to care. After a month, each PIDC was contacted by phone to provide quality control data. The data were confirmed by 90% of PIDC.
The prevalence based on individuals diagnosed and linked to care was calculated as the number of HIV-positive people diagnosed and linked to care divided by the number of residents in Italy in 2012, per 100 (all ages). The number of residents was obtained from the National Institute of Statistics (ISTAT).10
To estimate the total number of PLHIV in Italy in 2012, we added the estimated proportion of people either undiagnosed or diagnosed for HIV infection but not linked to care to the reported number of people diagnosed and linked to care. Based on previously published data, this proportion can range between 18% and 40%2,5,6,8,11–18 of all PLHIV. Therefore, we calculated two estimates of the total number of PLHIV: a minimum number considering 18% undiagnosed and a maximum number considering 40% undiagnosed.
To make data collection less burdensome for PIDC, no plasma viral load result or information about adherence to ART was requested in the survey. However, to obtain indirect information on these variables, we collected the proportion of individuals with a CD4 count above 350 cells/μl among those on ART, deemed as a proxy of those virologically suppressed on the basis of previous findings that show that viral suppression is associated with a high CD4 count.19,20
Results
In 2012, 173 PIDC were identified by the 20 regional health authorities and were included in the survey. Of these, 170 (98.3%) agreed to participate in the study. The three PIDC who did not participate were located in three small towns (Fermo, Foligno, and Ragusa) accounting for 0.9% of all Italian residents; the reason for not participating was due in all cases to operational problems (lack of time and/or dedicated personnel for collecting data). In 2012, these three towns reported one new AIDS case out of 715 reported at the national level.
Participating PIDC were geographically distributed as follows: 38.8% in Northern Italy, 27.6% in Central Italy, and 33.6% in Southern Italy. The number of PIDC per million residents was 2.2 per million in Northern Italy, 2.9 per million in Central Italy, and 2.6 per million in Southern Italy. An average number of 554 (SD±793) people diagnosed and linked to care by PIDC was reported: this average number was significantly higher for PIDC located in Northern Italy (908±1077) compared to those in Central (453±549) and Southern (226±214) Italy (Student's t test, p<0.001).
In 2012, 94,146 people HIV diagnosed and linked to care were reported by the 170 participating PIDC. The majority were males (70.1%), Italians (84.4%), and aged between 25 and 49 years (63.4%); 37.5% were HET, 28.1% IDU, 27.9% MSM, and 6.5% reported other modes of transmission. Among people linked to care, 10.5% had 200–349 CD4 cells/μl and 9.6% had less than 200 CD4 cells/μl (Table 1).
Table 1.
Characteristics of 94,146 People Living with HIV Diagnosed and Linked to Care per 100 Residents in Italy in 2012
| Characteristics | % |
|---|---|
| Gender | |
| Male | 70.1 |
| Female | 29.9 |
| Age, in years | |
| ≤24 | 3.2 |
| 25–49 | 63.4 |
| 50–59 | 25.1 |
| ≥60 | 8.3 |
| Nationality | |
| Italian | 84.4 |
| Non-nationals | 15.6 |
| Transmission route | |
| Heterosexual | 37.5 |
| IDU | 28.1 |
| MSM | 27.9 |
| Not determined | 6.5 |
| CD4 count (cells/μl) | |
| <200 | 9.6 |
| 200–349 | 10.5 |
| ≥350 | 79.9 |
| AIDS diagnosis | |
| No | 73.5 |
| Yes | 26.5 |
| People receiving ART | |
| Yes | 87.6 |
| No | 12.4 |
IDU, injecting drug use; MSM, men who have sex with men; ART, antiretroviral therapy.
Among HIV-positive people diagnosed and linked to care, 87.6% received ART in 2012; of these, 68.3% were males, 85.1% were Italians, and 62.3% were aged 25–49 years. A proportion of 37.3% HET, 28.4% IDU, 27.9% MSM, and 6.4% reporting other modes of transmission was on ART. Among people on ART, 9.4% had a CD4 cell count below 200 cells/μl, 11.2% had 200–349 CD4 cells/μl, and 62.4% had a CD4 higher than 350 cells/μl.
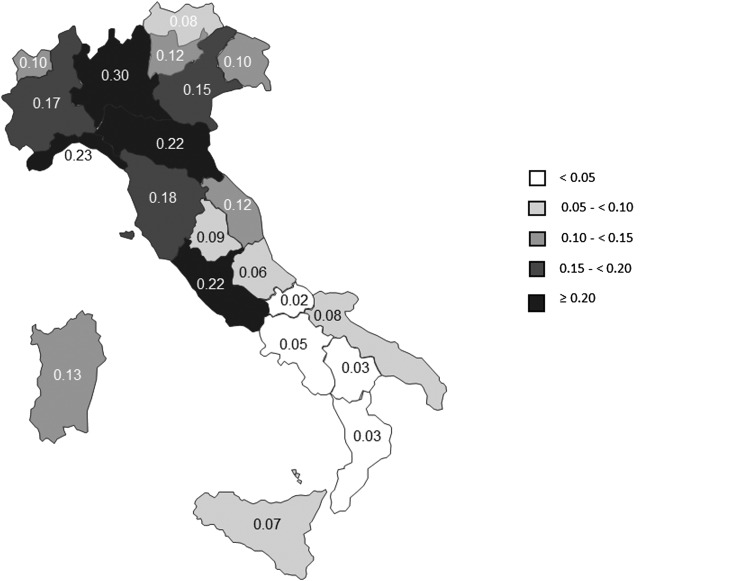
The overall estimated prevalence of PLHIV diagnosed and linked to care in 2012 was 0.16 per 100 residents (all ages). This proportion varied greatly by region ranging from a minimum in Molise (0.02 per 100 residents) to a maximum in Lombardia (0.30 per 100 residents) (Fig. 1). When adding the proportion of infected individuals living with an undiagnosed HIV infection or not linked to care,2,5,6,8,11–18 the total number of individuals HIV diagnosed and linked to care would range from a minimum of 114,812 (including 18% undiagnosed or not linked to care) to a maximum of 156,910 (including 40% undiagnosed or not linked to care) people. Consequently, the estimated HIV prevalence would range between 0.19 and 0.26 per 100 residents.
FIG. 1.
People living with HIV diagnosed and linked to care in Italy, by 100 residents—2012.
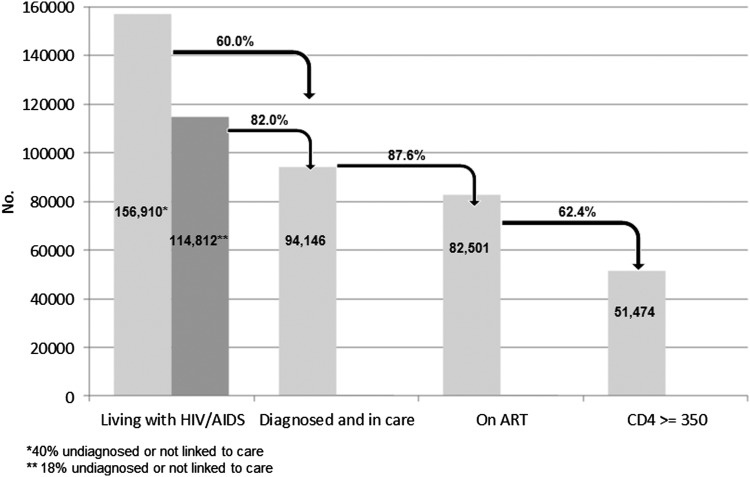
Figure 2 shows an estimate of PLHIV in Italy in 2012: the first bar (living with HIV) shows the two estimated numbers of PLHIV: 156,910 if 40% of PLHIV is undiagnosed and 114,812 if 18% of PLHIV is undiagnosed; of these, 94,146 people are diagnosed and linked to care; among these, 87.6% receive ART; and among these, 62.4% have more than 350 CD4 cells/μl (missing values were not considered). The proportion of PLHIV who have a CD4 <350 cell count ranges between 8.5% (when considering 156,910 PLHIV) and 11.6 % (when considering 114,812 PLHIV). The proportion is 14.1% when considering 94,146 PLHIV reporting by PIDC.
FIG. 2.
People living with HIV in Italy in 2012 by diagnosis and treatment.
Discussion
This is the first survey conducted in Italy aimed at defining the number and characteristics of HIV-positive individuals in care with the contribution of PIDC, providing an up-to-date description of individuals living with HIV infection in Italy. Given that PIDC treat almost all individuals diagnosed with HIV and are the only health services authorized to prescribe ART in Italy, the participation of almost all PIDC guarantees a very high completeness of data regarding individuals on ART.
Although the geographic density of PIDC is similar throughout the country, PIDC located in the north reported a higher number of HIV-positive people in care, which is in agreement with the higher number of new diagnoses of both HIV infection and AIDS observed since the beginning of the epidemic in the north compared to the center and south of Italy.21
The proportion of males found in this study is more than twice that of females, similar to what is observed for most years in the Italian surveillance of new HIV diagnoses where the number of males is about three times higher that of females,21 confirming that the HIV spread in Italy has been mainly driven by male IDU, at least in the first two decades of the epidemic.
Data obtained from the surveillance system of new HIV diagnoses show that the peak of the HIV epidemic in Italy occurred in the period 1986–1992,21 involving primarily individuals aged 25–30 years (these individuals would be older than 50 years in 2012), and the median age of newly diagnosed HIV cases steadily increased over time.21 When comparing these results with those obtained from this cross-sectional study, the finding that two-thirds of the reported population in care is younger than 50 years of age suggests a combined effect of a high mortality rate among older cohorts and a longer survival in the post-ART era among more recently infected cohorts.
These same two factors might also explain the low proportion of IDUs in care compared to both heterosexuals and MSM: in the Italian HIV surveillance data IDUs accounted for about 80% of all new HIV diagnoses in the period 1986–1992 but experienced a high mortality rate before ART introduction,22 whereas heterosexuals and MSM have become prominent after 1996 (reaching 80% of all new HIV diagnoses reported by the HIV surveillance system in 2011–2012) and could benefit from ART.
The HIV prevalence observed in the present survey is similar to that reported in France (0.14 per 100 residents)11 and the United Kingdom (0.15 per 100 residents),8 but lower than that reported in the United States6,7 and Canada,14 probably reflecting a higher spread of HIV in North America compared to Europe.
The estimated number of PLHIV of about 114,812–156,910 is similar to the estimate (110,000–140,000) obtained for Italy in 2012 using the EPP/Spectrum package of the UNAIDS23,24: this package incorporates surveillance data, results of prevalence surveys, and national demographic data in a mathematical model that generates estimates of prevalent cases and incidence trends over time. It must be stressed that in the HIV care continuum the estimated number of PLHIV depends on the proportion of people undiagnosed or not linked to care.
In Italy, the proportion of undiagnosed HIV-positive people has not been formally ascertained. A few recent studies conducted in various Italian populations undergoing routine HIV testing report the following proportion of HIV-positive individuals unaware of being HIV infected: 23% among pregnant women diagnosed in prenatal screening,25 37.5% among individuals with a sexually transmitted infection,26 and 57.1% among MSM tested in a survey conducted in gay venues.27 However, these proportions were found in specific subgroups and cannot be transferred to the population at large. In addition, the higher HIV prevalence and number of PIDC observed in Northern Italy as well as the relevant proportion of late diagnosis among migrants in Southern Italy4 suggest that the distribution of undiagnosed cases may be geographically uneven across the country. Therefore, additional information from ad hoc studies is needed to define more accurately the proportion of undiagnosed HIV-positive people.
Almost 90% of people diagnosed and linked to care receive ART, which is in agreement with recent Italian HIV treatment guidelines28 that recommend treatment initiation regardless of CD4 count to prevent HIV transmission and reduce the risk of disease progression. Other countries report similar proportions of ART: 89% in the United States,5,7,29 88% in the United Kingdom,8 83% in the Netherlands,16 and 79% in Canada.15
HIV plasma viral suppression among treated individuals is a crucial marker that defines the virologic success of ART, the immunological and clinical control of the disease, and implies a lower probability of transmitting the infection. The proportion of virally suppressed individuals among those on ART constitutes the last step of the HIV care continuum, but this information was not included in our survey to limit data collection to those variables that were more easily accessible by PIDC. Using the proportion of people with CD4 ≥350 cells/μl among those on ART as a proxy of adherence to treatment and consequent viral suppression, we found that two-thirds of individuals on ART had a CD4 count ≥350 cells/μl: this proportion is lower than that of virally suppressed individuals reported in other countries.5–7,18,29 However, aggregate data on the last CD4 count did not make it possible to determine if the CD4 measurement was taken prior to starting ART, when the CD4 count may be low, which may lead to an underestimate of the number of individuals on ART with a CD4 count ≥350 cells/μl. About 14.1% of PLHIV have a CD4 cell count below 350 cells/μl stressing the need to improve access to diagnosis and care of persons with HIV infection.
Some limitations of this study need to be mentioned. First, information requested to the PIDC was limited to the few variables that were considered more relevant and easier to be collected, which, however, hindered the depiction of all the steps of the HIV care continuum, forcing, for example, the replacement of the “virologically suppressed” proportion with that of “CD4 >350 cells/μl.” Second, data were collected as aggregate data, thus hampering the check of double counts and more detailed analyses in selected subgroups (such as IDU and migrants who have been shown to have a low access to HIV testing and care4). Third, the proportion of undiagnosed or not linked to care used for calculating the first bar of the HIV care continuum was arbitrarily defined based on information from the literature.
Our survey shows the first estimates of HIV prevalence and engagement in HIV care in Italy. A higher prevalence of PLHIV diagnosed and linked to care was observed in Northern Italy and among males; the majority of people diagnosed and linked to care received ART. A more accurate description of the HIV care continuum will provide appropriate information for improving the entire engagement in care; interventions should focus on test-and-treat strategies to substantially reduce the proportion of people still undiagnosed or with a detectable viral load, as well as improve the clinical outcomes and quality of life of PLHIV linked to care/on ART with an undetectable viral load or very low CD4 count.
Acknowledgments
We wish to thank Lucia Pugliese for technical support. This study received financial support from the Ministry of Health, Ricerca Finalizzata 2009, Grant RF-2009-1505025. The authors declare have no affiliation with any organization whose financial interest may be affected by material in the article or that may potentially bias it.
Sources of support: Collaborations: The CARPHA Study Group: Dante Di Giammartino, Giustino Parruti, Paola Di Stefano, Maurizio Paoloni, Margherita D'Alessandro, Alessandro Grimaldi, Maria Pina Sciotti, Eligio Pizzigallo, Jacopo Vecchiett, Carlo De Stefano, Angela La Gala, Giulio De Stefano, Angela Linzalone, Francesco Cesario, Lucio Cosco, Benedetto Caroleo, Giuseppe Foti, Nicola Serrao, Domenico Lucchino, Antonio Chirianni, Nicola Abrescia, Raffaele Pempinello, Crescenzo M. Izzo, Guglielmo Borgia, Pietro Filippini, Evangelista Sagnelli, Angelo Iodice, Angelo S. Megna, Giovanna D'Alessio, Nicola Acone, Maurizio Mazzeo, Daria Sacchini, Carlo Ferrari, Annamaria degli Antoni, Giacomo Magnani, Cristina Mussini, Vanni Borghi, Pierluigi Viale, Vincenzo Colangeli, Laura Sighinolfi, Marco Libanore, Alessandra Govoni, Claudio Cancellieri, Paolo Bassi, Massimo Arlotti, Roberto Luzzati, Matteo Bassetti, Umberto Tirelli, Emanuela Vaccher, Gianmichele Moise, Guido Palamara, Stefania Bernardi, Mario Falciano, Vincenzo Vullo, Gabriella d'Ettore, Vincenzo Renda, Cecilia Guariglia, Gloria Taliani, Ivano Mezzaroma, Francesca Paoletti, Camilla Ajassa, Roberta Gastaldi, Massimo Andreoni, Loredana Sarmati, Francesco Montella, Andrea Antinori, Alberto Giannetti, Nicola Pietrosillo, Enrico Girardi, Alfredo Pennica, Roberto Cauda, Manuela Colafigli, Simona Di Gianbenedetto, Antonio Caterini, Roberto Monarca, Stefano Aviani Barbacci, Gianpaolo Natalini Ramponi, Mauro Marchili, Enza Anzalone, Miriam Lichtner, Giuseppe Ferrea, Giovanni Cassola, Claudio Viscoli, Giovanni Mazzarello, Maurizio Setti, Stefania Artioli, Giovanni Riccio, Giorgetta Casalino Finocchio, Marco Anselmo, Marco Rizzi, Alfredo Scalzini, Francesco Castelli, Tiziana Quirino, Domenico Santoro, Angelo Pan, Alessia Zoncada, Paolo Bonfanti, Paolo Viganò, Massimo Villa, Marco Tinelli, Giorgio Perboni, Loredana Palvarini, Paolo Costa, Massimo Puoti, Massimo Galli, Giuliano Rizzardini, Antonella d'Arminio Monforte, Adriano Lazzarin, Antonella Castagna, Andrea Gori, Lorenzo Minoli, Gaetano Filice, Paolo Grossi, Andrea Giacometti, Marcello Tavio, Maria Montroni, Luca Butini, Patrizia Osimani, Enzo Petrelli, Alessandro Chiodera, Patrizio Vittucci, Paola Sabbatini, Chiara Pasqualini, Mauro Valle, Milena Zoppi, Eugenio Mantia, Giuliano Schettino, Massimo Deseraca, Davide Vitullo, Olivia Bargiacchi, Gianfranco Orofino, Caterina Bramato, Margherita Busso, Bernardino Salassa, Mariana Farenga, Stefano Bonora, Guido Leo, Federica Poletti, Mario Gobber, Giovanni Cristina, Clara Gabiano,Peter Mian, Oswald Moling, Claudio Paternoster, Nicoletta Dorigoni,Tommaso Fontana, Gioacchino Angarano, Nicoletta Ladisa, Domenico La Rovere, Cecilia Fico, Fabio Bulla, Teresa Santantonio, Benvenuto Grisorio, Piergiorgio Chiriacò, Pierpaolo Congedo, Paolo Tundo, Francesco Resta, Letizia Cristiano, Maria Stella Mura, Giordano Madeddu, Pietro Mesina, Sandro Piga, Marco Campus, Paolo Emilio Manconi, Francesco Ortu, Antonio Salvo, Camillo Baretti, Rosaria La Sala, Pietro Bellissima, Salvatore Bonfante, Salvatore Galvagna, Benedetto M. Celesia, Rosario La Rosa, Sebastiano Maiuzzo, Luigi Guarnieri, Salvatore Bruno, Isa Picerno, Nicola Tripodi, Enzo M. Farinella, Cecilia Occhino, Lucina Titone, Claudia Colomba, Tullio Prestileo, Marcello Saitta, Piera Dones, Rosa Boncoraglio, Antonio Davi, Antonina Franco, Vincenzo Portelli, Francesca Savalli, Consuelo Geraci, Maurilio Chimenti, Sauro Luchi, Corrado Catalani, Michele Trezzi, Donatella Aquilini, Spartaco Sani, Cesira Nencioni, Tiziana Carli, Francesco Mazzotta, Sergio Lo Caputo, Giuliano Zuccati, Riccardo Iapoce, Rita Consolini, Dario Bartolozzi, Alessandro Bartoloni, Filippo Bartalesi, Andrea De Luca, Maurizio De Martino, Danilo Tacconi, Sauro Tini, Franco Baldelli, Daniela Francisci, Renato F. Frongillo, Antonio Traverso, Ermenegildo Francavilla, Roberto Ferretto, Franco Marranconi, Vinicio Manfrin, Piero Cortese, Cristina Rossi, Francesca Cattelan, Andrea Petrucci, Pierluigi Brugnaro, Dino Sgarabotto, Renzo Scaggiante, Annamaria Cattelan, Oliviero Bosco, Ercole Concia, and Pierangelo Rovere.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Moreno S, Mocroft A, and D'Armino Monforte A: Medical and societal consequences of late presentation. Antivir Ther 2010;15(Suppl 1):9–15 [DOI] [PubMed] [Google Scholar]
- 2.Mugavero MJ, Amico KR, Horn T, et al. : The state of engagement in HIV care in the United States: From cascade to continuum to control. Clin Infect Dis 2013;57(8):1164–1171 [DOI] [PubMed] [Google Scholar]
- 3.Camoni L, Federico B, Capelli G, et al. : Few Italian drug users undergo HIV testing. AIDS Behav 2011;15(4):711–717 [DOI] [PubMed] [Google Scholar]
- 4.Camoni L, Raimondo M, Regine V, et al. : Late presenters among persons with a new HIV diagnosis in Italy, 2010–2011. BMC Public Health 20013;13:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall HI, Frazier EL, Rhodes P, et al. : Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med 2013;173(14):1337–1344 [DOI] [PubMed] [Google Scholar]
- 6.Gardner EM, McLees MP, Steiner JF, et al. : The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011;52(6):793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC: Vital signs: HIV prevention through care and treatment–Unites States. MMWR 2011;60(47) (www.cdc.gov/mmwr) [PubMed] [Google Scholar]
- 8.Health Protection Agency: HIV in United Kingdom: 2012. Report. London: Health Protection Services, Colindale; November2012 [Google Scholar]
- 9.Italia. Legge 135-5 Giugno 1990: Piano degli interventi urgenti in materia di prevenzione e lotta all'AIDS. G.U. n. 132, 8 giugno 1990
- 10.Istituto Nazionale di Statistica: ISTAT. Popolazione residente. 2012. (www.demo.istat.it)
- 11.Cazein F, Barin F, Le Start Y, et al. : Prevalence and characteristics of individuals with undiagnosed HIV infection in France: Evidence from a survey on hepatitis B and C seroprevalence. J Acquir Immune Defic Syndr 2012;60(4):e114–e116 [DOI] [PubMed] [Google Scholar]
- 12.Campsmith ML, Rhodes PH, Hall HI, et al. : Undiagnosed HIV prevalence among adults and adolescents in the United States at the end of 2006. J Acquir Immune Defic Syndr 2010;53:619–624 [DOI] [PubMed] [Google Scholar]
- 13.Ellis S, Curtis H, and Ong E: A survey of HIV care in the UK: Results of British HIV association (BHIVA) national audit 2010. Int J STD AIDS 2013;24(4):329–331 [DOI] [PubMed] [Google Scholar]
- 14.Yang Q, Boulos D, Yan P, et al. : Estimates of the number of prevalent and incident human immunodeficiency virus (HIV) infections in Canada, 2008. Can J Public Health 2010;101(6):486–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nosyk B, Montaner JSG, Colley G, et al. : The cascade of HIV care in British Columbia, Canada, 1996–2011: A population-based retrospective cohort study. Lancet Infect Dis 2014;14(1):40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Veen MG, Presanis AM, Conti S, et al. : National estimate of HIV prevalence in the Netherlands: Comparison and applicability of different estimation tools. AIDS 2011;25(2):229–237 [DOI] [PubMed] [Google Scholar]
- 17.Bayer R. and Oppenheimer GM: Routine HIV testing, public health, and the USPSTF—An end to the debate. New Engl J Med 2013;368:881–884 [DOI] [PubMed] [Google Scholar]
- 18.Lange JMA: “Test and Treat”: Is It Enough? Clin Infect Dis 2011;52(6):801–802 [DOI] [PubMed] [Google Scholar]
- 19.Holland CA, Ellenberg JH, Wilson CM, et al. : Relationship of CD4+ T cell counts and HIV type 1 viral loads in untreated, infected adolescents. AIDS Res Hum Retroviruses 2000;16(10):959–963 [DOI] [PubMed] [Google Scholar]
- 20.Marconi VC, Grandits G, Okulicz JF, et al. : Infectious Disease Clinical Research Program (IDCRP) HIV Working Group: Cumulative viral load and virologic decay patterns after antiretroviral therapy in HIV-infected subjects influence CD4 recovery and AIDS. PLoS One 2011;6(5):e17956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camoni L, Boros S, Regine V, et al. : Aggiornamento delle nuove diagnosi di infezione da HIV dei casi di AIDS in Italia al 31 dicembre 2012. Not Ist Super Sanità 2013;26(9, Suppl 1):3–47 [Google Scholar]
- 22.Serraino D, Zucchetto A, Suligoi B, et al. : Survival after AIDS diagnosis in Italy, 1999–2006: A population-based study. J Acquir Immune Defic Syndr 2009;52(1):99–105 [DOI] [PubMed] [Google Scholar]
- 23.WHO: Global report: UNAIDS report on the global AIDS epidemic 2013. UNAIDS 2013 [Google Scholar]
- 24.Camoni L, Regine V, Stanecki K, Salfa MC, Raimondo M, Suligoi B: Estimates of the number of people living with HIV in Italy. BioMed Res Int 2014. DOI: 10.1155/2014/209619 Epub 2014 Jul 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Floridia M, Polizzi C, Mattei A, et al. : HIV, gravidanza e terapia antiretrovirale. Not Ist Super Sanità 2008;21(2):11–14 [Google Scholar]
- 26.Salfa MC, Regine V, Ferri M, et al. : La sorveglianza delle malattie sessualmente trasmesse basata su una rete di centri clinici compie 21 anni (1991–2011). Not Ist Super Sanità 2013;26(6):3–9 [Google Scholar]
- 27.WHO Europe: SIALON project. Briefing Notes. 2009.
- 28.Ministero della Salute: Linee Guida Italiane sull'utilizzo dei farmaci antiretrovirali e sulla gestione diagnostico-clinica delle persone con infezione da HIV-1. 2013
- 29.Dombrowski JC, Kitahata MM, Van Rompaey SE, et al. : High levels of antiretroviral use and viral suppression among persons in HIV care in the United States, 2010. J Acquir Immune Defic Syndr 2013;63(3):299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]