Abstract
HIV is a pandemic disease, and many cellular and systemic factors are known to alter its infectivity and replication. Earlier studies had suggested that anemia is common in HIV-infected patients; however, higher iron was also observed in AIDS patients prior to the introduction of antiretroviral therapy (ART). Therefore, the relationship between iron and viral infection is not well delineated. To address this issue, we altered the levels of cellular iron in primary CD4+ T cells and showed that higher iron is associated with increased HIV infection and replication. In addition, HIV infection alone leads to increased cellular iron, and several ART drugs increase cellular iron independent of HIV infection. Finally, HIV infection is associated with increased serum iron in HIV-positive patients regardless of treatment with ART. These results establish a relationship between iron and HIV infection and suggest that iron homeostasis may be a viable therapeutic target for HIV.
Although great progress has been made in HIV treatment using antiretroviral therapy (ART), infection with this virus remains a major cause of mortality in the world. Thus, novel therapies involving new pathways are needed to eradicate this disease. Iron is essential for many cellular processes, but an increase in its levels leads to oxidative stress. In particular, high serum iron levels are associated with increased oxidative stress in HIV-infected men.1 As a result, the levels of cellular iron are tightly regulated. The role of iron in bacterial and fungal infections is well established2–4; however, changes in cellular or systemic iron after HIV infection is not well understood. It is known that anemia is associated with worse outcomes in HIV infection,5 but iron overload is also implicated as a risk factor for rapid progression of the disease. Observational studies prior to the introduction of ART demonstrated that patients with iron overload due to genetic polymorphisms had a more rapid progression of HIV infection.6 In addition, iron chelation in HIV-positive patients with thalassemia major slowed down the disease course while iron supplementation was associated with worse outcomes.7–9 In vitro studies also provided conflicting evidence linking iron with HIV progression. Higher cellular iron levels in HIV-infected macrophages are associated with increasing HIV transcription,10,11 and treatment of monocytes with the iron chelator deferoxamine (DFO) decreased NF-κB and HIV-1 reactivation by oxidative stress.12 However, another study found no change in NF-κB with iron chelation.13 A reduction in cyclin-dependent kinase (CDK) 2/cyclin E complex activity has also been suggested as one of the mechanisms of how iron deficiency reduces HIV replication,11,14 but changes in CDK2/cyclin E complex activity were not observed in another study using DFO to decrease cellular iron.15 Therefore, the interaction between HIV infection and cellular and systemic iron status, particularly in the post-ART era, remains unclear.
While the aforementioned observational studies focused on the link between iron and HIV, a few observational studies suggested that ART can also influence systemic iron levels. Treatment of pregnant HIV-positive women with ART in Botswana was associated with severe infant anemia.16 Also, lower ferritin levels were observed in immunosuppressed Thai HIV-positive patients with an interruption of ART,17 and cessation of ART exacerbated microcytic anemia in a parvovirus B19- and HIV-positive β-thalassemia patient.18 However, whether ART influences iron levels independent of HIV and whether ART corrects or worsens the altered iron homeostasis during HIV infection remain to be determined. In this article, we studied the effects of HIV infection and ART on cellular and systemic iron and the role of iron in HIV infection.
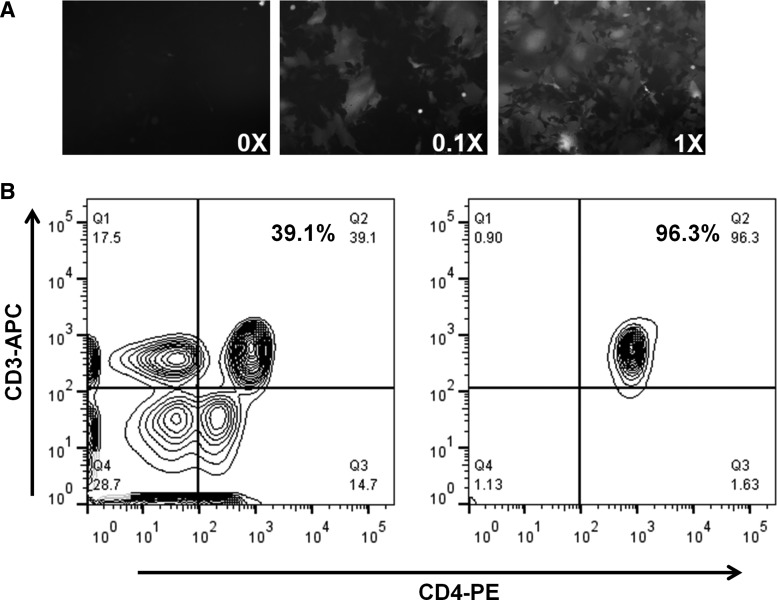
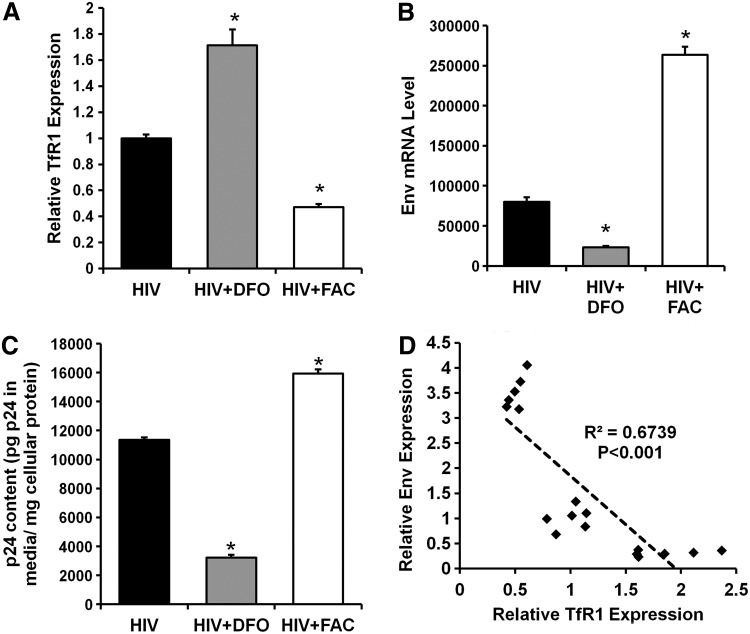
We first generated replication-competent HIV-1BaL virus and showed that the virus is capable of infecting GHOST cells, an HIV indicator cell line with CD4 and CXCR5 overexpression (Fig. 1A). Primary cultures of CD4+ T cells enriched from human peripheral blood mononuclear cells (PBMCs) were established using a magnetic bead-based depletion method (Fig. 1B). Using this virus and CD4+ cells, we then studied the effect of iron modulation on established HIV infection. Treatment of primary CD4+ T cells with the iron chelator DFO and ferric ammonium citrate (FAC) resulted in an increase and decrease in transferrin receptor 1 (TfR1) mRNA levels (which correlates with cellular iron levels),19–21 respectively, suggesting that these cells are responsive to iron modulation in vitro (Fig. 2A). HIV replication was slowed down in iron-depleted cells, while iron supplementation increased viral replication and release, as measured by Env mRNA inside the cells and p24 release into the media (Fig. 2B and C). The effect was independent of changes in cellular replication as the p24 release was normalized to cellular protein levels. The Env mRNA levels were negatively correlated with TfR1 mRNA levels, a surrogate for cellular iron levels (Fig. 2D). These observations suggest that HIV replication is accelerated in the presence of higher iron levels.
FIG. 1.
Production of HIV virus and isolation of primary human CD4+ T cells. (A) Infection of GHOST cells with HIV1BaL virus. 1X represents 0.5 MOI of virus. (B) Representative flow cytometry histograms of peripheral blood mononuclear cells (PBMCs) before (left) and after (right) isolation for CD4+ T cells. The percentage of CD4+ T cells after enrichment is routinely higher than 90%.
FIG. 2.
Iron modulation influences HIV replication in infected CD4+ T cells. (A) Cellular transferrin receptor 1 (TfR1) mRNA levels in response to iron chelation (DFO) and iron overload (FAC). Intracellular Env mRNA (B) and p24 release into media (C) in HIV-infected CD4+ T cells with iron modulation for 48 h. (D) Correlation between TfR1 expression (a marker for cellular iron levels) and Env expression for samples in A and B. N=6 for A–C. *p<0.05 vs. HIV infection alone.
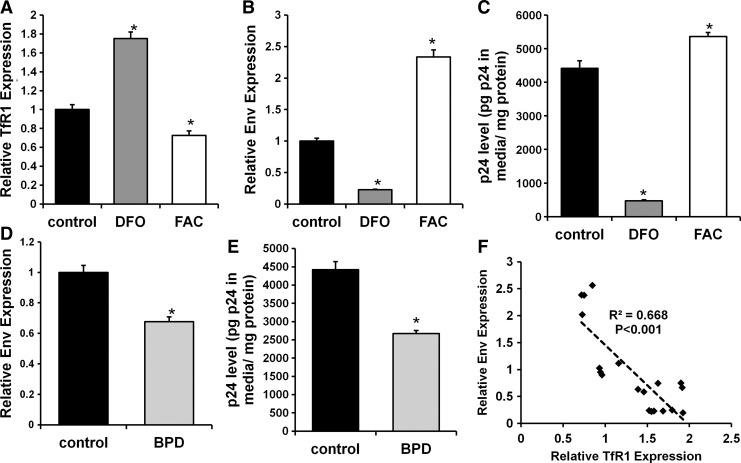
We next investigated whether changes in cellular iron had an impact on HIV infection. Cellular iron in primary CD4+ T cells was modulated with FAC or DFO prior to HIV infection, and the efficiency of iron modulation was confirmed by changes in TfR1 mRNA levels (Fig. 3A). Iron chelation reduced Env mRNA levels (Fig. 3B) and p24 release (Fig. 3C), while iron overload had opposite effects. These findings are consistent with reduced viral infection in iron-deficient cells. To ensure that the observed changes in viral infection were not due to the choice of iron chelator, we performed similar studies with a mechanistically different iron chelator 2,2′-bipyridyl (BPD). BPD pretreatment also reduced Env mRNA levels and p24 release after infection (Fig. 3D and E). We also observed a statistically significant negative correlation between cellular iron (measured by TfR1 mRNA levels) and Env mRNA levels (Fig. 3F). These findings indicate that a decrease in cellular iron is protective against HIV infection.
FIG. 3.
Iron modulation influences HIV infection of primary CD4+ T cells. (A) TfR1 mRNA level in response to iron modulation. Intracellular Env mRNA (B) and p24 release (C) in CD4+ cells with HIV infection after iron modulation. Intracellular Env mRNA (D) and p24 release (E) in CD4+ cells infected with HIV after being treated with 2,2′-bipyridyl (BPD). (F) Correlation between TfR1 expression (a marker for cellular iron levels) and Env expression for samples in A, B, D, and E. *p<0.05 vs. control. N=4–6 for A–E.
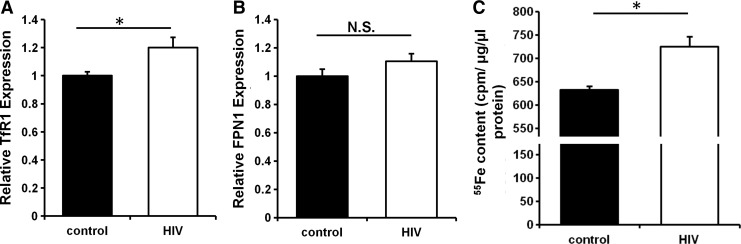
The above studies demonstrated that changes in cellular iron influence the replication and infection of HIV, but whether HIV infection alters cellular iron is unknown. We examined the effects of HIV infection on iron regulatory pathways in primary CD4+ T cells. HIV infection of primary CD4+ T cells resulted in a significant increase in TfR1 mRNA levels (Fig. 4A), while no significant changes were observed in the levels of ferroportin 1 (FPN1), the major cellular iron exporter (Fig. 4B). Consistently, we observed a significant increase in cellular iron content after HIV infection, as measured by intracellular 55Fe levels after incubating cells with 55Fe-nitrilotriacetic acid (NTA) for 48 h (Fig. 4C). These findings demonstrate that HIV infection results in cellular iron overload through increasing iron uptake.
FIG. 4.
HIV infection modulates iron levels in primary CD4+ T cells. mRNA levels of TfR1 (A) and ferroportin 1 (FPN1) (B) in CD4+ T cells in response to HIV infection. (C) Cellular 55Fe content in CD4+ T cells incubated with radioactive iron with or without HIV infection. N=6 for A and B and N=3–4 for C. *p<0.05.
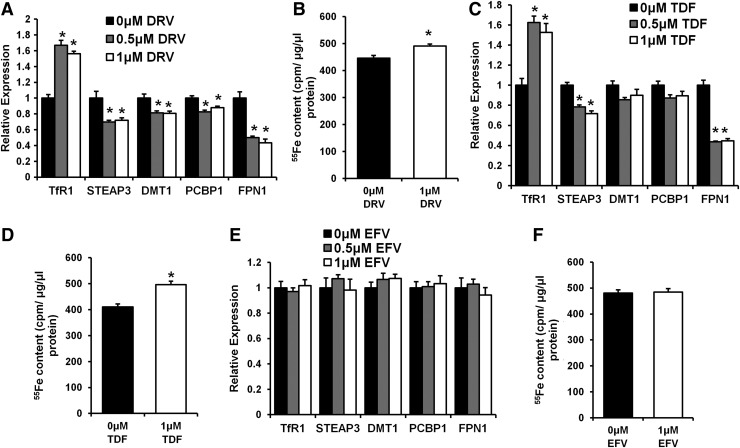
Limited numbers of studies examined the effect of ART on systemic and cellular iron and the data were not conclusive.16–18 Therefore, we next investigated the effect of three drugs from three different classes of ART—darunavir (protease inhibitor), efavirenz [non-nucleoside (tide) reverse transcriptase inhibitor (NNRTI)], and tenofovir [nucleoside (tide) reverse transcriptase inhibitor (NRTI)]—on cellular iron homeostasis. CD4+ T cells treated with darunavir or tenofovir alone without HIV infection had a significant increase in TfR1 mRNA levels and a concurrent reduction in FPN1 mRNA levels (Fig. 5A and 5C). Such changes resulted in a significant increase in cellular iron (Fig. 5B and 5D). On the other hand, efavirenz treatment did not alter cellular iron or the expression of iron regulatory genes (Fig. 5E and F). These findings show that some ART medications influence cellular iron independent of their effects on HIV virology.
FIG. 5.
Certain antiretroviral medications modulate cellular iron. Iron regulatory genes (A) and cellular iron levels (B) in CD4+ T cells treated with darunavir (DRV). Iron regulatory genes (C) and cellular iron levels (D) in CD4+ T cells treated with tenofovir (TDF). Iron regulatory genes (E) and cellular iron levels (F) in CD4+ T cells treated with efavirenz (EFV). N=6 for A–F. *p<0.05 vs. 0μM drug treatment.
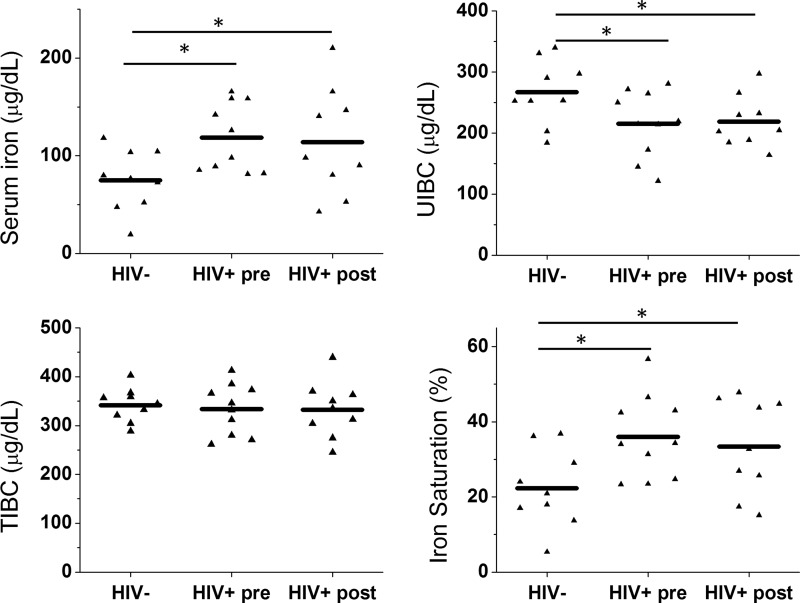
As our in vitro findings demonstrated that HIV independently influences cellular iron, we hypothesized that systemic iron levels are also altered in HIV patients. We analyzed iron parameters in serum samples of 10 age- and race-matched HIV-negative control and HIV-positive patients before ART therapies from the Multicenter AIDS Cohort Study. For HIV-positive patients, blood samples were drawn on average 4.2 years before the initiation of ART. Serum samples from HIV-positive patients prior to ART exposure displayed significant alteration in a number of iron parameters compared with HIV-negative controls, including a significant increase in serum iron level and transferrin saturation and a significant decrease in unsaturated iron-binding capacity (UIBC). Total iron-binding capacity (TIBC) did not differ between HIV-negative and HIV-positive samples (Fig. 6). The increase in iron saturation with HIV infection is consistent with an increase in systemic iron levels.
FIG. 6.
Antiretroviral therapy (ART) in HIV-positive patient does not correct the changes in serum iron profile associated with HIV infection. Serum iron, total iron-binding capacity (TIBC), unsaturated iron-binding capacity (UIBC), and iron saturation are measured in serum samples from HIV− control (HIV−) and HIV+ patients before (HIV+ pre) and after (HIV+ post) ART therapies. *p<0.05 vs. HIV−. N=9 in HIV− and HIV+ after ART. N=10 in HIV+ before ART.
Previous studies and our findings suggested that ART is capable of altering systemic and cellular iron, so we hypothesized that the changes in systemic iron persist even after ART treatment. To address this hypothesis, we measured iron parameters in the same HIV-positive patients on average 2.6 years after they were started on ART, at which time the serum viral load was below the detection limit (<50 copies ml−1). A summary of ART medications is included in Table 1. At the time of sample collection, all patients were on the same regimen for at least 6 months. Serum iron and iron saturation remained significantly elevated while UIBC remained significantly reduced in HIV-positive patients even after proper ART therapies (Fig. 6). Thus, HIV infection perturbs systemic iron homeostasis, and systemic iron derangement persists despite appropriate treatment with ART.
Table 1.
Antiviral Regimens of Study Subjects
| Treatment regimen | Count (% of total subjects) |
|---|---|
| Two NRTIs+two PIs | |
| TRU, RTV, ATZ | 2 (20%) |
| EPZ, RTV, ATZ | 1 (10%) |
| Two NRTIs+one PI | |
| TRU, RTV | 1 (10%) |
| Two PIs+one II | |
| RTV, DRV, IST | 1 (10%) |
| Two NRTIs+one NNRTI | |
| TDF, FTC, EFV | 3 (30%) |
| Two NRTIs+two PIs+one II | |
| TRU, RTV, DRV, IST | 1 (10%) |
| Two NRTIs+one II | |
| TRU, IST | 1 (10%) |
NRTI, nucleoside (tide) reverse trascriptase inhibitors; PI, protease inhibitors; II, integrase inhibitor; NNRTI, non-nucleoside (tide) reverse transcriptase inhibitors; TRU, truvada (combination of tenofovir and emtricitabine); RTV, ritonavir; ATZ, atazanavir; EPZ, epzicom (combination of abacavir and lamivudine); DRV, darunavir; IST, raltegravir; TDF, tenofovir; FTC, emtricitabine; EFV, efavirenz.
Our studies show that increased cellular iron facilitates both HIV infection and replication. Iron plays an important role in the synthesis of nucleic acids and is involved in DNA replication and repair.22 As a result, iron chelation might reduce the amount of available nucleic acids or directly impair viral protein function, both of which can decrease viral replication and release. Additionally, it is possible that iron chelation could downregulate the expression of adhesion molecules required for HIV attachment or proteins necessary for viral internalization. These changes in gene expression in response to a reduction in cellular iron can account for the protective effect of iron deprivation against HIV infection.
Increased intracellular iron content is required for the replication and pathogenesis of bacteria and fungus,2–4 and viruses may exhibit similar iron requirements for their replication. While HIV infection in primary CD4+ T cells resulted in only a modest change in cellular iron, even a small alteration in cellular iron and iron regulatory genes similar to our results has been shown to have significant physiological effects in other contexts.23–29 Normally, an increase in cellular iron inactivates iron regulatory proteins and decreases TfR1 mRNA levels. We observed increased TfR1 mRNA levels and a concurrent cellular iron overload with HIV infection, which suggests that HIV infection is driving the upregulation of TfR1 independent of other cellular iron regulatory machineries. This finding is contrary to other studies demonstrating that overexpression of HIV-1 Nef protein impairs the recycling of TfR1 to the cell surface,30,31 which would decrease cellular iron uptake and lead to cellular iron deficiency. However, one recent study suggested that the effect of Nef on TfR1 may be strain specific.32 The increased cellular iron levels observed in our studies may be a consequence of virus attempting to establish a favorable environment for its own replication. As tissue iron accumulation is seen in many HIV-associated diseases, including neurodegeneration and diastolic cardiac dysfunction,33–37 it is tempting to speculate that altered cellular iron homeostasis secondary to HIV infection may, at least partially, contribute to the organ damage associated with HIV infection.
Although ART medications are designed to target various aspects of HIV life cycles, many off-target effects have been described. NRTI such as tenofovir may target mitochondrial DNA polymerase γ and induce oxidative stress,38,39 and oxidative stress can lead to cellular iron overload.40 While mitochondrial toxicity is less associated with darunavir, various protease inhibitors were shown to inhibit mitochondrial proteases and cause oxidative stress.41,42 It is therefore tempting to speculate that these off-target effects may account for at least part of the alteration of cellular iron levels with HIV medications. However, these effects may be cell-type specific and require further investigation.
Systemic iron levels can change due to alterations in the absolute amount of iron in the blood or the serum iron-binding capacity. We observed an increase in systemic iron with HIV infection that persists despite proper ART treatment. A higher level of serum iron and a similar level of TIBC in HIV-positive patients suggest that HIV infection induces iron release from systemic iron storage organs and cells, such as the liver, intestine, and macrophages. The release of stored iron can be a direct effect of HIV virus on iron storage sites or an indirect consequence of HIV infection altering the expression of hepcidin, the major regulator of systemic iron. Altered hepcidin expression has been described in hepatitis B and hepatitis C infection,43,44 but observations of changes in hepcidin levels in HIV infection are conflicting. Hepcidin levels were shown to be increased in HIV infection with or without ART, which is consistent with reduced serum iron levels,45 but another study demonstrated reduced hepcidin levels in HIV-positive women, which can lead to increased iron release into the circulation.46 Our results are more consistent with reduced hepcidin levels leading to increased serum iron, although we did not measure hepcidin levels in our studies.
HIV infection is often associated with a heightened inflammatory state,47,48 and immune activation is a known modulator of cellular and systemic iron homeostasis.49–51 Increased immune activation in HIV patients is associated with lower hemoglobin levels,52 while ART treatment decreases immune activation and ameliorated the reduction of hemoglobin.53 Additionally, vitamin B deficiency during HIV infection can contribute to anemia in HIV-positive patients, and ART normalizes serum vitamin B12 levels.53,54 Our current study focused on serum iron level rather than hemoglobin level as many factors in addition to serum iron regulate the production of red blood cells. During acute infection and inflammation, increased hepcidin expression mediates the reduction of circulatory iron, which is considered a defensive mechanism.55 However, we observed an increase rather than a decrease in serum iron in HIV-positive patients, suggesting that HIV infection may influence systemic iron homeostasis independent of the immune activation state. This observation is also consistent with a recent report of increased serum iron levels in ART-naive HIV+ patients.56
Our results collectively demonstrate that altered iron status can have a profound impact on HIV infection and replication and that HIV infection influences both cellular and systemic iron levels. In addition, our data indicate that some ART drugs directly affect cellular iron homeostasis, and that HIV-induced changes in systemic iron levels persist despite adequate ART treatment. These observations establish a link between iron homeostasis and HIV infection in the post-ART era, and highlight iron regulation as a potential therapeutic target against HIV itself or HIV-associated pathologies that remain prevalent today.
Acknowledgments
We would like to thank Dr. Kelly Fahrbach for assistance in establishing the experiment system and Dr. Cheng-Shi Shiu for help with statistical analysis of human serum sample measurements. The patient serum samples were generous gifts of the Multicenter AIDS Cohort Studies. MACS centers: The Johns Hopkins Bloomberg School of Public Health (Joseph Margolick); Howard Brown Health Center and Northwestern University Medical School (John Phair, Steven Wolinsky); University of California, Los Angeles (Roger Detels, Oto Martinez-Maza); University of Pittsburgh (Charles Rinaldo); and Data Analysis Center (Lisa Jacobson). The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute and the National Heart, Lung, and Blood Institute: UO1-AI-35042, UL1-RR025005, UO1-AI-35043, UO1-AI-35039, UO1-AI-35040, and UO1-AI-35041. This work was supported by the Northwestern University Flow Cytometry Facility and a Cancer Center Support Grant (NCI CA060553). H.-C.C. is supported by the American Heart Association 12PRE12030002. H.A. is supported by NIH K02 HL107448, R01 HL104181, and 1PO1 HL108795.
Author Disclosure Statement
H.A. receives speaking honoraria from Merck and consulting honoraria from Takeda and Cubist Pharmaceuticals. B.T. has served as a consultant for ViiV Healthcare, Gilead Sciences, Pfizer, Janssen Pharmaceuticals, and GlazoSmithKline, and received research support through Northwestern University from ViiV Healthcare and Pfizer. F.J.P. is on the speakers' bureau and consults for the following commercial entities: Gilead Sciences, Bristol Myers Squibb, Janssen Pharmaceuticals, and Merck. G.P. and has participated in symposia organized by Gilead, Tibotec-Janssen BMS. The rest of authors declare no conflict of interests.
References
- 1.Crist MB, Melekhin VV, Bian A, et al. : Higher serum iron is associated with increased oxidant stress in HIV-infected men. J Acquir Immune Defic Syndr 2013;64(4):367–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foley TL. and Simeonov A: Targeting iron assimilation to develop new antibacterials. Expert Opin Drug Discov 2012;7(9):831–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porcheron G, Garenaux A, Proulx J, Sabri M, and Dozois CM: Iron, copper, zinc, and manganese transport and regulation in pathogenic Enterobacteria: Correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence. Front Cell Infect Microbiol 2013;3:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding C, Festa RA, Sun TS, and Wang ZY: Iron and copper as virulence modulators in human fungal pathogens. Mol Microbiol 2014;93(1):10–23 [DOI] [PubMed] [Google Scholar]
- 5.Drakesmith H. and Prentice A: Viral infection and iron metabolism. Nat Rev Microbiol 2008;6(7):541–552 [DOI] [PubMed] [Google Scholar]
- 6.Delanghe JR, Langlois MR, Boelaert JR, et al. : Haptoglobin polymorphism, iron metabolism and mortality in HIV infection. AIDS 1998;12(9):1027–1032 [PubMed] [Google Scholar]
- 7.de Monye C, Karcher DS, Boelaert JR, and Gordeuk VR: Bone marrow macrophage iron grade and survival of HIV-seropositive patients. AIDS 1999;13(3):375–380 [DOI] [PubMed] [Google Scholar]
- 8.Gordeuk VR, Delanghe JR, Langlois MR, and Boelaert JR: Iron status and the outcome of HIV infection: An overview. J Clin Virol 2001;20(3):111–115 [DOI] [PubMed] [Google Scholar]
- 9.Salhi Y, Costagliola D, Rebulla P, et al. : Serum ferritin, desferrioxamine, and evolution of HIV-1 infection in thalassemic patients. J Acquir Immune Defic Syndr Hum Retrovirol 1998;18(5):473–478 [DOI] [PubMed] [Google Scholar]
- 10.Xu M, Kashanchi F, Foster A, et al. : Hepcidin induces HIV-1 transcription inhibited by ferroportin. Retrovirology 2010;7(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nekhai S, Kumari N, and Dhawan S: Role of cellular iron and oxygen in the regulation of HIV-1 infection. Future Virol 2013;8(3):301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sappey C, Boelaert JR, Legrand-Poels S, Forceille C, Favier A, and Piette J: Iron chelation decreases NF-kappa B and HIV type 1 activation due to oxidative stress. AIDS Res Hum Retroviruses 1995;11(9):1049–1061 [DOI] [PubMed] [Google Scholar]
- 13.Debebe Z, Ammosova T, Breuer D, et al. : Iron chelators of the di-2-pyridylketone thiosemicarbazone and 2-benzoylpyridine thiosemicarbazone series inhibit HIV-1 transcription: Identification of novel cellular targets–iron, cyclin-dependent kinase (CDK) 2, and CDK9. Mol Pharmacol 2011;79(1):185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debebe Z, Ammosova T, Jerebtsova M, et al. : Iron chelators ICL670 and 311 inhibit HIV-1 transcription. Virology 2007;367(2):324–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucas J, Szepesi A, Domenico J, et al. : Effects of iron-depletion on cell cycle progression in normal human T lymphocytes: Selective inhibition of the appearance of the cyclin A- associated component of the p33cdk2 kinase. Blood 1995;86(6):2268–2280 [PubMed] [Google Scholar]
- 16.Dryden-Peterson S, Shapiro RL, Hughes MD, et al. : Increased risk of severe infant anemia after exposure to maternal HAART, Botswana. J Acquir Immune Defic Syndr 2011;56(5):428–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boom J, Kosters E, Duncombe C, et al. : Ferritin levels during structured treatment interruption of highly active antiretroviral therapy. HIV Med 2007;8(6):388–395 [DOI] [PubMed] [Google Scholar]
- 18.Furukawa Y, Hashiguchi T, Minami R, Yamamoto M, and Takashima H: Exacerbation of microcytic anemia associated with cessation of anti-retroviral therapy in an HIV-1-infected patient with beta thalassemia. J Infect Chemother 2014;20(6):387–389 [DOI] [PubMed] [Google Scholar]
- 19.Rao K, Harford JB, Rouault T, McClelland A, Ruddle FH, and Klausner RD: Transcriptional regulation by iron of the gene for the transferrin receptor. Mol Cell Biol 1986;6(1):236–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klausner RD, Rouault TA, and Harford JB: Regulating the fate of mRNA: The control of cellular iron metabolism. Cell 1993;72(1):19–28 [DOI] [PubMed] [Google Scholar]
- 21.Ponka P. and Lok CN: The transferrin receptor: Role in health and disease. Int J Biochem Cell Biol 1999;31(10):1111–1137 [DOI] [PubMed] [Google Scholar]
- 22.Bayeva M, Chang HC, Wu R, and Ardehali H: When less is more: Novel mechanisms of iron conservation. Trends Endocrinol Metab 2013;24(11):569–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayeva M, Khechaduri A, Puig S, et al. : mTOR regulates cellular iron homeostasis through tristetraprolin. Cell Metab 2012;16(5):645–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbeito AG, Levade T, Delisle MB, Ghetti B, and Vidal R: Abnormal iron metabolism in fibroblasts from a patient with the neurodegenerative disease hereditary ferritinopathy. Mol Neurodegene 2010;5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fillebeen C. and Pantopoulos K: Hepatitis C virus infection causes iron deficiency in Huh7.5.1 cells. PLoS One 2013;8(12):e83307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jellen LC, Lu L, Wang X, et al. : Iron deficiency alters expression of dopamine-related genes in the ventral midbrain in mice. Neuroscience 2013;252:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rathnasamy G, Ling EA, and Kaur C: Hypoxia inducible factor-1alpha mediates iron uptake which induces inflammatory response in amoeboid microglial cells in developing periventricular white matter through MAP kinase pathway. Neuropharmacology 2014;77:428–440 [DOI] [PubMed] [Google Scholar]
- 28.Yang L, Fan M, Du F, et al. : Hypoxic preconditioning increases iron transport rate in astrocytes. Biochim Biophys Acta 2012;1822(4):500–508 [DOI] [PubMed] [Google Scholar]
- 29.Yu X, Du T, Song N, et al. : Decreased iron levels in the temporal cortex in postmortem human brains with Parkinson disease. Neurology 2013;80(5):492–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madrid R, Janvier K, Hitchin D, et al. : Nef-induced alteration of the early/recycling endosomal compartment correlates with enhancement of HIV-1 infectivity. J Biol Chem 2005;280(6):5032–5044 [DOI] [PubMed] [Google Scholar]
- 31.Drakesmith H, Chen N, Ledermann H, Screaton G, Townsend A, and Xu X-N: HIV-1 Nef down-regulates the hemochromatosis protein HFE, manipulating cellular iron homeostasis. Proc Natl Acad Sci USA 2005;102(31):11017–11022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koppensteiner H, Hohne K, Gondim M, et al. : Lentiviral Nef suppresses iron uptake in a strain specific manner through inhibition of transferrin endocytosis. Retrovirology 2014;11(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Remick J, Georgiopoulou V, Marti C, et al. : Heart failure in patients with human immunodeficiency virus infection: Epidemiology, pathophysiology, treatment, and future research. Circulation 2014;129(17):1781–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brew BJ, Crowe SM, Landay A, Cysique LA, and Guillemin G: Neurodegeneration and ageing in the HAART era. J Neuroimmune Pharmacol 2009;4(2):163–174 [DOI] [PubMed] [Google Scholar]
- 35.Kremastinos DT. and Farmakis D: Iron overload cardiomyopathy in clinical practice. Circulation 2011;124(20):2253–2263 [DOI] [PubMed] [Google Scholar]
- 36.Ke Y. and Qian ZM: Iron misregulation in the brain: A primary cause of neurodegenerative disorders. Lancet Neurol 2003;2(4):246–253 [DOI] [PubMed] [Google Scholar]
- 37.Zecca L, Youdim MBH, Riederer P, Connor JR, and Crichton RR: Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci 2004;5(11):863–873 [DOI] [PubMed] [Google Scholar]
- 38.Kohler JJ, Hosseini SH, Hoying-Brandt A, et al. : Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Lab Invest 2009;89(5):513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canale D, de Bragança AC, Gonçalves JG, et al. : Vitamin D deficiency aggravates nephrotoxicity, hypertension and dyslipidemia caused by tenofovir: Role of oxidative stress and renin-angiotensin system. PLoS One 2014;9(7):e103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tampo Y, Kotamraju S, Chitambar CR, et al. : Oxidative stress–induced iron signaling is responsible for peroxide-dependent oxidation of dichlorodihydrofluorescein in endothelial cells: Role of transferrin receptor–dependent iron uptake in apoptosis. Circ Res 2003;92(1):56–63 [DOI] [PubMed] [Google Scholar]
- 41.Mukhopadhyay A, Wei B, Zullo SJ, Wood LV, and Weiner H: In vitro evidence of inhibition of mitochondrial protease processing by HIV-1 protease inhibitors in yeast: A possible contribution to lipodystrophy syndrome. Mitochondrion 2002;1(6):511–518 [DOI] [PubMed] [Google Scholar]
- 42.Chandra S, Mondal D, and Agrawal KC: HIV-1 protease inhibitor induced oxidative stress suppresses glucose stimulated insulin release: Protection with thymoquinone. Exp Biol Med 2009;234(4):442–453 [DOI] [PubMed] [Google Scholar]
- 43.Girelli D, Pasino M, Goodnough JB, et al. : Reduced serum hepcidin levels in patients with chronic hepatitis C. J Hepatol 2009;51(5):845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin D, Ding J, Liu J-Y, et al. : Decreased serum hepcidin concentration correlates with brain iron deposition in patients with HBV-related cirrhosis. PLoS One 2013;8(6):e65551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Armitage AE, Stacey AR, Giannoulatou E, et al. : Distinct patterns of hepcidin and iron regulation during HIV-1, HBV, and HCV infections. Proc Natl Acad Sci USA 2014;111(33):12187–12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malvoisin E, Makhloufi D, and Livrozet J-M: Serum hepcidin levels in women infected with HIV-1 under antiviral therapy. J Med Virol 2014;86(10):1656–1660 [DOI] [PubMed] [Google Scholar]
- 47.Shrestha S, Irvin MR, Grunfeld C, and Arnett DK: HIV, inflammation, and calcium in atherosclerosis. arteriosclerosis, thrombosis, and vascular biology. Arterioscler Thromb Vasc Biol 2014;34(2):244–250 [DOI] [PubMed] [Google Scholar]
- 48.Sandler NG. and Sereti I: Can early therapy reduce inflammation? Curr Opin HIV AIDS 2014;9(1):72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiss G: Modification of iron regulation by the inflammatory response. Best Pract Res Clin Haematol 2005;18(2):183–201 [DOI] [PubMed] [Google Scholar]
- 50.Weiss G: Iron and immunity: A double-edged sword. Eur J Clin Invest 2002;32:70–78 [DOI] [PubMed] [Google Scholar]
- 51.Nairz M, Haschka D, Demetz E, and Weiss G: Iron at the interface of immunity and infection. Front Pharmacol 2014;5:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuchs D, Zangerle R, Artner-Dworzak E, et al. : Association between immune activation, changes of iron metabolism and anaemia in patients with HIV infection. Eur J Haematol 1993;50(2):90–94 [DOI] [PubMed] [Google Scholar]
- 53.Sarcletti M, Quirchmair G, Weiss G, Fuchs D, and Zangerle R: Increase of haemoglobin levels by anti-retroviral therapy is associated with a decrease in immune activation. Eur J Haematol 2003;70(1):17–25 [DOI] [PubMed] [Google Scholar]
- 54.Hepburn MJ, Dyal K, Runser LA, Barfield RL, Hepburn LM, and Fraser SL: Low serum vitamin B12 levels in an outpatient HIV-infected population. Int J STD AIDS 2004;15(2):127–133 [DOI] [PubMed] [Google Scholar]
- 55.Drakesmith H. and Prentice AM: Hepcidin and the iron-infection axis. Science 2012;338(6108):768–772 [DOI] [PubMed] [Google Scholar]
- 56.Banjoko SO, Oseni F, Togun R, Onayemi O, Emma-Okon B, and Fakunle J: Iron status in HIV-1 infection: Implications in disease pathology. BMC Clin Pathol 2012;12(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]