Abstract
Systemic inflammation and immune activation may persist in HIV-infected persons on suppressive combination antiretroviral therapy (cART) and contribute to adverse health outcomes. We compared markers of immune activation, inflammation, and abnormal glucose and lipid metabolism in HIV-infected adults according to herpes simplex virus type 2 (HSV-2) serostatus in a 6-month observational cohort study in Toronto, Canada. HIV-infected adults on suppressive (viral load <50 copies/ml) cART were categorized as HSV-2 seropositive or seronegative using the HerpeSelect ELISA, and underwent study visits at baseline, 3 months, and 6 months. The primary outcome was the median percentage of activated (CD38+HLADR+) CD8 T cells. Secondary outcome measures included additional immune (activated CD4, regulatory T cells) and inflammatory (hsCRP, D-dimer, IL-1b, IL-6, MCP-1, TNF, sICAM-1, sVCAM-1, Ang1/Ang2 ratio) markers. Metabolic outcomes included the proportion with impaired fasting glucose/impaired glucose tolerance/diabetes, insulin sensitivity (calculated using the Matsuda index), insulin resistance (homeostasis model assessment of insulin resistance), and fasting lipids. The impact of HSV-2 on each outcome was estimated using generalized estimating equation regression models. Of 84 participants, 38 (45%) were HSV-2 seropositive. HSV signs and symptoms were uncommon. Aside from D-dimer, which was more often detectable in HSV-2 seropositives (adjusted odds ratio=3.58, 95% CI=1.27, 10.07), HSV-2 serostatus was not associated with differences in any other immune, inflammatory cytokine, acute phase reactant, endothelial activation, or metabolic markers examined in univariable or multivariable models. During the study, CD8 and CD4 T cell activation declined by 0.16% and 0.08% per month, respectively, while regulatory T cells increased by 0.05% per month. HSV-2 serostatus was not consistently associated with immune activation, inflammatory, or lipid and glucose metabolic markers in this cohort of HIV-infected adults on suppressive cART.
Introduction
HIV infection is characterized by chronic immune activation and systemic inflammation that are incompletely reversed by virologically suppressive combination antiretroviral therapy (cART).1 This systemic inflammatory response may contribute not only to HIV disease progression, but also to non-AIDS-related morbidity and mortality.2 For instance, inflammation may be a contributor to cardiovascular disease in HIV-infected persons, either directly or mediated through abnormal glucose and lipid metabolism. There is considerable interest in identifying underlying drivers and amplifiers of HIV-associated inflammation, as such knowledge could be harnessed to develop novel adjunctive treatment strategies for patients.
Herpes simplex virus type 2 (HSV-2) is a common coinfection found in more than half of HIV-infected adults,3,4 for which safe, affordable antiviral medications exist. Although we recently observed no significant impact of valacyclovir on attenuating inflammation in a randomized trial among HIV/HSV-2 coinfected adults,5 it remains unclear whether HSV-2 infection could nevertheless be a clinically important cause of HIV-related inflammation.
This complementary prospective cohort study therefore sought to determine whether HSV-2 coinfection is associated with increased immune activation and systemic inflammation, as well as abnormal glucose and lipid metabolism in HIV-infected adults on suppressive cART.
Materials and Methods
Objectives
The primary objective was to compare the median percentage of activated CD8+ T cells according to HSV-2 serostatus. Secondary analyses compared additional markers of immune activation, inflammatory cytokines, acute phase reactants, endothelial activation markers, glucose metabolism, and fasting lipids among HSV-2 seropositive and seronegative participants.
Study participants
HIV-infected adults were prospectively recruited from two tertiary care clinics in Toronto, Canada. Eligibility criteria included sustained plasma HIV RNA <50 copies/ml on cART for ≥12 months, absence of opportunistic infection for ≥12 months, and absence of recent (within 6 months) or anticipated chronic anti-HSV therapy during the course of the study. Individuals were excluded if they had active hepatitis B or C, had known previous cardiovascular events, were pregnant, or were receiving chemotherapy or immunomodulatory medications because the sample size was unlikely to be able to adequately account for these potential confounders.
Study procedures
Study participants underwent serial measurement of inflammatory biomarkers and fasting lipids [total/high-density lipoprotein (T/HDL) ratio, low-density lipoprotein (LDL), apolipoprotein B] at baseline, 3 months, and 6 months. At the baseline and 6-month visits, participants also underwent measurement of fasting blood glucose and fasting insulin levels, followed by a 75g oral glucose tolerance test (OGTT). HSV-2 status was determined by HerpeSelect gG-1 and gG-2 ELISA (Focus Technologies, Cypress, CA), with primary analyses employing the manufacturer's recommended index value threshold of 1.1 for defining seropositivity. Additional demographic, clinical, and laboratory data were obtained via interview and review of medical records. Written informed consent was obtained from all participants. The study was approved by the Research Ethics Boards of the University Health Network and St. Michael's Hospital.
T cell activation and regulatory T cells
Peripheral blood mononuclear cells (PBMCs) were cryopreserved at −150°C for batch testing. Samples were thawed and incubated overnight at 37°C in 5% carbon dioxide and then 1 million PBMCs were stained for T cell activation and regulatory T cells (Tregs). PBMCs were stained with CD3-PE, CD4-APC, CD8-V450 (BD Biosciences), CD25-PerCP-Cy5.5, HLADR-PE-Cy7, CD38-AF700 (eBioscience), and Live/Dead Aqua (Invitrogen), followed by a 30-min incubation with Fixation/Permeabilization (eBioscience), washed, and then stained for FoxP3-AF488. T cells were enumerated using LSR-2 flow cytometry (BD Biosciences) and analyzed using FlowJo 9.0 (Treestar). T cell subsets were defined as follows: expression of CD38+HLADR+ for activation and CD4+FoxP3+CD25+ for Tregs. Flow gates were drawn manually by blinded personnel.
Plasma biomarkers of inflammation and endothelial activation
Plasma was stored at −80°C prior to batch testing. Plasma concentrations (dilution factor) of IL-1β (1:2), IL-6 (1:2), MCP-1 (1:2), TNF (1:2), hsCRP(1:5,000), D-dimer (1:2), Ang-1 (1:5), Ang-2 (1:5), sICAM (1:1,000), and sVCAM (1:2,000) were measured by ELISA (DiaPharma, West Chester, OH for D-Dimer; R&D Systems, Minneapolis, MN for all others) according to the manufacturers' instructions, with the following changes (for all but D-dimer): assays were performed in volumes of 50 μl/well, plasma samples were incubated overnight at 4°C, and ELISAs were developed using extravidin-alkaline phosphatase (Sigma, 1:1,000 dilution, 45 min incubation) followed by the addition of p-nitrophenyl phosphate substrate (Sigma) and optical density readings at 405 nm. Concentrations were interpolated from 4-parameter-fit standard curves. Background levels were determined from blank wells included on each plate. Samples with optical densities below the lowest detectable standard were assigned the value of that standard. Lower limits of detection were as follows: IL-1β (0.98 pg/ml), IL-6 (2.34 pg/ml), MCP-1 (1.95 pg/ml), TNF (3.91 pg/ml), hsCRP (9.77 pg/ml), D-dimer (20 pg/ml), Ang-1 (39.07 pg/ml), Ang-2 (13.67 pg/ml), sICAM (7.81 pg/ml), and sVCAM (3.91 pg/ml).
Metabolic outcomes
Insulin sensitivity was quantified using the Matsuda index (=10,000/square root) [(fasting glucose×fasting insulin)×(mean glucose×mean insulin)] and insulin resistance using the homeostasis model assessment of insulin resistance (HOMA-IR) index.6 Definitions of diabetes and prediabetes and criteria for lipid-lowering therapy were applied as per 2013 Canadian guidelines.7,8
Statistical analysis
Generalized estimating equation (GEE) models using exchangeable correlation matrices and logit or identity link functions as appropriate were used to evaluate the effect of HSV-2 serostatus on the level of each outcome over time.9 The immune, inflammatory and metabolic markers were treated as continuous variables (with logarithmic transformations as appropriate) in linear regression models or as binary variables (detectable versus undetectable) in logistic regression models. Covariates included in multivariable analyses were selected due to prior literature suggesting them to have potential relevance to HIV-associated inflammation. For the immune and inflammatory outcomes, these included sex, duration of HIV infection, baseline CD4 cell count, and the number of days since the baseline visit (considered as a continuous variable); for the metabolic outcomes these included sex, duration of HIV infection, and time since baseline only.
Because some studies have recommended higher laboratory thresholds for defining HSV-2 seropositivity among HIV patients using the HerpeSelect 2 ELISA,10,11 sensitivity analyses were conducted using an index value cutoff of 3.5; participants with results equal to or less than 3.5 were excluded from this analysis. Statistical analyses were performed using SAS Version 9.3 (SAS Institute Inc., Cary, NC).
Sample size considerations
The enrollment target was 90 individuals, including provisions for 10% dropout. We assumed roughly equal group sizes, based on previous work documenting HSV-2 seroprevalence of 54.5% in Canadian HIV clinics.3 Using equations that account for correlation between repeated measures (rho, estimated at 0.5) and within-patient variance in the primary outcome measure (s2x, estimated at 1),12 a resulting sample size of 80 participants was expected to be able to detect a difference in the percentage of activated CD8+ T cells of 1.9% between HSV-2 seropositive and seronegative participants.
Results
Of 100 screened individuals, six did not meet eligibility criteria (two for anti-HSV therapy, two for detectable HIV RNA, one each for hepatitis B and cardiovascular disease), five withdrew consent, and five were lost to follow-up, leaving 84 participants in the analysis. Most (87%) were men, 70 (83%) were HSV-1 coinfected, and the median (interquartile range, IQR) baseline CD4 count was 493 (329, 622) cells/mm3. The majority of baseline characteristics were balanced between the 38 HSV-2 coinfected and 46 uninfected participants, except that female sex and black race were more common among HSV-2 seropositives (Table 1).
Table 1.
Participant Characteristics
| HSV-2 negative n=46 | HSV-2 positive n=38 | p | Test | |
|---|---|---|---|---|
| Demographics | ||||
| Age | 49 (40–53) | 49 (45–56) | 0.25 | Wilcoxon |
| Male | 45 (98%) | 28 (74%) | <0.01 | Fisher |
| Race | 0.04 | Fisher | ||
| White | 33 (72%) | 19 (50%) | ||
| Black | 4 (9%) | 12 (32%) | ||
| Asian | 6 (13%) | 3 (8%) | ||
| Other | 3 (7%) | 4 (11%) | ||
| Smoking status | 0.21 | Chi-square | ||
| Never | 17 (37%) | 15 (41%) | ||
| Former | 10 (22%) | 13 (35%) | ||
| Current | 19 (41%) | 9 (24%) | ||
| HIV-related variables | ||||
| HIV risk factors | Fisher | |||
| MSM | 33 (72%) | 25 (66%) | 0.56 | |
| Heterosexual | 15 (33%) | 17 (45%) | 0.25 | |
| Other | 9 (20%) | 3 (8%) | 0.13 | |
| Years since HIV Dx | 10 (7–18) | 12 (7–19) | 0.68 | Wilcoxon |
| Years since first ARV drug use | 9 (5–13) | 10 (3–15) | 0.89 | Wilcoxon |
| Years since first cART | 7 (4–12) | 8 (3–13) | 0.81 | Wilcoxon |
| Years on current regimen | 3 (1–4) | 2 (1–3) | 0.11 | Wilcoxon |
| Baseline regimen | 0.29 | Fisher | ||
| 2 NRTIs+NNRTI | 28 (61%) | 16 (43%) | ||
| 2 NRTIs+PI | 8 (17%) | 8 (22%) | ||
| Other | 10 (22%) | 13 (35%) | ||
| Baseline CD4 | Wilcoxon | |||
| Cell/mm3 | 466 (329–640) | 478 (342–621) | 0.92 | |
| Percentage | 29 (26–34) | 32 (22–37) | 0.87 | |
| Viral load <50 copies/ml | 46 (100%) | 35 (95%)a | 0.20 | Fisher |
| HSV-related variables | ||||
| HSV-1 seropositive | 39 (85%) | 31 (89%) | 0.75 | Fisher |
| Prior history of HSV symptoms | 25 (54%) | 17 (50%) | 0.70 | Chi-square |
| Clinically diagnosed comorbidities | ||||
| Hypertension | 7 (15%) | 7 (19%) | 0.65 | Chi-square |
| Hyperlipidemia | 12 (26%) | 10 (27%) | 0.92 | Chi-square |
| Diabetes | 1 (2%) | 3 (8%) | 0.32 | Fisher |
| Asthma | 4 (9%) | 2 (5%) | 0.69 | Fisher |
| Otherb | 4 (9%) | 1 (3%) | 0.37 | Fisher |
Does not equal 100% because participants with viral blips (defined as isolated low-level elevations in viral load no greater than 500 copies/ml that are both preceded and followed by viral load values <50 copies/ml) were eligible.
Other comorbidities: atrial fibrillation, chronic kidney disease, inflammatory bowel disease, tricuspid valvulopathy for HSV-2 negatives; Hashimoto's thyroiditis on thyroid replacement therapy for HSV-2 positive.
HSV-2, herpes simplex virus type 2; MSM, men who have sex with men; ARV, antiretroviral; cART, combination antiretroviral therapy; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Signs and symptoms of herpes reactivation (oral or anogenital burning, itching, or compatible skin changes) were uncommon during the study. Oral lesions consistent with HSV were identified on physical examination in three participants (4%) at baseline and one (1%) at the 6-month visit; no anogenital lesions were observed. Possible HSV-related symptoms were reported by only 11 participants (13%) between the baseline and 3-month visits and 12 (14%) between the 3- and 6-month visits; only one participant received short-course anti-HSV medication during the study.
At baseline, the median (IQR) level of the primary outcome measure, the percentage of activated (CD38+HLADR+) CD8+ T cells was 5.37% (3.64%, 7.88%) for the study cohort. Over the 6 months of the study, HSV-2 serostatus was not associated with this outcome in univariate or multivariable GEE models (Table 2). Similarly, HSV-2 coinfection was not associated with differences in any of the other immune parameters, inflammatory cytokines, endothelial activation markers, and acute phase reactants, aside from D-dimer, which was more likely to be detectable in HSV-2 seropositive participants (adjusted odds ratio, aOR=3.58, 95% confidence interval 1.27, 10.07) (Table 2).
Table 2.
Baseline Levels of Immune/Inflammatory Markers and Associations with Herpes Simplex Virus Type 2 Serostatus
| Parameter estimate—univariate modela | Parameter estimate—mult ivariable modelb | ||||
|---|---|---|---|---|---|
| Median baseline values (IQR) | (95% CI) | p | (95% CI) | p | |
| Immune outcomes | |||||
| CD38+CD8+ (%) | 6.77 (4.35, 9.16) | −0.02 (−1.33,1.29) | 0.98 | 0.26 (−1.20, 1.72) | 0.72 |
| CD38+CD4+ (%) | 2.06 (1.05, 3.28) | 0.00 (−0.47,0.47) | 0.99 | 0.05 (−0.45, 0.54) | 0.85 |
| Tregs (%) | 1.92 (1.34, 2.66) | 0.09 (−0.41,0.58) | 0.73 | 0.05 (−0.45, 0.56) | 0.84 |
| Soluble outcomes | |||||
| Ang1/Ang2 log10 | 1.03 (0.68, 1.32) | 0.13 (−0.12, 0.37) | 0.30 | 0.15 (−0.11, 0.40) | 0.26 |
| CRP (μg/ml) log10 | 0.03 (−0.41, 0.39) | 0.11 (−0.14, 0.36) | 0.40 | 0.06 (−0.17, 0.30) | 0.59 |
| D-Dimerc | 63 (75%) | 3.29 (1.30, 8.36) | 0.01 | 3.58 (1.27, 10.07) | 0.02 |
| sICAM (pg/ml) log10 | 5.08 (4.96, 5.22) | 0.04 (−0.04, 0.12) | 0.31 | 0.06 (−0.01,0.13) | 0.09 |
| IL-1βc | 13 (15%) | 0.95 (0.31, 2.98) | 0.94 | 1.01 (0.32, 3.18) | 0.98 |
| IL-6c | 16 (19%) | 0.84 (0.30, 2.36) | 0.75 | 0.83 (0.28, 2.48) | 0.74 |
| MCP-1c | 39 (46%) | 0.56 (0.25, 1.22) | 0.14 | 0.55 (0.24, 1.26) | 0.16 |
| TNFc | 16 (19%) | 0.96 (0.34, 2.71) | 0.94 | 0.85 (0.27, 2.61) | 0.77 |
| sVCAM (pg/ml) log10 | 5.43 (5.34, 5.57) | 0.05 (−0.03, 0.12) | 0.23 | 0.06 (−0.01, 0.13) | 0.11 |
Univariate parameter estimates estimate the relationship between HSV-2 serostatus and each outcome variable.
Multivariable parameter estimates estimate the relationship between HSV-2 serostatus and each outcome variable after adjustment for time, years since HIV diagnosis, baseline CD4, and sex.
Odds ratio of having a detectable value is presented.
The baseline burden of metabolic disease in the study cohort was moderate. Four individuals had a prior diagnosis of diabetes (including three HSV-2 seropositive participants). OGTT identified an additional three participants meeting criteria for diabetes, eight with impaired glucose tolerance and six with impaired fasting glucose. Nineteen participants were on lipid-lowering therapy at enrollment, and baseline lipid measurements identified 14 additional participants meeting the criteria for such therapy. The distribution of these glucose and lipid abnormalities at baseline was not significantly different between HSV-2 groups. Furthermore, no significant associations were observed between HSV-2 serostatus and any of the metabolic outcomes over the course of the study in GEE models (data not shown).
Immune, inflammatory cytokine, endothelial activation, acute phase reactant, and metabolic markers were qualitatively unchanged in sensitivity analyses employing a more stringent definition of HSV-2 seropositivity (data not shown), except that HSV-2 was no longer significantly associated with detectable D-dimer levels (aOR=1.97, 95% CI=0.70, 5.55).
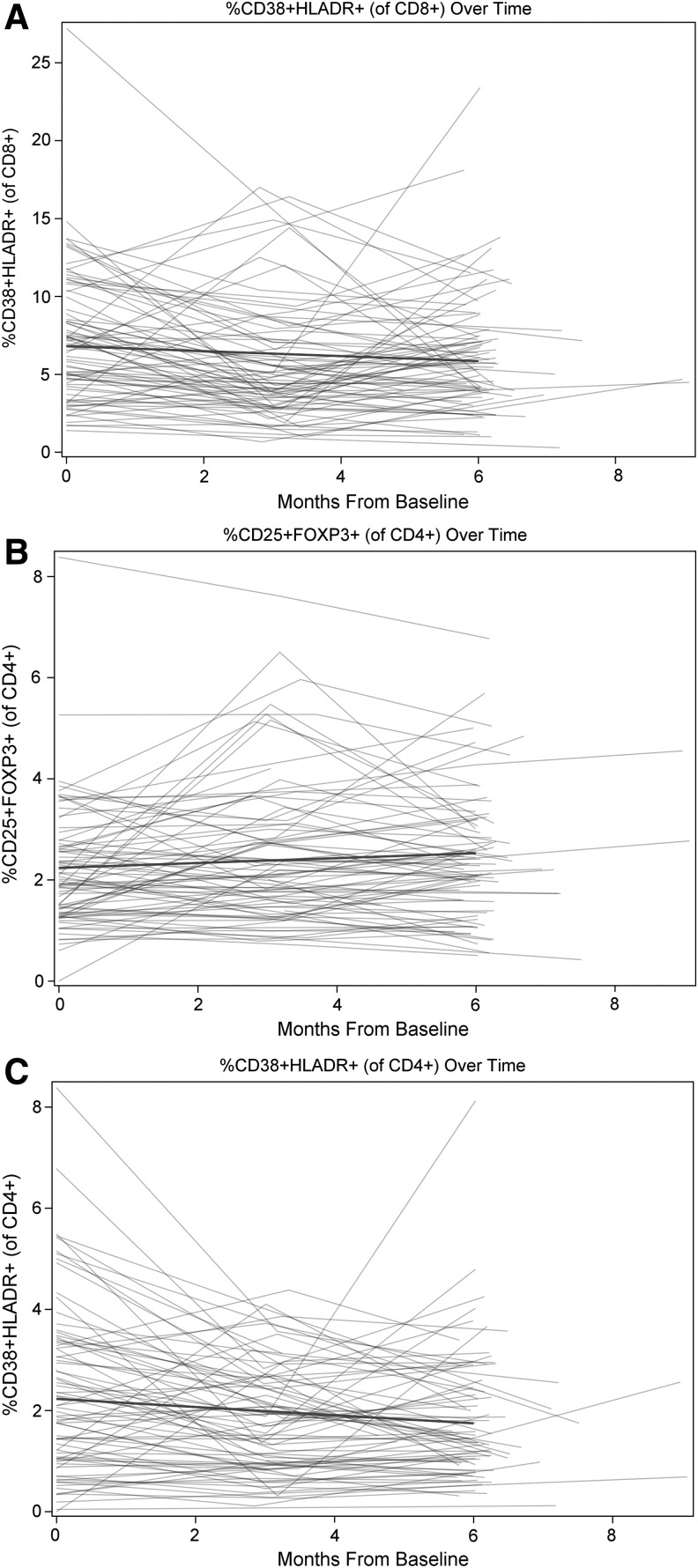
In post hoc analyses, we further explored whether immune and inflammatory parameters changed over time in univariable models (Fig. 1), and observed statistically significant reductions of −0.08 (−0.14, −0.02; p<0.01) and −0.16 (−0.31, −0.01; p=0.04) per month in the percentage of CD38+CD4+ and CD38+CD8+ T cells, respectively, and of −0.02 (−0.05, −0.00; p=0.04) in log10 hsCRP levels (μg/ml). A concomitant increase of 0.05 (0.02, 0.08; p<0.01) was also seen in the percentage of Tregs.
FIG. 1.
Immune parameters changed over time in this cohort of HIV-infected adults on suppressive antiretroviral therapy. The percentage of CD38+CD8+ T cells declined by −0.16 (−0.31, −0.01) per month (A), the percentage of CD38+CD4+ T cells declined by −0.08 (−0.14, −0.02) per month (B), and the percentage of Tregs increased by 0.05 (0.02, 0.08) per month (C).
Discussion
In this 6-month prospective cohort of 84 HIV-infected adults on suppressive cART, we generally observed no association between HSV-2 serostatus and various measures of immune activation, inflammatory cytokines, acute phase reactants, endothelial activation markers, glucose metabolism, and fasting lipids. While D-dimers were more often detectable in HSV-2 coinfected participants, this relationship was not maintained in sensitivity analyses employing a stricter definition of HSV-2 seropositivity. That HSV-2 was not associated with these inflammatory and metabolic suggests that HSV-2 therapies are unlikely to be useful in reducing cardiovascular risk in this context.
One possible explanation for these generally null results is that study participants had relatively inactive HSV-2 infection, as evidenced by the low frequency of clinical reactivations reported by participants and identified by study clinicians. Indeed, the natural history of HSV-2 involves a decreasing frequency of clinical reactivation over time.13–15 If a relationship exists between HSV-2 and increased inflammation, it might more readily be observed in patients experiencing more frequent subclinical or clinical reactivations, such as those with recently acquired HSV-2 or persons initiating cART.16,17
A related possible explanation may be that long-term cART use in the study cohort had already attenuated inflammation to the point that any incremental effect of HSV-2 infection was minimal. Prior work has shown HSV-2 infection to be associated with increased T cell activation among HIV-infected patients who are cART naive.18 The small decrements we observed in the percentage of CD38+CD4+ and CD38+CD8+ T cells over time in this study support the notion that time on cART alone reduces immune activation.
A third potential explanation for our findings could relate to the anatomic compartmentalization of HSV-2. In contrast to related herpesviruses such as cytomegalovirus, which is harbored in numerous cell types including circulating leukocytes and has been linked to increased T cell activation in HIV-infected persons particularly during long-term cART,19 HSV-2 infects only neurons innervating the genital and oral mucosae. While HSV-2 is associated with the recruitment of dendritic cells and activated CD4+ to the genital mucosa,20 it simply may not trigger a measurable systemic inflammatory response.
This study has limitations that warrant consideration. First, our sample size was sufficient to detect a difference of 1.9% in CD38+CD8+ T cells; hence we cannot exclude the possibility of a small impact of HSV-2 on some of the markers tested. Second, the degree of observed immune activation was lower than that upon which the sample size was based, so our ability to detect small changes was limited. Third, exclusion of patients with recent or anticipated chronic anti-HSV therapy may have selected for patients with less active herpes infection. Fourth, 83% of the participants were HSV-1 seropositive, although how this coinfection may have affected our results is unclear. Fifth, vaccinations and intercurrent illnesses did not preclude study participation and could have transiently increased inflammation. Finally, while we selected a range of biomarkers relevant to HIV pathogenesis, it is possible that HSV-2 may have effects on inflammatory pathways other than those studied herein.
The search for clinically relevant drivers and amplifiers of HIV-associated inflammation has proven challenging, with numerous interventional studies showing minimal therapeutic benefit.21–23 Prior work has suggested that HSV-2 might be a common and readily treatable instigator of such inflammation. We recently evaluated the effect of valacyclovir for attenuating inflammation among HIV, HSV-2 coinfected adults and observed no significant impact of the drug.5 Findings from the current study suggest that a likely explanation for those trial results is the lack of a significant underlying association between relatively inactive HSV-2 and systemic inflammation among cART-treated individuals.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research (CIHR) [grant number HC1-112573]. D.H.S.T. was supported by research fellowships from the CIHR and Ontario HIV Treatment Network (OHTN) during the conduct of this work. J.M.R., R.K., and S.L.W. were supported by Career Scientist Awards from the OHTN, T.J.Y. by a studentship from the OHTN, B.S. by a studentship from the CIHR, and W.C.L. by a Canada Research Chair in Infectious Diseases and Inflammation from CIHR.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hunt PW, Martin JN, Sinclair E, et al. : T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003;187:1534–1543 [DOI] [PubMed] [Google Scholar]
- 2.El-Sadr WM, Lundgren JD, Neaton JD, et al. : CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006;355:2283–2296 [DOI] [PubMed] [Google Scholar]
- 3.Romanowski B, Myziuk LN, Walmsley SL, et al. : Seroprevalence and risk factors for herpes simplex virus infection in a population of HIV-infected patients in Canada. Sex Transm Dis 2009;36:165–169 [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Warren T, and Wald A: Genital herpes. Lancet 2008;370:2127–2137 [DOI] [PubMed] [Google Scholar]
- 5.Yi TJ, Walmsley S, Szadkowski L, et al. : A randomized controlled pilot trial of valacyclovir for attenuating inflammation and immune activation in HIV/herpes simplex virus 2-coinfected adults on suppressive antiretroviral therapy. Clin Infect Dis 2013;57:1331–1338 [DOI] [PubMed] [Google Scholar]
- 6.Matthews DR, Hosker JP, Rudenski AS, et al. : Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg R. and Punthakee Z: Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Pharmacologic management of type 2 diabetes. Can J Diabetes 2013;37(Suppl 1):S61–S6824070965 [Google Scholar]
- 8.Anderson TJ, Gregoire J, Hegele RA, et al. : 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2013;29:151–167 [DOI] [PubMed] [Google Scholar]
- 9.Liang KY. and Zeger SL: Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22 [Google Scholar]
- 10.Ashley-Morrow R, Nollkamper J, Robinson NJ, et al. : Performance of focus ELISA tests for herpes simplex virus type 1 (HSV-1) and HSV-2 antibodies among women in ten diverse geographical locations. Clin Microbiol Infect 2004;10:530–536 [DOI] [PubMed] [Google Scholar]
- 11.Biraro S, Mayaud P, Morrow RA, et al. : Performance of commercial herpes simplex virus type-2 antibody tests using serum samples from Sub-Saharan Africa: A systematic review and meta-analysis. Sex Transm Dis 2011;38:140–147 [DOI] [PubMed] [Google Scholar]
- 12.Diggle PJ, Liang K-Y, and Zeger SL: Analysis of Longitudinal Data. Oxford University Press, Oxford, 1994 [Google Scholar]
- 13.Benedetti JK, Zeh J, and Corey L: Clinical reactivation of genital herpes simplex virus infection decreases in frequency over time. Ann Intern Med 1999;131:14–20 [DOI] [PubMed] [Google Scholar]
- 14.Benedetti J, Corey L, and Ashley R: Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med 1994;121:847–854 [DOI] [PubMed] [Google Scholar]
- 15.Wald A, Zeh J, Selke S, et al. : Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med 1995;333:770–775 [DOI] [PubMed] [Google Scholar]
- 16.Tobian AA, Grabowski MK, Serwadda D, et al. : Reactivation of herpes simplex virus type 2 after initiation of antiretroviral therapy. J Infect Dis 2013;208:839–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couppie P, Sarazin F, Clyti E, et al. : Increased incidence of genital herpes after HAART initiation: A frequent presentation of immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients. AIDS Patient Care Stds 2006;20:143–145 [DOI] [PubMed] [Google Scholar]
- 18.Sheth PM, Sunderji S, Shin LYY, et al. : Coinfection with herpes simplex virus type 2 is associated with reduced HIV-specific T cell responses and systemic immune activation. J Infect Dis 2008;197:1394–1401 [DOI] [PubMed] [Google Scholar]
- 19.Naeger DM, Martin JN, Sinclair E, et al. : Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One 2010;5:e8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shannon B, Yi TJ, Thomas-Pavanel J, et al. : Impact of asymptomatic herpes simplex virus type 2 infection on mucosal homing and immune cell subsets in the blood and female genital tract. J Immunol 2014;192:5074–5082 [DOI] [PubMed] [Google Scholar]
- 21.Paton NI, Goodall RL, Dunn DT, et al. : Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: A randomized controlled trial. JAMA 2012;308:353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta SK, Mi D, Dube MP, et al. : Pentoxifylline, inflammation, and endothelial function in HIV-infected persons: A randomized, placebo-controlled trial. PLoS One 2013;8:e60852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckard AR, Jiang Y, Debanne SM, et al. : Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. J Infect Dis 2014;209:1156–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]