Abstract
Recent studies have revealed the existence of hippocampal neurons that fire at successive moments in temporally structured experiences. Several studies have shown that such temporal coding is not attributable to external events, specific behaviours or spatial dimensions of an experience. Instead, these cells represent the flow of time in specific memories and have therefore been dubbed ‘time cells’. The firing properties of time cells parallel those of hippocampal place cells; time cells thus provide an additional dimension that is integrated with spatial mapping. The robust representation of both time and space in the hippocampus suggests a fundamental mechanism for organizing the elements of experience into coherent memories.
If I asked you to recall what you did when you woke up today, you might say that you got out of bed, showered and dressed, walked the dog, then sipped coffee and read the newspaper. Central to retelling this experience is remembering the order of events that composed the episode. The idea that recollection is temporally organized dates back at least to Aristotle and was described as the fundamental organizing dimension of episodic memory, as introduced by Tulving1. Interest in the temporal dimension of episodic memory is increasing2, as is a focus on mechanisms for encoding temporal relations among events in both simple and complex forms of associative learning3.
A large literature of studies in animals and humans has shown that the hippocampus plays a critical part in the organization of memories in time. A recent review summarized evidence from human and animal studies that used various physiological and behavioural approaches, and concluded that the hippocampus is essential to memory of the order of sequential events — even when it is not required for remembering specific events themselves — across a broad range of behavioural paradigms4. BOX 1 lists studies showing that damage to the hippocampus impairs memory of the temporal order of events in humans and animals, and that the hippocampus is activated in humans during encoding and recall of a sequence of events. Notably, the importance of temporal organization in hippo campal function extends even to simple conditioning and associative memory performance. For example, in classical trace conditioning (which is known to depend on the hippocampus) a tone is associated — across a temporal gap — with a shock, and the duration of the gap is a central component of the memory representation5.
Correspondingly, neurophysiological studies on classical conditioning have revealed that many hippocampal pyramidal neurons fire at specific moments that are timed to the conditioned response6,7. Also, in an experiment in which rats learned to associate the durations of different time intervals with particular stimuli, the hippocampus was essential for discriminating close temporal differences, suggesting a role in temporal pattern separation8. These findings indicate a strong link between the hippocampus and temporal dimensions of memory.
What is the fundamental mechanism for temporal representation within the hippocampal circuitry? As a point of reference for addressing this question, it is generally thought that the mechanism for spatial representation in the hippocampus involves place cells, which are the principal neurons of the hippocampus that fire when an animal occupies a particular location in an environment9. In striking similarity, recent evidence shows that there are also hippocampal ‘time cells’ that fire when an animal is at a particular moment in a temporally structured experience, encoding scalar time and controlled by temporal dimensions of the experience (FIG. 1). Could time cells be the key to understanding how memories are temporally organized? Here I evaluate this hypothesis, discussing first the evidence for the existence of hippocampal time cells, their characteristics and their role in memory. Then I assess the similarities between time cells and place cells, and discuss how representations of time and space cooperate to organize the contents of memories. Finally, I consider the possible sources of temporal information in the hippo campus, as well as the possible mechanisms by which time cells may arise within the hippocampus.
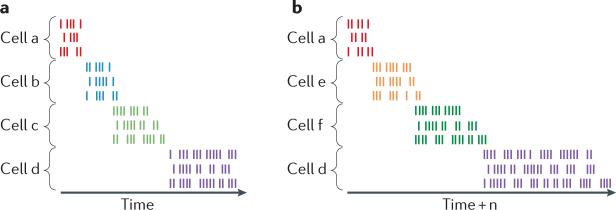
Figure 1. Key features of time-cell firing sequences.
a | A raster display of spiking activity from idealized, simultaneously recorded time cells (each shown in a different colour). For each cell, activity is shown as a raster of spikes for three example trials in which the cell fires for a brief period at approximately the same moment in each trial, with later-firing time cells being active for longer periods (indicating scalar coding of time). b | In the same recording session, when the time period is elongated (time + n), the cells shown at the top and bottom fire at the same moment relative to the beginning and end of the period, respectively (indicating that their activity is bound to those temporal boundaries), whereas the cells shown in the middle in part a have ceased firing and new cells (note the new colours in part b) fire to fill in the period, reflecting ‘re-timing’ in these cells to represent the altered temporal dimension. These characteristics parallel those of place cells, which typically fire at adjacent locations in space and, when critical spatial cues are altered, either remain bound to cues still present or ‘re-map’ to reflect the altered spatial dimensions.
Time cells
The earliest evidence of the representation of time by hippocampal neurons came from studies revealing that CA1 principal cells exhibit temporal coding in freely moving rats performing tasks that involve remembering sequences of events. These findings generated considerable interest but were initially criticized as being confounded by effects of variations in the animal's location across time and of distance travelled over time, so that the apparent temporal coding could reflect spatial rather than temporal dimensions of the animal's experience10,11.
More recent studies have addressed these concerns by tightly controlling the animal's location and movement. In addition, recent studies have found evidence of temporal representation by hippocampal neurons in non-human primates and humans. The brief descriptions of these experiments below provide an overview of the characteristics of temporal coding by the hippocampus (see BOX 1 for a summary).
Early evidence from freely moving animals performing memory tasks
The first direct evidence for temporal coding by hippocampal neurons emerged in an experiment in which ensembles of CA1 principal neurons were recorded in rats performing a task that required memory for the order of a sequence of odour stimuli. In the encoding phase of each trial, several odours were presented in sequence (each followed by a reward) at alternate sides of the enclosure. In the test phase of each trial, two odours were presented and the rat had to remember which one had occurred earlier in the preceding encoding phase12 (FIG. 2A). The pattern of activity of a CA1 neuron ensemble changed gradually across the encoding phase of a trial, and the strength of this change predicted memory performance in the subsequent test phase. This suggested that the activity pattern of the CA1 ensemble provided an evolving temporal context in the encoding of the memory of the odour presentations. The firing patterns of the same hippocampal ensemble also varied with the locations at which the odours were presented, but the differences in spatial representations were equivalent in correct trials and error trials, and therefore did not predict the accuracy of the memory performance. Notably, gradually changing patterns of hippocampal ensemble activity reflecting temporal contexts have been shown to cover periods of seconds and minutes12, hours to days13, and weeks14,15.
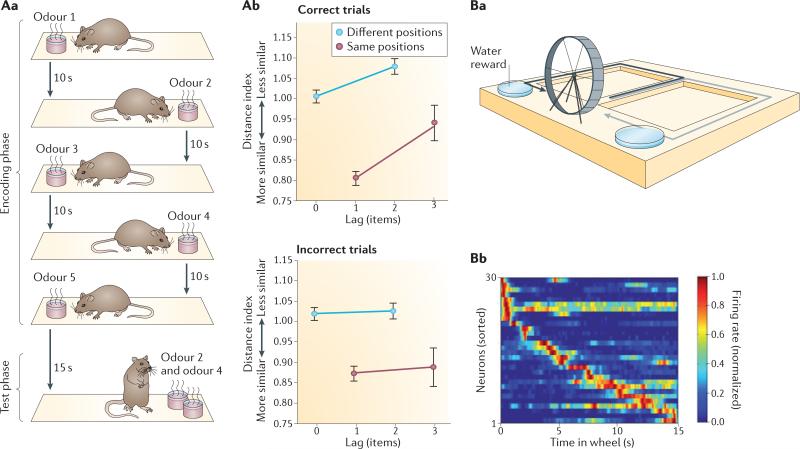
Figure 2. Early discoveries on temporal coding by hippocampal neurons.
Aa | Design of an odour sequence memory task. In the encoding phase of each trial, rats sampled a sequence of five odours, which were presented at alternating positions on a platform. In the test phase at the end of each trial, they were presented with two odours and had to judge which of the two had been presented earlier. Ab | Recordings from CA1 neural ensembles made during the test phase revealed that an index of distance (or dissimilarity) between periods surrounding odour representations in neural populations was larger for odours that had been sampled further apart (that is, they were separated by a larger number of intervening items) than for odours sampled close together (smaller lag) — but only on trials in which the order judgement was correct and not on error trials, regardless of whether the odours appeared at the same or different positions. By contrast, the distance between population representations of positions in space did not differentiate correct trials and errors. Error bars indicate the standard error of the mean for the variability across sessions. Ba | Design of a spatial alternation T-maze task. Rats travelled along two paths in a T-maze (indicated by black and grey arrows) in alternation, and in between they ran in a running wheel. Bb | The ensemble firing-rate plot shows the normalized firing rates of 30 neurons during the wheel running period (each row shows the activity of one neuron). The plot reveals that different hippocampal neurons fired at different times during wheel running, and that together, the neurons’ firing covered the entire period. These two studies revealed the existence of representations of a gradually changing temporal context signal in two very different memory tasks: one in which animals encoded unique sequences of odours and another in which they repeatedly ran on a running wheel in between memory judgements. Part A reprinted from Neuron, 56, Manns, J. R., Howard, M. W. and Eichenbaum, H. Gradual changes in hippocampal activity support remembering the order of events, 530–540, © (2007), with permission from Elsevier. Part Bb from Pastalkova, E., Itskov, V., Amarasingham, A., and Buzsáki, G. Internally generated cell assembly sequences in the rat hippocampus. Science 321, 1322–1327 (2008). Reprinted with permission from AAAS.
In the experiment discussed above12, each odour sequence occurred only once, and it was therefore not possible to determine whether the firing patterns observed in each trial were reliably associated with the specific odour sequence of that trial. A subsequent study16 recorded from single CA1 neurons in rats performing a spatial alternation task in a Tmaze. Here, the rats repeatedly alternated between left turns and right turns, and trials were separated by a fixed period of wheel running (FIG. 2B). This study showed that many neurons fired reliably at specific moments during wheel running, and the entire period of each wheel run was filled by a sequence of brief neuronal activations. Importantly, the firing sequences differed between trials in which the rat subsequently turned left or right — even though the rat was mostly in the same location (that is, in the running wheel) and performing the same behaviour (that is, running) — but they were consistent between left-turn trials and consistent between right-turn trials, suggesting that a sequence was linked to the content of the trial.
Another study17 examined the activity patterns of CA1 neurons in rats performing a place-reversal task. In the first half of each daily session, trials began at any of three arms of a plus-maze and the rats had to go to the remaining arm to obtain a reward; in the second half of the session, another arm became the ‘reward arm’ and trials started from any of the other three arms. In between trials, rats were placed on a small platform outside the maze for several seconds. During the course of training, time-specific firing patterns emerged during the inter-trial periods, and the firing sequences differed between the two sessions. The rats could move freely during the delay, but cells that had reliable place fields were excluded from the analysis, indicating that the measured activity patterns encoded time rather than place.
Another recent study18 examined whether CA1 neurons also fired at specific moments in a non-spatial task. In this task, rats learned to associate each of two visually distinct objects with one of two cups of scented sand (FIG. 3A). In each trial, rats approached and sampled one of the two objects and, after a fixed delay, were exposed to one of the two odour cups. If the odour matched the object, the rat had to dig in the sand to retrieve a buried reward. During the delay period, individual neurons fired at successive moments — filling out the entire period. The firing patterns differed depending on which object the rats had to remember and were consistent between trials in which the same object had to be remembered. Extensive general linear model analysis was used to distinguish activity patterns associated with the animal's location, speed and head direction during the delay period from activity patterns associated with the time elapsed. Although these spatial and behavioural parameters contributed to the activity patterns of many of the recorded cells, the analysis also revealed a contribution of time that was independent of these variables. Furthermore, the firing patterns of many of these neurons changed (that is, they ‘re-timed’) when the delay was increased. This happened even though the behaviour and locations of the animal during the initial period did not change (FIG. 3A; also see FIG. 1b), indicating that the firing patterns of these cells reflected the passage of time rather than variations in behaviour or place. Because these neurons fired at specific moments in a fixed period — much like place cells fire at specific locations in a fixed space — these neurons were dubbed ‘time cells’ in this study. Importantly, the authors18 observed that cells firing later in the delay period were active for longer durations (that is, they had larger ‘time-fields’) (FIG. 1), a phenomenon that was also observed in other studies19,20 (discussed below). This pattern suggests a scalar coding of time, which parallels a hallmark property of time judgements in humans and animals, as discussed below.
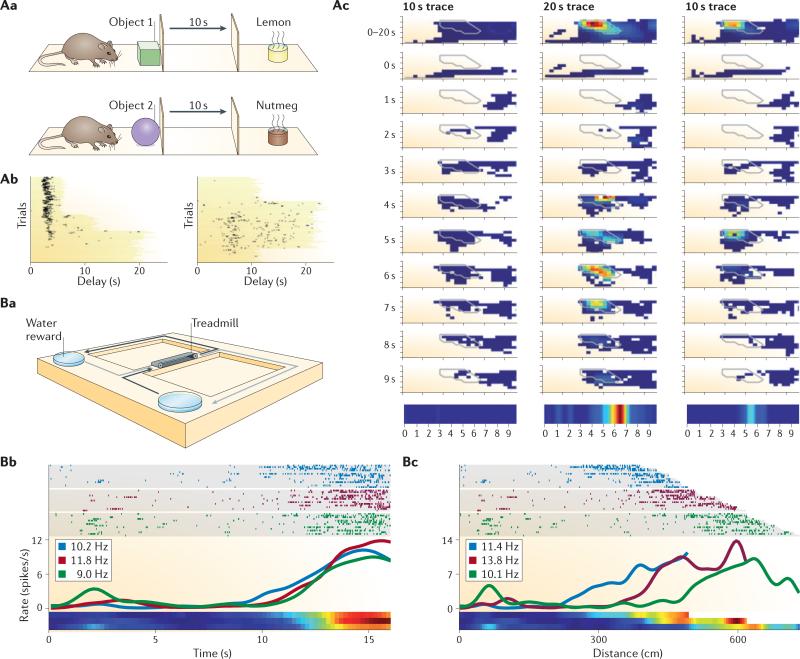
Figure 3. Time coding and spatial coding.
Aa | Design of a non-spatial task in which time cells were observed. Rats learned to associate each of two visually distinct objects with one of two cups of scented sand (for example, the green block was associated with the lemon-scented cup and the purple sphere was associated with the nutmeg-scented cup). In each trial, rats approached and sampled one of the two objects and, after a fixed (10 s or 20 s) delay, were exposed to one of the two odours. If the odour matched the object (for example, lemon odour following a green block), the rat had to dig in the scented sand to retrieve a reward. If the odour did not match the object (not shown), no reward was available in the odour cup and the rat did not dig. Ab | Two examples of cells that ‘re-timed’. On the left, a cell that progressively lost its time field as the delay was elongated from 5 s, to 10 s, to 20 s. On the right, a cell that did not fire when the delay period was 10 s but that did fire (at about t = 6–7 s) when the delay period was 20 s. Ac | The spatial activity pattern (place field) of the cell whose raster plot is shown on the right in part Ab. Activity is plotted for the entire delay period (top) and for the first ten successive seconds of the delay period in each trial block. Note that the cell fired at a particular location but only during the sixth to seventh second of the 20 s delay period (middle column) and not in trials with a 10 s delay period (left and right column; also see the normalized firing rates over time in the trace at the bottom). Ba | Design of a spatial alternation task. Rats ran on a treadmill in between alternating left turns and right turns on a T-maze (paths indicated by black and grey arrows). Bb,Bc | The firing patterns of a hippocampal neuron shown in raster plots (top), firing rate histograms (middle) and normalized firing rate (bottom) across a range of treadmill speeds. When firing is plotted according to time elapsed (Bb), the cell fired at about 14 s into treadmill running regardless of speed. Plotting the same data according to distance travelled (Bc) reveals that the cell fired at different distances depending upon speed. These experiments showed that hippocampal neurons can encode time and space conjointly (A) or can encode time only and not spatial dimensions (B). Parts Ab and Ac reprinted from Neuron, 71, MacDonald, C. J., Lepage, K. Q., Eden, U. T. and Eichenbaum, H. Hippocampal “time cells” bridge the gap in memory for discontiguous events, 737–749, © (2011), with permission from Elsevier. Parts Bb and Bc reprinted from Neuron, 78, Kraus, B. J., Robinson II, R. J., White, J. A., Eichenbaum, H. and Hasselmo, M. E. Hippocampal ‘time cells’: Time versus path integration, 1090–1101, © (2013), with permission from Elsevier.
Each of these studies provided evidence for the existence of an evolving temporal signal that takes the form of a succession of briefly firing neurons.
Temporal versus other dimensions of experience encoded by hippocampal neural activity
In all of the studies described so far, the rats could move freely during the periods when time-related activity was observed. Even though the neural activity patterns associated with elapsed time could be distinguished from the activity patterns associated with different locations, there was still the possibility that the apparent time-related activity was confounded by effects of the distance that the animal travelled during the recording periods. One study specifically addressed this possibility19. Here, rats performed a spatial alternation task in a maze. In between alternations, the animals ran on a treadmill placed in the segment overlapping the two paths through the maze (FIG. 3B; also see Supplementary information S1 (movie)). The authors varied the treadmill speed randomly on each trial, so that the effects of elapsed time and distance travelled on neural activity could be disentangled. A general linear model analysis that considered head position, time and distance revealed that the activity of over 80% of the neurons that were active during treadmill running was modulated by elapsed time. Position had little influence, but distance travelled was also strongly encoded in the activity of many neurons. The firing patterns of some neurons signalled only time or only distance, but the activity of most cells was influenced to differing degrees by both variables, indicating that most of these cells encoded both time and distance.
Further evidence supporting the existence of temporal signals that are independent of place or distance has come from recent studies of time cells in head-fixed animals in which the animal's location and behaviour were kept constant and movement was eliminated. For example, in one study20, rats performed an odour-cued delayed matching-to-sample task in which each trial began with the presentation of one of multiple sample odours for 1 s. Following a fixed delay, a test odour was presented. In order to receive a reward, the animal had to respond only to the test odour that matched the sample on that trial (FIG. 4A). In these rats, approximately 30% of hippocampal cells encoded successive moments during the delay. Another study in head-fixed animals used tw-photon calcium imaging to investigate the evolution of firing patterns among large ensembles of hippocampal neurons in mice undergoing classical conditioning21. On each trial, mice heard a brief tone that was followed, after a temporal gap, by an air puff to the eye (FIG. 4B). During acquisition of the conditioned eye-blink response, CA1 cells developed time-locked firing sequences throughout the trial, including during the temporal gap.
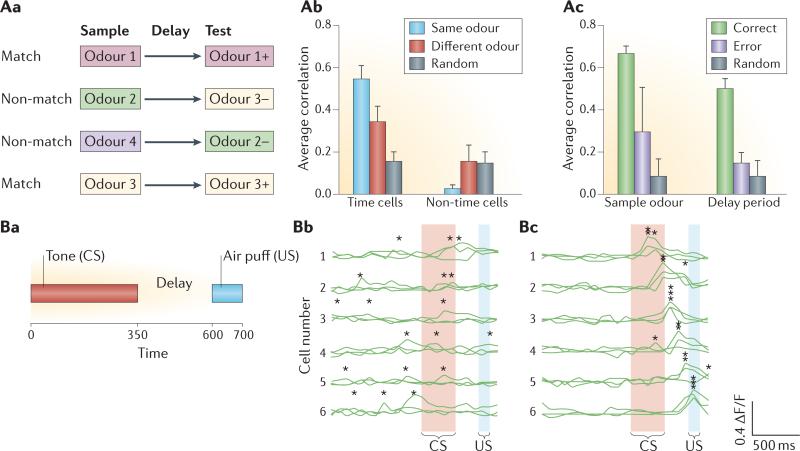
Figure 4. Time cells have a role in memory.
Aa | Design of an example series of trials used in immobilized (head-fixed) rats performing an odour-cued delayed matching-to-sample task. Each trial began with the presentation of one of up to four sample odours for 1 s. Following a 2–5 s delay, a test odour was presented. In ‘match’ trials (that is, when the test odour was the same as the sample odour), the rat could respond to the test odour to receive a reward (indicated by ‘+’). In ‘non-match’ trials (that is, when the test odour differed from the sample odour), no reward was available (indicated by ‘–’). Match and non-match trials were presented in a random order. Ab | Population vectors during the delay period for time cells were well correlated between trials that began with the same sample odour, and were less strongly correlated (but still above that of control (random) conditions) between trials with different sample odours; cells that were not temporally modulated did not code for different sample odours. Error bars indicate the standard error of the mean. Ac | Population vectors were similar between trials with the same sample when the match/non-match judgement was subsequently correct during both the sample period and the ensuing delay, but these correlations were reduced on error trials. Error bars indicate the standard error of the mean. Ba | Mice were given trace classical conditioning; in each trial, a 350 ms tone (the conditioned stimulus (CS); red bar) was followed by a 250 ms delay period, then a 100 ms air puff (the unconditioned stimulus (US); blue bar). Bb,Bc | Calcium signals (ΔF/F) in six example CA1 neurons (activity patterns on three example trials are shown for each neuron) from the same mouse before (Bb) and after (Bc) learning show the emergence of successive timing signals (‘*’ indicates the peak signal for each cell). Specifically, after conditioning, the timing of the CS (red bar), the delay and the US (blue bar) periods are encoded by the firing sequence of the six neurons. Part A is adapted with permission from REF. 20, Society for Neuroscience. Part B is adapted with permission from REF. 21, eLife Sciences.
Together, these observations provide compelling evidence that the principal cells of hippocampal area CA1 encode the temporal structure of events in remembered experiences independently of any effects of location and movement on neuronal activity.
Time cells in other species
There is also evidence of temporal coding by hippocampal neurons in monkeys and humans. For example, one study examined temporal modulation of hippocampal firing patterns in head-fixed monkeys performing a temporal-order memory task22. On each trial the monkey was shown two objects, separated by brief delay. When the animal was subsequently presented with an array of three objects composed of the same two objects and one other object, it had to identify the two previously encountered objects in the correct sequence in order to receive a reward. The firing rate of many hippocampal neurons increased or decreased during the delay between the two object presentations, such that the firing pattern of the whole ensemble consistently signalled the passage of time during the delay periods. Notably, only few hippocampal neurons encoded object identity. The proportion of cells encoding time decreased from entorhinal cortex to perirhinal cortex to infero-temporal cortex (upstream of the hippocampus), whereas the proportion of cells encoding object identity increased.
Other studies have involved recording of single-neuron activity in humans viewing video clips. These studies showed gradually changing patterns of activity of neurons in the medial temporal lobe that were repeated when specific clips were remembered23 or when specific memories were cued24. Also, the firing sequences of neurons in the hippocampus (but not in any other medial temporal areas) became more similar with repeated viewings of a particular clip segment — that is, while a memory of the clip was being formed25. Although these slowly changing activity patterns more closely resemble the gradually evolving time signal in the study by Manns et al.12 than the sequences of punctate time-cell activations in the above-described studies on rodents, these observations clearly demonstrate that temporal coding by neuronal activity related to memory also occurs in the human hippocampus.
The findings discussed in this section indicate that temporal coding by hippocampal neurons is prevalent across species and a broad range of memory paradigms. In each experiment, temporal coding was characterized by a gradual change in the activity of the neuronal population over time. Most prominent in some of the studies were individual neurons — called ‘time cells’ — that fired for brief periods consistently at successive moments within distinct repeated experiences. Several of these experiments provided compelling evidence that hippocampal neurons signal elapsed time independent of place, distance travelled and movement (FIG. 4), although these dimensions can also be encoded by the same neurons (FIG. 3).
The role of time cells in memory
Some of the studies discussed above have linked the temporal coding patterns of hippocampal neurons to memory performance, providing compelling evidence that the hippocampus creates a temporal organization of memories. Temporal coding has been linked to memory performance in three ways: first, temporal coding develops as animals acquire temporally structured memories; second, distinct temporal codes are associated with specific memories; and third, temporal coding predicts accurate memory judgements about individual events.
With regard to the development of temporal coding with learning, memory-specific firing sequences of time cells during wheel running were observed only after rats had been trained to remember particular paths through a maze and not during wheel running outside the memory task16. Furthermore, memory-specific time-cell firing sequences developed only over the course of learning a hippocampus-dependent place-reversal task and not during random runs on the same maze17. Moreover, in head-fixed mice undergoing classical trace eye-blink conditioning, reliable time-cell firing sequences appeared only after the conditioned response was established21 (FIG. 4B).
With regard to the specificity of memory coding, in several studies in which specific memories were repeated, distinct temporal codes were observed. For example, different consistent firing sequences of time cells during wheel running were observed that preceded the memory-specific judgements that rats subsequently made in a spatial alternation task16. Also, in head-fixed rats performing an odour-cued delayed matching-to-sample task, the time-cell firing sequences observed during the delay period following each odour cue involved both neurons that were specific to the preceding sample odour and neurons that were common to all of the odour memories (and that therefore probably represented the common temporal structure of all trials)20 (FIG. 4A). Moreover, in humans, neurons in the hippocampal region that fired in sequence as subjects watched individual video clips ‘replayed’ these sequential firing patterns as subjects mentally recalled the same individual clips23.
With regard to the accuracy of memory, the appearance of specific firing sequences predicts subsequent accurate memory judgements in individual trials. In an early study on temporal coding, successful memory for sequences of events that were presented only once was associated with a gradually changing firing pattern within a hippocampal neuronal population12 (FIG. 2A). Also, during performance of the odour-cued delayed matching-to-sample task, odour-specific time-cell firing sequences both during the sample period and during the delay of a particular trial predicted subsequent memory success on that trial20 (FIG. 4A). These studies show that time-cell sequences can reflect both unique memories and common elements between memories, and suggest that these patterns drive accurate memory performance. Finally, earlier studies have shown that neural ensemble activity in the hippocampus evolves over time during the delay in a recognition memory task, and the decay of these patterns predicts memory errors26.
Time cells and place cells
Time cells are the same neurons as place cells. One line of evidence supporting this conclusion is that, in freely moving animals, time cells are activated only when the animal is in a particular location (FIG. 3A). In rats performing a spatial alternation task including running on a treadmill between alternations19, time-specific firing could be identified when rats were running on the treadmill, and place-specific firing patterns could be identified when rats traversed other segments of the maze. Both time cells and place cells were observed within the same population of simultaneously recorded cells. Indeed, some of the same neurons that fired at particular moments in time (that is, time cells) on the treadmill also fired when the rat passed through specific locations on the maze outside the treadmill, showing that these cells also function as place cells (see Supplementary information S1 (movie)).
Additional characteristics of time cells closely paralleled the properties of place cells observed in other studies. For example, when a salient spatial cue in a familiar environment is altered, place cells either continue firing in association with the remaining spatial cues or ‘re-map’ (that is, alter the spatial firing pattern, or cease or begin firing)27. Similarly, when the duration of the interval between cues in an object–odour association task is altered, some time cells maintain firing at a specific time relative to the beginning or end of the interval, whereas many other cells ‘re-time’ — that is, fire at a different moment, or cease or begin firing18 (FIGS 1b,3A). Thus, the partial reorganization of firing patterns of time cells that occurs when a critical temporal cue is altered closely parallels the partial reorganization of place cells that occurs when spatial cues are altered.
In addition, both place cells and time cells encode other relevant dimensions of experience. So, under different conditions relevant in particular behavioural paradigms, place cells can encode a range of spatial features (for example, distance and direction28,29), as well as non-spatial stimuli (for example, an odour in a particular place30) and action events (for example, a learned ‘jump’ escape response31). Similarly, as described above, in freely moving animals, time cells can also encode place, distance and ongoing behaviour18–20. Thus, it may be more productive to think of hippocampal principal neurons as multidimensional, in the sense that they encode time and place along with other relevant spatial and non-spatial features of ongoing experience32.
Interactions between spatial and temporal codes
Particularly striking examples of hippocampal neurons encoding conjoint spatial and temporal dimensions of experience are evident when rats traverse routes in a maze. In this situation, space and time progress together as the rat moves through the maze, and the influences of both spatial and temporal coding can be observed. For example, many studies have reported that ensembles of simultaneously recorded place cells that fire in sequential locations as animals traverse a path through a maze subsequently ‘replay’ the corresponding sequence of firings during ‘off-line’ periods, including sleep and quiet wakefulness, when the animal is not moving through those locations33. Thus, spatial coding observed as rats actively run through a maze is recapitulated in temporally coded firing sequences when the rat is not moving. Disruption of these replay events impairs subsequent memory of the path34. Moreover, replays of sequences associated with alternative choice paths in a maze predict acquisition of a learned performance35. In addition, replay can be observed in sequential firing patterns associated with place-cell sequences that are about to occur as a rat takes a novel path in an open field36. These findings on replay strongly indicate a temporal coding of spatial representations relevant to memory.
In addition, the temporal context of an ongoing experience strongly influences place-cell activity. Thus, several studies have shown that when rats traverse the common segment of a maze that features two alternate paths, hippo campal place cells fire differentially depending on whether the animal is in the midst of following one path or the other, rather than firing in the same pattern at the common location37–40 (FIG. 5A). Furthermore, path-specific firing sequences predict the accuracy of memory performance; place cells exhibit path-specific firing sequences when subsequent memory choices are correct, but place cells in that sequence fire less or not at all prior to errors41 (FIG. 5B). The finding that a specific hippocampal firing sequence occurs on the overlapping segment of different routes through a maze is complemented by the report of memory-specific sequential firing patterns in the hippocampus in rats remembering overlapping sequences of odours42. These studies show that the broader temporal context of an entire episode strongly influences the coding of specific places and events.
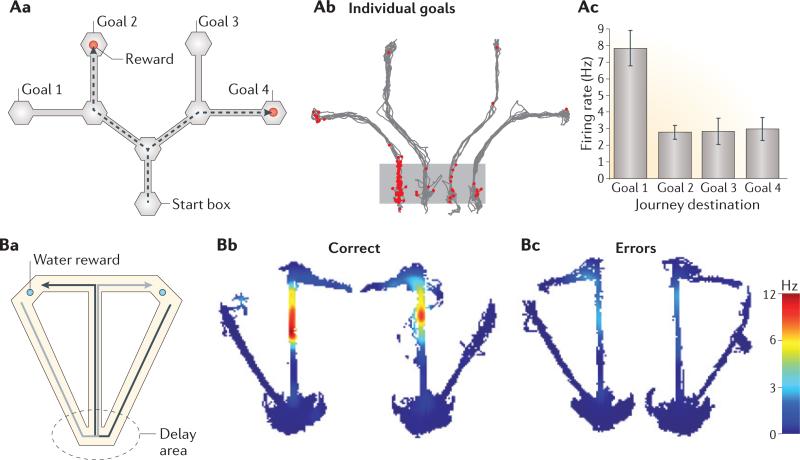
Figure 5. The influence of temporal context on spatial coding.
Aa |Rats were required to select one of four paths towards distinct goal boxes (two paths are shown as dashed lines). Ab,Ac | Plots of spikes (Ab) and firing rate (Ac) for an example cell. This cell fired maximally at the outset of the path towards goal 1, and much less so at the outset of paths towards the other three goals, even when the rat passed though the same location in all paths.
Error bars indicate the standard error of the mean across trials. Ba | Delayed spatial alternation task in which rats alternate between left-turn (black arrow) and right-turn (grey arrow) paths at a decision point on a maze. The two paths have a common arm, and both start with a 30 s delay period at the bottom of the common arm.
Bb,Bc | Normalized firing-rate plots of a hippocampal neuron. The plots show that this neuron fired maximally in the common maze segment on correct right-turn trials (Bb, left) and less so on correct left turn trials (Bb, right), and hardly at all on either type of error trial, regardless of the subsequent turn direction (Bc). In both of these experiments, the broader temporal context of the entire path through the maze determined which place cells were active at specific locations on the maze. Part A is adapted with permission from REF. 40, Society for Neuroscience. Part B reprinted from Behavioural Brain Research, 254, Robitsek, R. J., White, J. A. and Eichenbaum, H. Place cell activation predicts subsequent memory, 65–72, © (2013), with permission from Elsevier.
A different kind of interaction between spatial and temporal coding is observed in a situation in which hippo campal neurons can use either a spatial code or a temporal code when behavioural strategies associated with these codes compete. Thus, in a maze task43 in which mice must take novel routes towards a trained goal, the path that they take is sometimes guided by the known location of the goal as defined by distant spatial cues, whereas at other times it reflects the previously learned sequence of body turns. To determine which strategy was used, trials were started from a novel location in the maze and it was observed whether the animal went to the trained goal location or made the trained sequence of body turns. Analysis of neural activity revealed that firing sequences of hippocampal neurons reflected the strategy expressed on these probe trials — that is, when the mice ran towards the learned location, which required a new sequence of body turns, the neurons fired at consistent locations in the maze. By contrast, when mice reproduced the learned sequence of body turns, which resulted in moving to a different goal location, the neurons fired at consistent steps in the sequence.
Together, these findings provide compelling evidence that hippocampal neuronal ensembles represent the sequence — that is, the temporal organization — of events in memory. This includes memories for sequences of places traversed in a maze, sequential stimuli and action sequences.
To summarize the findings on time cells, there is now substantial evidence that hippocampal neurons encode elapsed time independently of, or along with, various other spatial and non-spatial variables, just as place cells can encode location independently of, or along with, other spatial variables and non-spatial events such as specific stimuli or behaviours. Time cells are controlled by the temporal cues (the beginning and end of an episode) and the temporal structure (critical intervals) of an experience, just as place cells are controlled by the spatial cues (landmarks) and the spatial structure (shape) of the environment. Also, there is now compelling evidence that firing sequences of time cells develop with learning, are memory specific and predict the accuracy of subsequent memory across a broad variety of tasks. Finally, time cells and place cells are the same neurons, and the same neuronal ensembles can encode the temporal and spatial organization of experiences.
Origins of timing in the hippocampus
What is the source of timing signals that are used to generate time cells in the hippocampus? It is possible that temporal information generated in the cerebral cortex and other brain areas provides signals to time cells within the hippocampus (FIG. 6a). Alternatively, the time-cell phenomenon might arise from the sequential activation of firing chains that are generated locally within the hippocampal circuitry (FIG. 6b). These two possibilities are considered below.
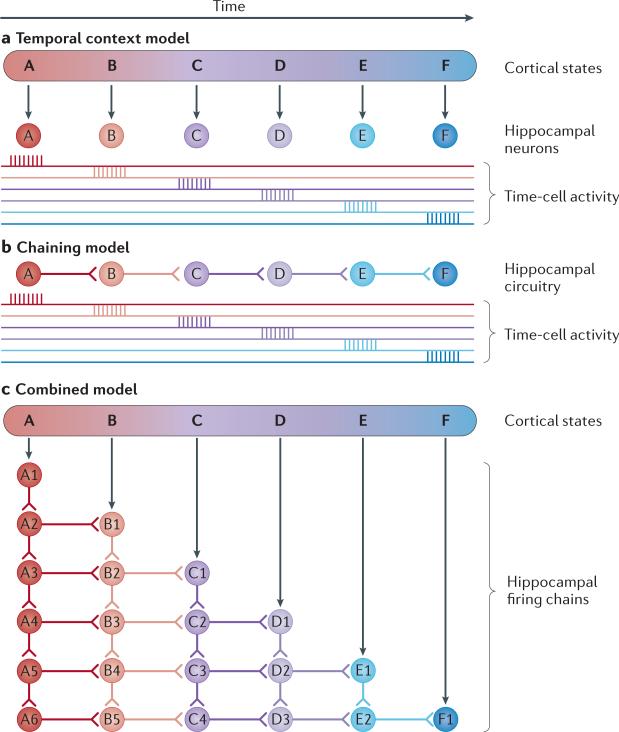
Figure 6. Temporal context versus chaining models.
a | In the temporal context model, each hippocampal neuron represents a unique state of cortical activity that corresponds to moments in the stream of events processed within the cortex69. b | According to the chaining model, hippocampal neurons increase sequential connection strengths with repeated experiences to produce hippocampal neuron firing sequences67. c | A combined model might include firing chains that are generated at successive steps as the temporal context gradually evolves, and also associations between neurons that fire at the same time across chains.
External sources of temporal information
There is a substantial literature on time perception in humans and animals, and a large number of recent studies have sought to identify brain areas that support the brain's ability to time in different tasks44–47. One prominent model suggests that the neural basis for interval timing involves interactions between multiple oscillatory patterns in prefrontal and parietal cortical areas, resulting in unique patterns of activation at different times that are integrated in the striatum48,49. Thus, possible external sources of temporal information to the hippo-campus include those prefrontal and parietal areas that provide some of the major inputs to the hippo-campus. Indeed, a study in rats showed that damage to the medial prefrontal area severely impaired interval timing and that neurons in the medial prefrontal cortex signalled elapsed time in a manner consistent with encoding of time on a logarithmic scale50. Studies in monkeys performing interval-time estimation showed that neurons in the lateral parietal area signal changes in a monkey's judgements about elapsed time (relative to a standard remembered duration)51,52. Furthermore, the parietal–temporal junction is activated in humans making temporal-order judgements53. Interestingly, in a prefrontal–parietal–medial-temporal network, different oscillation frequencies were associated with memory for the spatial location and memory for the temporal order of events54,55. These findings suggest that prefrontal areas and parietal areas that are classically considered part of the ‘where’ stream (that is, the visual stream for spatial processing) are also involved in the perception of time, and these areas have robust inputs to the hippocampus.
The major cortical input from the ‘where’ stream reaches the hippocampus via the parahippocampal cortex. This area, also known as the ‘parahippocampal place area’ because of its activation when humans look at scenes56, may also signal information about temporal context. For example, different parts of the parahippocampal cortex are activated when humans view images of objects that elicit memories of temporally co-occurring items than when they view images of objects that elicit memories of spatial arrangements57.
Moreover, the level of activation in the parahippocampal cortex during the viewing of scenes depends on the recent history of scene presentations, indicating that scene coding is embedded within a representation of temporal context58. In addition, during the retrieval of object sequences, activity patterns in the parahippocampal cortex and the perirhinal cortex were specifically associated with the temporal order of objects in sequences and with object identities, respectively, whereas those in the hippocampus encoded object identities within a temporal context59.
Following the ‘where’ stream further towards the hippocampus, lesions of the medial entorhinal cortex — an area commonly associated with spatial representation — impair memory of the temporal context of recently experienced stimuli60, and entorhinal inputs to the hippocampus are essential for bridging temporal gaps between associated events61,62. Furthermore, there is recent evidence that medial entorhinal cortex neurons that fire when a rat is in particular locations in space (known as ‘grid cells’) also fire at particular moments during treadmill running63. Combined, these findings suggest that signals about the temporal context of experience are sent via a network of cortical areas to the hippocampus, which then parses temporal context into discrete units (time fields) that represent the flow of sequential events64 (FIG. 6a). This view is consistent with a recently proposed distinction between the ability to judge temporal intervals, in which the hippocampus has a secondary role, and memory for temporal organization, in which the hippocampus is central65,66.
An internal mechanism?
An alternative mechanism for the time-cell phenomenon could be that the sequential activation of these cells reflects a strengthening of connections between neurons that are repeatedly activated in sequence within the hippocampus to create a firing chain (FIG. 6b). This model is consistent with the finding that when rats repeatedly run through a sequence of locations, the place fields of hippocampal neurons expand backwards from their original centre67. This backward expansion of place fields could reflect each place cell being driven by an earlier-firing place cell through enhanced connectivity that is generated by repeated sequential activation.
Evidence for both models
Several findings suggest that a strict chaining model, composed of a single sequence of cell activations, is unlikely to be the mechanism for time cells. As discussed above18, an experimental elongation of the critical temporal dimension (specifically, the delay between object exposure and odour exposure) caused most time cells to ‘re-time’. That is, the cells changed their temporal firing patterns even during the initial part of the delay (thus creating a new temporal context representation), rather than simply adding cell activations during the extended segment of the delay (that is, elongating the firing chain). Also, distinct firing sequences disambiguate overlapping memories — experiences that contain common elements — and two studies16,18 have shown that such distinct temporal firing patterns are associated with different memories even though the memories share identical events. Strict firing chains cannot ‘split’ to differently encode overlapping memories, although different initial elements in a sequence could specify a distinct sequence of succeeding elements that represent overlapping events68. In addition, firing sequences of time cells are usually scalar, so that time cells that fire later in a sequence also fire for a longer period (FIGS 1b,2B; but see REF. 16). This is inconsistent with firing chains involving uniform-duration neuronal activations but is consistent with a recent variation on the temporal context model known as the scalar temporal context model69.
When rats perform a maze task, sequential place-cell firing patterns that occur over seconds during maze learning ‘replay’ during subsequent quiet periods, and these replays are observed in accelerated form within about 100 ms33. Although it is easy to imagine the compression of firing chains by simply having fewer spikes per cell and the subsequent decompression as longer spike bursts, a reproduction of an externally generated representation of real-time context representations would require a more complex compression mechanism that allows decompression to reconstruct the original intervals. Furthermore, hippocampal neuronal firing sequences that can be observed prior to learning subsequently characterize the sequences of place-cell firing in maze performance70. Conversely, firing sequences persisted in a mouse model of neurodegeneration associated with a loss of spatial sequence coding, which is consistent with the existence of temporal firing chains prior to representations of events71. These findings suggest that firing chains are preconfigured within the hippocampus and are then co-opted to provide temporal organizations of events. However, one study examining trace eye-blink conditioning reported the emergence of new temporal patterns of neuronal firings associated with learning, rather than a co-opting of existing firing chains21. So, internally generated firing chains within the hippocampus might provide a temporal organization for spatial coding or might be modified by sequential events to represent their temporal organization. Combining the findings that support the existence of firing chains with the observations that a broader signal that defines entire memories is needed, perhaps the most likely network mechanism involves slowly evolving temporal context dynamics (that is, signals that spread across multiple sequential events) selecting specific firing chains that represent specific temporally organized memories72 (FIG. 6c).
It is important to note that studies of time cells have focused on hippocampal area CA1 because this area seems to be the most important for temporal organization73. However, there is some evidence that area CA2 (but not CA3) could also be important for temporal processing, suggesting that time cells might originate within the hippocampal circuitry74. It remains a central question whether temporal information represented within the hippocampus originates from its inputs or is generated internally, or whether a combination of mechanisms generates time cells.
Conclusions and perspective
Recent studies have revealed that in addition to place cells, which fire when rats are in a particular location in a spatially structured environment, the hippocampus contains time cells, which fire at particular moments in a temporally structured period. Place cells and time cells are the same neurons, so many of these cells encode both spatial and temporal dimensions, but the representations of place and time can be separately measured. Several recent papers, using diverse experimental approaches and multiple memory tasks, have established that temporal organization within the hippocampus occurs across animal species and humans. Furthermore, time-cell firing patterns appear during learning and can both distinguish specific memories and predict subsequent memory performance. The temporal information from which time cells are generated might result from earlier processing in other brain areas. It could be communicated to the hippocampus via the classic ‘where’ stream, along with internal mechanisms that might generate time-cell patterns of activity. Alternatively, or in addition, time cells might be a result of internal circuitry mechanisms. Although it is unlikely that time-cell sequences reflect simple firing chains, some evidence suggests that pre-existing firing sequences are enhanced and bound to moments in the evolving temporal context of experiences.
These findings have established temporal coding as a robust and prevalent aspect of hippocampal firing patterns. This challenges the view that the fundamental function of the hippocampus is spatial mapping and navigation75, and instead supports the notion that the hippocampus contributes to memory by organizing elements of experience in both spatial and temporal context. Finally, although both spatial and temporal coding are prevalent in the activity patterns of hippocampal neurons, and can be distinguished experimentally, it remains entirely possible that the common thread in both dimensions is ‘association by proximity’ in space or time. This would suggest that place cells and time cells are simply reflections of a basic function of the hippocampus — supporting associative networks that represent a broad range of relations among the elements of memories32,76.
Supplementary Material
Box 1 | Studies reporting hippocampal involvement in the temporal organization of memories.
Damage to the hippocampus in humans results in impairments in:
Reproducing the order of words in a list77
Recalling and recognizing the order of words and word pairs78
Remembering the order of objects visited in a virtual environment79
Retrieval of the order of presented objects82
Damage to the hippocampus in rodents results in impairments in:
Disambiguation of overlapping odour sequences85
Preferential exploration of earlier-presented odours in familiar sequences86
Memory for the order of odours presented in different places87
Preferential exploration of the earlier-presented object in a set of objects that had been presented once before74
Memory for the order of once-presented odour pairings88
Memory for the duration between presented odours8 • Serial reaction-time sequence learning89
Anticipatory selection of maze arms experienced in sequence90
Flexible expression of body-turn sequences in a maze91
Activation of the hippocampus in humans occurs during:
Encoding of overlapping face sequences92
Remembering the order of objects visited in a virtual environment93
Reconstruction of the order of scenes in a movie clip94
Retrieval of overlapping and non-overlapping face sequences95
Viewing of items out of order in a familiar sequence96
Encoding of word triplets predicting memory success97
Encoding during increased demand to bridge a temporal gap between stimuli98
Serial reaction-time sequence learning99
Disambiguation of overlapping routes through a virtual maze100
Successful retrieval of coarse temporal order101
Similar coding of consistently successive experienced objects102
Successful retrieval of the temporal positions of objects in learned sequences59
Differentiation of temporal context during successful sequence retrieval103
Similar coding of neighbouring objects predicting successful order memory104
Temporal coding by hippocampal neurons occurs during:
Encoding of once-presented odour sequences in rats12
Wheel running during the delay in maze alternation in rats16
Inter-trial intervals as rats learn a place reversal task17
Delay periods between paired-associate stimuli in rats18
Delay periods between sequentially presented stimuli in immobilized monkeys22
Treadmill running at different speeds during the delay in maze alternation in rats19
Delay periods in delayed non-match to sample in immobilized rats20
Trace eyelid conditioning in immobilized mice21
Acknowledgements
The author would like to acknowledge funding support from the US National Institutes of Mental Health (grants MH095297 and MH094263).
Footnotes
Competing interests statement The author declares no competing interests.
References
- 1.Tulving E. Elements of Episodic Memory. Oxford Univ. Press; 1983. [Google Scholar]
- 2.Templer VL, Hampton RR. Episodic memory in non-human animals. Curr. Biol. 2013;23:R801–R806. doi: 10.1016/j.cub.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallistel CR, Balsam PD. Time to rethink the neural mechanisms of learning and memory. Neurobiol. Learn. Mem. 2014;108:136–144. doi: 10.1016/j.nlm.2013.11.019. [This review proposes a reorientation of our views on learning away from the widely held view that learning is based on the temporal contiguity of associated events. Instead, the authors suggest that learning maps the temporal organization of sequential events.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichenbaum H. Memory on time. Trends Cogn. Sci. 2013;17:81–88. doi: 10.1016/j.tics.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Díaz-Mataix L, Ruiz Martinez RC, Schafe GE, LeDoux JE, Doyère V. Detection of a temporal error trigger reconsoklidation of amygdala-dependent memories. Curr. Biol. 2013;23:467–472. doi: 10.1016/j.cub.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger TW, Rinaldi PC, Weisz DJ, Thompson RF. Single unit analysis of different hippocampal cell types during classical conditioning of the rabbit nictitating membrane response. J. Neurophysiol. 1983;50:1197–1219. doi: 10.1152/jn.1983.50.5.1197. [DOI] [PubMed] [Google Scholar]
- 7.McEchron MD, Tseng W, Disterhoft JF. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (fear) conditioning in rabbit. J. Neurosci. 2003;23:1535–1547. doi: 10.1523/JNEUROSCI.23-04-01535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs NS, Allen TA, Nguyen N, Fortin NJ. Critical role of the hippocampus in memory for elapsed time. J. Neurosci. 2013;33:13888–13893. doi: 10.1523/JNEUROSCI.1733-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford Univ. Press; 1978. [Google Scholar]
- 10.Buszaki G. Time, space and memory. Nature. 2013;497:568–569. doi: 10.1038/497568a. [DOI] [PubMed] [Google Scholar]
- 11.Rowland DC, Moser M-B. Time finds its place in the hippocampus. Neuron. 2013;78:953–954. doi: 10.1016/j.neuron.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 12.Manns JR, Howard M, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [This is the first experimental study to reveal a gradually changing representation of context by hippocampal neuronal ensembles that is linked to memory for the order of events in unique experiences.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mankin EA, et al. Neuronal code for extended time in the hippocampus. Proc. Natl Acad. Sci. USA. 2012;109:19462–19467. doi: 10.1073/pnas.1214107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziv Y, et al. Long-term dynamics of CA1 hippocampal place codes. Nature Neurosci. 2013;16:264–266. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rangel LM, et al. Temporlly selective contextual encoding in the dentate gyrus of the hippocampus. Nature Comm. 2014 doi: 10.1038/ncomms4181. http://dx.doi.org/10.1038/ncomms4181. [DOI] [PMC free article] [PubMed]
- 16.Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [This study revealed the existence of hippocampal neurons that fire briefly in sequence as rats run in a running wheel between alternations on a T-maze. Different firing sequences distinguished left-turn and right-turn paths through the maze.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill PR, Mizumori SJ, Smith DM. Hippocampal episode fields develop with learning. Hippocampus. 2011;21:1240–1249. doi: 10.1002/hipo.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [This study revealed the existence of time cells in animals performing a non-spatial task in which paired stimuli were separated by a delay. The authors showed that temporally specific firing patterns during the delay are not explained by variations in location or behaviour but are controlled by the critical temporal parameter of delay duration.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraus BJ, Robinson RJ, 2nd, White JA, Eichenbaum H, Hasselmo ME. Hippocampal ‘time cells’: time versus path integration. Neuron. 2013;78:1090–1101. doi: 10.1016/j.neuron.2013.04.015. [This study directly compares the coding of elapsed time, distance travelled and location by hippocampal neurons in rats during running in place on a treadmill. The results show that a combination of time and distance strongly determine the firing patterns of most neurons, with some encoding only time or only distance. Also, the same neurons that are time cells on the treadmill have clear place fields at locations outside the treadmill, showing that time cells and place cells are the same neurons.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacDonald CJ, Carrow S, Place R, Eichenbaum H. Distinct hippocampal time cell sequences represent odor memories in immobilized rats. J. Neurosci. 2013;33:4607–14616. doi: 10.1523/JNEUROSCI.1537-13.2013. [This study revealed that time cells fire in sequence during the delay period in head-fixed rats performing a delayed matching-to-sample task, showing that time-cell sequences exist even when movement is completely prevented. Furthermore, the results showed that distinct time-cell sequences are associated with different memories and that these sequences predict accurate memory performance.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Modi MN, Dhawale AK, Bhalla US. CA1 cell activity sequences emerge after reorganization of network correlation structure during associative learning. eLife. 2014;3:e01982. doi: 10.7554/eLife.01982. [This study imaged calcium signals in hippocampal neurons of head-fixed mice during trace eye-blink classical conditioning. Hippocampal neural ensembles developed time-cell firing sequences, including during the trace period, associated with learning the conditioned response.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naya Y, Suzuki WA. Integrating what and when across the primate medial temporal lobe. Science. 2011;333:773–776. doi: 10.1126/science.1206773. [This study identifies time cells that fire as head-fixed monkeys learn the temporal order of pairs of visual stimuli separated by a delay. Notably, in this task, hippocampal neurons encoded elapsed time but not the visual stimuli, whereas neurons in upstream areas (entorhinal cortex, perirhinal cortex and inferotemporal cortex) progressively more strongly encoded information about the stimuli and less about elapsed time.] [DOI] [PubMed] [Google Scholar]
- 23.Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322:96–101. doi: 10.1126/science.1164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard MW, Viskontas IV, Shankar KH, Fried I. Ensembles of human MTL neurons “jump back in time” in response to a repeated stimulus. Hippocampus. 2012;22:1833–1847. doi: 10.1002/hipo.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paz R, et al. A neural substrate in the human hippocampus for linking successive events. Proc. Natl Acad. Sci. USA. 2010;107:6046–6051. doi: 10.1073/pnas.0910834107. [This study revealed the emergence of a gradually changing representation of events with repeated exposures to specific film clips in humans. These patterns, similar to those observed in rats (reference 12), emerged only in the hippocampus and not in other medial temporal areas.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deadwyler SA, Bunn T, Hampson RE. Hippocampal ensemble activity during spatial delayed non-match to sample performance in rats. J. Neurosci. 1996;16:354–372. doi: 10.1523/JNEUROSCI.16-01-00354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shapiro ML, Tanila H, Eichenbaum H. The cues that hippocampal place cells encode: dynamic and hierarchical representation of local and distal stimuli. Hippocampus. 1997;7:624–642. doi: 10.1002/(SICI)1098-1063(1997)7:6<624::AID-HIPO5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 28.Gothard KM, Skaggs WE, Moore KM, McNaughton BL. Binding of hippocampal CA1 neural activity to multiple reference frames in a landmark-based navigation task. J. Neurosci. 1996;16:823–835. doi: 10.1523/JNEUROSCI.16-02-00823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravassard P, et al. Multisensory control of hippocampal spatiotemporal selectivity. Science. 2013;340:1342–1346. doi: 10.1126/science.1232655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komorowski RW, Manns JR, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens. J. Neurosci. 2009;29:9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenck-Santini PP, Fenton AA, Muller RU. Discharge properties of hippocampal neurons during performance of a jump avoidance task. J. Neurosci. 2008;28:6773–6786. doi: 10.1523/JNEUROSCI.5329-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKenzie S, et al. Hippocampal representation of related and opposing memories develop within distinct, hierarchically-organized neural schemas. Neuron. 2014;83:202–215. doi: 10.1016/j.neuron.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nature Neurosci. 2011;14:147–153. doi: 10.1038/nn.2732. [This paper reviews several studies showing that hippocampal place cells that fire in sequence along previously travelled routes also ‘replay’ their sequential firing patterns in compressed time during subsequent periods of quiet wakefulness.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336:1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singer AC, Carr MF, Karlsson MP, Frank LM. Hippocampal SWR predicts correct decisions during the initial learning of an alternation task. Neuron. 2013;77:1163–1173. doi: 10.1016/j.neuron.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfeiffer BE, Foster DJ. Hippocampal place cell sequences depict future paths to remembered goals. Nature. 2013;497:74–79. doi: 10.1038/nature12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- 38.Wood ER, Dudchenko P, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro ML, Kennedy PJ, Ferbinteanu J. Representing episodes in the mammalian brain. Curr. Opin. Neurobiol. 2006;16:701–709. doi: 10.1016/j.conb.2006.08.017. [This paper reviews several studies showing that distinct ensembles of sequentially active hippocampal place cells map specific paths through a maze, including segments of paths that overlap. Thus, these ensembles represent the sequence of events that compose a specific route and not merely a set of adjacent locations in space.] [DOI] [PubMed] [Google Scholar]
- 40.Ainge JA, Tamosiunaite M, Woergoetter F, Dudchencko PA. Hippocampal CA1 place cells encode intended destination on a maze with multiple choice points. J. Neurosci. 2007;27:9769–9779. doi: 10.1523/JNEUROSCI.2011-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robitsek RJ, White J, Eichenbaum H. Place cell activation predicts subsequent memory. Behav. Brain Res. 2013;254:65–72. doi: 10.1016/j.bbr.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ginther MR, Walsh DF, Ramus SJ. Hippocampal neurons encode different episodes in an overlapping sequence of odors task. J. Neurosci. 2011;31:2706–2711. doi: 10.1523/JNEUROSCI.3413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cabral HO, et al. Oscillatory dynamics and place field maps reflect hippocampal ensemble processing of sequence and place memory under NMDA receptor control. Neuron. 2014;81:402–415. doi: 10.1016/j.neuron.2013.11.010. [This study shows that hippocampal firing patterns can signal either a series of positions traversed or a sequence of actions, depending upon the current behavioural strategy. Thus hippocampal representations can either be anchored to space or driven by the temporal organization of memories.] [DOI] [PubMed] [Google Scholar]
- 44.Gibbon J, Malpani C, Dale CL, Gallistel R. Toward a neurobiology of temporal cognition: advances and challenges. Curr. Opin. Neurobiol. 1997;7:170–184. doi: 10.1016/s0959-4388(97)80005-0. [DOI] [PubMed] [Google Scholar]
- 45.Mauk MD, Buonomano DV. The neural basis of temporal processing. Ann. Rev. Neurosci. 2004;27:307–340. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- 46.Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nature Rev. Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- 47.Yin B, Troger AB. Exploring the 4th dimension: hippocampus, time, and memory revisited. Front. Int. Neurosci. 2011;5:36. doi: 10.3389/fnint.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mattel MS, Meck WH. Neuropsychological mechanisms of interval timing behavior. BioEssays. 2000;22:94–103. doi: 10.1002/(SICI)1521-1878(200001)22:1<94::AID-BIES14>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 49.Lustig C, Matell MS, Meck WH. Not “just” a coincidence: frontal-striatal interactions in working memory and interval timing. Memory. 2005;13:441–448. doi: 10.1080/09658210344000404. [DOI] [PubMed] [Google Scholar]
- 50.Kim J, Ghim J-W, Lee JH, Jung MW. Neural correlates of interval timing in the prefrontal cortex. J. Neurosci. 2013;33:13834–13847. doi: 10.1523/JNEUROSCI.1443-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leon MI, Shadlen MN. Representation of time by neurons in the posterior parietal cortex of the macaque. Neuron. 2003;38:317–327. doi: 10.1016/s0896-6273(03)00185-5. [This study identifies neurons in the lateral parietal cortex that alter their firing rates according to elapsed time in monkeys performing a task in which they matched elapsed time to a remembered standard interval. Information used in the perception of temporal intervals in cortical areas (and elsewhere) might be the source of time signals to the hippocampus.] [DOI] [PubMed] [Google Scholar]
- 52.Janssen P, Shadlen MN. A representation of the hazard rate of elapsed time in macaque area LIP. Nature Neurosci. 2005;8:234–241. doi: 10.1038/nn1386. [DOI] [PubMed] [Google Scholar]
- 53.Davis B, Christie J, Rorden C. Temporal order judgments activate temporal parietal junction. J. Neurosci. 2009;29:3182–3188. doi: 10.1523/JNEUROSCI.5793-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watrous AJ, et al. Frequency-specific network connectivity increases underlie accurate spatiotemporal memory retrieval. Nature Neurosci. 2013;16:349–356. doi: 10.1038/nn.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts BM, Hsieh L-T, Ranganath C. Oscillatory activity during maintenance of spatial and temporal information. Neuropsychologia. 2013;51:349–357. doi: 10.1016/j.neuropsychologia.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- 57.Aminoff E, Gronau N, Bar M. The parahippocampal cortex mediates spatial and nonspatial associations. Cereb. Cortex. 2007;17:1493–1503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- 58.Turk-Browne NB, Simon MG, Sederberg PB. Scene representations in parahippocampal cortex depend on temporal context. J. Neurosci. 2012;32:7202–7207. doi: 10.1523/JNEUROSCI.0942-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hsieh LT, Gruber MJ, Jenkins LJ, Ranganath C. Hippocampal activity patterns carry information about objects in temporal context. Neuron. 2014;81:1165–1178. doi: 10.1016/j.neuron.2014.01.015. [This multivoxel pattern analysis of functional MRI scans in humans shows that activity patterns in the hippocampus carry information about the temporal positions of objects in learned sequences, whereas patterns in the parahippocampal cortex signal the temporal position only and patterns in the perirhinal cortex signalled object information only.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sauvage MM, Beer Z, Ekovich M, Ho L, Eichenbaum H. The caudal medial entorhinal cortex: a selective role in recollection-based recognition memory. J. Neurosci. 2010;30:15695–15699. doi: 10.1523/JNEUROSCI.4301-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suh J, Rivest AJ, Nakashiba T, Tominaga T, Tonegawa S. Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science. 2011;334:1415–1412. doi: 10.1126/science.1210125. [DOI] [PubMed] [Google Scholar]
- 62.Kitamura T, et al. Island cells control temporal association memory. Science. 2014;343:896–901. doi: 10.1126/science.1244634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kraus BJ, et al. Grid cells are time cells. Soc. Neurosci. Abstr. 2013;769.19 [Google Scholar]
- 64.Howard M, et al. A unified mathematical framework for coding time, space, and sequences in the medial temporal lobe. J. Neurosci. 2014;34:4692–4707. doi: 10.1523/JNEUROSCI.5808-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.MacDonald CJ. Prospective and retrospective duration memory in the hippocampus: is time in the foreground or background? Phil. Trans. R. Soc. B;2014;369:20120463. doi: 10.1098/rstb.2012.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MacDonald CJ, Fortin NJ, Sakata S, Meck WH. Retrospective and prospective views on the role of the hippocampus in interval timing and memory for elapsed time. Timing Time Percept. 2014;2:51–61. [Google Scholar]
- 67.Mehta MR, Quirk MC, Wilson MA. Experience-dependent asymmetric shape of hippocampal receptive fields. Neuron. 2000;25:707–715. doi: 10.1016/s0896-6273(00)81072-7. [DOI] [PubMed] [Google Scholar]
- 68.Itskov V, Curto C, Pastalkova E, Buzsáki G. Cell assembly sequences arising from spike threshold adaptation keep track of time in the hippocampus. J. Neurosci. 2011;31:2828–2834. doi: 10.1523/JNEUROSCI.3773-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Howard MW, Eichenbaum H. The hippocampus, time, and memory across scales. J. Exper. Psychol. General. 2013;142:1211–1230. doi: 10.1037/a0033621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dragoi G, Tonegawa S. Selection of preconfigured cell assemblies for representation of novel spatial experiences. Phil. Trans. R. Soc. B. 2014;369:20120522. doi: 10.1098/rstb.2012.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng J, Ji D. Rigid firing sequences undermine spatial memory codes in a neurodegenerative mouse model. eLife. 2013;2:e00647. doi: 10.7554/eLife.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wallenstein GV, Eichenbaum H, Hasselmo ME. The hippocampus as an associator of discontiguous events. Trends Neurosci. 1998;21:317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- 73.Kesner RP, Hunsaker MR, Gilbert PE. The role of CA1 in the acquisition of an object-trace-odor paired associate task. Behav. Neurosci. 2005;119:781–786. doi: 10.1037/0735-7044.119.3.781. [DOI] [PubMed] [Google Scholar]
- 74.DeVito LM, et al. Vasopressin 1b receptor knockout impairs memory for temporal order. J. Neurosci. 2009;29:2676–2683. doi: 10.1523/JNEUROSCI.5488-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eichenbaum H, Cohen NJ. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron. 2014;83:764–770. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shimamura AP. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28:803–813. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- 78.Mayes AR, et al. Memory for single items, word pairs, and temporal order of different kinds in a patient with selective hippocampal lesions. Cogn. Neuropsychol. 2001;18:97–123. doi: 10.1080/02643290125897. [DOI] [PubMed] [Google Scholar]
- 79.Spiers HJ, Burgess N, Hartley T, Vargha-Khadem F, O'Keefe J. Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus. 2001;11:715–725. doi: 10.1002/hipo.1087. [DOI] [PubMed] [Google Scholar]
- 80.McDonough L, Mandler JM, McKee RD, Squire LR. The deferred imitation task as a nonverbal measure of declarative memory. Proc. Natl Acad. Sci. USA. 1995;92:7580–7584. doi: 10.1073/pnas.92.16.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adlam A-L, Vargha-Khadem F, Mishkin M, de Haan M. Deferrred imitation of action sequences in developmental amnesia. J. Cogn, Neurosci. 2005;17:240–248. doi: 10.1162/0898929053124901. [DOI] [PubMed] [Google Scholar]
- 82.Konkel A, Warren DE, Duff MC, Tranel DN, Cohen NJ. Hippocampal amnesia impaires all manner of relational memory. Front. Hum. Neurosci. 2008;2:15. doi: 10.3389/neuro.09.015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fortin NJ, Agster KL, Eichenbaum H. Critical role of the hippocampus in memory for sequences of events. Nature Neurosci. 2002;5:458–462. doi: 10.1038/nn834. [This study revealed that the hippocampus in rats is essential for remembering the order of a unique sequence of objects, even though it is not required to remember the objects themselves.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kesner RP, Gilbert PE, Barua LA. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behav. Neurosci. 2002;116:286–290. doi: 10.1037//0735-7044.116.2.286. [DOI] [PubMed] [Google Scholar]
- 85.Agster KL, Fortin NJ, Eichenbaum H. The hippocampus and disambiguation of overlapping sequences. J. Neurosci. 2002;22:5760–5768. doi: 10.1523/JNEUROSCI.22-13-05760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Devito LM, Eichenbaum H. Memory for the order of events in specific sequences: contributions of the hippocampus and medial prefrontal cortex. J. Neurosci. 2011;31:3169–3175. doi: 10.1523/JNEUROSCI.4202-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ergorul C, Eichenbaum H. The hippocampus and memory for “what,” “where,” and “when”. Learn. Mem. 2004;11:397–405. doi: 10.1101/lm.73304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Farovik A, Dupont LM, Eichenbaum H. Distinct roles for dorsal CA3 and CA1 in memory for nonspatial sequential events. Learn. Mem. 2010;17:801–806. doi: 10.1101/lm.1616209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ergorul C, Eichenbaum H. Essential role of the hippocampal formation in rapid learning of higher-order sequential associations. J. Neurosci. 2006;26:4111–4117. doi: 10.1523/JNEUROSCI.0441-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.DeCoteau WE, Kesner RP. A double dissociation between the rat hippocampus and medial caudoputamen in processing two forms of knowledge. Behav. Neurosci. 2000;114:1096–1108. doi: 10.1037//0735-7044.114.6.1096. [DOI] [PubMed] [Google Scholar]
- 91.Fouqet C, et al. Complementary roles of the hippocampus and the dorsomedial striatum during spatial and sequence-based navigation behavior. PLoS ONE. 2013;8:e67232. doi: 10.1371/journal.pone.0067232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kumaran D, Maguire EA. The dynamics of hippocampal activation during encoding of overlapping sequences. Neuron. 2006;49:617–629. doi: 10.1016/j.neuron.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 93.Ekstrom AD, Bookheimer SY. Spatial and temporal episodic memory retrieval recruit dissociable functional networks in the human brain. Learn. Mem. 2007;14:645–654. doi: 10.1101/lm.575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lehn H, et al. A specific role of the human hippocampus in recall of temporal sequences. J. Neurosci. 2009;29:3475–3484. doi: 10.1523/JNEUROSCI.5370-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ross RS, Brown TI, Stern CE. The retrieval of learned sequences engages the hippocampus: evidence from fMRI. Hippocampus. 2009;19:790–799. doi: 10.1002/hipo.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kumaran D, Maguire EA. An unexpected sequence of events: mismatch detection in the human hippocampus. PLoS Biol. 2006;4:e424. doi: 10.1371/journal.pbio.0040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tubridy S, Davachi L. Medial temporal lobe contributions to episodic sequence encoding. Cereb. Cortex. 2011;21:272–280. doi: 10.1093/cercor/bhq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. J. Neurosci. 2006;26:9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schendan HE, Searl MM, Melrose RJ, Stern CE. An fMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- 100.Brown TI, Ross RS, Keller JB, Hasselmo ME, Stern CE. Which way was I going? Contextual retrieval supports the disambiguation of well-learned overlapping navigational routes. J. Neurosci. 2010;30:7414–7422. doi: 10.1523/JNEUROSCI.6021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jenkins LJ, Ranganath C. Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. J. Neurosci. 2010;30:15558–15565. doi: 10.1523/JNEUROSCI.1337-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schapiro AC, Kustner LV, Turke-Browne NB. Shaping of object representations in the human medial temporal lobe based on temporal regularities. Curr. Biol. 2012;22:1622–1627. doi: 10.1016/j.cub.2012.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Copara MS, et al. Complementary roles of human hippocampal subregions during retrieval of spatiotemporal context. J. Neurosci. 2014;34:6834–6842. doi: 10.1523/JNEUROSCI.5341-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ezzyat Y, Davachi L. Similarity breeds proximity: pattern similarity within and across contexts is related to later mnemonic judgments of temporal proximity. Neuron. 2014;81:1179–1189. doi: 10.1016/j.neuron.2014.01.042. [This study uses multivoxel pattern analysis of functional MRI scans in humans to show that the similarity of representations across a series of events predicts the likelihood of remembering the order of those events, similar to findings in rodents based on neural ensemble firing patterns in reference 12.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.