Abstract
Quantitation of lipid storage, desaturation, and oxidation in live C. elegans has been a long-standing obstacle. By hyperspectral stimulated Raman scattering imaging and multivariate analysis in fingerprint vibration region, we present a platform that allows quantitative mapping of fat distribution, degree of fat unsaturation, lipid oxidation, and cholesterol storage in vivo in whole worm. Our results reveal for the first time that lysosome related organelles in intestinal cells are sites for storage of cholesterol in C. elegans.
Keywords: Cell metabolism, C. elegans, Lipids, Stimulated Raman scattering imaging, Vibrational spectroscopy
C. elegans, an intact and simple multicellular animal, is extensively used for studying the impact of lipid metabolism on aging and disease.[1] Yet, tools for identification and quantitation of chemical composition of stored lipids in living animals remain to be developed. The “vital” dyes Nile red[2] and BODIPY[3] were historically adopted for fat staining through feeding live worms. Recent studies found that both Nile red and BODIPY largely stain the lysosome related organelles (LROs) rather than the fat stored in the intestinal cells, gonad, germ line, and hypodermis.[4] Thus, the lipid storages and phenotypes revealed by Nile red and BODIPY fluorescence do not correlate with fat level measured by fixative staining, such as Sudan black and Oil red O, nor with biochemical analysis.[3, 5] Attempts to identify the ubiquitous fat storage in C. elegans were made by using nonlinear vibrational imaging technologies[6] based on coherent anti-Stokes Raman scattering (CARS)[7] or stimulated Raman scattering (SRS).[8] Single-frequency CARS[9] and SRS[10] allowed visualization of lipid droplets in live C. elegans by exploiting signals from the intrinsic carbon-hydrogen (C-H) stretching vibration. Despite this chemical bond specificity for lipids, the mapped fat distribution in the body of C. elegans did not fully co-localize with the pattern produced by vital or fixative dyes.[9b, 10–11] In fact, as nearly all biomolecules including protein, cholesterol and triglyceride contribute to the C-H vibrational signals in the highly congested spectral window from 2800 to 3100 cm−1, single-frequency CARS or SRS is not able to distinguish protein-rich organelles or gut granules[12] from fat droplets in the body of C. elegans. In addition, the CARS/SRS contrast based on C-H stretch vibrations is dominated by the signal from lipid droplets, making it difficult to detect othter cellular compartments inside the worm.
In this work, we overcome the abovementioned limitations by integration of hyperspectral SRS imaging[13] (Supporting Information, Figure S1), k-means clustering, and multivariate curve resolution (MCR) analysis[14] (Supporting Information, Figure S2) to identify and quantitate the chemical contents of intracellular organelles in live C. elegans, which is out of reach by either fluorescence or single-frequency SRS microscopy. Using hyperspectral SRS images that cover a fingerprint vibration window and spectral groups identified by k-means clustering as MCR inputs, we show quantitative mapping of fat distribution, degree of fat unsaturation, lipid oxidation, and cholesterol storage in vivo in a whole worm. Our results reveal for the first time that lysosome related organelles are sites for storage of cholesterol.
We first performed hyperspectral SRS imaging in the Raman region from 1620 to 1800 cm−1 of wild-type N2 worms and daf-2 (e1370) mutants, which bear a mutation of insulin-like growth factor 1 (IGF-1) receptor in the C. elegans.[15] Figure 1a,b presents the MCR results of a single wild-type worm and a daf-2 mutant both at the L2 stage, in which development of intestinal cells dominates the worm growth. In all worms examined, three types of subcellular compartments are found in most intestinal cells, as shown by the higher magnification image of the daf-2 mutant (Figure 1c–f and Supporting Information, Movie S1). The concentration maps produced by the MCR algorithm clearly reveal the distributions of neutral fat droplets, protein-rich organelles, and oxidized lipids in distinct sites of intestinal cells. The content in the lipid droplets is primarily in the form of triglyceride (Figure 1e), confirmed by Raman peaks of acyl C=C bond at 1655 cm−1 and ester C=O bond at 1745 cm−1 (Figure 1h). The proteins are distributed more uniformly in the entire worm body including hypodermis, intestinal lumen and intestinal cells (Figure 1g), characterized by the broad amide I band peaked at 1650 cm−1 (Figure 1h). In addition to the fat stores, we discovered one type of protein-rich organelles in large amounts filling the intestinal cells (Figure 1d). Importantly, these spherical organelles are spectrally distinct from either lipid droplets or pure protein based on the MCR-retrieved Raman spectra. Compared to the amide I band of protein, the spectral peak of those organelles is shifted to higher wave numbers, implying that they contain a significant amount of cholesterol, which possesses the sterol C=C stretching band at 1669 cm−1 (Figure 1h, green curve).[13f] These cholesterol-containing compartments are assigned to be lysosome related organelles based on detailed evidence shown below.
Figure 1.
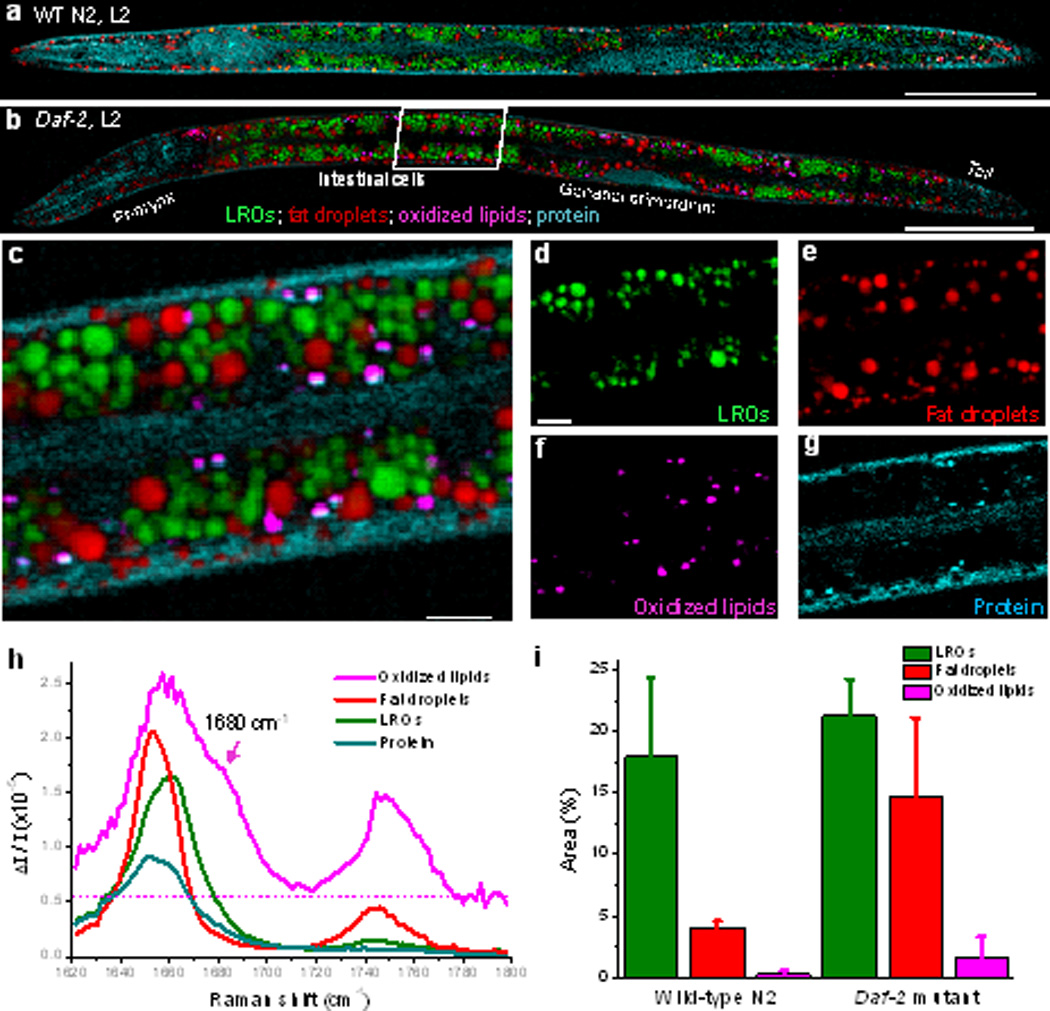
Compositional analysis of intracellular compartments in whole C. elegans by hyperspectral SRS imaging, k-means clustering and MCR analysis. a–b) MCR-retrieved concentration maps of neural fat droplets, lysosome related organelles (LROs), oxidized lipids, and protein in the body of whole wild-type and daf-2 mutants. Scale bar: 50 µm. c) Zoom-in of intestine cells indicated in (b). d–g) MCR-reconstructed concentration images of LROs, fat droplets, oxidized lipids, and protein, respectively. h) MCR-retrieved Raman spectra of corresponding compartments. The dotted line represents the pump-probe signal. Scale bar: 5 µm. i) Quantitation of amounts of LROs, fat droplets and oxidized lipids in wild-type N2 and daf-2 mutants (n ≥ 5) based on SRS spectral images of intestinal cells.
A third type of compartment found in the intestinal cells (Figure 1f) contains oxidized lipids, for which the SRS Raman spectrum showed a shoulder peak at 1680 cm−1 indicating the presence of aldehyde.[16] We note that a pump-probe signal, which is independent of Raman shift, was observed in the raw Raman spectrum, which implies that these compartments contain chromophores. The MCR algorithm is able to separate the SRS signal from the pump-probe background and retrieve background-free Raman spectra of detected chemicals (Figure 1h). To further explore the nature of the oxidized lipids, hyperspectral SRS imaging was performed in the region from 1520 to 1700 cm−1 (Supporting Information, Figure S3). A specific Raman peak at 1555 cm−1 was identified and assigned to the retinal C=C stretching band.[17] Together, these characteristic Raman peaks and the pump-probe signal suggest that we detected and located retinaldehyde, a known aging-related pigment stored in lipofuscin.[17]
To quantify the amount of fat, LROs and oxidized lipids in worms, we performed SRS imaging of intestinal cells in multiple wild-type worms (Supporting Information, Figure S4, n = 5) and daf-2 mutants (Supporting Information, Figure S5, n = 6). Areas in the intestinal cells occupied by fat, LROs, and oxidized lipids were normalized by the total imaged area of the worm (Supporting Information, Figure S6). In wild-type worms, 18% area of the intestinal cells is filled by cholesterol-rich LROs and 4% by neutral fat. Oxidized lipids appear much less in the whole body and the occupied area is <0.3% (Figure 1i). Compared to wild-type, the daf-2 insulin/IGF-1 receptor mutants show increased LROs and neutral fat storages by 1.2 and 3.6 folds, respectively. In addition, the amount of oxidized lipids is 6-fold greater than wild-type, suggesting that the insulin/IGF-1 signaling pathway regulates not only neutral fat storage but also lipid oxidation. The measured fold change difference of neutral fat between wild-type and daf-2 mutants is in agreement with earlier results by single-frequency SRS and thin-layer chromatography-gas chromatography.[10]
To investigate whether the observed cholesterol-containing organelles are LROs, we performed hyperspectral SRS imaging on adult wild-type worms fed LysoTracker, a marker for LROs (Supporting Information, Movie S2). The MCR concentration map in Figure 2a clearly shows that the neutral lipid droplets are distributed in both intestinal cells and undifferentiated germ cells in the gonad arm located above the intestine. The content of these lipid droplets is primarily in the form of triglyceride, confirmed by the presence of acyl C=C and C=O stretching band (Figure 2f). The retrieved protein map (Figure 2b) not only shows the protein distribution in regions of intestinal cells and lumen, but also reveals many compartments having similar size of lipid droplets. These protein-rich organelles do not overlap with lipid droplets, but co-localized with the MCR-retrieved third component shown in Figure 2c. The corresponding Raman spectrum derived from the MCR (Figure 2g, green curve) indicates a characteristic Raman peak of cholesterol at 1670 cm−1, and therefore suggests that these protein-rich organelles store a significant amount of cholesterol.[13f] Spontaneous Raman spectrum acquired at the position of LROs (Supporting Information, Figure S7) confirmed this finding. We further performed two-photon-excited fluorescence (TPEF) imaging of LysoTracker in the same area of the worm (Figure 2e). Comparing the MCR color-overly image (Figure 2d) and the TPEF image of LysoTracker highlights the co-localization of LROs with the cholesterol-containing compartments other than the lipid droplets. Figure 2f presents the raw SRS spectra of lipid droplets, LROs, and background protein at pixels indicated by arrows in Figure 2d. Figure 2g presents the MCR decomposed SRS spectra for neutral lipids, cholesterol, and protein. These data collectively suggest that LROs store both protein represented by amide I band and cholesterol represented by the sterol C=C band. To provide additional evidence that these cholesterol-rich compartments are indeed LROs, wild-type worms bearing a transgenic marker for LROs[4c] (hjIs9 [glo-1::gfp]) were imaged by hyperspectral SRS (Figure 2h) and TPEF signals (Figure 2i). As expected, all spherical LROs structures recognized by MCR were encircled by GLO-1::GFP fluorescence.
Figure 2.
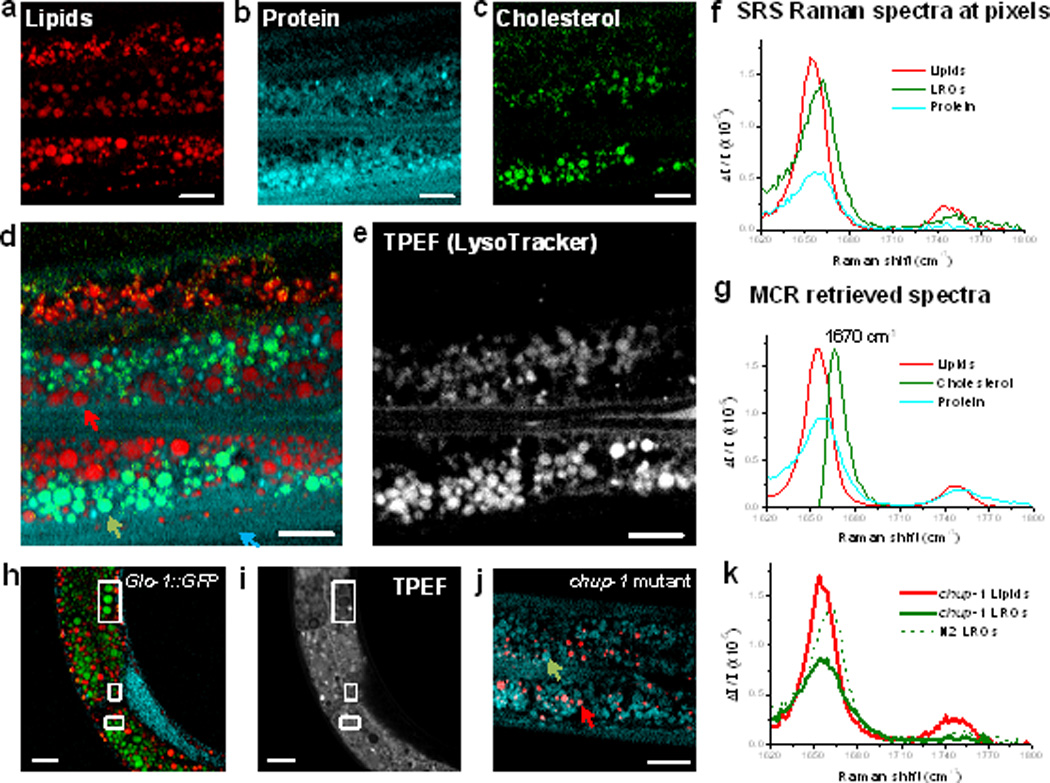
Hyperspectral SRS imaging and multivariate data analysis identify cholesterol storage compartments. a–c) MCR-reconstructed concentration maps of neutral lipid droplets, protein, and cholesterol in LROs. d) Color-overlay image of (a-c). e) TPEF image of the same area stained with vital LysoTracker. f) SRS spectra at pixels indicated by arrows in (d). g) MCR-retrieved Raman spectra of lipids, cholesterol and protein. h-i) MCR-concentration maps and TPEF image of LRO-specific GLO-1::GFP worm. j) MCR image of chup-1 mutant. k) SRS spectra at indicated compartments in (j). Dashed spectrum of LROs in wild-type is provided for comparison. Scale bar: 10 µm.
It is known that worms cannot synthesize sterols de novo[18] and rely on exogenous sources of cholesterol. We next asked whether mutations in genes implicated in trafficking of cholesterol to lysosomes would alter the cholesterol storage in LROs. To do this, we performed hyperspectral SRS imaging of chup-1(gk245) mutants, which lack the cholesterol uptake protein CUP-1.[19] MCR-reconstructed image (Figure 2j) and spectra (Figure 2k) showed the disappearance of the cholesterol peak at 1669 cm−1. Though LROs in worms were considered as fat storage compartments for a long time, it is shown here, for the first time by SRS spectral imaging, that LROs are actually the sites for cholesterol storage regulated by the CUP-1 protein. Consistently, it is worth noting that all fat stores are comprised of triglyceride and none contains esterified cholesterol based on the MCR spectra.
Hyperspectral SRS imaging in the fingerprint vibration region further allowed in situ analysis of lipids unsaturation. Quantitative chemical maps based on the intensity ratio of acyl C=C and ester C=O Raman bands, generated by MCR analysis, were used to determine the degree of unsaturation of the fat in different compartments. Figure 3a shows a MCR-reconstructed image of the gonad arm in a daf-2 mutant. The nucleus and cytoplasm, indicated in cyan, represent the area in the cell that is protein enriched. Four oocytes with a dense population of small size lipid droplets are visualized and highlighted in yellow. Below the oocytes, a few hypodermal lipid droplets were spotted. In the intestinal lumen, we found a large amount of lipid droplets (yellow) together with oxidized lipids (magenta). We also observed the gonadal sheath overlying the undifferentiated germ cells above the intestinal lumen (Supporting Information, Movie S3). SRS spectra at indicated pixels shown in Figure 3a, are given in Figure 3b to highlight the difference in the intensity of the C=C band normalized to the C=O band. These two bands were then used to quantify the degree of lipid unsaturation as follows: First, the hyperspectral SRS image stack was decomposed into two concentration maps (Figure 3d,e), based on the internal reference spectra of acyl C=C and ester C=O bands acquired from glyceryl trioleate (Figure 3c).[13f] Then, the image of unsaturation (Figure 3f) was generated by the intensity ratio between the acyl C=C and ester C=O bands at each pixel and interpreted as average number of C=C bonds per fatty acid chain in a triglyceride molecule. Figure 3f illustrates the apparent difference in degree of unsaturation among hypodermal fat, oocyte fat and gut deposits in the lumen of the intestine. Statistically, the hypodermal fat exhibits the highest degree of unsaturation ~ 2.0 and appears to be in the less-ordered liquid phase (Figure 3g). In contrast, the fat stored in oocytes are analogous to glyceryl trioleate which bears on average one C=C bond per fatty acid chain. The fat stored in the lumen resembles gel-like saturated lipid with the average degree of unsaturation being ~0.54. In separate experiments, we also checked the degree of unsaturation of lipids in the intestinal cells (Figure 3h) and the germ cells, and found their degree of unsaturation was close to 1.0, similar to lipids stored in oocytes. To explore the enabling applications of in vivo unsaturation measurement, we evaluated the hypodermal fat in fat-6; fat-5 double mutants C. elegans. Here, fat-6 is a stearoyl-CoA-Δ9-desaturase that converts stearic to oleic acid while fat-5 is a palmitoyl-CoA-Δ9-desaturase that converts palmitic to palmitoleic acid. From the unsaturation image shown in Figure 3i, the fat-6; fat-5 double mutants bear a lower degree of unsaturation in hypodermal fat compared to either wild-type or daf-2 mutants. Statistical measurements show their degree of unsaturation is about 1.3 (Figure 3g). Collectively these data show that SRS imaging of acyl C=C and ester C=O bands is capable of in vivo lipid unsaturation analysis with submicron spatial resolution.
Figure 3.
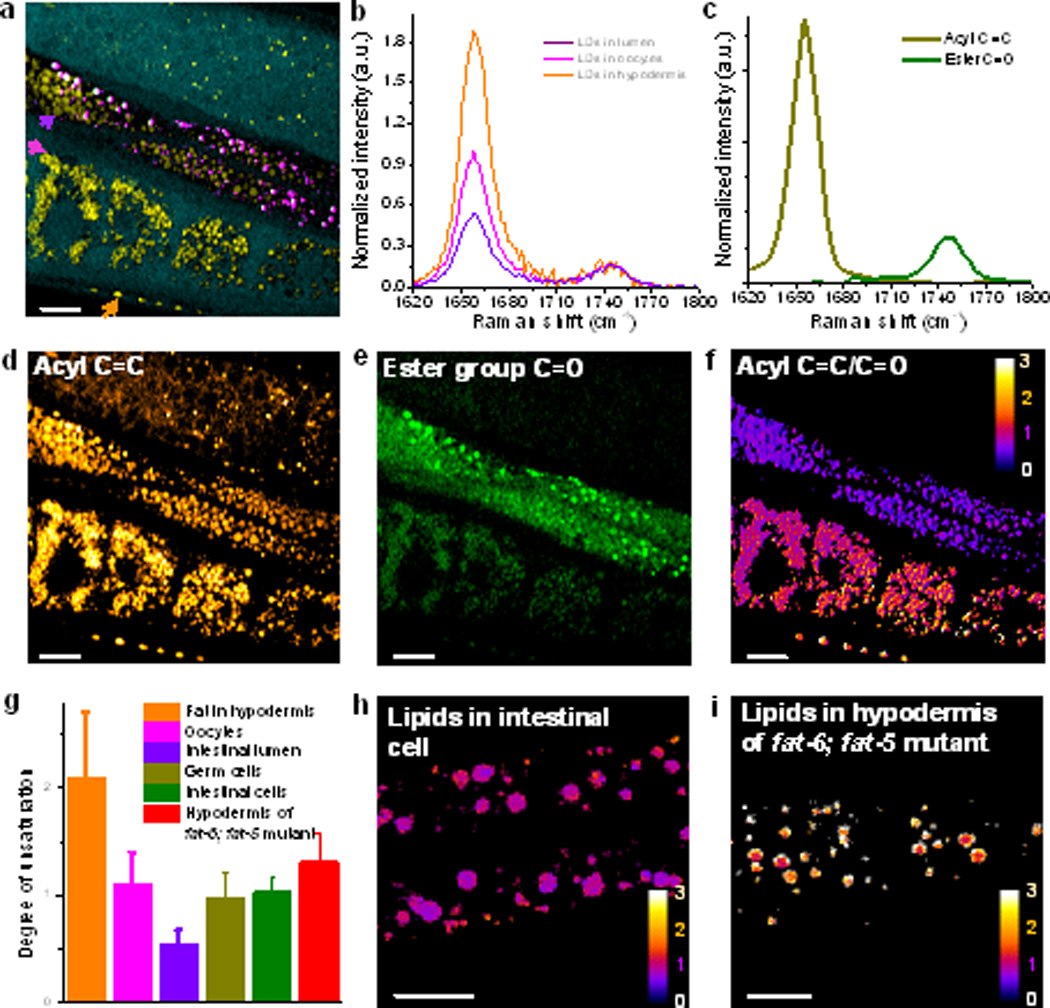
Mapping the degree of unsaturation of fat distributed in different segments of C. elegans. a) Color-overly image of neutral fat, proteins, and oxidized lipids in gonad arms of adult daf-2 worm. b) SRS spectra of three types of neutral fat at indicated position of worm in (a). c) MCR reference spectra of acyl C=C bond and ester group C=O bond. d-e) MCR-decomposed concentration maps corresponding to acyl C=C and ester C=O bonds. f) Degree of unsaturation map based on the ratio of (d) and (e). g) Statistical comparison of unsaturation of fat stored in the oocytes, lumen, germ cells, intestinal cells and hypodermis tissue of a fat-6; fat-5 double mutant. h) Unsaturation degree map of fat in intestinal cells of Figure. 1c. i) Unsaturation degree map of fat in hypodermis tissue in a fat-6; fat-5 double mutant. Scale bar: 10 µm.
In summary, hyperspectral SRS imaging in fingerprint vibration region, followed by k-means clustering and MCR analysis, allowed in situ identification and quantitation of three distinct lipid compartments in live worms. In particular, we found that LROs are sites for storage of cholesterol in C. elegans. With capability of mapping the degree of unsaturation, lipid oxidation, and cholesterol storage in vivo with subcellular spatial resolution, further studies by our platform promise to yield new insight into the impact of diet and the insulin/IGF-1 signaling pathway on obesity, diabetes, and longevity from C. elegans to mammals.
Methods
Details about experimental setup, specimen preparation, and MCR algorithm can be found in Supporting Information.
Supplementary Material
Footnotes
Some of the strains were kindly provided by the Caenorhabditis Genetics Center funded by NIH Office of Research Infrastructure Programs (P40 OD010440). This work was supported by National Institutes of Health grant GM104681 to JXC and grant AG031237 to HAT and an endowment from the William Randolph Hearst Foundation to HAT.
Supporting information for this article is available on the WWW under http://www.angewandte.org.
Contributor Information
Ping Wang, Email: jcheng@purdue.edu, Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN 47907 (USA), Fax: (+1) 765 496 1902.
Bin Liu, Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN 47907 (USA), Fax: (+1) 765 496 1902.
Delong Zhang, Department of Chemistry, Purdue University, West Lafayette, IN 47906 (USA).
Micah Y. Belew, Program in Gene Function and Expression, University of Massachusetts Medical School, Worcester, MA 01605 (USA)
Heidi A. Tissenbaum, Email: Heidi.Tissenbaum@umassmed.edu, Program in Gene Function and Expression, University of Massachusetts Medical School, Worcester, MA 01605 (USA).
Ji-Xin Cheng, Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN 47907 (USA), Fax: (+1) 765 496 1902; Department of Chemistry, Purdue University, West Lafayette, IN 47906 (USA).
References
- 1.a) Kaletta T, Hengartner MO. Nat. Rev. Drug Discovery. 2006;5:387. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]; b) Jones KT, Ashrafi K. Dis. Models & Mech. 2009;2:224. doi: 10.1242/dmm.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Watts JL. Trends Endocrinol. Metab. 2009;20:58. doi: 10.1016/j.tem.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenspan P, Fowler SD. J. Lipid Res. 1985;26:781. [PubMed] [Google Scholar]
- 3.Mak HY, Nelson LS, Basson M, Johnson CD, Ruvkun G. Nat. Genet. 2006;38:363. doi: 10.1038/ng1739. [DOI] [PubMed] [Google Scholar]
- 4.a) Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. Nature. 2003;421:268. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]; b) O'Rourke EJ, Soukas AA, Carr CE, Ruvkun G. Cell Metab. 2009;10:430. doi: 10.1016/j.cmet.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zhang SBO, Trimble R, Guo FL, Mak HY. BMC Cell Biol. 2010;11 doi: 10.1186/1471-2121-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Wang MC, O'Rourke EJ, Ruvkun G. Science. 2008;322:957. doi: 10.1126/science.1162011. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Brooks KK, Liang B, Watts JL. PLoS One. 2009;4:e7545. doi: 10.1371/journal.pone.0007545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng JX, Xie XS. Coherent Raman Scattering Microscopy. New York: Taylor & Francis Group; 2012. [Google Scholar]
- 7.Evans CL, Xie XS. Annu. Rev. Anal. Chem. 2008;1:883. doi: 10.1146/annurev.anchem.1.031207.112754. [DOI] [PubMed] [Google Scholar]
- 8.a) Ploetz E, Laimgruber S, Berner S, Zinth W, Gilch P. Appl. Phys. B: Lasers Opt. 2007;87:389. [Google Scholar]; b) Min W, Freudiger CW, Lu S, Xie XS. Annu. Rev. Phys. Chem. 2011;62:507. doi: 10.1146/annurev.physchem.012809.103512. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zhang D, Wang P, Slipchenko MN, Cheng JX. Acc. Chem. Res. 2014 doi: 10.1021/ar400331q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Hellerer T, Axang C, Brackmann C, Hillertz P, Pilon M, Enejder A. Proc. Natl. Acad. Sci. USA. 2007;104:14658. doi: 10.1073/pnas.0703594104. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yen K, Le TT, Bansal A, Narasimhan D, Cheng JX, Tissenbaum HA. PLoS One. 2010;5:e12810. doi: 10.1371/journal.pone.0012810. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Le TT, Duren HM, Slipchenko MN, Hu CD, Cheng JX. J. Lipid Res. 2010;51:672. doi: 10.1194/jlr.D000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang MC, Min W, Freudiger CW, Ruvkun G, Xie XS. Nat. Methods. 2011;8:135. doi: 10.1038/nmeth.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klapper M, Ehmke M, Palgunow D, Bohme M, Matthaus C, Bergner G, Dietzek B, Popp J, Doring F. J. Lipid Res. 2011;52:1281. doi: 10.1194/jlr.D011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laufer JS, Bazzicalupo P, Wood WB. Cell. 1980;19:569. doi: 10.1016/s0092-8674(80)80033-x. [DOI] [PubMed] [Google Scholar]
- 13.a) Ozeki Y, Umemura W, Otsuka Y, Satoh S, Hashimoto H, Sumimura K, Nishizawa N, Fukui K, Itoh K. Nat. Photonics. 2012;6:844. [Google Scholar]; b) Suhalim JL, Chung CY, Lilledahl MB, Lim RS, Levi M, Tromberg BJ, Potma EO. Biophys. J. 2012;102:1988. doi: 10.1016/j.bpj.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Fu D, Holtom G, Freudiger C, Zhang X, Xie XS. J. Phys. Chem. B. 2013;117:4634. doi: 10.1021/jp308938t. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Zhang D, Wang P, Slipchenko MN, Ben-Amotz D, Weiner AM, Cheng JX. Anal. Chem. 2013;85:98. doi: 10.1021/ac3019119. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Mansfield J, Moger J, Green E, Moger C, Winlove CP. J. Biophotonics. 2013;6:803. doi: 10.1002/jbio.201200213. [DOI] [PubMed] [Google Scholar]; f) Wang P, Li J, Wang P, Hu CR, Zhang D, Sturek M, Cheng JX. Angew. Chem. 2013;125:13280–13284. Angew. Chem. Int. Ed.2013, 52, 13042–13046. [Google Scholar]
- 14.Jaumot J, Gargallo R, de Juan A, Tauler R. Chemom. Intell. Lab. Syst. 2005;76:101. [Google Scholar]
- 15.Kimura KD, Tissenbaum HA, Liu YX, Ruvkun G. Science. 1997;277:942. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 16.Mukai Y, Koyama Y, Ito M, Tsukida K. J. Raman Spectrosc. 1986;17:387. [Google Scholar]
- 17.Eldred GE, Lasky MR. Nature. 1993;361:724. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- 18.Hieb WF, Rothstein M. Science. 1968;160:778. doi: 10.1126/science.160.3829.778. [DOI] [PubMed] [Google Scholar]
- 19.Valdes VJ, Athie A, Salinas LS, Navarro RE, Vaca L. PLoS One. 2012;7:e33962. doi: 10.1371/journal.pone.0033962. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


