Abstract
The specificity of estrogen signaling in brain is defined at one level by the types and distributions of receptor molecules that are activated by estrogens. At another level, as our understanding of the neurobiology of the estrogen synthetic enzyme aromatase has grown, questions have emerged as to how neuroactive estrogens reach specific target receptors in functionally relevant concentrations. Here we explore the spatial specificity of neuroestrogen signaling with a focus on studies of songbirds to provide perspective on some as-yet unresolved questions. Studies conducted in both male and female songbirds have helped to clarify these interesting facets of neuroestrogen physiology.
Introduction
The past 40 years have seen rise to an enormous body of research demonstrating that the expression and activity of the estrogen synthetic enzyme aromatase is a conserved property of the vertebrate brain. These studies have demonstrated brain aromatase in an extraordinary diversity of species from virtually every vertebrate lineage, that aromatase can be expressed in a wide variety of neural circuits, that aromatase is expressed in a diversity of cell types, is present in neurons in somata, processes and in terminals, that the enzyme is subject to diverse regulatory mechanisms, and that the substrates for brain aromatization can arise from peripheral or central steroidogenesis. On top of this, we also know estrogen receptors (ER) can be distributed in numerous subcellular locations, from nuclei to membrane sites potentially quite distal to ER-positive somata. The breadth and importance of this rather complex field of neuroestrogen synthesis has recently been covered quite extensively (Balthazart and Ball, 2013; Micevych, 2012), but questions remain. A crucially important set of issues involve how, within the brain’s highly heterogeneous steroidal environment, is spatial specificity of estrogen provision achieved (Schmidt et al., 2008). Here, we focus on three outstanding questions and on studies of neuroestrogen synthesis in the songbird brain to provide perspective on some as-yet unresolved questions.
A brief description of the traditional view of brain aromatization is needed to launch this discussion. The bulk of early studies on the role of brain aromatase focused on males and established the principle that testosterone secreted by the testes reached the brain where, in or near discrete regions that expressed aromatase, locally produced 17β-estradiol masculinized (or defeminized) neural circuits developmentally and, in adults, activated circuits to produce a masculine behavioral phenotype. The key features that are crucial here are a) that the testosterone was produced peripherally in males in which there was little or no peripherally produced neuroactive estradiol (a condition that stood in stark contrast with females that secreted ample estradiol from their ovaries to activate feminine behaviors); b) steroids were thought to diffuse liberally in brain so gonadal testosterone was available to the whole brain but was only locally converted to estradiol where aromatase was found; c) that aromatase was close to target neurons expressing receptors for estrogens; and d) during development, and possibly also in adults, the brains of both males and females were protected from inappropriate exposure to any peripherally produced estrogen, that is ovarian or maternal, by binding proteins in blood. Whereas much of this foundational work remains undisputed, recent studies regarding direct neurosteroidogenesis, diverse functions for brain aromatase outside of the control of reproductive behavior and physiology, and the rapid neuromodulatory roles for estrogens, force expansion of some of these basic concepts. The following are three questions that are unresolved but which bear strongly on our concepts of the spatial specificity of neuroestrogen action:
When aromatase is present in brain and estradiol is available from the periphery, how do neural estrogen targets restrict or balance their responses to peripheral vs centrally produced estradiol?
As steroids are lipophyllic molecules, they are often conceived as diffusing relatively freely in brain. How then are estrogen actions spatially restricted near aromatase-expressing cells? In other words, how do estrogen-dependent neural circuits preserve the spatio-temporal fidelity of the estrogen signal?
The two previous questions focus solely on neuroactive estrogens. As estrogen synthesis requires androgenic substrates, how do neural circuits balance their access to peripherally derived androgens with those potentially derived from nearby neurosteroidogenic circuits?
Songbird Brain Aromatase
As we will highlight work on songbirds, a description of brain aromatization in these birds is essential before expanding on these basics in subsequent sections. Much early work on avian aromatase focused on non-songbirds, like doves and quail (Schlinger and Balthazart, 2013), and showed that, as in some mammals, aromatase was expressed at its highest levels in regions of the hypothalamus and other parts of the animal’s “social brain” (Goodson, 2005; Newman, 1999). Songbirds differ taxonomically from these other species and are attractive animal models in neurobiological research because they a) sing complex songs, acoustic signals used for reproductive and non-reproductive communication; b) they learn these songs from their father (or other tutor) developmentally; and c) they possess a unique complex set of sex-steroid sensitive neural circuits that enable song learning and production. Investigation of brain aromatase in these birds showed that the enzyme was expressed widely, in “social brain” sites as in non-songbirds, as well as in many other brain regions (Saldanha et al., 2013). Aromatase in the songbird brain was present in neuronal somata, as well as in processes that projected locally or to more distal brain regions t hat lacked somal aromatase (Saldanha et al., 2000). Aromatase-positive synaptic terminals were seen to contact aromatase-positive and negative dendrites, axons and somas (Peterson et al., 2005).
One site of especially high aromatase in songbirds was found in the caudal nidopallium. Recent studies demonstrate that estrogens formed in this region, that includes an auditory processing area called NCM, fluctuate independently of estrogens in blood and do so in response to appropriate auditory and/or visual stimuli (Remage-Healey et al., 2012; Remage-Healey et al., 2008). These estrogens produced in the NCM rapidly boost local auditory responses to song stimuli and appear to improve auditory perception (Remage-Healey et al., 2010).
Another site of especially high aromatase in several songbird species is the hippocampus (HP) (Saldanha et al., 1998). This region, strongly associated with spatial learning and memory capabilities (Patel et al., 1997; Watanabe & Bischof, 2004), is structurally elongated rostro-caudally in birds and caudally lies just dorso-medially to the NCM, though separated by the lateral ventricle. Estrogens impact spatial learning and memory in many species, including in songbirds (Oberlander et al., 2004; Rensel et al., 2013), and can do so by direct actions on the HP (Bailey et al., 2013).
Aromatase is constitutively expressed in neurons in the brains of mammals and birds, though in teleost fish, radial glia can be the dominant aromatase-positive cell (Forlano et al., 2001; Pellegrini et al., 2013). After neural injury in mammals and birds, aromatase-expression is up-regulated in reactive astrocytes (in mammals) and in astrocytes and radial glia (in songbirds) (Duncan et al., 2013; Peterson et al., 2004; Spence et al., 2009). Studies of songbirds show that injury-induced aromatase limits the spread of the injury and assists wi th some neural repair (Wynne & Saldanha, 2004; Saldanha et al., 2005; Peterson et al., 2007).
Thus, in the uninjured songbird brain, aromatase is present in numerous sites with known reproductive functions as well as in sites not traditionally associated with reproduction. Some of these estrogen-dependent regions are in close proximity to one another, like the NCM and the HP. Some brain regions contain aromatase-positive somata, fibers and terminals whereas other regions, or even sub-regions, contain just aromatase-positive fibers and terminals. Superimposed on this distribution, injury induces aromatase in new populations of glial cells. With this background, we now return to address questions about the spatial specificity of estrogen action on brain.
Question 1: When aromatase is present in brain and estradiol is available from the periphery, how do neural estrogen targets restrict or balance their responses to peripheral vs centrally produced estradiol?
The ovaries of female vertebrates secrete estradiol, in regular as well as periodic episodes, from sexual maturity until they become post-reproductive, and these estrogens are seen as activating neural circuits that yield the feminine behavioral phenotype. However, in the face of a peripheral supply of estrogens, there is still evidence that brain aromatase in females is functional, suggesting that central-produced and peripherally-produced estrogens have unique functions in brain. At least two examples have been demonstrated in songbirds. In one case, estrogen-dependent, auditory-induced neuronal responses in the NCM of female zebra finches are inhibited by local application of fadrozole, an inhibitor of the aromatase enzyme (Remage-Healey et al., 2012). In a second case, estrogen-dependent gene regulation associated with neural repair/neuroprotection in females is reduced by neural application of an aromatase inhibitor (Walters and Saldanha, 2008). An important common theme is that functional estrogen synthesis was inhibited locally, within the brain, in these two observations in females. Consequently, in both of these cases, despite the likelihood that estrogens are present in blood, ovarian estrogens seem to play little role in these neural estrogen-dependent actions.
Results like these raise several questions. Are ovarian estrogens somehow restricted from reaching some, but not all, neural target sites? Are the concentrations of estrogen in blood sufficient to activate some neural circuits, like those involved in female sexual behavior, but not others, such as circuits involved in auditory processing or in neural repair? Are these examples of widespread and ever present physiological systems that routinely function to allow circulating estrogens to activate only some neural pathways while allowing locally produced estrogens to only activate other cells and circuits?
Circulating steroids are thought to readily cross the blood-brain-barrier (Pardridge, 1981; Banks, 2012) or are thought to circulate bound to proteins that restrict or enhance their delivery to the brain. According to the free hormone hypothesis (Mendel, 1989), only ‘free’ or unbound steroid hormone can reach receptors on target tissues. Recent work with sex hormone binding globulins (SHBGs, which bind androgens and estrogens) and corticosteroid binding globulins (CBGs, which bind corticosteroids, progesterone, and testosterone) suggests that, in contrast to this view, hormone binding globulins act to maintain access of steroid to receptors through multiple mechanisms. First, CBGs in mammals reduce clearance rates of circulating corticosterone, ensuring that stress-induced concentrations are adequate to reach targets in the brain and impact behavior (Minni et al., 2012; Moisan et al., 2013). Second, both CBGs and SHBGs are locally produced in several mammalian brain regions (Herbert et al., 2003; 2004; Jirikowski et al.,2007; Mopert et al., 2006). SHBGs localized in the brain appear to facilitate uptake or sequestration of hormones, possibly through interaction with the ERβ receptor (Caldwell et al., 2007). Finally, uptake of estradiol in the hamster ovary is dependent on the presence of SHBG (Caldwell & Jirikowski, 2013). Therefore it appears that hormone binding globulins, both in the circulation and within distinct neural circuits, may be important for determining the local concentrations of hormone reaching and acting at target cells.
Unlike in mammals, songbirds do not possess a circulating SHBG (Deviche et al., 2001; Wingfield et al, 1984). It is therefore unlikely that hormone binding globulins alter the ability of ovarian-produced estradiol to access distinct regions of the songbird brain (although circulating albumins may non-specifically bind estradiol and other steroid hormones with lower affinity). However, circulating testosterone and dihydrotestosterone (5α-DHT), bind to CBGs in songbirds (no specific binding globulin for estradiol has been identified). While it is unknown whether the songbird brain synthesizes CBGs, it is possible that circulating CBGs play a role in the delivery of T to the brain, which can then be aromatized into estradiol in regions with abundant aromatase expression.
Despite the fact that circulating estradiol is not bound to hormone binding globulin in songbird blood, there is supportive evidence for the notion that neural targets associated with reproduction in females are more responsive to blood concentrations of free estradiol than areas not directly linked to reproduction. First, many of these ‘reproduction-related’ areas were the first to be described as neural target sites using the earliest methods to identify estrogen receptors in brain. Across many species, including in songbirds, biochemical techniques of steroid binding and radioactive steroid autoradiography clearly revealed estrogen-binding or estrogen receptors in brain regions associated with reproduction such as the hypothalamus, pre-optic area, and bed nucleus of the stria terminalis, amongst others (e.g. Gahr et al., 1987; Metzdorf et al., 1999). Discovery of a second estrogen receptor (ERβ; Kuiper et al., 1996; 1998) and the use of more sensitive techniques such as immunocytochemistry and in situ hybridization confirmed these earlier studies but also revealed more widespread expression of ER (Ball et al., 1999). Evidence for the existence of membrane ER was dismissed as artifact and often ignored (Pietras and Szego, 1999), though we now know of their presence in a wide range of species, including in songbirds (see below). It is possible, therefore, that in females, the higher concentrations of receptor enable relatively low circulating concentrations of estradiol to activate specific circuits associated with reproduction. Similarly, in males, these regions would be activated by concentrations of estradiol produced from local aromatization of circulating testosterone, but only when circulating T and local aromatase were elevated in reproductively active birds (Schlinger and Callard, 1989).
Brain regions with lower expression levels of ERs might have limited responses to ovarian-derived levels of estradiol but might require higher local concentrations of estradiol provided by targeted, nearby estrogen synthesis. In addition to binding the classical nuclear receptors, recent work suggests that locally produced estrogens may act via a non-traditional mechanism, through binding to a membrane receptor. There is convincing evidence that estrogens act via membrane receptor-dependent processes (Micevych, 2012), and one membrane ER has recently been characterized in a songbird (Acharya & Veney, 2012). In songbirds, aromatase is present within some synaptic boutons, where its activity is regulated by depolarization and Ca+-dependent phosphorylation (Cornil et al., 2012b; Remage-Healey et al., 2011) and where estrogen concentrations might be especially elevated in the proximity of membrane ER. This appears to be the case in the NCM of the zebra finch where these locally produced estrogens impact auditory responses and processing via a membrane-dependent receptor system (Remage-Healey et al., 2012). It may well be that the concentrations of estradiol produced at or near the synapse exceed those that arrive from the circulation and are able to activate membrane receptors that are left inactivated by circulating estradiol concentrations. It is worth remembering that there was a lengthy history of evidence for rapid, non-genomic effects of steroids that were largely ignored in part because the concentrations employed by researchers were considered pharmacological, exceeding what is normally present in blood (Cornil et al., 2012a). It is likely that these pharmacological concentrations were indeed physiological with respect to local brain regions but not with respect to the periphery.
We cannot dismiss the possibility that ovarian androgens are converted in some brain regions into neuroactive estrogens. Remarkably, there is still some debate about the true concentrations of free androgen and estrogen in blood across ovulatory cycles as different assays give varying results (Fogle et al., 2007; Morley and Perry, 2003; Yasui et al., 2012). Typically, androgens circulate in blood of adult females at levels below what is required to promote expression of masculine characters by actions on peripheral androgen receptors. Nevertheless, given the high affinity aromatase has for its androgenic substrate (Lephart, 1996), these concentrations of androgen in blood may be sufficient to serve as substrate for functional aromatization in brain. Ovariectomy is commonly utilized to eliminate circulating estrogens. When ovariectomy eliminates a neural effect that is restored by estrogen replacement, invariably, the conclusion is that it was the circulating estrogens that were replaced. Experiments that do not also test replacement with aromatizable androgen ignore the possibility that some circulating androgens are aromatized in the brains of females to locally synthesize bioactive estrogen. Androgen replacement does not always replicate effects of ovariectomy (Saldanha et al., 2009), so it is likely that plasma androgens are functionally aromatized in the female brain under specific conditions.
2) As steroids are lipophyllic molecules, they are often conceived as diffusing relatively freely in brain. How then are estrogen actions spatially restricted near aromatase-expressing cells? In other words, how do estrogen-dependent neural circuits preserve the spatio-temporal fidelity of the estrogen signal?
Circulating steroids are typically seen as moving relatively freely through cellular and extracellular compartments to reach all their neural targets including behaviorally-relevant target neurons as well as circuits of the hypothalamic-pituitary-gonadal axis on which estrogens provide negative feedback. The question of just how freely steroids move and how far they can go has, to our knowledge, received little experimental attention. In many cases, aromatase expressing cells are themselves targets of estrogen or their somas are positioned in close proximity to the somas of ER-expressing cells (e.g. in birds (Saldanha and Coomaralingam, 2005). Thus, the neuroactive estrogens need only diffuse a short distance through intra- or extracellular space to reach their target receptor molecules. Aromatase is bound to endoplasmic reticulum which can be dispersed throughout the cytoplasm, including in processes and, as we shall see, in terminals. Thus, when there is a high concentration of aromatase-positive neurons in close proximity within a brain nucleus, there is also an abundance of aromatase-positive fibers (dendrites and axons), giving aromatase a significant cellular surface area in which to synthesize estrogen from any aromatizable androgen that reaches these nuclei. This perspective raises some questions however. Do these locally produced estrogens diffuse to other brain areas in neuroactively sufficient concentrations (i.e. can one brain nucleus communicate with another via the diffusion of the steroid it produces)?
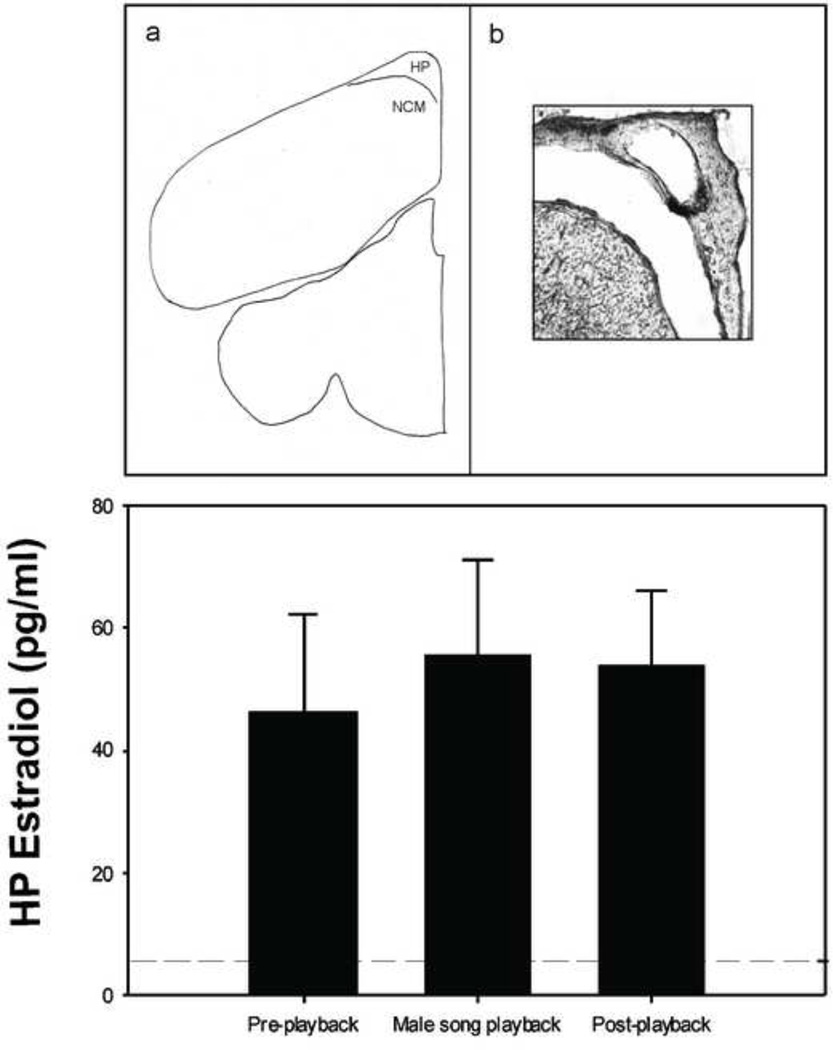
Work on the songbird brain points to relatively limited diffusion of significant quantities of estrogen from neural sites of synthesis lending support for the notion that steroids do not readily diffuse through the brain as has been widely believed. First, we have evidence that aromatase expressing cells project into regions that lack aromatase-expressing neuronal soma (Saldanha et al., 2000). This leads to a condition where some areas of the brains have somas whereas others have only fibers and terminals with aromatase (Peterson et al., 2005). Thus, one region can control the estrogen content of another by projecting afferents in that area. Second, we can measure estrogens in the auditory NCM of zebra finch males and females using in vivo microdialysis. Using this technique, we reliably detect estradiol in brain, including acute fluctuations in response to appropriate sensory stimulation (Remage-Healey et al., 2008) These changes are not detected in adjacent areas of the nidopallium that lack cellular aromatase (Remage-Healey et al., 2008). Thus, with the possible exception of the bloodstream, locally produced estrogens are not diffusing in significant amounts outside of the NCM. This observation has been confirmed recently by performing microdialysis of the aromatase rich HP directly dorsal to NCM. While estradiol levels in the NCM of male zebra finches increase in response to playback of male song (Remage-Healey et al., 2008), male song did not elicit any changes in estradiol levels in the HP just dorsal to the NCM (Fig. 1). Third, studies that microdissect specific regions of the zebra finch brain find that concentrations vary widely with some regions matching levels seen in blood whereas other differ markedly (Charlier et al., 2011; Chao et al., 2011; Fokidis et al., 2013). Finally, we have collected surprising results in acute experiments in which two microdialysis probes are positioned within the NCM at a ~ 500 µm distance from eachother. When one probe is perfused with estradiol (110 µM) and the other is perfused with aCSF, the latter is unable to register a detectable increase in baseline estradiol (as would be expected from passive diffusion away from the former) in a limited sampling window (60 min), as measured by ELISA (Remage-Healey and Schlinger, unpubl. obs.). While there are certainly procedural issues associated with this experiment, one viable explanation is that local estradiol levels are spatially restricted in the songbird brain.
Figure 1.
Top panel: a) coronal section schematic showing location of caudal telencephalon targeted for microdialysis in the HP and NCM (figure adapted from zebra finch brain atlas; coordinates = 1.35mm rostral to the bifurcation of the midsaggital sinus (Nixdorf-Bergweiler & Bischof, 2007; Remage-Healey et al., 2008). The HP and NCM lie adjacent to one another separated by the lateral ventricle. b) Photomicrograph showing typical damage to the left HP caused by the microdialysis probe. Note the artificially enlarged distance between the HP and underlying caudal telencephalon (lateral ventricle) caused by section mounting. Bottom panel: mean HP estradiol levels (± 1 SEM) in male zebra finch HP (n= 4) for 30 minutes before, during, and after male song playback. Unlike in the adjacent NCM (Remage-Healey et al., 2008), there was no increase in HP estradiol levels in response to playback (F2,6 = 0.55; P = 0.605). Microdialysis details: during guide cannula implantation surgery, a CMA7 microdialysis probe was aimed at the following coordinates: 1.6mm rostral to the bifurcation of the midsagittal sinus and 0.5mm lateral to the midline. The guide cannula was placed on the surface of the brain and secured in place with dental cement. To begin collection, the microdialysis probe was implanted and secured with cyanoacrylate, then constantly perfused with artificial cerebral spinal fluid at a rate of 2µl/min. Individuals were housed singly in soundproof chambers with ad lib food and water throughout microdialysis sample collection. Estradiol levels were assessed in HP dialysate using the Cayman Chemical Estradiol EIA kit. For more details on microdialysis procedures, see Remage-Healey et al., 2008.
Taken together, these observations suggest that despite the relatively enhanced capacity to synthesize estrogens in the songbird brain due to the widespread expression of aromatase, the estrogens synthesized in brain exert their actions locally, retaining the integrity of their actions over estrogen-dependent targets. Interestingly, the restricted spatial spread (~ 500–1000 µm) of estrogens in the brain has been observed in a variety of preparations in the past (e.g., Davis et al., 1982; Delville and Blaustein, 1991). We, and others, have postulated that estradiol functions as a true, intrinsic neuromodulator in brain (Balthazart and Ball, 2006; Saldanha et al., 2011). Spatial specificity of action is a core feature of any neuromodulatory system, thus this perspective contributes to this important view about fast-acting neuroestrogens. We do not know if specificity is achieved by simple laws of diffusion whereby estrogen concentrations rapidly decline as a function of distance from sites of synthesis or, as mentioned previously, whether hormone binding globulins could sequester neurosteroids. Along these same lines, it is also possible that neuroestrogens undergo fairly rapid enzyme catalyzed biochemical modifications to limit their actions to discrete neural circuits adjacent to their site of synthesis.
We cannot exclude the possibility that some estrogen concentrations in some brain regions are limited by the active secretion of estrogen into the circulation for their subsequent elimination by excretion. Estrogens made in the songbird brain can be found exiting in jugular blood (Schlinger and Arnold, 1992), but whether this stems from passive diffusion down a concentration gradient or active secretion from brain is unknown. Studies addressing these various possibilities are needed to help ascertain how local estrogen concentrations in brain are achieved.
3) The two previous questions focus solely on neuroactive estrogens. As estrogen synthesis requires androgenic substrates, how do neural circuits balance their access to peripherally derived androgens with those potentially derived from nearby neurosteroidogenic circuits?
Aromatase catalyzes the synthesis of bioactive estrogens from androgenic substrates. Thus, to fully understand properties of neuroestrogen synthesis the source of the substrate for the aromatase reaction and the mechanisms that alter the local concentrations of the androgenic substrate must be considered. We have evidence in songbirds for three possible sources of androgens for neural aromatization: secretion of the aromatizable gonadal androgens testosterone and androstenedione (AE), secretion of the active androgen precursor dehydroepiandrosterone (DHEA) by the adrenals (or gonads) followed by its conversion in brain into an aromatizable androgen, and the synthesis of aromatizable androgens by the brain itself (Schmidt et al., 2008). Do birds actually utilize all of these sources and, if so, how and where are they utilized? Also, does neural aromatase impact local androgen levels?
There is little doubt that circulating T, and possibly also AE, serve as substrates for brain aromatization in reproductively active male songbirds (Balthazart, 1983; Wingfield and Farner, 1993). Less is known about the sources of androgen utilized by brain aromatase when males are not breeding, i.e. when the testes are regressed and not steroidogenic, or in females, whose peripheral testosterone production is often lower than that of males (but see Jawor, 2007; Ketterson et al., 2005).
One possible source involves adrenal (or gonadal) synthesis and secretion of DHEA. DHEA can be acted on by the enzyme 3β-hydroxysteroid dehydrogenase (3β-HSD) to produce AE, which can then undergo aromatization into estrone (E1) or undergo conversion into T via 17β-hydroxysteroid dehydrogenase (17β-HSD) (Type 1). As we know, T serves as the substrate for aromatization to produce estradiol, or 17β-HSD can convert E1 into estradiol. Thus, regions that co-express 3β-HSD, 17β-HSD and aromatase are potential targets for neuroestrogen production, and can be active when T in blood is basal, but DHEA is present in blood.
There is convincing evidence that both 3β-HSD and 17β-HSD are expressed and active in the zebra finch brain, and we have additional evidence that these enzymes can work coordinately with aromatase to synthesize active estrogens (Soma et al., 2004; Tam and Schlinger, 2007; Vanson, 1996). Note that although 17β-HSD Type 1 has not been mapped in the songbird brain, its activity has been detected in many regions of the zebra finch brain (B. Schlinger, personal obs) and we assume it is present throughout as discussed below. There is also good evidence that the songbird adrenals secrete DHEA during the non-breeding season (Newman et al., 2008; Spinney et al., 2006) that can activate masculine territorial behavior (Shah et al., 2011; Soma and Wingfield, 2001). Female songbirds also secrete DHEA (Chin et al., 2008; Hau et al., 2004; Shah et al., 2011; Soma & Wingfield, 2001; Spinney et al., 2006), although the function of this circulating DHEA has not been well-explored. Thus, when DHEA is available from the periphery, some spatial specificity for estrogen signaling can be achieved by those brain regions that express 3β-HSD and aromatase. Such specificity could be achieved by neurons that express all the enzymes, or by aromatase-positive terminals residing within regions that make aromatizable androgenic substrate available from DHEA. For example, nucleus taeniae (Tn) expresses aromatase and 3β-HSD, but not all other steroidogenic factors, so it is a region that might utilize DHEA as a substrate for the synthesis of estrogens (Table 1).
TABLE 1.
Presence or absence of upstream steroidogenic factor expression in brain regions expressing aromatase (mRNA or protein) in the zebra finch1
| Brain Area | StAR | CYP11A1 | 3β-HSD | CYP17 |
|---|---|---|---|---|
| NC | + | + | + | ? |
| HP | + | + | + | ? |
| HVC | + | + | + | + |
| RA | + | + | + | − |
| Tn | − | + | + | ? |
| nSt | ? | ? | ? | + |
| mPOA | + | + | + | + |
| PVN | + | + | + | + |
| VMN | + | + | + | + |
| Tu | − | + | − | ? |
| SNc | + | + | − | ? |
| VTA | + | + | − | ? |
Data compiled from (London et al., 2006; London et al., 2003; Peterson et al., 2005; Saldanha et al., 2000; Shen et al., 1995) Symbols indicate presence (+), absence (−) or undescribed (?) expression. NC, caudal nidopallium; HP, hippocampus; HVC, HVC; RA, robust nucleus of the arcopallium; Tn, nucleus taeniae; nSt, nucleus of the stria terminalis; mPOA, medial preoptic nucleus; PVN, paraventricular nucleus; VMN, ventromedial nucleus; Tu, nucleus tuberis; SNc, substantia nigra pars compacta; VTA, ventral tegmental area.
This latter issue is further complicated by the possibility that some brain regions may synthesize DHEA independent of the adrenals and it is this DHEA that serves as the initial substrate for subsequent reactions that produce neuroactive estrogens. We have documented expression in the zebra finch brain of a complete set of genes involving cholesterol transport and steroidogenesis that could produce the neuroactive androgens and estrogens (Steroidogenic acute regulatory protein, StAR; Cytochrome P450 (CYP) side-chain cleabage enzyme; CYP11A1; 17a-hydroxylase (CYP17), 3β-HSD) ; London et al., 2003; London et al., 2006). Similar to the data for 3β-HSD alone, these enzymes/transporters have unique distributions likely producing a variety of steroidal molecules only some of which may lead to the formation of estrogens. Interestingly, two hypothalamic nuclei (mPOA, medial preoptic nucleus; PVN, paraventricular nucleus) seem to express a complete set of genes to synthesize estradiol from cholesterol (Table 1). The song system nucleus HVC expresses genes for the steroidogenic factors, but lacks aromatase expression (Table 1). Nevertheless, there are aromatase-positive projections into HVC as well as aromatase-positive terminals (Peterson et al., 2005; Saldanha et al., 2000) suggesting that estrogen synthesis occurs at some synapses in the zebra finch HVC from locally produced substrates. This kind of spatial heterogeneity adds new layers of complexity involving steroidal micro-environments in brain.
This latter point is ultimately key to this overall discussion. Do steroidal micro-environments exist in brain or is the brain somewhat awash with steroids diffusing helter-skelter from distal and proximal sites of synthesis? Studies from in vivo microdialysis provide some perspective. Somewhat curiously, when an aromatase inhibitor is locally applied to the songbird NCM and estrogen levels decline, as measured during 30mins intervals of in vivo microdialysis, local levels of testosterone show a concomitant rise (Remage-Healey et al., 2008). If the brain was being continuously “perfused” with relatively stable levels of testosterone from the periphery, and this T that was serving as substrate for local synthesis of estradiol, we might not expect to see a significant change in T levels. It appears more as though there is a somewhat stable “pool” of T that is altered by local aromatization. Together with measures of estradiol involving microdialysis, as well as direct measures of estradiol in microdissected brain regions, these studies suggest that there are complex dynamics of androgen and estrogen in brain that are highly localized.
Summary and Conclusions
Although it may not be possible to fully answer the principal questions that have motivated this review, we believe these observations stimulate a shift in some concepts regarding neuroestrogen physiology, certainly in the songbird brain, and perhaps also in the brains of other vertebrates. First, there is evidence for distinct functions within specific brain regions for estrogens produced peripherally versus those produced centrally. It would be useful to determine whether there are discrete mechanisms that limit or enhance access of circulating estrogens to brain. Moreover, we would benefit from increasing the resolution with which we can measure actual concentrations of estrogen in discrete brain regions as well as determining what concentrations are needed to activate distinct receptor populations. Second, diffusion of estrogens in brain is limited, so only ER in close proximity to aromatase-positive cells or projections are subject to activation. This site-fidelity of action enables estrogens to function as true neuromodulators within discrete neural circuits. Finally, the cellular and subcellular localization of steroidogenic enzymes that provide the substrates for aromatization create a heterogeneous steroidal environment such that some estrogen- producing brain regions only derive androgens from the periphery, while others receive substrate from circulating precursors, like DHEA, and others synthesize androgenic substrate de novo. Added to this, aromatase-expressing somata may reside within an area that has androgen-synthetic capabilities while some neurons may project aromatase-positive terminals into androgen synthetic sites. When considered altogether, it is clear that there is still a long way to go before we will fully comprehend brain estrogen dynamics and there remains ample room for future empirical and theoretical advances on this topic.
Highlights.
Neuroestrogens are synthesized by aromatase in the songbird brain
Neuroestrogen actions are locally regulated in the brain
The songbird brain balances peripheral and central androgens and estrogens
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharya KD, Veney SL. Characterization of the G-protein-coupled membrane bound estrogen receptor GPR30 in the zebra finch brain reveals a sex difference in gene and protein expression. Dev Neurobiol. 2012;72:1433–1446. doi: 10.1002/dneu.22004. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Ma C, Soma KK, Saldanha CJ. Inhibition of hippocampal aromatization impairs spatial memory performance in a male songbird. Endocrinol. 2013;154:4707–4714. doi: 10.1210/en.2013-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball GF, Bernard DJ, Foidart A, Lakaye B, Balthazart J. Steroid sensitive sites in the avian brain: does the distribution of the estrogen receptor alpha and beta types provide insight into their function? Brain Behav Evol. 1999;54:28–40. doi: 10.1159/000006609. [DOI] [PubMed] [Google Scholar]
- Balthazart J. Hormonal Corrleates of Behavior. In: Farner DS, King James R, Parkes Kenneth C, editors. Avian Biology. New York: Academic Press; 1983. pp. 221–365. [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Neuroendocrinology. Oxford: Oxford Univ. Press; 2013. Brain Aromatase, Estrogens and Behavior, Behavioral; p. 530. [Google Scholar]
- Banks WA. Brain meets body: the blood-brain barrier as an endocrine interface. Endocrinol. 2012;153:4111–4119. doi: 10.1210/en.2012-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JD, Jirikowski GF. Sex hormone binding globulin and corticosteroid binding globulin as major effectors of steroid action. Steroids. 2013 doi: 10.1016/j.steroids.2013.11.010. in press. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Shapiro RA, Jirikowski GF, Suleman F. Internalization of sex hormone-binding globulin into neurons and brain cells in vitro and in vivo. Neuroendocrinol. 2007;86:84–93. doi: 10.1159/000107072. [DOI] [PubMed] [Google Scholar]
- Chao A, Schlinger BA, Remage-Healey L. Combined liquid and solid-phase extraction improves quantification of brain estrogen content. Front Neuroanat. 2011;5:57. doi: 10.3389/fnana.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Po KW, Newman AE, Shah AH, Saldanha CJ, Soma KK. 17beta-Estradiol levels in male zebra finch brain: combining Palkovits punch and an ultrasensitive radioimmunoassay. Gen Comp Endocrinol. 2010;167:18–26. doi: 10.1016/j.ygcen.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier T, Newman A, Heimovics S, Po K, Saldanha C, Soma K. Rapid effects of aggressive interactions on aromatase activity and oestradiol in discrete brain regions of wild male white-crowned sparrows. J Neuroendocrinol. 2011;23:742–753. doi: 10.1111/j.1365-2826.2011.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin EH, Shah AH, Schmidt KL, Sheldon LD, Love OP, Soma KK. Sex differences in DH EA and estradiol during development in a wild songbird: Jugular versus brachial plasma. Horm Behav. 2008;54:194–202. doi: 10.1016/j.yhbeh.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J. Rapid control of male typical behaviors by brain-derived estrogens. Front Neuroendocrinol. 2012a;33:425–446. doi: 10.1016/j.yfrne.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Leung CH, Pletcher ER, Naranjo KC, Blauman SJ, Saldanha CJ. Acute and specific modulation of presynaptic aromatization in the vertebrate brain. Endocrinol. 2012b;153:2562–2567. doi: 10.1210/en.2011-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PG, Krieger MS, Barfield RJ, McEwen BS, Pfaff DW. Implants in Feminine Sexual Behavior: An Autoradiographic Analysis. Endocrinol. 1982;111:1581–1586. doi: 10.1210/endo-111-5-1581. [DOI] [PubMed] [Google Scholar]
- Delville Y, Blaustein JD. A site for estradiol priming of progesterone-facilitates sexual receptivity in the ventrloateral hypothalamus of female guinea pigs. Brain Res. 1991;559:191–199. doi: 10.1016/0006-8993(91)90002-d. [DOI] [PubMed] [Google Scholar]
- Deviche P, Breuner C, Orchinik M. Testosterone, corticosterone, and photoperiod interact to regulate plasma levels of binding globulin and free steroid hormone in dark-eyed juncos, Junco hyemalis. Gen Comp Endocrinol. 2001;122:67–77. doi: 10.1006/gcen.2001.7613. [DOI] [PubMed] [Google Scholar]
- Duncan KA, Walters BJ, Saldanha CJ. Traumatized and inflamed--but resilient: glial aromatization and the avian brain. Horm Behav. 2013;63:208–215. doi: 10.1016/j.yhbeh.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007;92:3040–3043. doi: 10.1210/jc.2007-0581. [DOI] [PubMed] [Google Scholar]
- Fokidis HB, Prior NH, Soma KK. Fasting increases aggression and differentially modulates local and systemic steroid levels in male zebra finches. Endocrinol. 2013;154:4328–4339. doi: 10.1210/en.2013-1171. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Myers DA, Bass AH. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: aromatase enzyme and mRNA expression identify glia as source. J Neurosci. 2001;21:8943–8955. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahr M, Flügge G, Güttinger HR. Immunocytochemical localization of estrogen-binding neurons in the songbird brain. Brain Res. 1987;402:173–177. doi: 10.1016/0006-8993(87)91063-8. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau M, Stoddard ST, Soma KK. Territorial aggression and hormones during the non-breeding season in a tropical bird. Horm Behav. 2004;45:40–49. doi: 10.1016/j.yhbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Herbert Z, Jirikowski GF, Petrusz P, Englöf I, Caldwell JD. Distribution of androgen-binding protein in the rat hypothalamo-neurohypophyseal system, co-localization with oxytocin. Brain Res. 2003;992:151–158. doi: 10.1016/j.brainres.2003.08.041. [DOI] [PubMed] [Google Scholar]
- Herbert Z, Pollák E, Molnár L, Caldwell JD, Jirikowski GF. Co-transport of sex hormone-binding globulin/SHBG with oxytocin in transport vesicles of the hypothalamo-hypophyseal system. Horm Metab Res. 2006;38:291–293. doi: 10.1055/s-2006-925350. [DOI] [PubMed] [Google Scholar]
- Jawor JM. Testosterone in Northern Cardinals (Cardenalis cardinalis): possible influence of prolonged territorial behavior. The Auk. 2007;124:331–338. [Google Scholar]
- Jirikowski GF, Pusch L, Möpert B, Herbert Z, Caldwell JD. Expression of corticosteroid binding globulin in the rat central nervous system. J Chem Neuroanat. 2007;34:22–28. doi: 10.1016/j.jchemneu.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan JV, Sandell M. Testosterone in females: mediator of adaptive triats, contraint of sexual dimporphism, or both? Am Nat. 2005;166:585–598. doi: 10.1086/444602. [DOI] [PubMed] [Google Scholar]
- Kuiper G, Enmark E. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Nat Acad Sci. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, Van der Saag PT, Van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinol. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lephart ED. A review of brain aromatase cytochrome P450. Brain Res Rev. 1996;22:1–26. [PubMed] [Google Scholar]
- London S, Monks DA, Wade J, Schlinger BA. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinol. 2006;147:5975–5987. doi: 10.1210/en.2006-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Boulter J, Schlinger BA. Cloning of the zebra finch androgen synthetic enzyme CYP17: A study of its neural expression throughout posthatch development. J Comp Neurol. 2003;467:496–508. doi: 10.1002/cne.10936. [DOI] [PubMed] [Google Scholar]
- Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. 1989;10:232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- Metzdorf R, Gahr M, Fusani L. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J Comp Neurol. 1999;407:115–129. [PubMed] [Google Scholar]
- Micevych P. Membrane-initiated estradiol signaling regulates the central nervous system. Front Neuroendocrinol. 2012;33:329–330. doi: 10.1016/j.yfrne.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Minni AM, Dorey R, Pierard C, Dominguez G, Helbling J-C, Foury A, Beracochea D, Moisan M-P. Critical role of plasma corticosteroid-binding-globulin during stress to promote glucocorticoid delivery to the brain:impact on memory retrieval. Endocrinol. 2012;153:4766–4774. doi: 10.1210/en.2012-1485. [DOI] [PubMed] [Google Scholar]
- Moisan MP, Minni AM, Dominguez G, Helbling JC, Foury a, Henkous N, Dorey R, Béracochéa D. Role of corticosteroid binding globulin in the fast actions of glucocorticoids on the brain. Steroids. 2013 doi: 10.1016/j.steroids.2013.10.013. in press. [DOI] [PubMed] [Google Scholar]
- Mopert B, Herbert Z, Caldwell J, Jirikowsi G. Expression of corticosterone-binding globulin in the rat hypothalamus. Horm Metab Res. 2006;38:246–252. doi: 10.1055/s-2006-925344. [DOI] [PubMed] [Google Scholar]
- Morley JE, Perry HM., 3rd Androgens and women at the menopause and beyond. J Gerontol A Biol Sci Med Sci. 2003;58:M409–M416. doi: 10.1093/gerona/58.5.m409. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior - A node in the mammalian social behavior network. Advancing From The Ventral Striatum To The Extended Amygdala: Implications For Neuropsychiatry And Drug Abuse: In Honor Of Lennart Heimer. 1999:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Newman AEM, Pradhan DS, Soma KK. Dehydroepiandrosterone and corticosterone are regulated by season and acute stress in a wild songbird: jugular versus brachial plasma. Endocrinol. 2008;149:2537–2545. doi: 10.1210/en.2007-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixdorf-Bergweiler BE, Bischof HJ. Transverse and Sagittal Sections. Bethesda (MD): National Center for Biotechnology Information (US); 2007. A Stereotaxic Atlas Of The Brain Of The Zebra Finch, Taeniopygia Guttata: With Special Emphasis On Telencephalic Visual And Song System Nuclei. [Internet]. [Google Scholar]
- Oberlander JG, Schlinger BA, Clayton NS, Saldanha CJ. Neural aromatization accelerates the acquisition of spatial memory via an influence on the songbird hippocampus. Horm Behav. 2004;45:250–258. doi: 10.1016/j.yhbeh.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Pardridge W. Transport of nutrients and hormones through the blood-brain barrier. Diabetologia. 1981;20:246–254. doi: 10.1007/BF00254490. [DOI] [PubMed] [Google Scholar]
- Patel SN, Clayton NS, Krebs JR. Hippocampal tissue transplants reverse lesion-induced spatial memory deficits in zebra finches (Taeniopygia guttata) J Neurosci. 1997;17:3861–3869. doi: 10.1523/JNEUROSCI.17-10-03861.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini E, Vaillant C, Diotel N, Benquet P, Brion F, Kah O. Expression, regulation, and potential functions of aromatase in radial glial cells of the fish brain. In: Balthazart J, Ball GF, editors. Brain aromatase, estrogens and behavior. Oxford: Oxford Univ. Press; 2013. pp. 115–137. [Google Scholar]
- Peterson RS, Lee DW, Fernando G, Schlinger BA. Radial glia express aromatase in the injured zebra finch brain. J Comp Neurol. 2004;475:261–269. doi: 10.1002/cne.20157. [DOI] [PubMed] [Google Scholar]
- Peterson R, Fernando G, Day L, Allen T, Chapleau J, Menjivar J, Schlinger B, Lee D. Aromatase expression and cell proliferation following injury of the adult zebra finch hippocampus. Dev Neurobiol. 2013;67:1867–1878. doi: 10.1002/dneu.20548. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Roy Soc B. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras RJ, Szego CM. Cell membrane estrogen receptors resurface. Nature Med. 1999;5:1330. doi: 10.1038/70877. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci. 2010;107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong S, Maidment NT, Schlinger BA. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J Neurosci. 2011;31:10034–10038. doi: 10.1523/JNEUROSCI.0566-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol. 2012;107:1621–1631. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nature Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensel MA, Salwiczek L, Roth J, Schlinger BA. Context-specific effects of estradiol on spatial learning and memory in the zebra finch. Neurobiol Learning Memory. 2013;100:41–47. doi: 10.1016/j.nlm.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Coomaralingam L. Overlap and co-expression of estrogen synthetic and responsive neurons in the songbird brain - a double-label immunocytochemical study. Gen Comp Endocrinol. 2005;141:66–75. doi: 10.1016/j.ygcen.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Rohmann KN, Coomaralingam L, Wynne RD. Estrogen provision by reactive glia decreases apoptosis in the zebra finch (Taeniopygia guttata) J Neurobiol. 2005;64:192–201. doi: 10.1002/neu.20147. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Duncan KA, Walters BJ. Neuroprotective actions of brain aromatase. Front Neuroendocrinol. 2009;30:106–118. doi: 10.1016/j.yfrne.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Popper P, Micevych PE, Schlinger BA. The passerine hippocampus is a site of high aromatase: Inter- and intraspecies comparisons. Horm Behav. 1998;34:85–97. doi: 10.1006/hbeh.1998.1447. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev. 2011;32:532–549. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, Schlinger BA. Neuroanatomical distribution of aromatase in birds: Cellular and subcellular analyses. In: balthazart J, Ball GF, editors. Brain aromatase, estrogens and behavior. Oxford: Oxford Univ. Press; 2013. pp. 100–114. [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Arnold AP. Circulating estrogens in a male songbird originate in the nrain. Proc Nat Acad Sci. 1992;89:7650–7653. doi: 10.1073/pnas.89.16.7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Balthazart J. Aromatase and behavior: Concepts gained from studies of aromatase in the avian brain. In: Balthazart J, Ball GF, editors. Brain aromatase, estrogens and behavior. Oxford: Oxford Univ. press; 2013. pp. 169–198. [Google Scholar]
- Schlinger BA, Callard GV. Estrogen receptors in quail brain: a functional relationship to aromatase and aggressiveness. Biol Reprod. 1989;40:268–275. doi: 10.1095/biolreprod40.2.268. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Pradhan DS, Shah AH, Charlier TD, Chin EH, Soma KK. Neurosteroids, immunosteroids, and the Balkanization of endocrinology. Gen Comp Endocrinol. 2008;157:266–274. doi: 10.1016/j.ygcen.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Shah AH, Chin EH, Schmidt KL, Soma KK. DHEA and estradiol levels in brain, gonads, adrenal glands, and plasma of developing male and female European starlings. J Comp Physiol A. 2011;197:949–958. doi: 10.1007/s00359-011-0655-4. [DOI] [PubMed] [Google Scholar]
- Shen P, Schlinger BA, Campagnoni AT, Arnold AP. An atlas of aromatase mRNA expression in the zebra finch brain. J Comp Neurol. 1995;360:172–184. doi: 10.1002/cne.903600113. [DOI] [PubMed] [Google Scholar]
- Soma KK, Alday NA, Schlinger BA. DHEA metabolism by 3b-HSD in adult zebra finch brain: sex difference and rapid effect of stress. Endocrinol. 2004;145:1668–1677. doi: 10.1210/en.2003-0883. [DOI] [PubMed] [Google Scholar]
- Soma KK, Wingfield JC. Dehydroepiandrosterone in songbird plasma: seasonal regulation and relationship to territorial aggression. Gen Comp Endocrinol. 2001;123:144–155. doi: 10.1006/gcen.2001.7657. [DOI] [PubMed] [Google Scholar]
- Spence RD, Zhen Y, White S, Schlinger BA, Day LB. Recovery of motor and cognitive function after cerebellar lesions in a songbird: role of estrogens. Eur J Neurosci. 2009;29:1225–1234. doi: 10.1111/j.1460-9568.2009.06685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinney LH, Bentley GE, Hau M. Endocrine correlates of alternative phenotypes in the white-throated sparrow (Zonotrichia albicollis) Horm Behav. 2006;50:762–771. doi: 10.1016/j.yhbeh.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Tam H, Schlinger BA. Activities of 3B-HSD and aromatase in slices of the adult and developing zebra finch brain. Gen Comp Endocrinol. 2007;150:26–33. doi: 10.1016/j.ygcen.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanson A, Arnold AP, Schlinger BA. 3B-Hydroxysteroid dehydrogenase/isomerase and aromatase activity in primary cultures of developing zebra finch telencephalon: dehydroepiandrosterone as substrate for synthesis of androstenedione and estrogens. Gen Comp Endocrinol. 1996;102:342–350. doi: 10.1006/gcen.1996.0077. [DOI] [PubMed] [Google Scholar]
- Walters BJ, Saldanha CJ. Glial aromatization increases the expression of bone morphogenetic protein-2 in the injured zebra finch brain. J Neurochem. 2008;106:216–223. doi: 10.1111/j.1471-4159.2008.05352.x. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Bischof H. Effects of hippocampal lesions on acquisition and retention of spatial learning in zebra finches. Behav Brain Res. 2004;155:147–152. doi: 10.1016/j.bbr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Matt KS, Farner DS. Physiologic properties of steroid hormone-binding proteins in avian blood. Gen Comp Endocrinol. 1984;53:281–292. doi: 10.1016/0016-6480(84)90254-5. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Farner D. Endocrinology of Reproduction in Wild Species. In: Farner DS, King JR, Parkes KC, editors. Avian Biology. New York: Academic Press; 1993. pp. 163–327. [Google Scholar]
- Wynne RD, Saldanha CJ. Glial aromatization decreases neural injury in the zebra finch (Taeniopygia guttata): influence on apoptosis. J Neuroendocrinol. 2004;16:676–683. doi: 10.1111/j.1365-2826.2004.01217.x. [DOI] [PubMed] [Google Scholar]
- Yasui T, Matsui S, Tani A, Kunimi K, Yamamoto S, Irahara M. Androgen in postmenopausal women. J Med Invest. 2012;59:12–27. doi: 10.2152/jmi.59.12. [DOI] [PubMed] [Google Scholar]