Abstract
PRO131921 is a third-generation, humanized anti-CD20 monoclonal antibody with increased antibody-dependent cytotoxicity and complement-dependent cytotoxicity compared to rituximab. In this phase I study, PRO131921 was administered as a single agent to patients with CD20+, relapsed or refractory, indolent non-Hodgkin lymphoma (NHL) who had been treated with a prior rituximab-containing regimen. The primary aim of this study was safety and tolerability of PRO131921. The secondary aim of the study, and focus of this report, was to determine the pharmacokinetics (PK) profile of PRO131921 and establish a correlation between drug exposure and clinical efficacy. Patients were treated with PRO131921 by intravenous infusion weekly for 4 weeks and the dose was escalated based on safety in a 3 + 3 design. Twenty-four patients were treated with PRO131921 at doses from 25 mg/m2 to 800 mg/m2. Analysis of PK data demonstrated a correlation between higher normalized drug exposure (normalized AUC) and tumor shrinkage (p = .0035). Also, normalized AUC levels were higher among responders and subjects displaying tumor shrinkage versus subjects progressing or showing no regression (p = 0.030). In conclusion, PRO131921 demonstrated clinical activity in rituximab-relapsed and refractory indolent NHL patients. The observation that higher normalized AUC may be associated with improved clinical responses has potential implications in future trials of monoclonal antibody-based therapies, and emphasizes the importance of early PK studies to optimize antibody efficacy.
Keywords: Monoclonal antibody, Pharmacokinetics, Area under the curve, Efficacy
1. Introduction
Monoclonal antibodies have become critical components of the successful treatment of both Hodgkin (HL) and non-Hodgkin lymphomas (NHLs) [1]. Since the 1980's, over 40 monoclonal antibodies and derivatives have been approved for therapeutic use [2]. The most widely used therapeutic antibodies in NHL target the CD20 molecule, a cell surface antigen expressed by most normal and malignant human B-lymphocytes. Rituximab is a type I IgG1 chimeric (mouse/human) monoclonal antibody against CD20, which became the first antibody approved for treatment of NHL in 1997. It is currently indicated for the treatment of both follicular and aggressive B-cell NHLs [3–5]. Rituximab mediates B-cell depletion by triggering natural killer cell mediated antibody dependent cellular cytotoxicity (ADCC), through programmed cell death, and through complement dependent cytotoxicity (CDC) [6].
In addition to Rituximab, several other anti-CD20 monoclonal antibodies are in development [7]. Based on their mechanisms of action, anti-CD20 antibodies can be classified as type I, with superior CDC and ADCC activity, and type II, which have little CDC activity but are effective at inducing direct cell death [8]. Clinical studies using type I [veltuzumab, ocrelizumab (both humanized), and ofatumumab (human)] and type 2 anti-CD20 humanized antibodies (obinutuzumab, ocaratuzumab) are in progress [9,10]. Of these, ofatumumab has been approved by the FDA for fludarabine-refractory disease and for patients who have failed trials of alemtuzumab, as well as in the first line setting in chronic lymphocytic leukemia (CLL) [11,12]. Obinutuzumab has also been approved in combination with chlorambucil for patients with CLL in the first line setting [13]. It currently remains unclear to what extent each mechanism impacts the therapeutic activity of the antibody, and whether other modifications such as dose and infusion schedule can enhance efficacy [14].
Beyond glycoengineering and enhancement of affinity, pharmacokinetics (PK) strategies to optimize the dose and infusion schedule of monoclonal antibodies by disease type are becoming increasingly important in order to increase treatment effect. Early PK studies of rituximab demonstrated a consistent relationship between drug concentration and response [15–21]. However, the minimum concentration of rituximab required to induce clinical activity in lymphoma has never been established. Moreover, consistent correlations between rituximab exposure and objective response in specific lymphoma histologies are lacking. For newer generations of anti-CD20 antibodies, PK analyses have been unable to establish a correlation between area under the curve (AUC) pharmacokinetics, with the exception of ofatumumab in CLL [22–24].
Several studies have reported higher rituximab concentrations in responding patients compared to non-responders, yet none of these studies included evaluation based on AUC [12–18]. In fact, few data exist on rituximab exposure affecting responses. We had a unique opportunity to evaluate AUC and response to monoclonal antibody treatment of patients with follicular lymphoma (FL). PRO131921 is a third-generation, humanized, IgG1 anti-CD20 antibody, engineered to increase FcλR and C1q binding. In preclinical models, PRO131921 was found to facilitate increased ADCC and CDC compared to rituximab. Using a transgenic mouse model expressing human CD20 and human CD16, PRO131921 was also observed to cause more B cell depletion in the blood and spleen compared to rituximab (unpublished data). In this study, we report on the observed relationship between PK data and clinical response to PRO131921 in patients with relapsed and refractory indolent lymphomas. To our knowledge no other anti-CD20 antibody has demonstrated a similar correlation between higher normalized drug exposure, tumor shrinkage, and clinical efficacy in FL. This analysis may inform future clinical trial design and the optimization of other antibodies for anti-tumor efficacy.
2. Methods
This open-label, multicenter, phase I/II study evaluated the safety of escalating doses of single-agent PRO131921 in patients with relapsed or refractory, CD20-positive, indolent NHL. The original study design included two phases: a phase I dose-escalation portion for patients with indolent NHL and a phase II portion with enrollment of additional patients with follicular NHL into two expanded treatment cohorts. The study was terminated after phase I because of sponsor decision to pursue development of a different anti-CD20 molecule. No patients were enrolled in the phase II cohorts.
Eligible patients were required to sign an informed consent, be >18 years of age, have histologically confirmed CD20+ relapsed/refractory indolent NHL defined as grade I, II, or IIIa follicular lymphoma, as defined by the Revised European American Lymphoma/World Health Organization (REAL/WHO) classification, small lymphocytic lymphoma, or marginal zone lymphoma. Relapsed disease was required to have a documented history of response of >6 months to a rituximab-containing regimen; refractory disease was defined as progression on treatment, stable disease (SD), partial response (PR) (or better), with progression <6 months after the last administration of rituximab containing therapy. Patients were required to have bi-dimensionally measurable disease (>1.5 cm in longest dimension) defined radiographically, absolute B cell count > the lower limit of normal (as determined by flow cytometry), Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2, normal kidney and liver function, platelet count > 100,000/μL, hemoglobin > 9 g/dL, and, if of reproductive potential, use contraception or other measures to avoid pregnancy. Patients were excluded if they had prior use of an anti-CD20 monoclonal antibody therapy other than rituximab within 6 months, lymphoma treatment within 4 weeks of study enrollment, history of severe allergic anaphylactic reactions to humanized, chimeric, or murine monoclonal antibodies; evidence of severe uncontrolled concomitant illness, or any evidence or myelodysplasia.
2.1. Study design
This open label phase I study used a 3 + 3 dose escalation design. The primary objectives were to evaluate safety and tolerability. The secondary objective, and focus of the current study, was to determine the PK and preliminary efficacy of PRO131921 after the first infusion, and following subsequent weekly infusions. Unless withdrawn, each enrolled patient was to receive four weekly infusions of PRO131921 at the assigned dose on days 1, 8, 15, and 22. The starting dose was 25 mg/m2. Patients received premedication with acetaminophen (650 mg–1000 mg orally) and diphenhydramine (25 mg–50 mg orally) before each infusion. Assessments of the tolerability of escalating doses of PRO131921 in the first and subsequent infusions were conducted in parallel. Escalation to the next cohort was to occur after the last patient of the cohort has been followed for >7 days after the fourth dose and the escalation criteria for the cohort had been satisfied. PK samples were obtained pre- and post-infusion on days 1, 8, 15, and 22, and once each on days 2, 23, 29, 50, and 78 (and at later time points for up to a year). Anti-therapeutic antibodies (ATAs) against PRO131921 were assessed in blood samples obtained at defined time points on the study using a bridging electrochemiluminescence assay (ECLA). This assay employed PRO131921 conjugated to biotin and PRO131921 conjugated to BV-TAG™ label (BioVeris Corp.; Gaithersburg, MD) as assay reagents to form immune complexes with ATAs, and the complex was captured by streptavidin-coated paramagnetic beads and detected on a BioVeris M384 analyzer (BioVeris Corp.). The screening assay decision threshold was determined based on serum samples from patients naive to PRO131921, and the specificity of the screened positive samples was confirmed by competitive binding with unlabeled PRO131921. Adverse events were assessed using the National Cancer Institute Common Toxicity Criteria version 3.0. The study was conducted in accordance with the Declaration of Helsinki protocol, and all patients signed the informed consent prior to enrollment. The trial was registered at Clinicaltrials.gov (NCT00452127).
2.2. Pharmacokinetics
PRO131921 was given in 4 weekly IV infusions on the first day of Weeks 1, 2, 3, and 4. PRO131921 serum levels were evaluated at pre-infusion (0–2 h prior to start of infusion), post infusion (0–30 min after the end of the infusion), and then on designated days after the first infusion. PK data were available from a total of 23 patients who received PRO131921 at six dose levels. Pharmacokinetic parameters were estimated using non-compartmental methods. Serum PRO131921 concentrations and pharmacokinetic parameters were summarized by descriptive statistics (mean, standard deviation, coefficient of variation, minimum, maximum). Serum PRO131921 concentrations were evaluated using an exploratory research assay. The pharmacodynamics of PRO131921 were characterized by the extent and duration of B-cell depletion at each dose level. B-cell depletion and recovery were assessed by determining both the absolute counts and percentage of baseline peripheral blood CD19+ B-cell counts at post-treatment time points. The analysis included estimating the time to normal B-cell recovery to within 50% of baseline value for patients with B-cell depletion. Peripheral blood CD19+ B-cell depletion and recovery profiles for each patient were summarized descriptively for each dose level.
2.3. Statistics and sample size calculation
Progression-free survival was defined as the time from start of treatment to the earlier of documented disease progression or death due to any cause within 30 days of the last infusion of PRO131921. If the specified event (disease progression, death) did not occur, progression-free survival for the purpose of the analysis was censored at the time of the last tumor evaluation. The progression-free survival (PFS) curve and the median time to the event were estimated using Kaplan–Meier methodology [25].
The sample size for the dose-escalation phase of this trial was based on dose-escalation rules as follows: If none of the 3 patients enrolled in a cohort experienced a dose limiting toxicity (DLT) within 7 days after completing the fourth infusion, then dose escalation occurred to the next dose level. If 1 of the 3 patients in a cohort experienced a DLT, then 3 additional patients were added to that cohort. If no other patient experienced a DLT within 7 days after completing the fourth infusion, then escalation occurred to the next dose level. If 2 or more patients experienced an infusion-related DLT only with their first infusions at any given dose level, then the maximum tolerated dose (MTD) was considered exceeded and dose escalation for the first infusion would be stopped.
In the event that two Grade 2 events or one Grade ≥ 3 event was observed within a given cohort, excluding reversible infusion-related toxicities and transient cytopenias, dose escalation was to be modified from a 100% increase for each cohort to a slower dose-escalation scheme following a modified Fibonacci design, such that the increment of dose escalation after the cohort at which toxicity was observed would be 50%, then 40%, followed by 30% increments until the MTD is identified or the 2700 mg/m2 per course cohort was enrolled. Response assessment was made by the investigator, based on physical examinations, computerized tomography scans, and bone marrow examinations, using the Standardized Response Criteria of the National Cancer Institute International Working Group (IWG) at day 78 [26]. Additionally, 18F-fluorodeoxyglucose (FDG) positron-emission tomography (PET) scans were obtained to evaluate response using the Revised IWG Response Criteria if needed to confirm response [27].
3. Results
Twenty-four patients previously treated with rituximab received PRO131921. Patient characteristics are noted in Table 1. The study population consisted of 11 males (46%) and 13 females (54%), with a median age of 58 years (range 38–78). Histologies included follicular lymphoma (N = 20), small lymphocytic lymphoma (N = 3), and marginal zone lymphoma (N = 1). Median number of prior regimens was 2 (range: 1–6). Thirteen patients (54%) had an ECOG score of 0, and 10 patients (42%) a score of 1. Four patients had B symptoms at baseline.
Table 1.
Patient demographics and baseline characteristics among treated subjects (n = 24).
| Treated subjects | N = 24 |
|---|---|
| Age at baseline, years | |
| n | 24 |
| Mean, (range) | 58 (38-78) |
| Sex | |
| Male | 11 (46%) |
| Female | 13 (54%) |
| Race | |
| White | 21 (88%) |
| Black or African-American | 2 (8%) |
| Not available | 1 (4%) |
| ECOG score | |
| 0 | 13 (54%) |
| 1 | 10 (42%) |
| 2 | 0 (0%) |
| missing | 1 (4%) |
| Histology | 20 (83%) |
| fNHL | |
| SLL | 3 (13%) |
| MZL | 1 (4%) |
| Prior regimens | |
| n | 24 |
| median (range) | 2(1-6) |
Abbreviations: fNHL = follicular non-Hodgkin's lymphoma; SLL = small lymphocytic lymphoma; MZL = marginal zone lymphoma.
3.1. Safety outcomes
PRO131921 was generally well tolerated, and no maximum tolerated dose was reached. The most common adverse events were grade 1 or 2 (CTCAE V3.0) chills, flushing, itching, fatigue, reactions (limited to the first infusion). These events responded well to slowing or interruption of the infusion, as well as symptomatic treatment. Two patients were unable to receive all 4 doses of therapy due to DLTs. One DLT was observed in the 200/400 mg/m2 dose cohort due to significant infusion reaction (grade 3 hypoxia), and a second was observed at the 300/800 mg/m2 dose cohort due to grade 3 joint pain and fatigue following 2 infusions. One serious adverse event related to PRO131921 occurred (hypoxia), and subsequently resolved. Adverse events related to PRO131921 are listed in Table 2. The serious adverse effects are noted in Table 3.
Table 2.
Adverse events related to PRO131921 experienced by more than one patient among treated subjects (N = 24).
| AE preferred term | Patient worst grade AEs |
|
|---|---|---|
| All grades | Grade > 3 | |
| Chills | 10 (41%) | 0 |
| Fatigue | 9 (38%) | 1 (4%) |
| Flushing | 9 (38%) | 0 |
| Pruritus | 9 (38%) | 0 |
| Nausea | 7 (29%) | 0 |
| Pyrexia | 7 (29%) | 0 |
| Chest discomfort | 5 (21%) | 0 |
| Dizziness | 5 (21%) | 0 |
| Feeling hot | 5 (21%) | 0 |
| Headache | 5 (21%) | 0 |
| Neutropenia | 5 (21%) | 3 (13%) |
| Urticaria | 5 (21%) | 0 |
| Hypotension | 4 (17%) | 0 |
| Rash | 4 (17%) | 0 |
| Throat irritation | 4 (17%) | 0 |
| Throat tightness | 4 (17%) | 1 (4%) |
| Anemia | 3 (13%) | 0 |
| Erythema | 3 (13%) | 0 |
| Vomiting | 3 (13%) | 0 |
| Bronchospasm | 2 (8%) | 1 (4%) |
| Constipation | 2 (8%) | 0 |
| Diarrhea | 2 (8%) | 0 |
| Dyspnea | 2 (8%) | 0 |
| Hypoxia | 2 (8%) | 2 |
| Oropharyngeal pain | 2 (8%) | 0 |
| Pain in extremity | 2 (8%) | 0 |
| Paraesthesia | 2 (8%) | 0 |
| Paraesthesia oral | 2 (8%) | 0 |
| Rhinorrhea | 2 (8%) | 0 |
| Tachycardia | 2 (8%) | 0 |
| Thrombocytopenia | 2 (8%) | 0 |
CTC v3.0 adverse events.
Adverse events are summarized by worst grade of AEPT per patient.
Table 3.
Serious adverse events among treated subjects (N = 24).
| Cohort (mg/m2) | NHL Subtype | SAE | Related to PRO131921a | Resolved |
|---|---|---|---|---|
| 200/400 | Follicular | Gr 3 hypoxia | Yes | Yes |
| 25/50 | Follicular | Gr 3 PNEUMONIA | No | Yes |
| 50/100 | Follicular | Gr 4 PE | No | Yes |
| 200/400 | Follicular | Gr 3 DVT | No | Yes |
Abbreviations: PE = pulmonary embolism; DVT = deep venous thrombosis.
As assessed by investigator.
3.2. Pharmacokinetics and pharmacodynamics
There was a rapid and sustained depletion of CD20+ B cells after the first PRO131921 infusion, leaving a reduced number of CD20+ B cells available for the antibody to bind at subsequent infusions. The vast majority of patient's B cell count did not return to baseline levels during the study. However, a number of patients exhibited an increase in B cell counts following an initial decline on study. PK studies of PRO131921 demonstrated a dose-dependent increase in exposure, but with significant inter- and intra-patient variability. As a result, following 4 weekly infusions, the median of individual estimates of PRO131921 terminal elimination half-life was 24.5 days (range, 16.5 to 37.8 days), a half-life typical of IgG antibodies. After 4 weekly IV infusions, PRO131921 clearance and volume of distribution were 144 mL/day and 3.93 L respectively. No gender differences were observed.
3.3. Efficacy
The best investigator-assessed responses to treatment in the 22 evaluable patients were 6 responders (27%) including complete response (CR) in 1 patient, and PR in 5 patients. There was SD in 13 patients and PD in 3 patients. Five of 10 evaluable patients in the two highest dose cohorts responded. PK data demonstrated a correlation between higher normalized drug exposure (normalized AUC) and adenopathy shrinkage at day 78 with respect to baseline (Spearman correlation r = −0.62, p = 0.0035). Additionally, normalized AUC values were significantly higher among responders and patients with tumor shrinkage at day 78 versus subjects with progression or no tumor burden reduction at day 78 (Wilcoxon Rank Sum, p = 0.03) (see Table 4, Figs. 1–3). PRO131921 was highly effective in rituximab-refractory B-cell NHL patients; 50% of follicular NHL patients responded to treatment with the highest doses of antibody. The median PFS in the whole group was 11 months (Fig. 4) with a median follow-up duration of 5.9 months.
Table 4.
Best response to PRO131921 by cohort among treated subjects (N = 24).
| Cohort (dose) and diagnosis (n) | Response |
||||
|---|---|---|---|---|---|
| CR | PR | SD | PD | DLT | |
| A (25/25 mg/m2) fNHL (3) | 2 | 1 | |||
| B (25/50 mg/m2) fNHL (2), SLL (1) | 3 | ||||
| C (50/100 mg/m2) fNHL (3) | 1 | 2 | |||
| D (100/200 mg/m2) fNHL (2), SLL (1) | 3 | ||||
| E (200/400 mg/m2) fNHLa (5), MZL (1) | 2 | 2 | 1 (MZL) | 1 | |
| F (300/800 mg/m2) fNHL (5), SLL (1) | 1b | 2 | 1 | 1 (SLL) | 1 |
Abbreviations: CR = complete response; PR = partial response; SD = stable disease; PD = progressive disease; DLT = dose limiting toxicity; fNHL = follicular non-Hodgkin's lymphoma; SLL = small lymphocytic lymphoma; MZL = marginal zone lymphoma.
1 fNHL patient was refractory to prior R-CHOP.
CR subject (1026) is unconfirmed and will be reclassified as PR.
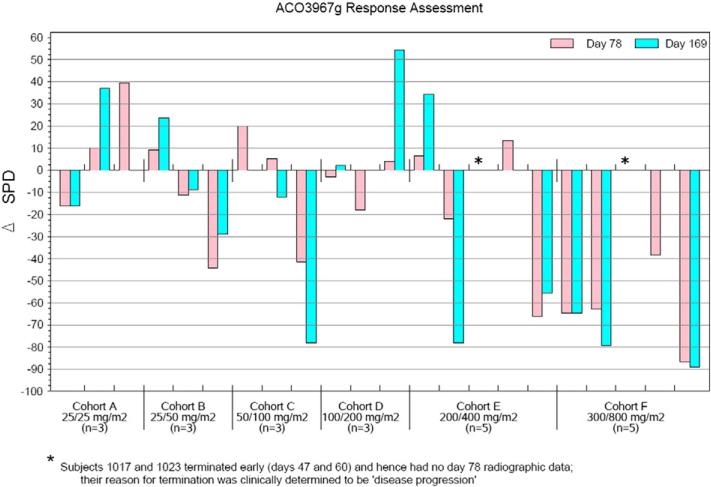
Figure 1.
Change in the sum of the product of the greatest diameters (SPD) assessed at days 78 and 169 of study by cohort. Asterisks indicate disease progression by day 50.
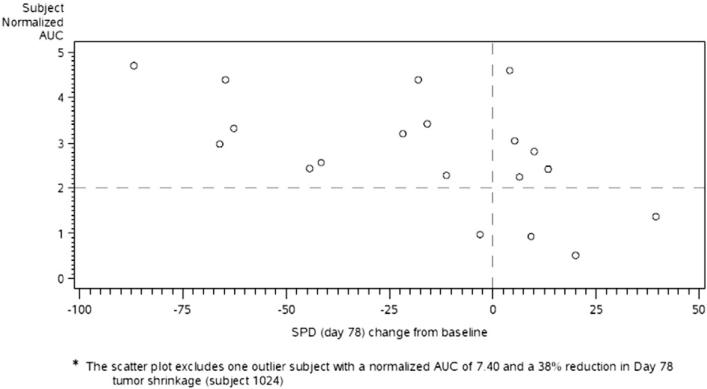
Figure 3.
Scatter plot of subject normalized AUC values against Day 78 tumor burden percent change from baseline. * The scatter plot excludes one outlier subject with a normalized AUC of 7.40 and a 38% reduction in Day 78 tumor burden (subject 1024).
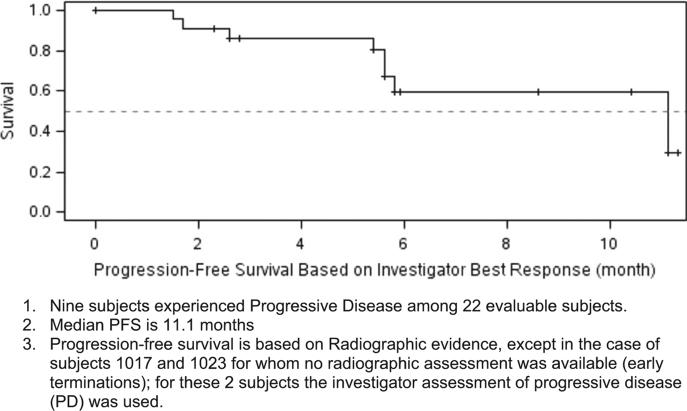
Figure 4.
Kaplan–Meier progression-free survival among evaluable subjects.
1. Nine subjects experienced progressive disease among 22 evaluable subjects.
2. Median PFS is 11.1 months
3. Progression-free survival is based on radiographic evidence, except in the case of subjects 1017 and 1023 for whom no radiographic assessment was available (early terminations); for these 2 subjects the investigator assessment of progressive disease (PD) was used.
4. Discussion
In our study, we determined that PRO131921, a novel fully humanized anti-CD20 monoclonal antibody, administered in four weekly doses to patients with relapsed or rituximab-refractory CD20 positive indolent NHL, is well tolerated, and no MTD was achieved. The most common adverse reactions were Grade 1 or 2 (CTCAE V3.0) chills, flushing, pruritus, and fatigue, mostly related to the first infusion, similar to that observed with rituximab. Moreover, this humanized anti-CD20 antibody was highly effective in rituximab-refractory B-cell NHL patients; 50% of follicular NHL patients responded to treatment with the highest doses of antibody. These results are similar to observed responses to other monoclonal antibodies in lymphoma [11,28–30]. Importantly, we also demonstrated a significant correlation between normalized AUC and tumor regression seen at day 78; approximately one third of the variability in tumor shrinkage (day 78) is accounted for by normalized AUC. Additionally, we showed a significantly higher normalized AUC in subjects with any tumor shrinkage versus those without. To our knowledge, this is the first demonstration of AUC correlating with response in patients with non-Hodgkin lymphoma treated with a single agent monoclonal antibody.
Use of the first approved monoclonal anti-CD20 antibody rituximab is now standard in the care of patients with both indolent and aggressive B-cell NHL based on its significant clinical activity and favorable toxicity profile. There is a paucity of data regarding the mechanisms involved in rituximab distribution and elimination, and AUC pharmacokinetics. Rituximab pharmacokinetics were first described in 15 relapsed NHL patients treated with rituximab doses between 10 and 500 mg/m2. In this study, the antibody half-life was difficult to establish because of antibody binding to tumor burden [31]. In a subsequent pivotal study in NHL by Berinstein et al., rituximab was administered at 375 mg/m2 in four consecutive doses. Three months following treatment, an increase in the median serum level of rituximab was detected in responders compared to non-responders [19]. This correlation has been observed by others as well [3,17,32]. Importantly, while these studies have suggested that prolonged rituximab use may be beneficial to patients with NHL, there is significant uncertainty regarding the optimal duration and exposure to rituximab due to limited phase I information in NHL, and no PK or AUC pharmacokinetics for its use in CLL [33,34]. Moreover, correlations between single agent rituximab concentration over time, or AUC, have never been previously demonstrated. This association may be relevant in dose optimization, since a long, low concentration exposure may be as important as shorter but higher concentration, given pleiotropic mechanisms.
For other humanized anti-CD20 monoclonal antibodies, PK analyses have not successfully established correlations between AUC and disease responses in NHL [22–24]. A pilot study of rituximab in combination with fludarabine and mitoxantrone in FL found differences in AUC between men and women, as well as between patients with and without bone marrow infiltration, but these were not statistically significant [35]. In CLL, where CD20 surface expression is expressed at a lower density, only one study identified that response to the antibody ofatumumab correlated with AUC [36]. Responses to intravenous and subcutaneous administration of the anti-CD52 monoclonal antibody alemtuzumab have also been reported to correlate with its pharmacokinetic profile in several CLL studies [37–39] Indeed, CLL is a different disease, where response rates to single agent monoclonal antibodies are lower, and circulating tumor bulk can be a “sink” for antibodies. This represents a challenge when extrapolating CLL data to NHL.
In our study, we found a rapid and sustained depletion of CD20+ B cells after the first PRO131921 infusion, leaving a reduced number of CD20+ cells available for the antibody to bind at subsequent infusion, consistent with that observed by Coiffier et al. in a CLL study of ofatumumab [36]. This depletion indicates that there was prompt binding of PRO131921 to its target CD20+ B cells. As a result, following 4 weekly infusions, the median of individual estimates of PRO131921 terminal elimination half-life was 24.5 days (range, 16.5 to 37.8 days), which is typical for IgG antibodies. PK studies of PRO131921 were broadly similar to rituximab, with a dose dependent increase in exposure, but with significant inter- and intra-patient variability. This is not unlike other studies of rituximab in indolent NHL that have been unable to establish a relationship between objective clinical response and drug exposure. In our study the variability in PK parameters was unlikely due to anti-PRO131921 antibodies, as no patients with serum anti-drug antibodies were observed. These differences may well reflect differences in patient body surface area or NHL tumor burden.
There are aggressive development programs in NHL for several 3rd generation anti-CD20 antibodies, including large phase III studies in upfront and relapsed DLBCL and FL (www.clinicaltrials.gov, NCT01200589; NCT01332968) [40,41]. Based upon our findings, the development strategy for these antibodies should incorporate the use of AUC as a decision point in dosing either as single agents or in combination with cytotoxic therapy. To our knowledge, dosing and regimens for anti-CD20 antibodies currently in development have not yet incorporated the use of AUC pharmacokinetics. The AUC of a drug is an estimate of the total concentration of a drug over time, and is a reflection of a drug's bioavailability. AUC, unlike other measures of circulating drug, is the primary measure of drug exposure [42]. PK parameters provide important information on the most efficacious dosing and metabolism, and contribute to the success and cost of a drug. However AUC pharmacokinetics are particularly important, since understanding the minimum AUC required for efficacy will allow optimization of dose selection and dosing regimens. We observed statistically significant positive correlations between objective response and normalized AUC. This suggests that high exposure to PRO131921 is important for the attainment of clinical response.
In summary, our data indicate a relationship between response to PRO 131921 and AUC. One hypothesis is that higher tumor burden in excess of drug decreases efficacy. Alternatively, PK may correlate with drug efficacy independently. A tumor sensitive to a monoclonal antibody may remove the ‘antigen sink’, resulting in slower drug clearance. The precise mechanism is not understood. Improved exposure may be based upon saturation of tumor burden and achievement of sustained drug levels. A better understanding of mechanisms of action, pharmacokinetics, and other patient factors is needed to optimize dosing of monoclonal antibodies. These data should augment our current understanding of PK profiling of anti CD20 monoclonal antibodies, and consequently may lead to more rapid and effective treatment strategies involving novel dosing and schedule. These data suggest that an adjusted schedule of antibody according to serum level could improve clinical outcomes of patients receiving monoclonal antibodies for cancer and other diseases.
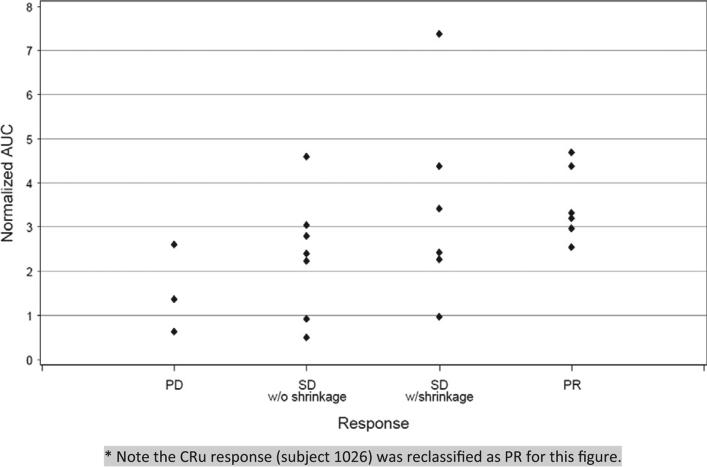
Figure 2.
Correlation between dose-normalized AUC and clinical response (P = .03). * Note the CRu response (subject 1026) was reclassified as PR for this figure.
Acknowledgments
This project was funded with assistance from Roche and Genentech. Assistance with statistical support and data collection were provided by Bernard Fine and Denis Boisvert. Jonathan W. Friedberg is a Scholar in Clinical Research from the Leukemia and Lymphoma Society.
The data analysis and figures for this paper were generated using SAS software. Copyright, SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA.
Footnotes
Conflict of interest statement
The author(s) declare that there are no conflicts of interest.
References
- 1.Winter MC, Hancock BW. Ten years of rituximab in NHL, Expert Opin. Drug Saf. 2009;8:223–235. doi: 10.1517/14740330902750114. [DOI] [PubMed] [Google Scholar]
- 2.Vacchelli E, Aranda F, Eggermont A, Galon J, Sautes-Fridman C, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: tumor-targeting monoclonal antibodies in cancer therapy. Oncoimmunology. 2014;3:e27048. doi: 10.4161/onci.27048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maloney DG, Grillo-Lopez AJ, Bodkin DJ, White CA, Liles TM, Royston I, Varns C, Rosenberg J, Levy R. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin's lymphoma. J. Clin. Oncol. 1997;15:3266–3274. doi: 10.1200/JCO.1997.15.10.3266. [DOI] [PubMed] [Google Scholar]
- 4.Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, Janakiraman N, Foon KA, Liles TM, Dallaire BK, Wey K, Royston I, Davis T, Levy R. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood. 1997;90:2188–2195. [PubMed] [Google Scholar]
- 5.Cartron G, Blasco H, Paintaud G, Watier H, Le Guellec C. Pharmacokinetics of rituximab and its clinical use: thought for the best use? Crit. Rev. Oncol. Hematol. 2007;62:43–52. doi: 10.1016/j.critrevonc.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Rudnicka D, Oszmiana A, Finch DK, Strickland I, Schofield DJ, Lowe DC, Sleeman MA, Davis DM. Rituximab causes a polarization of B cells that augments its therapeutic function in NK-cell-mediated antibody-dependent cellular cytotoxicity. Blood. 2013;121:4694–4702. doi: 10.1182/blood-2013-02-482570. [DOI] [PubMed] [Google Scholar]
- 7.Czuczman MS, Gregory SA. The future of CD20 monoclonal antibody therapy in B-cell malignancies. Leuk. Lymphoma. 2010;51:983–994. doi: 10.3109/10428191003717746. [DOI] [PubMed] [Google Scholar]
- 8.Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol. Immunol. 2007;44:3823–3837. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- 9.Tobinai K, Ogura M, Kobayashi Y, Uchida T, Watanabe T, Oyama T, Maruyama D, Suzuki T, Mori M, Kasai M, Cronier D, Wooldridge JE, Koshiji M. Phase I study of LY2469298, an Fc-engineered humanized anti-CD20 antibody, in patients with relapsed or refractory follicular lymphoma. Cancer Sci. 2011;102:432–438. doi: 10.1111/j.1349-7006.2010.01809.x. [DOI] [PubMed] [Google Scholar]
- 10.Salles G, Morschhauser F, Lamy T, Milpied N, Thieblemont C, Tilly H, Bieska G, Asikanius E, Carlile D, Birkett J, Pisa P, Cartron G. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood. 2012;119:5126–5132. doi: 10.1182/blood-2012-01-404368. [DOI] [PubMed] [Google Scholar]
- 11.Wierda WG, Kipps TJ, Mayer J, Stilgenbauer S, Williams CD, Hellmann A, Robak T, Furman RR, Hillmen P, Trneny M, Dyer MJ, Padmanabhan S, Piotrowska M, Kozak T, Chan G, Davis R, Losic N, Wilms J, Russell CA, Osterborg A. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J. Clin. Oncol. 2010;28:1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.R.T., Hillmen P, Janssens A, et al. Ofatumumab + chlorambucil versus chlorambucil alone in patients with untreated chronic lymphocytic leukemia (CLL): results of the phase III study complement 1 (OMB110911) abstract # 528. American Society of Hematology Annual Meeting. 2013 [Google Scholar]
- 13.Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, Chagorova T, de la Serna J, Dilhuydy MS, Illmer T, Opat S, Owen CJ, Samoylova O, Kreuzer KA, Stilgenbauer S, Dohner H, Langerak AW, Ritgen M, Kneba M, Asikanius E, Humphrey K, Wenger M, Hallek M. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N. Engl. J. Med. 2014;370:1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 14.Cang S, Mukhi N, Wang K, Liu D. Novel CD20 monoclonal antibodies for lymphoma therapy. J. Hematol. Oncol. 2012;5:64. doi: 10.1186/1756-8722-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez J, Gutierrez A. Pharmacokinetic properties of rituximab. Rev. Recent Clin. Trials. 2008;3:22–30. doi: 10.2174/157488708783330495. [DOI] [PubMed] [Google Scholar]
- 16.Mangel J, Buckstein R, Imrie K, Spaner D, Franssen E, Pavlin P, Boudreau A, Pennell N, Combs D, Berinstein NL. Pharmacokinetic study of patients with follicular or mantle cell lymphoma treated with rituximab as ‘in vivo purge’ and consolidative immunotherapy following autologous stem cell transplantation. Ann. Oncol. 2003;14:758–765. doi: 10.1093/annonc/mdg201. [DOI] [PubMed] [Google Scholar]
- 17.Piro LD, White CA, Grillo-Lopez AJ, Janakiraman N, Saven A, Beck TM, Varns C, Shuey S, Czuczman M, Lynch JW, Kolitz JE, Jain V. Extended rituximab (anti-CD20 monoclonal antibody) therapy for relapsed or refractory low-grade or follicular non-Hodgkin's lymphoma. Ann. Oncol. 1999;10:655–661. doi: 10.1023/a:1008389119525. [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, Jain V, Ho AD, Lister J, Wey K, Shen D, Dallaire BK. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J. Clin. Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 19.Berinstein NL, Grillo-Lopez AJ, White CA, Bence-Bruckler I, Maloney D, Czuczman M, Green D, Rosenberg J, McLaughlin P, Shen D. Association of serum rituximab (IDECC2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin's lymphoma. Ann. Oncol. 1998;9:995–1001. doi: 10.1023/A:1008416911099. [DOI] [PubMed] [Google Scholar]
- 20.Tobinai K, Igarashi T, Itoh K, Kobayashi Y, Taniwaki M, Ogura M, Kinoshita T, Hotta T, Aikawa K, Tsushita K, Hiraoka A, Matsuno Y, Nakamura S, Mori S, Ohashi Y. Japanese multicenter phase II and pharmacokinetic study of rituximab in relapsed or refractory patients with aggressive B-cell lymphoma. Ann. Oncol. 2004;15:821–830. doi: 10.1093/annonc/mdh176. [DOI] [PubMed] [Google Scholar]
- 21.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N. Engl. J. Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 22.Morschhauser F, Leonard JP, Fayad L, Coiffier B, Petillon MO, Coleman M, Schuster SJ, Dyer MJ, Horne H, Teoh N, Wegener WA, Goldenberg DM. Humanized anti-CD20 antibody, veltuzumab, in refractory/recurrent non-Hodgkin's lymphoma: phase I/II results. J. Clin. Oncol. 2009;27:3346–3353. doi: 10.1200/JCO.2008.19.9117. [DOI] [PubMed] [Google Scholar]
- 23.Morschhauser F, Marlton P, Vitolo U, Linden O, Seymour JF, Crump M, Coiffier B, Foa R, Wassner E, Burger HU, Brennan B, Mendila M. Results of a phase I/II study of ocrelizumab, a fully humanized anti-CD20 mAb, in patients with relapsed/refractory follicular lymphoma. Ann. Oncol. 2010;21:1870–1876. doi: 10.1093/annonc/mdq027. [DOI] [PubMed] [Google Scholar]
- 24.Cheson BD. Ofatumumab, a novel anti-CD20 monoclonal antibody for the treatment of B-cell malignancies. J. Clin. Oncol. 2010;28:3525–3530. doi: 10.1200/JCO.2010.27.9836. [DOI] [PubMed] [Google Scholar]
- 25.Lawless J. Statistical Methods for Lifetime Data. 1982 [Google Scholar]
- 26.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J. Clin. Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 27.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V. Revised response criteria for malignant lymphoma. J. Clin. Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 28.Salles GA, Morschhauser F, Solal-Celigny P, Thieblemont C, Lamy T, Tilly H, Gyan E, Lei G, Wenger M, Wassner-Fritsch E, Cartron G. Obinutuzumab (GA101) in patients with relapsed/refractory indolent non-Hodgkin lymphoma: results from the phase II GAUGUIN study. J. Clin. Oncol. 2013;31:2920–2926. doi: 10.1200/JCO.2012.46.9718. [DOI] [PubMed] [Google Scholar]
- 29.Morschhauser FA, Cartron G, Thieblemont C, Solal-Celigny P, Haioun C, Bouabdallah R, Feugier P, Bouabdallah K, Asikanius E, Lei G, Wenger M, Wassner-Fritsch E, Salles GA. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large b-cell lymphoma or mantle-cell lymphoma: results from the phase II GAUGUIN study. J. Clin. Oncol. 2013;31:2912–2919. doi: 10.1200/JCO.2012.46.9585. [DOI] [PubMed] [Google Scholar]
- 30.Czuczman MS, Fayad L, Delwail V, Cartron G, Jacobsen E, Kuliczkowski K, Link BK, Pinter-Brown L, Radford J, Hellmann A, Gallop-Evans E, DiRienzo CG, Goldstein N, Gupta I, Jewell RC, Lin TS, Lisby S, Schultz M, Russell CA, Hagenbeek A. Ofatumumab monotherapy in rituximab-refractory follicular lymphoma: results from a multicenter study. Blood. 2012;119:3698–3704. doi: 10.1182/blood-2011-09-378323. [DOI] [PubMed] [Google Scholar]
- 31.Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo-Lopez A, Levy R. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84:2457–2466. [PubMed] [Google Scholar]
- 32.Davis TA, White CA, Grillo-Lopez AJ, Velasquez WS, Link B, Maloney DG, Dillman RO, Williams ME, Mohrbacher A, Weaver R, Dowden S, Levy R. Single-agent monoclonal antibody efficacy in bulky non-Hodgkin's lymphoma: results of a phase II trial of rituximab. J. Clin. Oncol. 1999;17:1851–1857. doi: 10.1200/JCO.1999.17.6.1851. [DOI] [PubMed] [Google Scholar]
- 33.Gordan LN, Grow WB, Pusateri A, Douglas V, Mendenhall NP, Lynch JW. Phase II trial of individualized rituximab dosing for patients with CD20-positive lymphoproliferative disorders. J. Clin. Oncol. 2005;23:1096–1102. doi: 10.1200/JCO.2005.12.171. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien SM, Kantarjian H, Thomas DA, Giles FJ, Freireich EJ, Cortes J, Lerner S, Keating MJ. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J. Clin. Oncol. 2001;19:2165–2170. doi: 10.1200/JCO.2001.19.8.2165. [DOI] [PubMed] [Google Scholar]
- 35.Jager U, Fridrik M, Zeitlinger M, Heintel D, Hopfinger G, Burgstaller S, Mannhalter C, Oberaigner W, Porpaczy E, Skrabs C, Einberger C, Drach J, Raderer M, Gaiger A, Putman M, Greil R. Rituximab serum concentrations during immuno-chemotherapy of follicular lymphoma correlate with patient gender, bone marrow infiltration and clinical response. Haematologica. 2012;97:1431–1438. doi: 10.3324/haematol.2011.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coiffier B, Losic N, Ronn BB, Lepretre S, Pedersen LM, Gadeberg O, Frederiksen H, van Oers MH, Wooldridge J, Kloczko J, Holowiecki J, Hellmann A, Walewski J, Robak T, Petersen J. Pharmacokinetics and pharmacokinetic/pharmacodynamic associations of ofatumumab, a human monoclonal CD20 antibody, in patients with relapsed or refractory chronic lymphocytic leukaemia: a phase 1–2 study. Br. J. Haematol. 2010;150:58–71. doi: 10.1111/j.1365-2141.2010.08193.x. [DOI] [PubMed] [Google Scholar]
- 37.Elter T, Molnar I, Kuhlmann J, Hallek M, Wendtner C. Pharmacokinetics of alemtuzumab and the relevance in clinical practice. Leuk. Lymphoma. 2008;49:2256–2262. doi: 10.1080/10428190802475303. [DOI] [PubMed] [Google Scholar]
- 38.Elter T, Kilp J, Borchmann P, Schulz H, Hallek M, Engert A. Pharmacokinetics of alemtuzumab in combination with fludarabine in patients with relapsed or refractory B-cell chronic lymphocytic leukemia. Haematologica. 2009;94:150–152. doi: 10.3324/haematol.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montagna M, Montillo M, Avanzini MA, Tinelli C, Tedeschi A, Visai L, Ricci F, Vismara E, Morra E, Regazzi M. Relationship between pharmacokinetic profile of subcutaneously administered alemtuzumab and clinical response in patients with chronic lymphocytic leukemia. Haematologica. 2011;96:932–936. doi: 10.3324/haematol.2010.033159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matasar MJ, Czuczman MS, Rodriguez MA, Fennessy M, Shea TC, Spitzer G, Lossos IS, Kharfan-Dabaja MA, Joyce R, Fayad L, Henkel K, Liao Q, Edvardsen K, Jewell RC, Fecteau D, Singh RP, Lisby S, Moskowitz CH. Ofatumumab in combination with ICE or DHAP chemotherapy in relapsed or refractory intermediate grade B-cell lymphoma. Blood. 2013;122:499–506. doi: 10.1182/blood-2012-12-472027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dyer MJ, Grigg A, Gonzalez M. Obinutuzumab (GA101) in combination with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) or bendamustine in patients with previously untreated follicular lymphoma (FL): results of the phase Ib GAUDI study (BO21000) Blood. 2012 [Google Scholar]
- 42.H.D., Bonate P. Pharmacokinetics in Drug Development: Clinical Study Design and, Analysis. 2004 [Google Scholar]