Abstract
Background and Purpose
Lobar microbleeds suggestive of cerebral amyloid angiopathy (CAA) are often identified on MRI in the absence of lobar intracerebral hemorrhage (ICH). We compared the baseline characteristics and risk of subsequent ICH among such patients to those presenting with CAA-related lobar ICH.
Methods
Clinical data (demographics, risk factors), apolipoprotein E genotype, neuroimaging markers of CAA severity (microbleed counts, leukoaraiosis volume), and clinical outcomes (incidence rates of ICH and death during a mean follow-up of 5.3±3.8 years) were compared between 63 patients enrolled because of incidentally found microbleeds and 316 with CAA-related ICH, in our prospectively enrolled cohort. Predictors of incident ICH were explored in the microbleed-only patients using multivariable Cox regression models.
Results
Microbleed-only patients shared similar demographic, apolipoprotein E, and vascular risk profiles with lobar ICH patients, but had more lobar microbleeds (median, 10 versus 2; P<0.001) and higher leukoaraiosis volumes (median, 31 versus 23 mL; P=0.02). Microbleed-only patients had a nontrivial incidence rate of ICH, not different from patients presenting with ICH (5 versus 8.9 per 100 person-years; adjusted hazard ratio, 0.58; 95% confidence interval, 0.31–1.06; P=0.08). Microbleed-only patients had a higher mortality rate (hazard ratio, 1.67; 95% confidence interval, 1.1–2.6) compared with ICH survivors. Warfarin use and increasing age were independent predictors of future ICH among microbleed-only patients after correction for other covariates.
Conclusions
Patients presenting with isolated lobar microbleeds on MRI have a genetic, neuroimaging, and hemorrhagic risk profile suggestive of severe CAA pathology. They have a substantial risk of incident ICH, potentially affecting decisions regarding anticoagulation in clinical situations.
Keywords: cerebral amyloid angiopathy, cerebral hemorrhage, cerebral microbleeds, magnetic resonance imaging
Cerebral amyloid angiopathy (CAA) represents amyloid β-peptide deposition in small- and medium-sized blood vessels in the brain, leading to hemorrhagic and ischemic injury.1–5 Classically, CAA patients are diagnosed when they develop lobar intracerebral hemorrhage (ICH), a severe type of stroke resulting in high rates of mortality and disability.6,7 Lobar microbleeds on T2*-weighted MRI have also been identified as a marker of CAA severity and constitute an important component of the Boston criteria, a validated set of clinical-radiological features that showed high accuracy in CAA diagnosis.8–10 The Boston criteria were originally validated in patients presenting with lobar ICH. With growing use of T2*-weighted MRI and increasing awareness of this condition, however, the diagnosis of CAA is now often considered in the setting of isolated lobar microbleeds in patients with neurological symptoms not related to ICH.11–13 Detection of lobar micro-bleeds in large proportions of stroke-free, community-dwelling older individuals13–15 also raises the question of whether many or most of them have advanced CAA or are at risk of future ICH. This issue is particularly important for individuals needing long-term anticoagulation, as there are few data about the risk of ICH in the setting of isolated lobar microbleeds.
We explored these questions in a prospective observational cohort of patients diagnosed with CAA in the absence or the presence of prior ICH. We hypothesized that patients without symptomatic lobar ICH but otherwise meeting Boston criteria for CAA (aged >55 years with strictly lobar microbleeds and no other cause of hemorrhage)8,16,17 would demonstrate similar vascular risk factors and genetic/radiological characteristics as lobar ICH patients diagnosed with definite/probable CAA and an appreciable risk of future ICH.
Methods
Study Population
We have analyzed prospectively collected baseline and follow-up data from consecutive patients presenting to Massachusetts General Hospital with neurological symptoms and enrolled in a longitudinal cohort study of the natural history of CAA.18–20 Patients were enrolled with definite or probable CAA according to the previously validated Boston criteria,8,21 by which individuals aged ≥55 years with multiple hemorrhagic lesions restricted to lobar, cortical, or cortico-subcortical regions (cerebellar hemorrhage allowed) and no other definite cause (trauma, ischemic stroke, tumor, vascular malformation, vasculitis, coagulopathy, anticoagulation with international normalized ratio >3.0) are diagnosed as probable CAA. For the current analysis, we grouped the patients into 2 categories: those presenting with (1) ≥2 lobar microbleeds in the absence of lobar ICH (microbleed-only patients) or (2) those presenting with a lobar ICH with ≥1 lobar microbleed (ICH patients). Patients with a diagnosis of inflammatory CAA22 or autosomal dominant hereditary CAA23 were not included into this analysis. A full history was obtained, a neurological examination was performed, and head computed tomography, brain MRI, and computed tomography angiography or magnetic resonance angiography of the brain were performed to exclude an underlying vascular abnormality or other structural causes of hemorrhage. Microbleed-only patients underwent neurological and cognitive testing including mini mental state examination as part of the research protocol on which they scored ≥27. For the survival analysis, day zero for the ICH group was taken as the date of ICH. For the microbleed-only subjects who entered the prospective study without a prior ICH, day zero for survival analyses was taken as the date of study enrollment.
Data Collection
Subject enrollment, baseline data collection, and MRI acquisition and analysis were performed as described previously.24 Baseline characteristics were compared between ICH patients and microbleed-only patients among all patients enrolled. Individuals who consented to longitudinal follow-up and ICH patients who survived the first 90 days after their index event were studied for incident lobar ICH or death as described.1,24 Forty-six patients who died within the first 3 months after their index ICH were not included into the longitudinal analyses. Thirty-three patients who did not consent for the longitudinal study were older (P=0.003), but other baseline characteristics (sex, vascular risk factors, apolipoprotein E [APOE], number of microbleeds, and leukoaraiosis volume) did not differ from the longitudinal cohort (n=300; all P>0.2). Information on antithrombotic medication use, incident lobar ICH, and occurrence and cause of death was obtained by follow-up phone calls at 3 months after enrollment and every 6 months thereafter.20 Chart review was performed when needed to adjudicate the nature of an event reported as a new lobar ICH. We accrued the date of death by consulting the Social Security Death Index as described previously.6 All patients were followed from their date of enrollment until the occurrence of ICH, death, or the end of follow-up in June 2012.
This study was performed with the approval of and in accordance with the guidelines of the institutional review board of Massachusetts General Hospital and with informed consent of all subjects or authorized family members. Radiological and genetic analyses were performed by separate study personnel and the results recorded without the knowledge of the subjects’ clinical information.
Clinical and Laboratory Data
Data on demographics (age, sex) and vascular risk factors (hypertension, diabetes mellitus, and hypercholesterolemia) were obtained by interviewing the patients (or their families or surrogates) at enrollment. APOE genotype was determined in a large subset of patients who provided research blood samples.25
MRI Acquisition and Analysis
Images were obtained using a 1.5-T magnetic resonance scanner (GE Signa). Whole-brain axial gradient-echo images (repetition time/echo time, 750/50 ms; 5 mm slice thickness; 1 mm interslice gap) and flu-id-attenuated inversion recovery images (repetition time/echo time, 10 000/140 ms; inversion time, 2200 ms; number of excitations, 1; 5 mm slice thickness; 1 mm interslice gap) were performed.
Lobar microbleeds were classified as punctate, hypointense foci (<5 mm in diameter) selectively involving the cortex and underlying white matter on gradient-echo images, distinct from vascular flow voids and leptomeningeal hemosiderosis.11 White matter hyper-intensity (WMH or leukoaraiosis) volume was quantified as previously validated18 using a computer-assisted algorithm that involves MRicron, a freely available tool.26 All MRI analyses were performed and recorded by investigators blinded to clinical and genetic data.
Statistical Analysis
Univariate analyses were used to compare clinical characteristics, frequencies of the APOE ε2 and ε4 alleles, and radiological markers between the 2 groups. Subsequently, multivariate analyses were performed to look for independent associations between these predictors and diagnostic categories. For multivariate models, APOE genotype was analyzed as a categorical variable according to the presence or the absence of the ε2 and ε4 alleles. As blood samples for genotyping were not available in 28% of subjects, multivariate models were built with and without APOE; addition of this variable did not change the associations observed among other variables. In the follow-up cohort, the mean follow-up time was calculated and the incidence rates of ICH and death were determined using the incidence per 100 person-years of follow-up. We used multivariable Cox regression analyses to calculate the crude and adjusted hazard ratios for occurrence of ICH and death. For the adjusted Cox regression model, patient group (with ICH as the control group), age, sex, hypertension, WMH volume, and lobar microbleed counts were entered in the model. In the microbleed-only patients, a multivariable Cox regression model was built to test the association between anticoagulant use and incident ICH after adjustment for demographics, hypertension, WMH, and microbleeds. All analyses were performed with SPSS 22.0 (released 2012, IBM SPSS Statistics for Windows, version 22.0, IBM Corp, Armonk, NY). All tests of significance were 2 tailed.
Results
We analyzed a total of 379 patients who were enrolled between January 1993 and January 2012, of whom 63 patients presented with lobar microbleeds only and 316 with lobar ICH. Of the 63 microbleed-only patients, 26 patients underwent their index MRI for evaluation of symptoms suggestive of an ischemic event, 27 because of mild cognitive symptoms, 4 because of a gait disorder, and 6 because of transient sensory spells. None of the microbleed-only patients was found to have ischemic stroke, dementia, mass lesion, or other neurodegenerative conditions after complete evaluation.
Demographics (age, sex), vascular risk factors, and APOE genotype did not differ significantly between microbleed-only and ICH groups (Table 1). The lobar microbleed count was significantly higher in microbleed-only patients (median, 10; interquartile range, 4–30) compared with the ICH patients (median, 2; interquartile range, 1–9; P<0.001). This difference remained significant after adjusting for demographics and vascular risk factors (P<0.001). Within the lobar microbleed-only group, no significant correlation was found between micro-bleed counts and other factors such as demographics, vascular risk factors, or APOE. Microbleed-only patients had a larger median WMH volume compared with the patients with ICH (31 versus 23 mL; P=0.02; Table 1). Higher WMH volume remained independently associated with the microbleed-only category (P=0.04) after correction for age, sex, and vascular risk factors.
Table 1.
Clinical and Radiological Characteristics of the 2 Patient Groups
| Variable | No. of Patients Presenting With
|
||
|---|---|---|---|
| Lobar Microbleed- Only (n=63) | Lobar ICH (n=316) | P Value | |
| Definite/probable CAA | All | All | |
| Clinical variables | |||
| Male sex (%) | 40 (63) | 162 (51) | 0.1 |
| Age, y | 73.6±8.3 | 73.6±9 | >0.2 |
| Hypertension (%) | 34 (54) | 194 (61) | >0.2 |
| Hypercholesterolemia (%) | 26 (41) | 136 (43) | >0.2 |
| Diabetes mellitus (%) | 9 (14) | 55 (17) | >0.2 |
| Genotype | |||
| APOE ε2 frequency | 15.6% | 12% | >0.2 |
| APOE ε4 frequency | 25% | 23% | >0.2 |
| Radiological markers | |||
| Lobar microbleed count | 10 (4–30) | 2 (1–9) | <0.001 |
| WMH volume, mL | 31 (18–46) | 23 (12–40) | 0.02 |
Values are displayed as mean ± SD, median (25th–75th quartile), or n (%). APOE genotypes were available in 48 subjects with microbleed-only and 224 with ICH. APOE indicates apolipoprotein E; CAA, cerebral amyloid angiopathy; ICH, intracerebral hemorrhage; and WMH, white matter hyperintensity.
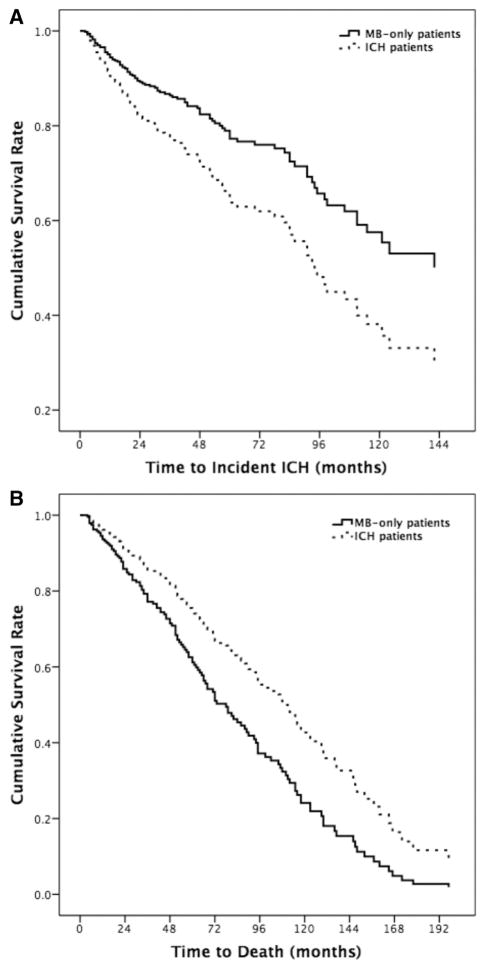
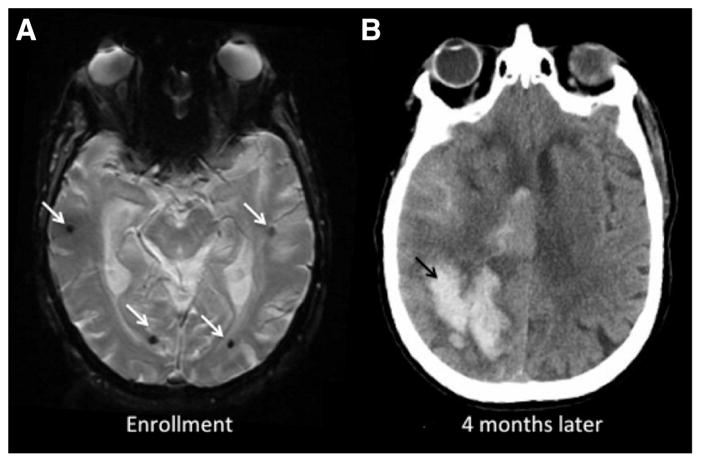
Three hundred patients (240 ICH who survived the first 90 days after their index ICH and 60 microbleed-only) were followed longitudinally for 5.3±3.8 years after their index event. Twelve microbleed-only patients (20% of the microbleed-only group, 5 per 100 person-years) developed a lobar ICH during follow-up versus 86 patients (36%) presenting with ICH (8.9 per 100 person-years). Details of the incident event rates, hazard ratios, and confidence intervals are presented in Table 2. Cox regression analysis showed a mildly lower risk of incident ICH for the microbleed-only versus the lobar ICH patients, but this difference did not reach statistical significance (Figure 1A; hazard ratio, 0.58; 95% confidence interval, 0.31–1.06; P=0.08). The ICH rate observed in either group of CAA patients was orders of magnitude greater than that of the general elderly population (estimated at 0.015–0.05 incident ICHs per 100 people per year).16,17 Figure 2 shows the baseline and follow-up imaging of an microbleed-only patient who later developed a symptomatic ICH.
Table 2.
Incidence Rates and Hazard Ratios for the Occurrence of ICH and Death in Both Groups During Follow-Up
| Event Rate | No. of Patients Presenting With
|
||
|---|---|---|---|
| Lobar Microbleed- Only (n=60) | Lobar ICH (n=240) | P Value | |
| Event: occurrence of lobar ICH | |||
| Observed person-years | 241 | 968 | … |
| No. of occurrence (%) | 12 (20) | 86 (36) | … |
| Incidence of ICH per 100 person-years (95% CI) | 5 (2.6–8.7) | 8.9 (7.1–11) | … |
| Crude hazard ratio (95% CI) | 0.57 (0.3–1.04) | Ref | 0.07 |
| Adjusted hazard ratio* (95% CI) | 0.58 (0.31–1.06) | Ref | 0.08 |
| Event: occurrence of death | |||
| Observed person-years | 261 | 1316 | … |
| No. of occurrence (%) | 31 (52) | 105 (44) | … |
| Incidence of death per 100 person-years (95% CI) | 11.9 (8–16.8) | 8 (6.5–9.7) | … |
| Crude hazard ratio (95% CI) | 1.8 (1.2–2.8) | Ref | 0.005 |
| Adjusted hazard ratio* (95% CI) | 1.67 (1.1–2.6) | Ref | 0.02 |
CI indicates confidence interval; ICH, intracerebral hemorrhage; and ref, reference for hazard ratios.
Adjusted for age, sex, hypertension, microbleed count, and white matter hyperintensity volume.
Figure 1.
Survival curves of the 2 groups for occurrence of intracerebral hemorrhage (ICH; A) and death (B). MB indicates microbleed.
Figure 2.
Baseline and follow-up imaging of a lobar microbleed-only patient who later developed intracerebral hemorrhage (ICH). An 85-year-old woman with no prior stroke, who presented with cognitive symptoms, was enrolled after finding of multiple isolated lobar microbleeds on MRI (white arrows, A). Four months later, the patient presented to the emergency department with acutely altered mental status. Her head computed tomography showed a right-sided posterior lobar ICH with ventricular extension (black arrow, B).
We analyzed the predictors of incident ICH in the microbleed-only group. Warfarin use (P=0.02) and older age (P=0.04) were independently associated with time to incident ICH in a multivariable Cox regression model that also included sex, hypertension, WMH volume, and microbleed count as covariates. These associations did not change when aspirin use was introduced into the model, and aspirin was not associated with increased ICH risk (P>0.2).
Thirty-one microbleed-only patients (11.9 per 100 person-years) died during follow-up versus 105 patients in the ICH group (8 per 100 person-years). After adjusting for age, sex, hypertension, WMH volume, and microbleed counts, the case-fatality rate was higher in microbleed-only patients (Table 2 and Figure 1B; adjusted hazard ratio, 1.67; 95% confidence interval, 1.1–2.6; P=0.02). Introduction of APOE status into multivariate models did not change any of the associations presented under the Results section. Two microbleed-only and 9 lobar ICH patients underwent autopsy. Presence of moderate-to-severe CAA was pathologically confirmed in all of these patients.
Discussion
In this study, we have identified similar genetic and radiological characteristics at presentation between individuals with nonspecific symptoms who had lobar microbleeds on T2* MRI and patients with CAA diagnosed after a lobar ICH. Compared with the lobar ICH CAA patients, the patients with isolated lobar microbleeds were in the same age range and had similar vascular risk factors. APOE genotypes were also similar, with relatively high frequencies for the ε2 and ε4 alleles as previously observed in nontraumatic lobar ICH.27 There also seemed to be notable differences between the 2 groups. The microbleed-only group demonstrated higher microbleed counts, a finding that might in part reflect a higher likelihood that patients with large numbers of microbleeds would be identified and referred to our longitudinal research study. Patients with lobar microbleed-only in this study demonstrated increased WMH volume, a previously identified consequence of severe CAA pathology,28 as well as a substantial risk of subsequent ICH, and an overall higher mortality than CAA patients presenting with ICH.
The Boston criteria for diagnosis of CAA during life originally assumed the presence of ≥1 lobar hemorrhage, the presence of lobar microbleeds strengthening the diagnosis. The ongoing question in the field has been the diagnostic and prognostic importance of finding multiple lobar microbleeds on MRI of an older adult without any symptomatic ICH and without other causes for microbleeds. The results of our baseline comparisons that show similar demographic, genetic, and vascular risk profiles between the groups support the view that the lobar microbleed-only pattern can reliably be considered as probable CAA. The finding of a more severe marker of CAA-related cerebral damage (high WMH volume) also suggests vascular amyloid-related small vessel dysfunction as the principal pathological mechanism in these cases.
The current data bear on the important question of which patients should receive anticoagulant therapy. Individuals with isolated lobar microbleeds are being increasingly detected by more frequent use of sensitive MRI techniques, with prevalences in the range of 11% to 24% of the community-dwelling elderly.13,14 As the risk of ICH in these patients is largely unknown, however, there has been insufficient evidence to conclude that the presence of lobar microbleeds alone should preclude anticoagulant therapy.29 A neuropathologic study also suggested that CAA patients with multiple microbleeds might have different vessel pathology (with thicker amyloid-positive vessel walls) compared with CAA patients with few lobar microbleeds, suggesting that the ICH risk might be different across these groups.30 For these reasons, it has not been possible to extrapolate ICH risk estimates in a population with isolated lobar microbleeds based on prior studies of patients with past ICH.1,24 The current study suggests that microbleed-only CAA patients, though at mildly lower risk for future ICH than those with past ICH, are nonetheless at substantial risk.
Despite our relatively small sample size, we have also found that coumadin use was independently associated with the risk of incident symptomatic lobar ICH. An important area for future research will therefore be to determine, either by observational analysis or by randomized clinical trial, whether this risk of future ICH is sufficient to tip the risk versus benefit calculation away from anticoagulant treatment in specific clinical situations. Such a study will need to be powered to analyze the contribution of multiple ICH risk factors.31 A previous decision analysis suggested that the particularly high risk of future ICH among CAA patients with past ICH weighed strongly against anticoagulation, even in patients with nonvalvular atrial fibrillation.32
In light of the substantial risk for future ICH observed among the microbleed-only subjects, it is reasonable to consider this condition as a potential precursor or early form of CAA-related ICH. In other respects, however, the microbleed-only patients in the current study demonstrate markers of CAA equal to or greater than those in the ICH group. Among these markers were increased microbleed counts (possibly a reflection of referral preferences as noted above), higher WMH burden, and earlier mortality. We were unable to determine the potential causes of increased mortality in patients with microbleed-only CAA, but this finding is in line with recent studies that show higher mortality in older adults with microbleeds.33,34 Although clearly requiring further analysis, the current data suggest that microbleed-only CAA may represent an alternative pathway by which this pathology can cause progressive neurological damage, even in the absence of major hemorrhagic stroke. Pathological confirmation of the CAA diagnosis in all 11 patients who underwent autopsy also supports the view that Boston criteria can accurately establish this diagnosis during life.
Our study has limitations. It is indeed likely that many of the microbleed-only patients were referred to our clinic because of finding relatively high number of lobar micro-bleeds, an issue that might be related to higher WMH load and mortality in this particular cohort. We do note, however, that the number of lobar microbleeds was not related to the risk of incident ICH in these subjects, suggesting that this possible referral bias did not account for the relatively high incidence of future hemorrhage in microbleed-only patients. The question of CAA diagnosis is typically raised when a brain MRI obtained for neurological complaints in an older adult shows lobar microbleeds. In that sense, our study population is similar to patients seen in clinical practice. None of our microbleed-only patients had dementia, stroke, or other neurodegenerative conditions at enrollment, limiting the contribution of potential confounders to the outcomes observed. A second limitation was our sample size, which limited our ability to assess the risk of antithrombotic use in better multivariate models. A larger study would be necessary to address the risk of ICH with or without antithrombotic use, in patients with isolated lobar microbleeds who are at high risk of ischemic events because of the presence of atrial fibrillation, deep venous thrombosis, or pulmonary embolism. Data from randomized clinical trials are unlikely to be forthcoming, however, as such trials to rule out harmful medication effects in high-risk subjects are difficult to justify and perform.
Conclusions
The vascular risk factors as well as genetic and radiological characteristics of patients with isolated lobar microbleeds are similar to patients with CAA diagnosed after a lobar ICH, therefore suggestive of substantial CAA pathology. In this sense, lobar microbleeds on MRI, a common finding in otherwise healthy elderly individuals with or without nonspecific symptoms, seem to be a promising diagnostic marker of advanced CAA. We also find that patients presenting with isolated lobar microbleeds are at considerable risk of future lobar ICH, a risk made worse by the use of warfarin. Given the high prevalence of isolated lobar microbleeds in the elderly, our findings support the importance of developing early detection markers for CAA in asymptomatic individuals, studying its impact in this population, and determining the feasibility of treating CAA before it becomes symptomatic.
Acknowledgments
Sources of Funding
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke (K23 NS083711, T32 NS048005, R01 NS070834), the National Institute on Aging (R01 AG26484), the Department of Radiology at Leiden University, and Dutch Alzheimer Foundation.
Footnotes
Disclosures
Dr Rosand serves as a consultant for Boehringer Ingelheim. Drs Rosand, Greenberg, and Gurol receive research support from National Institutes of Health. The other authors report no conflicts.
References
- 1.Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004;35:1415–1420. doi: 10.1161/01.STR.0000126807.69758.0e. [DOI] [PubMed] [Google Scholar]
- 2.Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18:311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- 3.Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP., Jr Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991;30:637–649. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 4.Gregoire SM, Charidimou A, Gadapa N, Dolan E, Antoun N, Peeters A, et al. Acute ischaemic brain lesions in intracerebral haemorrhage: multicentre cross-sectional magnetic resonance imaging study. Brain. 2011;134(pt 8):2376–2386. doi: 10.1093/brain/awr172. [DOI] [PubMed] [Google Scholar]
- 5.Gurol ME, Viswanathan A, Gidicsin C, Hedden T, Martinez-Ramirez S, Dumas A, et al. Cerebral amyloid angiopathy burden associated with leukoaraiosis: a positron emission tomography/magnetic resonance imaging study. Ann Neurol. 2013;73:529–536. doi: 10.1002/ana.23830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164:880–884. doi: 10.1001/archinte.164.8.880. [DOI] [PubMed] [Google Scholar]
- 7.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 9.Smith EE, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Curr Atheroscler Rep. 2003;5:260–266. doi: 10.1007/s11883-003-0048-4. [DOI] [PubMed] [Google Scholar]
- 10.Gurol ME, Dierksen G, Betensky R, Gidicsin C, Halpin A, Becker A, et al. Predicting sites of new hemorrhage with amyloid imaging in cerebral amyloid angiopathy. Neurology. 2012;79:320–326. doi: 10.1212/WNL.0b013e31826043a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Microbleed Study Group. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Rooden S, van der Grond J, van den Boom R, Haan J, Linn J, Greenberg SM, et al. Descriptive analysis of the Boston criteria applied to a Dutch-type cerebral amyloid angiopathy population. Stroke. 2009;40:3022–3027. doi: 10.1161/STROKEAHA.109.554378. [DOI] [PubMed] [Google Scholar]
- 13.Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 14.Sveinbjornsdottir S, Sigurdsson S, Aspelund T, Kjartansson O, Eiriksdottir G, Valtysdottir B, et al. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry. 2008;79:1002–1006. doi: 10.1136/jnnp.2007.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero JR, Preis SR, Beiser AS, DeCarli C, Lee DY, Viswanathan A, et al. Lipoprotein phospholipase A2 and cerebral microbleeds in the Framingham Heart Study. Stroke. 2012;43:3091–3094. doi: 10.1161/STROKEAHA.112.656744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labovitz DL, Halim A, Boden-Albala B, Hauser WA, Sacco RL. The incidence of deep and lobar intracerebral hemorrhage in whites, blacks, and Hispanics. Neurology. 2005;65:518–522. doi: 10.1212/01.wnl.0000172915.71933.00. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 18.Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66:23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- 19.Smith EE, Gurol ME, Eng JA, Engel CR, Nguyen TN, Rosand J, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intra-cerebral hemorrhage. Neurology. 2004;63:1606–1612. doi: 10.1212/01.wnl.0000142966.22886.20. [DOI] [PubMed] [Google Scholar]
- 20.Biffi A, Halpin A, Towfighi A, Gilson A, Busl K, Rost N, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2010;75:693–698. doi: 10.1212/WNL.0b013e3181eee40f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg SM. Cerebral amyloid angiopathy: prospects for clinical diagnosis and treatment. Neurology. 1998;51:690–694. doi: 10.1212/wnl.51.3.690. [DOI] [PubMed] [Google Scholar]
- 22.Kinnecom C, Lev MH, Wendell L, Smith EE, Rosand J, Frosch MP, et al. Course of cerebral amyloid angiopathy-related inflammation. Neurology. 2007;68:1411–1416. doi: 10.1212/01.wnl.0000260066.98681.2e. [DOI] [PubMed] [Google Scholar]
- 23.Zhang-Nunes SX, Maat-Schieman ML, van Duinen SG, Roos RA, Frosch MP, Greenberg SM. The cerebral beta-amyloid angiopathies: hereditary and sporadic. Brain Pathol. 2006;16:30–39. doi: 10.1111/j.1750-3639.2006.tb00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Donnell HC, Rosand J, Knudsen KA, Furie KL, Segal AZ, Chiu RI, et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med. 2000;342:240–245. doi: 10.1056/NEJM200001273420403. [DOI] [PubMed] [Google Scholar]
- 25.Brouwers HB, Biffi A, Ayres AM, Schwab K, Cortellini L, Romero JM, et al. Apolipoprotein E genotype predicts hematoma expansion in lobar intracerebral hemorrhage. Stroke. 2012;43:1490–1495. doi: 10.1161/STROKEAHA.111.643262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- 27.Biffi A, Sonni A, Anderson CD, Kissela B, Jagiella JM, Schmidt H, et al. International Stroke Genetics Consortium. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol. 2010;68:934–943. doi: 10.1002/ana.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holland CM, Smith EE, Csapo I, Gurol ME, Brylka DA, Killiany RJ, et al. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008;39:1127–1133. doi: 10.1161/STROKEAHA.107.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckman MH, Wong LK, Soo YO, Lam W, Yang SR, Greenberg SM, et al. Patient-specific decision-making for warfarin therapy in nonvalvular atrial fibrillation: how will screening with genetics and imaging help? Stroke. 2008;39:3308–3315. doi: 10.1161/STROKEAHA.108.523159. [DOI] [PubMed] [Google Scholar]
- 30.Greenberg SM, Nandigam RN, Delgado P, Betensky RA, Rosand J, Viswanathan A, et al. Microbleeds versus macrobleeds: evidence for distinct entities. Stroke. 2009;40:2382–2386. doi: 10.1161/STROKEAHA.109.548974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 32.Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke. 2003;34:1710–1716. doi: 10.1161/01.STR.0000078311.18928.16. [DOI] [PubMed] [Google Scholar]
- 33.Akoudad S, Ikram MA, Koudstaal PJ, Hofman A, van der Lugt A, Vernooij MW. Cerebral microbleeds and the risk of mortality in the general population. Eur J Epidemiol. 2013;28:815–821. doi: 10.1007/s10654-013-9854-3. [DOI] [PubMed] [Google Scholar]
- 34.Altmann-Schneider I, Trompet S, de Craen AJ, van Es AC, Jukema JW, Stott DJ, et al. Cerebral microbleeds are predictive of mortality in the elderly. Stroke. 2011;42:638–644. doi: 10.1161/STROKEAHA.110.595611. [DOI] [PubMed] [Google Scholar]