Abstract
Herbal medicine, including traditional Chinese medicine, has been used for the prevention, treatment, and cure of disorders or diseases for centuries. In addition to being used directly as therapeutic agents, medicinal plants are also important sources for pharmacological drug research and development. With the increasing consumption of herbal products intended to promote better health, it is extremely important to assure the safety and quality of herbal preparations. However, under current regulation surveillance, herbal preparations may not meet expectations in safety, quality, and efficacy. The challenge is how to assure the safety and quality of herbal products for consumers. It is the responsibility of producers to minimize hazardous contamination and additives during cultivation, harvesting, handling, processing, storage, and distribution. This article reviews the current safety obstacles that have been involved in traditional Chinese herbal medicine preparations with examples of popular herbs. Approaches to improve the safety of traditional Chinese medicine are proposed.
1 Introduction
According to a US survey in 2007, approximately 38 % of adults and 12 % of children used some form of complementary and alternative medicine (CAM) with a total of USD$33.9 billion out-of-pocket spending. The spending on non-vitamin, non-mineral, natural products including botanicals was USD$14.8 billion. The top three products for adults were fish oil, omega 3, or docosahexaenoic acid (DHA 37.4 %), glucosamine (19.9 %), and Echinacea (19.8 %) while Echinacea (37.2 %), fish oil, omega 3, or DHA (30.5 %), and the combination herb pill (17.9 %) were the top products for children. Ginseng was the most commonly used traditional Chinese medicine (TCM) in the survey [1, 2]. Other studies also reported that there was more CAM used among cancer patients than patients with benign disease [3]. The World Health Organization reported the annual sales of herbal medicines in Germany reached USD$2.432 billion in 2002. According to China's National Bureau of Statistics, the value of industrial output from TCM reached USD$68 billion (RMB418 billion) in 2011 with an annual growth rate of 37.9 %. Worldwide, the TCM market is increasing by 10–20 % annually [4].
TCM uses have been documented since 200AD to prevent, treat, and cure symptoms or diseases and promote good health. Yellow Emperor's Inner Canon (Huangdi Neijing) and Materia Medica of Deity of Agriculture (Shen Nong Ben Cao Jing) were two of the most influential, earliest, Chinese medicine books, depicting the pharmacological efficacy of the TCM formulation, derived from empirical observations with 365 entries on medicaments listed [5, 6]. Before the introduction of Western medicine to China in the 19th century, TCM had been the major treatment for various diseases in Chinese communities. According to a 2009 Department of Health in Taiwan report, approximately 27.8 % of the Taiwanese population aged older than 15 years had received TCM treatment at least once in their life [7]. With the general mild nature and long historical use, TCM uses have been viewed as safe treatments for preventive and/or chronic disease. However, because of increasing fatal incidences, regulatory agencies in the US, Europe, and Asia have gradually tightened their regulations on herbal products in the market. One example is the removing of Ephedra-containing dietary supplements from the market in 2004 by the US Food and Drug Administration (FDA) [8–10]. Indeed, the European Union (EU) has recently changed its regulation to allow only long-established and quality-controlled medicines to be sold in the EU to ensure the safety of TCM [11, 12]. With the establishment of a voluntary adverse drug reaction (ADR) reporting system by the Chinese State Food and Drug Administration (SFDA) in 1989, the awareness of TCM drug safety and risk management is gradually improving [13].
According to FDA regulations, a botanical may be marketed as a dietary supplement, through an over-the-counter drug monograph, or through the approval of a New Drug Application. Under the Dietary Supplement Health and Education Act of 1994 (DSHEA), dietary supplements, including botanicals, are considered to be a food, which does not need pre-market approval by the FDA, and not as a food additive, which needs a pre-market approval by the authority. It is the responsibility of the dietary supplement manufacturer to ensure safety before a dietary supplement is marketed. The DSHEA regulations do not allow statements that claim “to diagnose, mitigate, treat, cure or prevent a specific disease or class of diseases”. If a botanical product is intended for use in diagnosing, mitigating, treating, curing, or preventing disease, it must be marketed under an approved New Drug Application or via the FDA's over-the-counter drug monograph system [14, 15]. The FDA's Guidance for Industry: Botanical Drug Products describes regulatory approaches that must be followed to market botanicals as drugs in the USA [16]. In contrast to the policies of the European Medicines Evaluation Agency or Canadian regulatory authorities, the FDA considers botanical drugs to be in the same category as non-botanical drugs in clinical trials [11, 16, 17]. There are currently only two FDA-approved botanical drugs: Veregen® (sinecatechins ointment) for the treatment of genital warts [18], and Fulyzaq® (crofelemer), an anti-diarrheal drug for HIV/AIDS [19]. In most cases, TCM has been catalogued as dietary supplements in the USA.
No matter whether targeting TCM as botanical drugs or dietary supplements, besides efficacy, safety and consistent quality are the two most important factors in TCM formulations. There are several factors that may contribute to the safety concerns of TCM products: the intrinsic toxicities of herbs, environmental contaminations (such as air pollution, soil contaminations, and heavy metals), cultivation practices (such as pesticides, fungicides, heavy metals, microorganisms, and endotoxins), and manufacture processing and handling (including storage, additives, microorganisms, endotoxins, and human adulteration). Other safety concerns such as long-term toxicity, dose-dependent toxicity, treatment duration, herb–drug interactions, and herb–herb interactions also need to be carefully evaluated in the use of TCM.
2 Intrinsic Toxicities of TCM
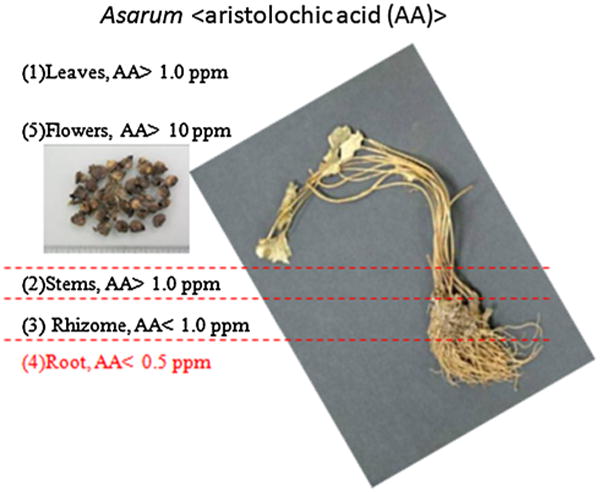
There are misconceptions about the safety of particular herbs. Indeed, several herbs have been reported to have severe intrinsic hepatologic, renal, cardiologic, neurologic, carcinogenic, and gastrointestinal toxicities [20–29]. These herbs with their prospective toxicities are listed in Table 1. Many species of Aristolochia, including A. debilis, A. contorta, A. manshuriensis, and A. fangchi, have been documented and used in TCM for hundreds of years to treat symptoms such as acute arthritis and edema [30]. Between 1990 and 1992, more than 100 women in Belgium and France were found to have extensive interstitial fibrosis of the kidneys after using a weight-loss regimen involving TCM. The incidences resulted in many kidney transplants and cancer cases. The investigation into these incidences found that one of the herbs in the pills, Stephania tetrandra (Fen Fang Ji or Han Fang Ji) was inadvertently replaced by A. fangchi (Guang Fang Ji) during a manufacturing error. A nephrotoxic and carcinogenic agent, aristolochic acid (AA), was found to have contributed to nephropathy in this incidence. It raised the attentions of AA nephropathy to the Western world [25–29]. The incidences involving Aristolochia fangchi resulted in a ban of the Aristolochia family in most countries in 2003; however, some countries, such as India and China, still use Aristolochia as part of their traditional herbal medicines [28]. Asarum heterotropoides Fr. Schmidt var. mandshuricum (Maxim.) Kitag also belongs to the family of Aristolochiaceae. The root of Asarum (Asari Radix et Rhizoma or Xi Xin), which has a low level of aristolochic acid (less than 0.5 ppm), has been commonly used in TCM formulation as an analgesic to treat headache, toothache, and other inflammatory diseases; however, the whole Asarum plant has a high level of AA (1–150 ppm) and can be mistakenly used in manufacturing to yield a herbal product with high toxicity [30] as shown in Fig. 1. A recent study in Taiwan also revealed that AA-containing TCM contributed significantly to the incidence of upper urothelial cancer [28]. Another example involving the toxicity of AA was the retrospective study of a Taiwan health insurance database on hepatitis B virus patients treated with the traditional TCM formula Long-Dan-Xie-Gan-Tang, used for hepatitis jaundice [31]. Long-Dan-Xie-Gan-Tang consists of 10 herbs including Lardizabalaceae (Mu Tong) and Radix bupleuri (Chai Hu). Lardizabalaceae (Mu Tong), dried stems of Akebia quinata (Thunb.) Decne. (Akebia quinata), Akebia trifoliata (Thunb.) Koidz. (Akebia trifoliata), or Akebia trifoliata (Thunb.) Koidz. var. and australis (Diels) Rehd were originally documented at the Materia Medica of Deity of Agriculture (Shen Nong Ben Cao Jing) for the improvement of liver functions. However, Caulis aristolochiae manshuriensis (Guang Mu Tong), which contains AA, inadvertently replaced Mu Tong (Lardizabalaceae) in the formula, causing nephropathy in patients [32]. This type of mistake could be avoided if the herb was authenticated by experienced herbalists and confirmed with modern analytical technologies.
Table 1. Traditional Chinese medicine with severe intrinsic toxicities.
| Common name | Scientific name | Intrinsic toxicity | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Hepato | Renal | Cardiologic | Neurologic | Gastrointestinal | Carcinogenic | ||
| Ma Huang | Ephedra sinica [9, 10] | ✓ | ✓ | ✓ | |||
| Fu Zi | Aconitum species [21, 22] | ✓ | ✓ | ✓ | |||
| Guang Fang Ji | Aristolochia fangchi [23, 26] | ✓ | ✓ | ||||
| Chai Hu | Radix bupleuri [24, 73] | ✓ | |||||
| Xi Xin | Asarum [30] | ✓ | ✓ | ||||
| Guang Mu Tong | Caulis aristolochiae [32] | ✓ | ✓ | ||||
| Jin Bu Huan | Lycopodium serratum [65, 66] | ✓ | |||||
Fig. 1. Distribution of aristolochic acid (AA) in different parts of Asarum (XiXin).
The Aconitum species (Fu Zhi, Chuan Wu, Cao Wu) of herbs is more toxic owing to its high content of aconitine alkaloids. It is required to perform detoxifying processes post-harvesting to remove the intrinsic cardiologic, neurolgoic, and gastrointestinal toxins [21–23]. Aconitum has been documented in “Shang han lun” since 200AD for its therapeutic effects as a stimulant to dispel chills and increase vitality. These pharmacological properties have been translated into modern terminology of diseases or conditions such as rheumatoid arthritis, gastroenteritis, and bronchial asthma [33]. Although the SFDA in China stipulates that only processed and detoxified roots of Aconitum species can be used in clinical decoctions or in pharmaceutical manufacturing, these herbs remain a constant source of poisoning [34].
Other botanical toxins such as pyrrolizidine alkaloids, produced by plants as defense agents against insect herbivores, can cause toxic reactions in humans, primarily in veno-occlusive liver disease. Certain TCMs, such as Lithospermum erythrorhizon (Zi Cao), Flos Tussilaginis Farfarae (Kuan dong hua), Herba Senecionis Scandens (Qian li quang), and Eupatorium fortune (Pei Lan) also contain liver-damaging pyrrolizidine alkaloids [35].
3 Extrinsic Toxicities of TCM
3.1 Toxicities Evolved During Cultivation
While some TCM has been recognized for its intrinsic toxicities, harmful events can usually be prevented if the herbs are inspected by experienced TCM doctors/practitioners, or analyzed by analytical instruments. The major challenge for most TCM consumers is the assurance of obtaining a non-harmful, non-contaminated, and quality-consistent herbal raw material or product. The increasing prevalence of environmental pollutions, such as high heavy metal-contaminated soils, has become one of the hard-to-avoid obstacles for global acceptance of TCM. Another preventable hurdle is contamination caused by the high use of chemicals in agriculture during herbal cultivation (pesticides, fungicides), handling, manufacture processing, and distribution (microorganisms, toxins, preservatives, additives, adulteration). Since 2004, adulteration with banned pharmaceutical ingredients in dietary supplements led to approximately half of the FDA class I drug recalls [36].
3.1.1 Heavy Metals
Heavy metal contamination in soil usually comes from industrial waste, but it is also contributed from minerals in the soil. Studies found that rice, one of the most popular foods in the world, is a natural heavy metal scavenger for arsenic (As) and cadmium (Cd). With traditional flood field agriculture, rice has been shown to be the dominant source of inorganic arsenic, which is a class one non-threshold carcinogen (in ppb ranges). The FDA has recommended that the general population eat a variety of grains to avoid adverse reactions from the excessive eating of any single food [37–39]. Accumulation and magnification of heavy metals [Cd, Pb, As, and mercury (Hg)] in human tissues through consumption of herbal remedies can cause hazardous results. Many regulatory authorities from different countries have set regulations on herbal products, but the maximum allowable limits of As, Cd, Pb, and Hg in raw herbs or herbal products are different (Table 2). Although US Pharmacopeia (USP) convention has restricted the limit of heavy metal levels in dietary supplement dosage forms, many herbal products on the US market do not follow regulations and often surpass the heavy metal limit levels [40, 41].
Table 2. Limitation on heavy metal contaminants in herbal medicine and products in various countries.
| Heavy metal | Arsenic (As) | Lead (Pb) | Cadmium (Cd) | Chromium (Cr) | Mercury (Hg) | |
|---|---|---|---|---|---|---|
| Canadaa | Raw herbs | 5 ppm | 10 ppm | 0.3 ppm | 2 ppm | 0.2 ppm |
| Finished herbal products | 0.01 mg/day | 0.02 mg/day | 0.006 mg/day | 0.02 mg/day | 0.02 mg/day | |
| Chinaa | Herbal materials | 2 ppm | 10 ppm | 1 ppm | 0.5 ppm | |
| Singaporea | Finished herbal products | 5 ppm | 20 ppm | 0.5 ppm | ||
| EP 8.0b | Herbal drug | 5 ppm | 1 ppm | 0.1 ppm | ||
| USP35c | Drug substance and excipients | 1.5 ppm | 0.5 ppm | 2.5 ppm | 1.5 ppm | |
| USP2232d | Dietary supplements | 1.5 ppm | 1 ppm | 0.5 ppm | 1.5 ppm | |
| Taiwane | Herbal products | 3 ppm | 10 ppm | 0.5 ppm | 0.5 ppm |
The individual components limits are based on a maximum daily intake of 10 g of a dietary supplement and are intended for use only with options for compliance with limits of elemental contaminants under individual component option
ppm parts per million, USP US Pharmacopeia, WHO World Health Organization
WHO guidelines for assessing quality of herbal medicines with reference to contaminants and residues
European Pharmacopoeia 8.0
USP 35-NF30
USP2232
Regulations for Registration of Medicinal Products (in Chinese)
Cordyceps sinensi is one of the most rare and expensive TCM in the world. Although it has been long been believed to have beneficial effects in disease prevention and treatments such as night sweating, hyposexuality, hyperglycemia, hyperlipidemia, asthenia after severe illness, respiratory disease, renal dysfunction/failure, arrhythmias, and other heart and liver diseases, there are very limited clinical data to support these claims [42]. Cordyceps sinensi is a rare combination of a caterpillar and a fungus (winter worm summer grass) and can only be found at altitudes above 3,800 m sea level in cold grassy alpine meadows of the Himalayan mountains. Zuo et al. reported their analyses of the heavy metal contents in 24 natural C. sinensi samples, five soil samples from the surface of these C. sinensi samples, as well as 61 soil samples collected from their habitats in the provinces of Sichuan and Tibet. The results indicated that most of the Pb, Cu, Cd, and Hg levels met the Chinese Pharmacopoeia requirements in these natural C. sinensi samples. However, most of As levels in these C. sinensi samples were over the limit, which was affected by the soil of the immediate growing areas [43]. The findings suggest the importance of the agricultural environment and removing the soil during the harvest of natural C. sinensi. Because of the high price of C. sinensi (varying from 100 to 500 RMB per gram), the adulteration with Pb is a common practice to increase weight [44].
Notoginseng (Panax notoginseng, San Qi) is another popular herb used in TCM formulas to treat blood disorders such as blood stasis and bleeding. It is also the major ingredient of a famous Chinese hemostatic proprietary herbal remedy, Yunnan Baiyao. A joint effort among several institutions has reported recently on the heavy metal contents of notoginseng in Wenshan and Baise areas of Yunnan Province in China [45, 46]. It was reported that 80 % of Radix Panax notoginseng planting soil in Wenshan area is not suitable for continuous cropping. Studies indicated the infection of the rhizosphere microorganism, such as Sinomonas notoginsengisoli sp. nov, could contribute to crop failure [46, 47]. Among 12 Radix notoginseng samples collected from different habitats, nearly half of the samples were over the heavy metal limits of USP specification in dietary and herbal supplements. As and Cd were the most common elements exceeding USP limits in 3 out of 10 raw herbal samples collected. In addition, As and Hg were found exceeding the limits in five and two samples out of 11 commercial samples, respectively [45]. One major issue of the safe level of heavy metals in herbal medicine is the diversification of current guidelines. The toxicity established is based on inorganic heavy metals. The toxicity of heavy metals in herbal medicine could be very different because it could chelate with organic chemicals in the herb. Research should be encouraged to address this issue.
3.1.2 Pesticides
Besides the contents of heavy metal, pesticides are another concern in TCM preparations. A recent report entitled “Chinese Herbs: Elixir of Health or Pesticide Cocktail” from the environmental group, Greenpeace, revealed some shocking information on contaminations in TCM [48]. Thirty-six dry herbs and herbal products, including chrysanthemum, wolfberry, honeysuckle, dried lily bulb, san qi, Chinese date, and rosebud were sampled. These herbal materials were originally exported from China to the Western world such as Canada, England, France, Germany, Italy, Netherlands, and the USA. Among these 36 samples studied, 32 samples were found to contain at least three types of pesticides. Moreover, 17 samples were classified as highly or extremely hazardous pesticide residues by World Health Organization standards (Table 3). In addition, 26 out of 29 well-categorized samples showed pesticide residue levels exceeded the European maximum level for safety. Because only a small percentage of pesticides or fungicides could reach the target plants, the majority of pesticides or fungicides are believed to end up in the environment (soil, water, and air). The heavy pesticide use is not only directly damaging the health of farmers, but also has negative impacts on the ecosystem. The global disappearance of bees is one of the negative results from the heavy use of pesticides and neonicotinoids destroying the ecological balance of the surrounding environment [49].
Table 3. Presence of pesticide residue classified as WHO Ia and Ib.
| Hazardous | Hazardous WHO class | Pesticides | Number of products, where found | Range of residue levels found (ppb) | Countries where the samples were bought |
|---|---|---|---|---|---|
| Extremely | Ia | Ethoprophos | 1 | 90 | Canada |
| Phorate | 3 | 6–10 | Canada, Italy, France | ||
| Highly | Ib | Omethoate | 5 | 10 | France, Germany, Canada |
| Methamidophos | 1 | 140 | Canada | ||
| Methomyl | 2 | 5–8 | Germany, Canada | ||
| Triazophos | 4 | 20–170 | US, Germany, The Netherlands |
[48] Greenpeace, “Chinese Herbs: Elixir of Health or Pesticide Cocktail”. Available from: http://www.greenpeace.org/eastasia/campaigns/food-agriculture/Chinese-Herbs-Elixir-of-Health/
ppb parts per billion, WHO World Health Organization
3.2 Toxicities Raised During Processing, Storage, and Distribution
3.2.1 Processing
Prior to use in TCM formulae, raw herbs must undergo physical and/or chemical pretreatment processes (Paozhi) after harvest for preservation, detoxification, or enhancing efficacy [50]. These processes include sun drying, stir frying, roasting, honey frying, wine frying, soil frying, vinegar frying, steaming, fumigation, and calcination. Adding postharvest additives to herbs is common in TCM processing. The key issue is how to ensure that the use of additives does not compromise herbal safety. In general, the processing methods, including adding safe doses of additives and conditions, have been evolved from decades of experience and documentation in Chinese Pharmacopeia.
Sun drying is the most popular conventional approach to handling TCM post-harvesting. It has been recently reported that herbal farmers or wholesalers use hazardous sulfur-fumigated processing for the purpose of keeping moisture, preserving color, preventing insects and molds, and bleaching. High doses of sulfur fumigation are not only destroying the chemical and biological properties of herbs, sulfur dioxide is released to the environment and causes toxic effects [51, 52]. Some herbs have been treated directly with sulfites such as sodium or potassium sulfite, bisulfite, and metabisulfite. Sulfite-contaminated herbal material could have potential adverse reactions, including severe asthmatic attacks and anaphylactic reactions [53]. Although China Pharmacopeia has prohibited using sulfur fumigation technique for bleaching TCM herbs since 2005, there are no well-defined acceptable levels of sulfur dioxide in herbs.
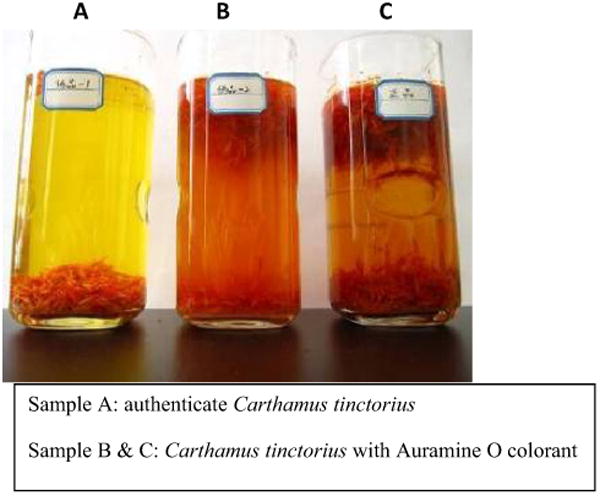
Detoxification is a necessary procedure for some TCM raw material; however, there is a major health issue if carcinogenic agents or harmful materials have been applied during TCM processing. It is a rising concern that some businesses, either intentionally or unintentionally, are putting their profits as the priority and ignoring the potential damage caused to humans from the harmful chemicals. China's Ministry of Health has marked the additives Auramine O, talc, and lead. Auramine O, a carcinogenic industrial dye that can cause liver and kidney toxicity, as “non-edible” substances. Carthamus tinctorius, an herb used as a purgative, analgesic, antipyretic, and an antidote to poisoning, has recently been found with Auramine O dye, which was used to help keep its color so that it could be sold for a higher retail price [54, 55] (Fig. 2). Talc and magnesium sulfate are other common additives used in TCM handling to keep moisture away and to add extra weight to raise prices of precious herbs.
Fig. 2. Illegal additive, Auramine O, in Carthamus tinctorius.
All TCMs sold in the USA as dietary supplements are regulated by the FDA with a different set of regulations than drug products. It is the responsibility of manufacturers to assure the safety of the herbal products by manufacturing from qualified herbal raw material or substances. Nonetheless, the FDA will take action against any adulterated or misbranded dietary herbal supplement product after it reaches the market. Regardless of the popularity of a dietary supplement, the FDA has removed some herbal products because of safety concerns about pharmaceutical ingredient adulteration from the process of dose production. PC-SPES is a well-known adulteration story in the herbal industry [56, 57].
PC-SPES was commercially available from November 1996 until 2002 as a dietary supplement. Preclinical studies suggested PC-SPES might have beneficial effects in reducing prostate specific antigen (PSA) levels, improving pain management, and enhancing the quality of life for those with hormone-refractory prostate cancer [58–63]. PC-SPES was a combination of eight different herbs: Chrysanthemum morifolium, Ganoderma lucidum, Glycyrrhiza glabra, Isatis indigotica, Panax pseudoginseng, Rabdosia rubescens, Scutellaria baicalensis, and Serona repens. Four prospective PC-SPES clinical trials with promising results were reported in patients with prostate cancer [57]. Small et al. conducted the largest PC-SPES clinical study in 70 patients to compare the androgen-dependent and -independent groups. Each patient received up to nine capsules of PC-SPES per day (each capsule contained 320 mg). It was reported that all 33 of the androgen-dependent group experienced a reduction in PSA of >80 % and that 19 of the 37 (54 %) with androgen-independent prostate cancer experienced a >50 % decrease in their PSA [60]. Attempts to identify the active compounds in PC-SPES have yielded incongruous results. Moreover, warfarin was identified in the serum of a patient taking PC-SPES who experienced a bleeding disorder. Sovak et al. [63] analyzed PC-SPES lots manufactured from l996 through mid-2001 and found that the phytochemical composition of PC-SPES varied by lot, and chemical analyses detected various amounts of the synthetic drugs diethylstilbestrol, indomethacin, warfarin, and several natural products. Clinical trials were halted and PC-SPES was removed from the market because of adulteration.
Jin Bu Huan (Lycopodium serratum) is a Chinese herb used for centuries as a mild sedative and analgesic. It has been recently marketed for insomnia, arthritic and orthopedic pain, and gastrointestinal complaints. Jin Bu Huan contains levo-tetrahydropalmatine, a controlled substance in the US and EU for its opiate-like sedating analgesic effect as a dopamine receptor or calcium channel antagonist. Jin Bu Huan product (sold as Jin Bu Huan Anodyne Tablets) was reported in more than a dozen cases of acute and chronic liver injury in the USA. Acute life-threatening neurologic and cardiovascular manifestations developed in children, while chronic hepatitis was induced in adults with long-term Jin Bu Huan use [64, 65]. In addition, the constituents of Jin Bu Huan were misidentified on the package, resulting in significant delay in identifying the plant alkaloid responsible for its toxicity. This product was therefore blacklisted by US and European health authorities [66].
3.2.3 Toxins and Microorganisms
Traditional Chinese medicines have been reported to contain excessive levels of mycotoxins including aflatoxins and ochratoxin A. Mycotoxins are toxic secondary metabolites produced by molds such as Aspergillus, Fusarium, and Alternaria, Penicillium which are carcinogenic, neurotoxic, teratogenic, and immunotoxic [67–69]. These toxins can occur at any stage of herbal production, from raw herb harvest to storage. TCMs that are manufactured or stored under inadequate conditions such as high humidity could become susceptible to mold growth and mycotoxin production. Mycotoxin production can be enhanced by ecological conditions such as drought, damage by insects, or mechanical harvesting during cultivation and storage. The common microbial contaminations contain Salmonella, Escherichia coli, Enterobacteria, TAMC, yeasts, and molds [70]. Maximum limits of aflatoxins and microbials have been set by the USP and the European Pharmacopoeia, as listed in Table 4.
Table 4. Maximum limits of microbials and aflatoxins.
| Item | US Pharmacopoeia 32 | European Pharmacopoeia 7.0 |
|---|---|---|
| Microbial limits | ||
| Total aerobic microbial | 105 CFU/g | 104 CFU/g |
| Total combined yeasts/molds | 103 CFU/g | 102 CFU/g |
| Enterobacterial count (bile-tolerant Gram-negative bacteria) | 103 CFU/g | 102 CFU/g |
| Escherichia coli | Absence in 10 g | Absence |
| Salmonella | Absence in 10 g | Absence |
| Aflatoxins | ||
| Aflatoxin B1 | ≤2 ppb | ≤2 ppb |
| Aflatoxin B2 | ≤0.54 ppb | |
| Aflatoxin G1 | ≤1.7 ppb | |
| Aflatoxin G2 | ≤0.4 ppb | |
| Sum of Aflatoxin B1, B2, G1, G2 | ≤4 ppb | |
CFU colony-forming units, ppb parts per billion
3.3 Dose-Dependent and Long-Term Exposure Toxicities
In addition to the human experience, nonclinical pharmacology and toxicity research studies, such as repeat-dose general toxicity studies, nonclinical pharmacokinetic/toxicokinetic studies, reproductive toxicology, genotoxicity studies, or carcinogenicity studies, provide the safety profiles of products. Because TCM is classified as dietary supplements in the US, the regulatory requirements for TCM products are not as strict as for botanical drugs, which require FDA premarket approval. Therefore, there is no motivation for the TCM industry to perform nonclinical safety assessments. Without systemic clinical or preclinical studies on varying herbal doses and duration of treatment, especially on long-term exposure, it is very difficult to assess the safety of TCM products.
Chinese herbal products containing Radix bupleuri (Chai Hu) are often prescribed for the treatment of chronic hepatitis. A retrospective study using the database of the National Health Insurance in Taiwan during 1997–2004 suggested a significant dose-dependent correlation between liver injury and the Radix bupleuri containing herbal remedy, Xiao-Chai-Hu-Tang (a traditional formula to treat jaundice, hepatitis, liver fibrosis, and liver cancer) in HBV patients [24, 71–73]. The data suggested that the TCM regimen containing more than 19 g of Radix bupleuri in HBV-infected patients might increase their risks of liver injury. This conclusion is consistent with a traditional claim that this formula should not be taken for a prolonged time as it could cause headache, dizziness, and bleeding of the gums.
3.4 Herb–Drug Interactions
A recent report indicated that cancer patients had a higher use in CAM, especially in herbs or supplements, than patients with benign disease. The study also indicated that CAM is more commonly used among patients with unre-sectable cancer than resectable disease (51 vs. 22 %, p <0.001) [74]. A study reviewed almost 3,000 cancer clinical trials in China and found 72 % of cancer patients reported using TCM with their active cancer therapies [75]. The top three TCM users were patients with lung cancer, liver cancer, and stomach cancer. It is clear that TCM is one of the global solutions to either improve the active therapy or quality of life of patients with unmet medical conditions. Drug–drug interactions have long been considered important safety factors during multiple therapeutic treatments. However, there is not much information available on herb–drug interactions. It is important for cancer patients to disclose their herbal use to their physicians.
Drug metabolism has to pass through phase I (such as cytochrome 450 isoenzyme, 3A4, 2C9), phase II metabolizing enzymes (such as glutathione s-transferases), and phase III efflux transporters (such as p-glycoprotein). The impacts of botanicals on these drug-metabolizing enzymes would provide important information to predict the toxicity and efficacy outcome with therapeutic drugs. Because of the early lack of herb–drug information with protease inhibitors treatments, HIV patients who took St. John's wort (Hypericum perforatum) as an anti-depressant agent incurred negative clinical outcomes on their HIV treatment. The adverse effects were later explained by the induction of St. John's wort on phase 1 metabolic enzymes CYP P450 (CYP3A4 and CYP2C9) and p-glycoprotein (Pgp) efflux transporter. As an inducer of CYP3A4, St. John's wort increased the metabolism of HIV protease inhibitors and reduced their bioavailability. In addition, it also increased HIV protease inhibitor clearance, led to lower plasma concentrations, and resulted in significant reductions in the bioavailability of P-gp substrates (e.g., HIV-1 protease inhibitors) [76]. Therefore, it is very important to set up a database of herb–drug interactions to avoid serious negative incidences.
4 Future Prospective to Improve the Safety of Traditional Chinese Medicine
With a long history of use, TCM is gaining popularity and being marketed as dietary supplements or being developed into herbal drugs. Regardless of the differences in regulatory standards, safety and quality are the common requirements for herbal drugs and herbal dietary supplements. However, with the complexity in ingredients, uncertainty in safety, and a lack of statistical meaning in the claims, there is continuous controversy about herbal dietary supplements. To overcome these hurdles, there are several factors that need to be addressed with the joint efforts of governments, research institutions, herbal industrials, and consumers: (1) establishing a global surveillance system to track the origin, characteristics, and processing of TCM; (2) use advanced technologies as quality-control standards during TCM production and processing; and (3) promoting evidence-based TCM. A non-profit organization, the Consortium for the Globalization of Chinese Medicine (www.tcmedicine.org), started at the University of Hong Kong in 2003, is serving these functions.
4.1 Establishing a Global Surveillance System to Track the Origin, Characteristics, and Processing of TCM
Ecological farming and manufacturing are the concepts of GAP and GMP (Good Manufacture Practice) for TCM-based botanical drugs and dietary supplements to ensure the quality of TCM products. The requirements on the certificate of authenticity of the plant and plant parts for botanical drugs are much stricter than those of herbal dietary supplements or foods. Aiming to reduce the incidences of adulteration and toxic additives in herbal products, China's SFDA has required TCM manufacturers to follow GMP regulations established by the SFDA for herbal materials and products. With increasing recalls on herbal products in the USA, it is not surprising that the FDA would tighten regulations on the herbal industry in the near future. It is important to have good tracking on herbal origins, information on cultivation conditions, harvesting, handling, processing, labeling, packaging, and distribution. The newly developed DNA barcoding system provides a reliable approach for herbal authentication. A group of scientists in China has developed a DNA barcoding system, BOMMD (Barcode of Medicinal Materials Database), to integrate TCM taxonomy and pharmacological properties. A web DNA barcode multimedia information platform-Medicinal Materials DNA Barcode Database (MMDBD) integrated herbal resources, medical parts, adulterant information, photographs, and the primers used for obtaining barcodes and key references. MMDBD contains over 1,600 species of medicinal materials listed in the Chinese Pharmacopoeia and American Herbal Pharmacopoeia up to May 2014 [77, 78]. Because the properties of botanicals vary through different harvesting environments, conditions, and time, if the MMDBD were to extend its database to include the details of herbal origins, cultivation conditions, harvesting, handling, and processing of each herbal batch studied, the corresponding clinical data will provide additional benefit to TCM development. Another new technology evolving is “TCM network pharmacology”. By taking advantage of powerful computational tools and “Omics” technologies, TCM network pharmacology is receiving increased popularity towards studying and analyzing the relationships of TCM components, active substance, and biological activities [79]. Current existing surveillance systems, such as the ADR reporting system in China and the FDA MedWatch program provide good information resources on the adverse events of herbal products. However, some countries still lack such surveillance programs. Establishing a global herbal surveillance system that integrates the different reporting systems around the world would raise the overall quality of TCM.
4.2 Use Advanced Technologies as Quality-control Standards During TCM Production and Processing
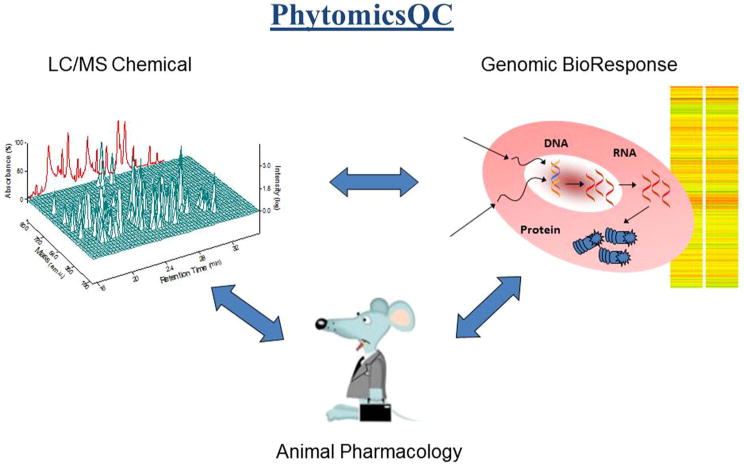
Technologies play important roles in TCM anatomy. Traditionally, raw herbs were authenticated morphologically by a botanist. Microscope and simple chemical analyses such as thin layer chromatography (TLC), high-pressure liquid chromatography (HPLC), and gas chromatography (GC) were gradually introduced for validation. Nevertheless, these macro-analytical technologies were not able to satisfy the complicated polychemical properties in TCM. With advanced technology evolving, more molecular level analytical tools, such as mass chromatography (MS), LC-MS-MS, UHPLC/MS, GC-MS, ICP-MS, and ATP-FTIR would provide molecular resolution in chemical fingerprints. DNA microarray or other “Omics” technologies, including genomics, transcriptomics, proteomics, and metabolomics could serve as biological fingerprints of botanicals. “PhytomicsQC” technology that integrated chemical fingerprint, biological fingerprint, animal pharmacology, and advanced statistical analysis formed a quality control platform for botanicals [15, 80, 81] (Fig. 3). Because extraction procedure of botanicals could alter the outcome of analysis, solid state detection such as MALDI and Infrared should be included in the consideration. Any additives or adulteration in TCM would be easy to detect with the advance of technologies. In addition to understanding the impact of TCM on the metabolism of active therapy, the metabolites of TCM in humans were detectable with the advanced technologies [82].
Fig. 3. PhytomicsQC: a platform integrating chemical fingerprints, bioresponse fingerprints, and in vivo animal pharmacology validation. Copied from Tilton et al. [81].
Although the analytical methods for the authentication of botanicals and additives are maturing in academic settings, the validated methods listed, in both Chinese Pharmacopoeia and US Pharmacopoeia, are old fashioned. More advanced analytical methods need to be validated and included in the official guidelines. The specification limits in toxin, heavy metal, microbial, and pesticide and fungicide residues in herbal products are too diversified, which could cause confusion in defining the quality of botanical products in different countries. In addition to the herbal specification analysis, it is also important to perform safety evaluations in animals, especially long-term toxicity. The current long-term studies in conventional drugs require at least a 30-day evaluation in animals. Because most TCMs are used as preventive medicine with months or years of administration, studies in different species of animals with longer than 30 days evaluation will be more adequate. A recent 2-year toxicity and pathology study in rodents involving several common herbs such as Panax ginseng, Aloe vera, Ginkgo biloba, goldenseal, kava, milk thistle, and turmeric oleoresin provided a very solid prospective on long-term toxicity. Ginseng was found to be safe at doses of 1,250–5,000 mg/kg with continuously oral gavage (5 days per week) for up to a 2-year period [83].
4.3 Promoting Evidence-based TCM
Although TCM is derived from empirical practice in humans, it did not go through the standard, double-blind, placebo-controlled, clinical settings used in conventional drug development. TCM does not undergo any phase of clinical trials before being given to patients. To make TCM more globally accepted, it is important to use scientific proof combined with statistically significant results to claim the health benefits of TCM. By definition, “evidence-based medicine” is the integration of best research evidence with clinical expertise and patient values, from Sackett [84], there was not enough evidence-based toxicology profile as well as the clinical outcome in TCM uses. A well-designed, double-blind, placebo-controlled, clinical trial with scientifically defined outcome measurements is necessary for an evidence-based botanical [85].
Several TCM formulations, such as Dantonic®, and PHY906, have been conducted under GCP (good clinical practice) guidelines to advance towards regulatory approval as prescription drugs. Dantonic® (T89) consists of containing three Chinese herbal material medica, Danshen (Radix Salviae Miltiorrhizae), Sanqi (Radix Notoginseng), and Borneolum syntheticum, and has been approved as a drug in 26 countries, except the USA, to prevent and treat patients with chronic stable angina pectoris and other cardiovascular disease-related conditions [86]. In recent clinical trials in the USA, B. syntheticum has been replaced with the single-compound borneol as a transporting enhancer [87].
PHY906, a four-herb formulation used for over 1,800 years to treat gastrointestinal distress, has been studied intensively as an adjuvant therapy to increase the therapeutic index of chemotherapy, target therapy, and radiation therapy. Preclinical studies suggested PHY906 could enhance the therapeutic indices of a broad spectrum of anticancer agents without changing the pharmacokinetics of the chemotherapeutic agents studied. The mechanisms of action of PHY906 have been studied extensively [88, 89]. Various doses of PHY906 have been tested in phase I and II clinical trials in combination with escalating doses of irinotecan or capecitabine in a variety of solid tumors, and apecitabine for pancreatic and hepatocellular carcinomas. With five completed and three on-going early-stage clinical trials in colorectal cancer, hepatocellular carcinoma, advanced pancreatic cancer, and radiation therapy, PHY906 is one of a few TCM formulas developed with an evidence-based approach [90–95]. A comprehensive platform, PhytomicsQC, which integrates chemical and biological fingerprints together with a novel biostatistical methodology, has been developed to assess the quality of different batches of PHY906. This multiplex technology has been used to show batch-to-batch consistency of PHY906 production over a 10-year period (Fig. 3).
5 Conclusions
One of the main approaches of TCM is to restore or strengthen immunity and resistance to disease, which is similar to the philosophy of preventive medicine. Another approach is to improve quality of life, which is similar to functional medicine. Achieving the goal of making evidence-based TCM will require a wide-scale effort from farmers and industries in herbal cultivation, harvesting, processing, manufacturing, and distribution. It also requires government effort in regulating botanical drug, dietary supplements, and food. The technologies developed from research institutions provide major contributions to understanding the characteristics and potentials of each botanical and formulation. Modern agriculture technology should be explored for future herbal cultivation. Self-policing by industry is critical to upgrading the quality of the herbal industry. Consumer education is also critical to ensure the safe administration of TCM.
Key Points.
Safety, quality, and efficacy of traditional Chinese medecine (TCM) are essential criteria
Safety surveillance strengthens confidence in TCM
Acknowledgments
The authors thank Christopher C. Chen for scientific editorial assistance with this article. This work was supported by the National Cancer Institute (Grant No. 1PO1CA154295-01A1). Yung-Chi Cheng is a fellow of the National Foundation for Cancer Research, USA.
Footnotes
Disclaimer The views expressed in this article are personal and do not reflect the official position or policies of the authors' respective organizations.
Conflict of interest Yung-Chi Cheng, Shwu-Huey Liu, and Zaoli Jiang are the co-inventors of PHY906 patents. Wu-Chang Chuang and Wing Lam have no conflicts of interest that are directly relevant to the content of this article.
Contributor Information
Shwu-Huey Liu, Department of Pharmacology, Yale University School of Medicine, New Haven, CT 06510, USA.
Wu-Chang Chuang, Brion Research Institute of Taiwan, New Taipei City, Taiwan, ROC.
Wing Lam, Department of Pharmacology, Yale University School of Medicine, New Haven, CT 06510, USA.
Zaoli Jiang, Department of Pharmacology, Yale University School of Medicine, New Haven, CT 06510, USA.
Yung-Chi Cheng, Email: yccheng@yale.edu, Department of Pharmacology, Yale University School of Medicine, New Haven, CT 06510, USA.
References
- 1.Barnes PM, Bloom B, Nahin R. CDC National Health Statistics Report #12. Complementary and alternative medicine use among adults and children: United States, 2007. 2008 Dec 10; [PubMed] [Google Scholar]
- 2.Nahin R, Barnes PM, Stussman BJ, et al. CDC National Health Statistics Report #18. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. 2009 Jul 30; [PubMed] [Google Scholar]
- 3.Fouladbakhsh JM, Stommel M, Given BA, Given CW. Pedictors of use of complementary and alternative therapies among patients with cancer. Oncol Nurs Forum. 2005;32(6):1115–22. doi: 10.1188/05.ONF.1115-1122. [DOI] [PubMed] [Google Scholar]
- 4.Friesen D. Traditional Chinese Medicine: Business blockbuster or false fad? CKGSB Knowledge. 2013 Jan 8; http://knowledge.ckgsb.edu.cn/2013/01/08/china/traditional-chinese-medicine-business-blockbuster-or-false-fad/
- 5.Xiwen tr Luo. Bencao Gangmu: compendium of materia medica. Vol. 6. Foreign Languages Press; 2003. [Google Scholar]
- 6.Maoshiny N. The yellow Emperor's Classic of Medicine: a new translation of the Neijing suwen with commentary. Shambhala Publications, Inc.; 1995. [Google Scholar]
- 7.Division of Health Statistics. Statistical Annual Report of Medical Care, National Health Insurance. Taipei: The ROC Department of Health; 2009. [Google Scholar]
- 8.U.S. Food and Drug Administration Web site. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2004/ucm108379.htm.
- 9.Borum ML. Fulminant exacerbation of autoimmune hepatitis after the use of ma huang. Am J Gastroenterol. 2001;96(5):1654–5. doi: 10.1111/j.1572-0241.2001.03827.x. [DOI] [PubMed] [Google Scholar]
- 10.Powell T, Hsu FF, Turk J, Hruska K. Ma-huang strikes again: ephedrine nephrolithiasis. Am J Kidney Dis. 1998;32(1):153–9. doi: 10.1053/ajkd.1998.v32.pm9669437. [DOI] [PubMed] [Google Scholar]
- 11.Fan TP, Deal G, Koo HL, et al. Future development of global regulations of Chinese herbal products. J Ethnopharmacol. 2012;140(3):568–86. doi: 10.1016/j.jep.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 12.Hughes D. New EU regulations on herbal medicines come into force. BBC News. 2011 [Google Scholar]
- 13.Zhang L, Yan J, Liu X, et al. Pharmacovigilance practice and risk control of traditional Chinese medicine drugs in China: current status and future perspective. J Ethnopharmacol. 2012;10(140):519–25. doi: 10.1016/j.jep.2012.01.058. [DOI] [PubMed] [Google Scholar]
- 14.Dietary supplements. U S Food and Drug Administration Web site. Accessed at www.fda.gov/Food/DietarySupplements.
- 15.Liu SH, Cheng YC. Old formula, new Rx:the journey of PHY906 as cancer adjuvant therapy. J Ethnopharmacol. 2012;140(3):614–23. doi: 10.1016/j.jep.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 16.FDA. Guidance for Industry. Botanical Drug Products (FDA, DC, June 2004) http://www.fda.gov/cder/guidance/4592fnl.pdf.
- 17.Wu KM, Farrelly J, Birnkrant D, et al. Regulatory toxicology perspectives on the development of botanical drug products in the United Sates. Am J Ther. 2004;11:213–7. doi: 10.1097/00045391-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Veregen: a botanical for treatment of genital warts. Obstet Gynecol. 2008;112(3):691–2. [PubMed] [Google Scholar]
- 19.Tradtrantip L, Namkung W, Verkman AS. Crofelemer, an anti-secretory antidiarrheal proanthocyanidin oligomer extracted from Croton lechleri, targets two distinct intestinal chloride channels. Mol Pharmacol. 2010;77(1):69–78. doi: 10.1124/mol.109.061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teschke R, Wolff A, Frenzel C, Schulze J. Review article: herbal hepatotoxicity—an update on traditional Chinese medicine preparations. Aliment Pharmacol Ther. 2014;40(1):32–50. doi: 10.1111/apt.12798. [DOI] [PubMed] [Google Scholar]
- 21.Chen SP, Ng SW, Poon WT, Lai CK, et al. Aconite poisoning over 5 years: a case series in Hong Kong and lessons towards herbal safety. Drug Saf. 2012;35(7):575–87. doi: 10.2165/11597470-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Nyirimigabo E, Xu Y, Li Y, et al. A review on phytochemistry, pharmacology and toxicology studies of Aconitum. J Pharm Pharmacol. 2014 doi: 10.1111/jphp.12310. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Shaw D. Toxicological risks of Chinese herbs. Planta Med. 2010;76(17):2012–8. doi: 10.1055/s-0030-1250533. [DOI] [PubMed] [Google Scholar]
- 24.Haller CA, Dyer JE, Ko R, Olson KR. Making a diagnosis of herbal-related toxic hepatitis. West J Med. 2002;176(1):39–44. doi: 10.1136/ewjm.176.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanherweghem JL, Depierreux M, Tielemans C, et al. Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. Lancet. 1993;341:387–91. doi: 10.1016/0140-6736(93)92984-2. [DOI] [PubMed] [Google Scholar]
- 26.Nortier JL, Martinez MC, Schmeiser HH, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi) N Engl J Med. 2000;342(23):1686–92. doi: 10.1056/NEJM200006083422301. [DOI] [PubMed] [Google Scholar]
- 27.Vanherweghem LJ. Misuse of herbal remedies: the case of an outbreak of terminal renal failure in Belgium (Chinese herbs nephropathy) J Altern Complement Med. 1998;4(1):9–13. doi: 10.1089/acm.1998.4.1-9. [DOI] [PubMed] [Google Scholar]
- 28.Chen CH, Dickman KG, Huang CY, et al. Aristolochic acid-induced upper tract urothelial carcinoma in Taiwan: clinical characteristics and outcomes. Int J Cancer. 2013;133(1):14–20. doi: 10.1002/ijc.28013. [DOI] [PubMed] [Google Scholar]
- 29.Hoang ML, Chen CH, Sidorenko VS, et al. Mutational signature of aristolochic acid exposure as revealed by whole-exome sequencing. Sci Transl Med. 2013;5:197ra102. doi: 10.1126/scitranslmed.3006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drew AK, Whyte IM, Bensoussan A, et al. Chinese herbal medicine toxicology database: monograph on Herba Asari, “xi xin”. J Toxicol Clin Toxicol. 2002;40(2):169–72. doi: 10.1081/clt-120004405. [DOI] [PubMed] [Google Scholar]
- 31.Lee CH, Wang JD, Chen PC. Risk of liver injury associated with Chinese herbal products containing Radix bupleuri in 639,779 patients with Hepatitis B virus infection. PLoS One. 2011;6(1):e16064. doi: 10.1371/journal.pone.0016064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue X, Xiao Y, Gong L, et al. Comparative 28-day repeated oral toxicity of Longdan Xieganwan, Akebia trifoliate (Thunb.) koidz., Akebia quinata (Thunb.) Decne. and Caulis aristolochiae manshuriensis in mice. J Ethnopharmacol. 2008;119(1):87–93. doi: 10.1016/j.jep.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 33.Hsu HY, Hsu CS. Commonly used Chinese herb formulas with illustrations. Los Angeles: Oriental Healing Art Institute; 1980. [Google Scholar]
- 34.Pharmacopoeia of the People's Republic of China. 1997;1 [Google Scholar]
- 35.Fu PP, Yang YC, Xia Q, et al. Pyrrolizidine alkaloids-tumorigenic components in Chinese herbal medicines and dietary supplements. J Food Drug Anal. 2002;10(4):198–211. [Google Scholar]
- 36.Cohen PA, Maller G, DeSouza R, et al. Presence of banned drugs in dietary supplements following FDA recalls. JAMA. 2014;312(16):1691–3. doi: 10.1001/jama.2014.10308. [DOI] [PubMed] [Google Scholar]
- 37.Blum D. The trouble with rice. N Y Times. 2014 http://nyti.ms/1eIX0T4.
- 38.Meharg AA. Arsenic in rice—understanding a new disaster for South-east Asia. Trends Plant Sci. 2004;9:415–7. doi: 10.1016/j.tplants.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Moreno-Jime´nez E, Meharg AA, Smolders E, et al. Sprinkler irrigation of rice fields reduces grain arsenic but enhances cadmium. Sci Total Environ. 2014;1(485–486):468–73. doi: 10.1016/j.scitotenv.2014.03.106. [DOI] [PubMed] [Google Scholar]
- 40.Harris ESJ, Cao S, Littlefield BA, et al. Heavy metal and pesticide content in commonly prescribed individual raw Chinese herbal medicines. Sci Total Environ. 2011;409(20):4297–305. doi: 10.1016/j.scitotenv.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Genuis SJ, Schwalfenberg G, Siy AKJ, Rodushkin I. Toxic element contamination of natural health products and pharmaceutical preparations. In: Sem DS, editor. PLoS One. 11. Vol. 7. 2012. p. e49676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee KH, Morris-Natschke SL, Yang X, et al. Recent progress of research on medicinal mushrooms, foods, and other herbal products used in traditional Chinese medicine. J Tradit Complement Med. 2012;2(2):84–95. [PMC free article] [PubMed] [Google Scholar]
- 43.Zuo HL, Chen SJ, Zhang DL, et al. Quality evaluation of natural Cordyceps sinensis from different collecting places in China by the contents of nucleosides and heavy metals. Anal Methods. 2013;5:5450–6. [Google Scholar]
- 44.Wu TN, Yang KC, Wang CM, et al. Lead poisoning caused by contaminated Cordyceps, a Chinese herbal medicine: two case reports. Sci Total Environ. 1996;182(1–3):193–5. doi: 10.1016/0048-9697(96)05054-1. [DOI] [PubMed] [Google Scholar]
- 45.Chen LL, Zhou X, Zhang YX, et al. Chemical composition and Heavy metal content of radix of Panax notoginseng from different sources. Proceedings of the 13th Meeting of Consortium for Globalization of Chinese Medicine. 2014 [Google Scholar]
- 46.Zhang MY, Xie J, Zhang TY, et al. Sinomonas notoginsengisoli sp. nov., isolated from the rhizosphere of Panax notoginseng. Antonie Van Leeuwenhoek. 2014;106(4):827–35. doi: 10.1007/s10482-014-0252-y. [DOI] [PubMed] [Google Scholar]
- 47.Bhattacharyya P, Jha DK. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotech-nol. 2012;28:1327–50. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- 48.Greenpeace. Chinese Herbs: elixir of health or pesticide cocktail [report on the internet] Available from: http://www.greenpeace.org/eastasia/campaigns/food-agriculture/Chinese-Herbs-Elixir-of-Health/
- 49.Michael W. Mystery malady kills more bees, heightening worry on farms. N Y Times. 2013 http://www.nytimes.com/2013/03/29/science/earth/soaring-bee-deaths-in-2012-sound-alarm-on-malady.html.
- 50.Simon RA. Adverse reactions to drug additives. J Allergy Clin Immunol. 1984;74:623. doi: 10.1016/0091-6749(84)90116-7. [DOI] [PubMed] [Google Scholar]
- 51.Kan WL, Ma B, Lin G. Sulfur fumigation processing of traditional Chinese medicinal herbs: beneficial or detrimental? Front Pharmacol. 2011;27(2):84. doi: 10.3389/fphar.2011.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu J, Shen H, Xu J, et al. Detection of Sulfur-fumigated Paeo-niae Alba Radix in complex preparations by high performance liquid chromatography tandem mass spectrometry. Molecules. 2012;17:8938–54. doi: 10.3390/molecules17088938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asgarpanah J, Kazemivash N. Phytochemistry, pharmacology and medicinal properties of Carthamus tinctorius L. Chin J Integr Med. 2013;19(2):153–9. doi: 10.1007/s11655-013-1354-5. [DOI] [PubMed] [Google Scholar]
- 54.Rao WW, Jiang L, Zhao CY, et al. Identification of chemical industry dyes in adulteration of Chinese medicinal materials. J Pharm Anal. 2007;27(11):1742–5. [Google Scholar]
- 55.Liu SH, Cheng YC, Saif MW. Chapter 7 of “Supportive Cancer Care with Chinese Medicine”. Springer Science; 2010. Controlling chemotherapy-related side effects with Chinese medicine. [Google Scholar]
- 56.White J. PC-SPES-A Lesson for future dietary supplement research. J Natl Cancer Inst. 2002;94(17):1261–2. doi: 10.1093/jnci/94.17.1261. [DOI] [PubMed] [Google Scholar]
- 57.Olaku O, White JD. Herbal therapy use by cancer patients: a literature review on case reports. Eur J Cancer. 2011;47(4):508–14. doi: 10.1016/j.ejca.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meyer JP, Gillatt DA. PC-SPES: a herbal therapy for the treatment of hormone refractory prostate cancer. Prostate Cancer Prostatic Dis. 2002;5(1):13–5. doi: 10.1038/sj.pcan.4500563. [DOI] [PubMed] [Google Scholar]
- 59.DiPaola RS, Zhang H, Lambert GH, et al. Clinical and biologic activity of an estrogenic herbal combination (PC-SPES) in prostate cancer. N Engl J Med. 1998;339(12):785–91. doi: 10.1056/NEJM199809173391201. [DOI] [PubMed] [Google Scholar]
- 60.Small EJ, Frohlich MW, Bok R, et al. Prospective trial of the herbal supplement PC-SPES in patients with progressive prostate cancer. J Clin Oncol. 2000;18:3595–603. doi: 10.1200/JCO.2000.18.21.3595. [DOI] [PubMed] [Google Scholar]
- 61.De la Taille A, Hayek OR, Burchardt M, et al. Role of herbal compounds (PC-SPES) in hormone-refractory prostate cancer: two case reports. J Altern Complement Med. 2000;6:449–51. doi: 10.1089/acm.2000.6.449. [DOI] [PubMed] [Google Scholar]
- 62.De la Taille A, et al. Effects of a phytherapeutic agent, PC-SPES, on prostate cancer: a preliminary investigation on human cell lines and patients. BJU Int. 1999;84:845–50. doi: 10.1046/j.1464-410x.1999.00285.x. [DOI] [PubMed] [Google Scholar]
- 63.Sovak M, Seligson AL, Konas M, et al. Herbal composition PC-SPES for management of prostate cancer: identification of active principles. J Natl Cancer Inst. 2002;94(17):1275–81. doi: 10.1093/jnci/94.17.1275. [DOI] [PubMed] [Google Scholar]
- 64.Woolf GM, Petrovic LM, Rojter SE, Wainwright S, et al. Acute hepatitis associated with the Chinese herbal product jin bu huan. Ann Intern Med. 1994;121(10):729–35. doi: 10.7326/0003-4819-121-10-199411150-00001. [DOI] [PubMed] [Google Scholar]
- 65.Picciotto A, Campo N, Brizzolara R, et al. Chronic hepatitis induced by jin bu huan. J Hepatol. 1998;28:165–7. doi: 10.1016/s0168-8278(98)80217-1. [DOI] [PubMed] [Google Scholar]
- 66.Horowitz RS, Feldhaus K, Dart RC. The clinical spectrum of Jin Bu Huan toxicity. Arch Intern Med. 1996;156(8):899–903. [PubMed] [Google Scholar]
- 67.FAO (Food and Agriculture Organization of United Nations) Manual on the Application of the HACCP System in Mycotoxin Prevention and Control. Vol. 73. FAO Food and Nutrition Paper; Rome: 2001. [Google Scholar]
- 68.Zain ME. Impact of mycotoxins on humans and animals. J Saudi Chem Soc. 2011;15:129–44. [Google Scholar]
- 69.Ashiq S, Hussain M, Ahmad B. Natural occurrence of myco-toxins in medicinal plants: a review. Fungal Genet Biol. 2014;66:1–10. doi: 10.1016/j.fgb.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 70.Kneifel W, Czech E, Kopp B. Microbial contamination of medicinal plants—a review. Planta Med. 2002;68(1):5–15. doi: 10.1055/s-2002-20060. [DOI] [PubMed] [Google Scholar]
- 71.Melchart D, Linde K, Weidenhammer W, Hager S, Shaw D, et al. Liver enzyme elevations in patients treated with traditional Chinese medicine. JAMA. 1999;282(1):28–9. doi: 10.1001/jama.282.1.28. [DOI] [PubMed] [Google Scholar]
- 72.Itoh S, Marutani K, Nishijima T, Matsuo S, Itabashi M. Liver injuries induced by herbal medicine, syo-saiko-to (xiao-chai-hu-tang) Dig Dis Sci. 1995;40(8):1845–8. doi: 10.1007/BF02212712. [DOI] [PubMed] [Google Scholar]
- 73.Seeff L, Stickel F, Navarro VJ. Hepatotoxicity of herbals and dietary supplements. In: Kaplowitz N, DeLeve LD, editors. Drug-induced liver disease. 3rd. Amsterdam: Elsevier; 2013. pp. 631–58. [Google Scholar]
- 74.Schieman C, Rudmik LR, Dixon E, et al. Complementary and alternative medicine use among general surgery, hepatobiliary surgery and surgical oncology patients. Can J Surg. 2009;52(5):422–6. [PMC free article] [PubMed] [Google Scholar]
- 75.Li X, Yang G, Li X, et al. Traditional Chinese medicine in cancer care: a review of controlled clinical studies published in Chinese. PLoS One. 2013;8(4):e60338. doi: 10.1371/journal.pone.0060338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gurley BJ, Swain A, Williams DK, et al. Gauging the clinical significance of P-glycoprotein-mediated herb-drug interactions: Comparative effects of St. John's wort, echinacea, clarithromy-cin, and rifampin on digoxin pharmacokinetics. Mol Nutr Food Res. 2008;52(7):772–9. doi: 10.1002/mnfr.200700081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lou SK, Wong KL, Li M, et al. An integrated web medicinal materials DNA database: MMDBD (Medicinal Materials DNA Barcode Database) BMC Genomics. 2010;24(11):402. doi: 10.1186/1471-2164-11-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song J, Yao H, Li Y, Li X, Lin Y, Liu C, Han J, Xie C, Chen S. Authentication of the family Polygonaceae in Chinese pharmacopoeia by DNA barcoding technique. J Ethnopharmacol. 2009;124:434–9. doi: 10.1016/j.jep.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 79.Yan M, Chen JL, Xu LW, et al. Navigating traditional Chinese medicine network pharmacology and computational tools. Evid Based Complement Alternat Med. 2013;2013:731969. doi: 10.1155/2013/731969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ye M, Liu SH, Jiang Z, et al. Liquid chromatography/mass spectrometry analysis of PHY906, a Chinese medicine formulation for cancer therapy. Rapid Commun Mass Spectrom. 2007;21(22):3593–607. doi: 10.1002/rcm.2832. [DOI] [PubMed] [Google Scholar]
- 81.Tilton R, Paiva AA, Guan JQ, et al. A comprehensive platform for quality control of botanical drugs (PhytomicsQC): a case study of Huangqin Tang (HQT) and PHY906. Chin Med. 2010;20(5):30. doi: 10.1186/1749-8546-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang W, Saif MW, Dutschman GE, et al. Identification of chemicals and their metabolites from PHY906, a Chinese medicine formulation, in the plasma of a patient treated with irino-tecan and PHY906 using liquid chromatography/tandem mass spectrometry (LC/MS/MS) J Chromatogr A. 2010;1217(37):5785–93. doi: 10.1016/j.chroma.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dunnick JK, Nyska A. The toxicity and pathology of selected dietary herbal medicines. Toxicol Pathol. 2013;41(2):374–86. doi: 10.1177/0192623312466451. [DOI] [PubMed] [Google Scholar]
- 84.Sackett DL, Straus SE, Richardson WS, Rosenberg W, Haynes RB. Evidence-based medicine: how to practice and teach EBM. Edinburgh: Churchill Livingstone; 2000. [Google Scholar]
- 85.Pelkonen O, Xu Q, Fan TP. Why is research on herbal medicinal products important and how can we improve its quality. J Tradit Complement Med. 2014;4(1):1–7. doi: 10.4103/2225-4110.124323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O'Brien KA, Ling S, Abbas E, et al. A chinese herbal preparation containing radix salviae miltiorrhizae, radix notoginseng and borneolum syntheticum reduces circulating adhesion molecules. Evid Based Complement Alternat Med. 2011;2011:790784. doi: 10.1093/ecam/nen060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phase III Trial of Dantonic® (T89) Capsule to Prevent and Treat Stable Angina (CAESA) ClinicalTrials.gov Identifier: NCT01659580
- 88.Lam W, Bussom S, Guan F, et al. PHY906, a four-herb Chinese medicine formula first described 1,800 years ago, reduces irino-tecan-induced intestinal damage through anti-inflammatory effects and by promotion of the repopulation of intestinal progenitor cells. Sci Transl Med. 2010;2(45):45ra59. doi: 10.1126/scitranslmed.3001270. [DOI] [PubMed] [Google Scholar]
- 89.Wang E, Bussom S, Chen J, et al. Interaction of a traditional chinese medicine (PHY906) and CPT-11 on the inflammatory process in the tumor microenvironement. BMC Med Genomics. 2011;4:38. doi: 10.1186/1755-8794-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yen Y, So S, Rose M, et al. Phase I/II study of PHY906/cape-citabine in advanced hepatocellular carcinoma. Anticancer Res. 2009;29(10):4083–92. [PubMed] [Google Scholar]
- 91.Saif MW, Lansigan F, Ruta S, et al. Phase I study of the botanical formulation PHY906 with capecitabine in advanced pancreatic and gastrointestinal malignancies. Phytomedicine. 2010;17(3–4):161–9. doi: 10.1016/j.phymed.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 92.Kummar S, Copur MS, Rose M, et al. A phase I study of the Chinese herbal medicine PHY906 as a modulator of irinotecan-based chemotherapy in patients with advanced colorectal cancer. Clin Colorectal Cancer. 2011;10(2):85–96. doi: 10.1016/j.clcc.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 93.Saif MW, Li J, Lamb L, et al. First-in-human phase II trial of the botanical formulation PHY906 with capecitabine as second-line therapy in patients with advanced pancreatic cancer. Cancer Chemother Pharmacol. 2014;73(2):373–80. doi: 10.1007/s00280-013-2359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Villanueva T. Therapy: good old herbs. Nature Rev Cancer. 2010;10:664. doi: 10.1038/nrc2937. [DOI] [PubMed] [Google Scholar]
- 95.Eng C. Are herbal medicines ripe for the cancer clinic? Sci Transl Med. 2010;2(45):45ps41. doi: 10.1126/scitranslmed.3001517. [DOI] [PubMed] [Google Scholar]


