Abstract
Introduction
Thrombosis remains the most common preventable cause of mortality in cancer patients receiving chemotherapy. Whilst the prophylaxis and treatment of this condition is well understood, the patient experience and subsequent behavioral factors are not.
Methods
Patients receiving treatment for cancer-associated thrombosis (CAT) were interviewed about their experiences of CAT within the context of their cancer journey. Twenty interviews were transcribed and analyzed using framework analysis.
Results
Chemotherapy patients were well informed about the risks of febrile neutropenia, how to recognize it, and when to seek medical attention. However, they had limited knowledge about CAT and received no information about the condition. Red flag symptoms suggestive of CAT were attributed to chemotherapy or the underlying cancer, resulting in delayed presentation to hospital, and diagnosis. The CAT journey was considered a distressing one, with limited support or information, in complete juxtaposition with the treatment they received for their cancer. Patients felt there was little ownership for the management of CAT, which further added to their distress.
Conclusion
CAT is a common occurrence and patients view their experiences of it within the context of their overall cancer journey. However, patients receive little information to help recognize CAT and access timely treatment on the development of symptoms. Whilst other cancer complications have clear treatment pathways, thrombosis does not appear to have been afforded the same priority. A proactive approach to increase patient awareness, coupled with established CAT pathways is likely to reduce mortality, morbidity, and long-term psychological distress.
Keywords: venous thromboembolism, qualitative, patient journey, low-molecular-weight heparin, patient experience
Introduction
Venous thromboembolism (VTE), comprising of deep vein thrombosis (DVT) and pulmonary embolus (PE) is a common phenomenon, which occurs in one in 1,000 patients and annually affects 6.5 million people worldwide.1,2 The rate is higher in the cancer population, accounting for 18% of all VTE cases.3 Up to 20% of patients with malignancy will develop cancer-associated thrombosis (CAT) during the course of their disease.4 Of these, over 50% will occur in the first 3 months of diagnosis, thus complicating cancer treatments, as well as conferring additional symptom burden. In addition to acute and long-term morbidity, CAT remains the number one cause of death during chemotherapy and the most common cause of all cancer deaths second only to disease progression.5,6 The challenges of managing CAT are also well recognized; cancer patients are at greater risk of both recurrent VTE and bleeding complications compared to those without malignancy.6–8
Clinical guidelines recommend that the first-line treatment of CAT require 3–6 months anticoagulation with weight-adjusted low-molecular-weight heparin (LMWH).9–11 Despite the high-quality evidence informing treatment options, there is limited data on how CAT and its treatment impacts on the patient experience or quality of life, with the majority of research focusing on VTE in the non-cancer setting.12–17 This is of particular relevance, since experiences of CAT may have an additional impact on the preexisting cancer journey, and ultimately, the cancer outcome. Qualitative research in the non-cancer population has identified that VTE has a significant negative psychological impact, including symptoms suggestive of posttraumatic stress disorder being reported.18 Specific to CAT, three qualitative studies have been published; two studied the acceptability of long-term LMWH in its treatment and one explored the experiences of patients receiving cancer treatment who developed a VTE.19–21 Emerging data from these studies suggested that patients felt that there were several areas of unmet need within the diagnosis and treatment of CAT.21 We therefore undertook a qualitative study to explore the wider patient experience of living with CAT. Specifically, the study aims were to explore:
the CAT journey through the lived patient experience;
the meaning of CAT to patients within the context of the cancer journey;
the impact of the treatment for CAT;
the emotional impact of CAT;
unmet areas of need within the CAT journey.
Methods
Data collection
Ethical approval was granted by the National Research Ethics Service (NRES) Committee South Central – Oxford B (reference 13/SC/041). Patients attending a dedicated CAT clinic, within a regional cancer center and district general hospital, were sequentially screened for inclusion into the study. The lead author (SN), who was also a clinician managing patients with CAT, undertook screening. All eligible patients were invited to participate. Where patients attended with their partner or next of kin, they were also given the opportunity to be interviewed.
Inclusion criteria included:
histologically confirmed cancer;
receiving LMWH for a proven new VTE (DVT or PE) and having received such treatment for at least two consecutive months;
able to consent and participate in a 40-minute interview. Exclusion criteria included:
non-melanoma skin cancers;
Patients whose physical level of functioning meant that they were unable to converse for up to 40 minutes without fatigue.
All participants provided written consent, which was taken by the researcher at the patients’ homes prior to the interview commencing. Semi-structured interviews were carried out over a 5-month period between November 2013 and March 2014 by an experienced qualitative researcher (HP). Interviews were guided by a prompt list to ensure that the same issues were discussed at each interview.
The researcher, who was from a nursing background, had no prior relationship with participants or declared clinical interest in CAT management. Data were elicited on the following:
their experience of being diagnosed with CAT;
the physical and emotional impact of CAT and its treatment;
how their care could be improved.
To facilitate this, questions were open-ended, with the use of prompts to probe further into issues that arose as significant or meaningful to the participant. Interviews were digitally recorded and transcribed verbatim. Field notes were also taken. Interviews took approximately 40 minutes each. No repeat interviews were necessary; neither did any aspects of transcripts require additional checking with participants.
Analysis
Transcripts were typed into a Word document and uploaded to NVivo 10 computer software for data management.22 Data analysis was undertaken using a methodology increasingly used in health care research, framework analysis (FA). This was considered the most appropriate analytic method to enable a deductive approach toward creating an analytic framework based on the interview schedule, whilst also allowing room for inductive observations.23 Analysis was undertaken using Ritchie and Spencer’s five interconnected stages inherent in FA:
Familiarization with data: listening to recordings, re-reading field notes and transcripts, and listing key ideas and recurrent themes.
Identifying a thematic framework: reviewing the data and identifying key issues, concepts, and themes to develop an index of themes informed by the research aims and the issues raised by the respondents themselves.
Indexing the data: systematically applying the thematic framework or index to each of the transcripts. This framework may be adjusted as it is applied to subsequent transcripts.
Charting: lifting the data from the transcripts and rearranging it according to its thematic reference. At this stage, a chart can be drawn up.
Mapping and interpretation: comparing and contrasting themes, searching for a structure, and using diagrams and tables to explore relationships.
Results
Participant characteristics
Of 25 eligible patients invited to participate, 20 consented (ten males, ten female). Patient characteristics are summarized in Table 1. Patients were aged between 53 and 81 years old (mean: 68 years) representing seven different primary cancers including lung (n=4), colorectal (n=4), breast (n=4), prostate (n=2), and ovarian (n=2). Participants had been receiving LMWH for between 2 and 20 months (mean: 8 months, median: 6 months).
Table 1.
Characteristics of study participants including age, sex, cancer details, VTE details, and length of time on treatment
| Code | Age (years) | Sex M/F | Cancer diagnosis | VTE details | Injection given by self or carer | LMWH Rx duration |
|---|---|---|---|---|---|---|
| VCC01 | 53 | M | Metastatic lung | PE (Sym) | Wife | 8 months |
| VCC02 | 64 | F | Metastatic breast | PE (Sym) | Self | 20 months |
| VCC03 | 61 | F | Metastatic lung | DVT (Sym) | Self | 5 months |
| VCC04 | 74 | M | Colorectal | PE (Inc) DVT (Sym) |
Self | 4 months |
| VCC05 | 64 | F | Metastatic breast | PE (Sym) | Self | 20 months |
| VCC06 | 72 | M | Mesothelioma | PE (Sym) DVT (Sym) |
Self | 10 months |
| VCC07 | 73 | F | Metastatic colorectal | PE (Sym) | Self | 7 months |
| VCC08 | 80 | F | Breast | DVT (Sym) | Self | 2 months |
| VCC09 | 66 | M | Lung | DVT (Sym) | Sister, and then self | 7 months |
| VCC10 | 81 | F | Metastatic ovarian | PE (Sym) | Self | 6 months |
| VCC11 | 74 | M | Metastatic prostate | PE (Sym) | Self | 14 months |
| VCC12 | 69 | M | Pancreas | PE (Sym) DVT (Sym) |
Self | 3 months |
| VCC13 | 57 | M | Colorectal | PE (Sym) | Wife | 4 months |
| VCC14 | 58 | F | Metastatic ovarian | DVT (Sym) | Daughter | 9 months |
| RG01 | 77 | M | Metastatic unknown primary | DVT (Sym) | Self | 5 months |
| RG02 | 57 | F | Ovarian | PE (Sym) | Husband | 6 months |
| RG03 | 69 | M | Metastatic colorectal | PE (Inc) | Self | 6 months |
| RG04 | 73 | F | Metastatic colorectal | DVT (Sym) | Husband | 8 months |
| RG05 | 80 | M | Metastatic prostate | DVT (Sym) | Self | 4 months |
| RG06 | 67 | F | Breast | DVT (Sym) | Self | 6 months |
Abbreviations: DVT, deep vein thrombosis; Inc, incidental; F, female; LMWH, low-molecular-weight heparin; M, male; PE, pulmonary embolus; Rx, prescription; Sym, symptomatic; VTE, venous thromboembolism.
Principal findings
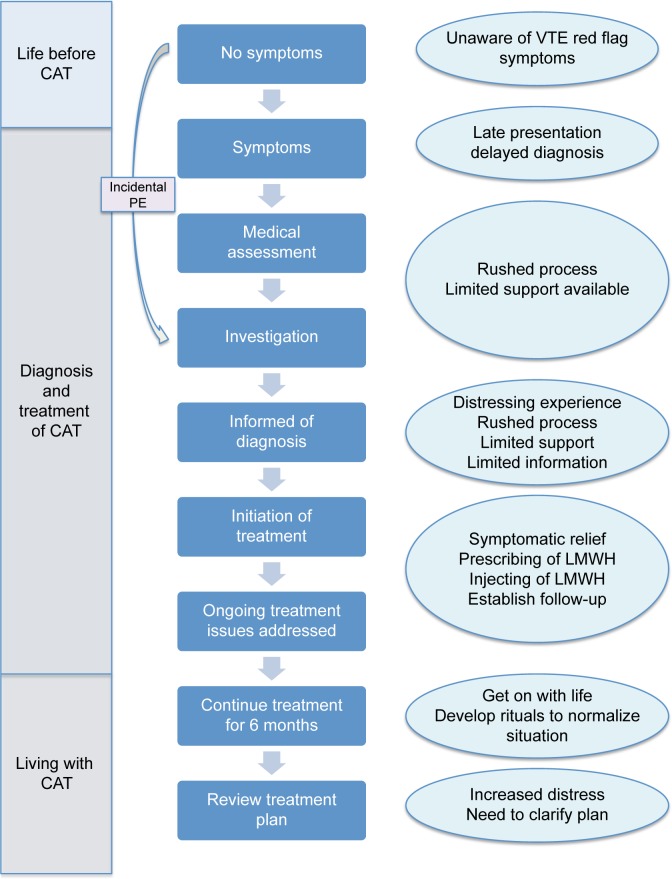
Analysis of the data identified three major themes, illustrating key phases in the CAT journey, namely: life before CAT, diagnosis and treatment of CAT, and living with CAT. These, along with associated subthemes, are outlined in Figure 1 and discussed below with accompanying interview excerpts. Excerpts of interviews were selected on the basis of two criteria. Firstly, they illustrate the issue being discussed and secondly, an attempt was made to use a spread of participants rather than rely on a few individuals. Figure 2 further illustrates participants’ subjective account of the CAT journey, identifying points at which key interventions were made during the diagnosis, treatment, and ongoing management.
Figure 1.
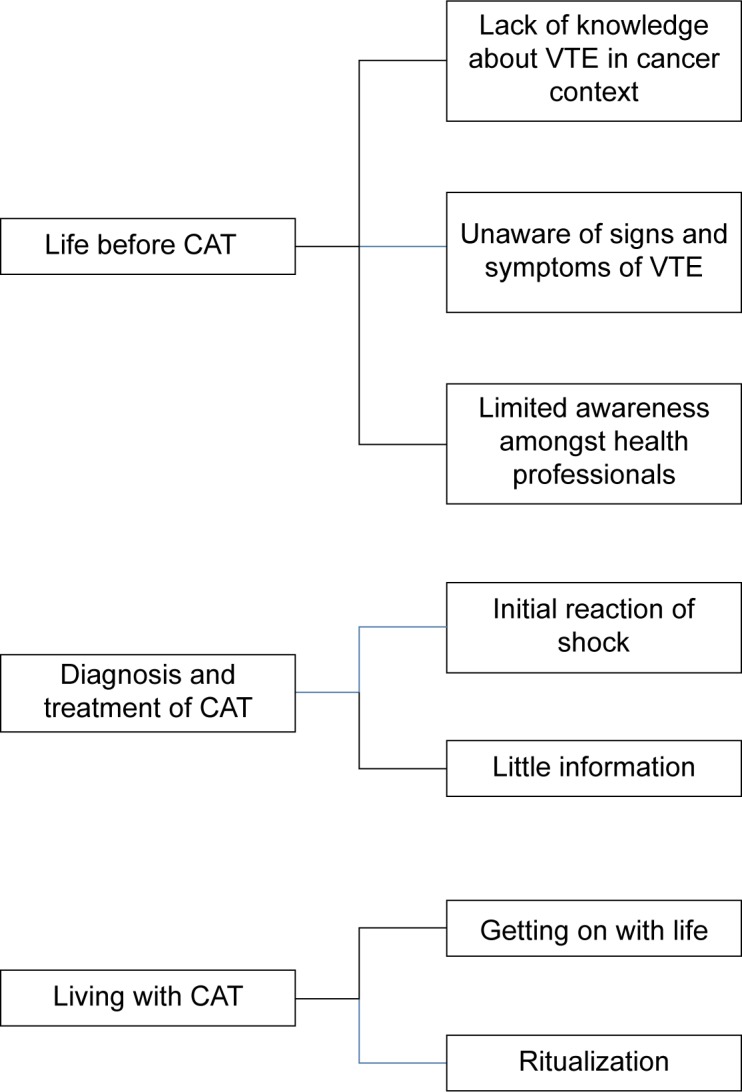
Major themes and associated subthemes.
Abbreviations: CAT, cancer-associated thrombosis; VTE, venous thromboembolism.
Figure 2.
Illustration of CAT journey with experiences at respective phases.
Abbreviations: CAT, cancer-associated thrombosis; LMWH, low-molecular-weight heparin; PE, pulmonary embolus; VTE, venous thromboembolism.
Life before CAT
In non-cancer patients, VTE usually presents subsequent to a short period of medical illness or surgery in patients who normally enjoy good health. However, CAT most commonly occurs within the first 3 months of a cancer journey and is as such experienced within the context of an already diagnosed life-threatening condition.
Lack of knowledge of VTE in the context of cancer
Whilst most participants had heard of VTE, none had been aware of an association with cancer. Long-haul flights were most commonly cited as a risk factor:
[…] cos I do long-haul flights […] I do all the things, the long socks, I walk up and down the plane, you know so I’m aware of the seriousness of a blood clot. [VCC02]
[…] they’re in the news quite a bit with people dropping dead when getting off an aeroplane and things like that. [VCC12]
Specifically, none knew of the association between VTE and cancer:
[…] that it could happen yeah, cos you’re half expecting it then, in a way, but er, and I have learnt that more people die from clots in the lungs and everything else after cancer than they do from cancer, and you’re never told that, so I thought well it is a major point, you know these clots. [VCC07]
Patients unaware of risks of thrombosis or symptoms to look out for
Patients reported little prior knowledge of the association between thrombosis with cancer or warning signs that they had developed VTE. Whilst patients receiving chemotherapy were advised on what they should do if they developed signs and symptoms of neutropenic sepsis, none recalled being told of the increased risk of VTE and associated symptoms, which would necessitate seeking medical attention.
[…] but they don’t tell you you’re gonna get clots after chemo, that’s the one thing they haven’t, they never said but we, we just put it down to, it’s just my breathing […] just that one item of information that we weren’t aware of. [VCC07]
Consequently, when patients developed symptoms suggestive of DVT or PE, several attributed them to the chemotherapy and did not consider thrombosis as a cause.
[…] but um this time again first set of chemo, she felt terrible and the thing is, when we went back to hospital really desperate, the only problem we thought was that it was the chemotherapy that was causing it. [RG02]
[…] but I didn’t realize that that was what causing it like, obviously a clot like you know, I didn’t have any pain or anything, I just thought I was getting short of breath anyway like, do you know what I mean, because of the chemo and everything. [VCC04]
Some patients also attributed symptoms of VTE to the worsening of preexisting comorbidities.
If I didn’t have the mild asthma, I would have picked it, would pick up quicker […] But I suppose somebody that didn’t have asthma in the same thing would pick up on that quicker maybe and would have gone to the GP and maybe they would have taken a different route […] cause maybe they [the GPs] were a little bit side-lined by the asthma as well. [RG03]
Limited awareness amongst health professionals
The limited awareness of CAT was not restricted to patients. Several patients perceived a lack of knowledge amongst health care professionals. When patients presented with symptoms attributable to DVT or PE, alternative diagnoses were often considered first. Some perceived that this led to a delay in definitive diagnosis. One patient presenting with unilateral leg swelling was treated for several months with escalating doses of diuretics even when this did not lead to improvement.
It just got bigger and bigger and bigger, over months really […] then they doubled them (diuretics), and then they trebled them. [RG05]
Patients with new or increased dyspnea with chest pain were often treated first with antibiotics for a presumed chest infection.
I went to the doctor and she listened and whatever and said it was probably pleurisy. [VCC12]
They said it was probably pleurisy, gave me antibiotics […] it was a pain I’d never had before. [VCC11]
Diagnosis and treatment of CAT
Participants described two distinct pathways leading to the diagnosis of CAT;
-
Diagnosis of incidental thrombosis
A small proportion of patients had their CAT diagnosed as an incidental finding on a scan undertaken for another purpose (usually to stage the cancer). Patients were usually asymptomatic for thrombosis and not expecting an additional diagnosis to their cancer.
-
Diagnosis of symptomatic VTE
The majority of participants reported presenting with distressing symptoms that prompted investigation for VTE. These included dyspnea, chest pain and hemoptysis (for PE) and leg swelling, pain, and erythema (for DVT). These patients were expecting an explanation for their unpleasant symptoms.
Initial reaction of shock
Previous research in non-cancer patients has identified the feeling of shock at the diagnosis of VTE.18 This was also experienced by those diagnosed during their cancer journey.
[…] like everything else, it’s a shock at first. [RG05]
Distress appeared to be augmented in cancer patients as they viewed their VTE in the context of their overall cancer journey. As such, they perceived the CAT to be a complication of the cancer, which consequently inferred a worse overall prognosis.
[…] and you think ah crumbs, what’s next, you know, what’s going to happen next? [RG06]
I think we both thought oh God, what else? […] what else is going to happen? [VCC11]
[…] having the cancer and then the thrombosis on top of it, erm, not knowing how bad it was when I went in, I know I was in terrific pain with my chest and that erm, it was frightening to be honest. [VCC01]
Little information
Patients reported the diagnostic process to be rushed, with little explanation as to what was happening. When the diagnosis of CAT was made, patients described being started on LMWH and discharged quickly with limited explanation or support.
Nobody really explained, […] ‘coz they need the bed, you know. So you don’t feel as though erm, you know, I think if it was a little bit more relaxed er, they probably would’ve got somebody you know, from a department to come and explain it more. [VCC05]
[…] in the beginning, it’s just in and out sort of thing innit, take this, take that, don’t get a lot of information. [RG05]
When faced with limited information, patients would seek answers from the Internet, thereby learning of their potentially fatal condition without the necessary support or answers to questions they may have.
[…] it’s only when you start reading up about it, you sort of realize just how serious erm, you know, sort of blood clots are […] I was very lucky that you know, it was a fatal, you know attack, so er which is a little bit erm, scary. [VCC05]
Participants identified a desire for information about their condition. They did not require extensive details; rather, an overview of the condition, a treatment plan, and indicator of prognosis.
I don’t need, you know graphic details and chemical things. As long as they tell me, you know, they think that’s what caused it. That’s the treatment we’re going to give you and it should sort it out and this is what you need to look out for in the future like, you know. [VCC07]
Living with CAT
Getting on with life
Subsequent to the initial shock of VTE diagnosis and treatment initiation, participants described ‘getting on with life’ and settling back into their cancer journey. Over time, any distress attributable to CAT became less pronounced and even daily reminders such as the administration of LMWH became a routine part of daily life.
You want to try to recover and get back to some sort of um, you know, some sort of normality. [RG06]
Because I do it as soon as I get up and then if I’ve got to go anywhere it’s all done. Done and dusted. [VCC02]
I mean I just treat it as one illness to be honest […] because you know, you’ve got it at the same time and the one was caused by the other. [RG03]
Ritualization
Many patients had their LMWH initially administered by the district nurse, who in time trained them to self-inject. Self-injecting gave patients greater freedom since they did not have to wait at home each day for the nurse to call; furthermore, they were able to get the injection ‘over and done with’ so they could get on with their day. As such, the daily injection appeared to cause minimal distress or inconvenience.
[…] they give me the option of doing ‘em myself, that’s when I decided to do ‘em myself. And now I do ‘em myself, I do ‘em, you know, it’s to suit me. So I do ‘em in the morning. And the day’s my own then like, do you know what I mean like? [VCC05]
Many participants described the development of systems and rituals around their daily LMWH injection.
[…] is a ritual now. [VCC10]
They described the development of specific personalized routines, which they would strictly adhere to.
Right we sit here and I say I’ve got to have me jab. I go in the bedroom […] shut the door, ‘coz I got to pull this up, pull that down. It’s only a little thing like that. [VCC01]
I do it on the toilet normally, on the toilet seat. You’ve gotta wash the tummy first of all, get the circulation going right, and then you dry it, you wash your own hands then. [VCC08]
The development of rituals increased compliance with medicines but more importantly empowered patients to regain control over their life and not remain in the house waiting for a district nurse visit.
I do ‘em, you know, it’s to suit me. So I do ‘em in the morning. And the day’s my own then like, do you know what I mean like? [VCC05]
Discussion
The PELICAN study is the first qualitative study to explore the CAT journey through the patients’ lived experience and within the context of their ongoing cancer journey. It has identified three stages in the journey, namely: life before CAT, initial diagnosis and treatment of CAT, and living with CAT. Each stage is associated with specific patient needs, with respect to clinical intervention and process, information, training, and psychological support. These are discussed below.
Life before CAT
As identified in previous studies, patients have limited awareness about the risks of VTE beyond that associated with long-haul flights. Whilst PELICAN, similarly, identified limited patient knowledge about the risk of VTE, it also suggested this lack of awareness may delay the diagnosis and subsequent treatment of CAT. It is commonplace for chemotherapy patients to be advised of the signs and symptoms of neutropenic sepsis and to be given clear instructions of what to do in such an event.20 Since CAT is the most common cause of chemotherapy-related mortality, it would seem appropriate to afford similar emphasis on CAT awareness as is seen with neutropenic sepsis.5 In addition to earlier recognition and treatment of CAT, the knowledge that VTE is an anticipated consequence of chemotherapy may result in lower distress levels at diagnosis.
Diagnosis and treatment of CAT
The emotional impact of a diagnosis of cancer is well recognized and an emphasis is placed on training for skills required to break bad news, offer emotional support, and provide necessary information. A key worker, usually a specialist nurse, is often standard, allowing patients and families access to a named professional to navigate them though the sometimes complex diagnostic and therapeutic journey. Informing a patient of a diagnosis of CAT is, to all intents and purposes, breaking further bad news, and yet patients receive insufficient information and support when CAT is diagnosed.
A diagnosis of CAT does not solely bring an increased medical burden on the patient; it should also alert health care professionals to additional support and information requirements additional to those of the cancer alone. Failure to recognize and address these needs may lead to increased distress, misunderstanding, and poor compliance with treatment.
Living with CAT
The treatment of CAT brings with it symptomatic relief, reassuring patients that their condition is improving. This reduces distress and allows patients, over time, to get back to ‘some sort of normality’; normality now being their ongoing cancer journey. Likewise, the initial concerns regarding injecting LMWH appear to quickly abate as patients develop a ritualistic approach to daily injections. Previous research suggested reminders of the thrombotic episode event may increase distress and contribute to the development of chronic psychological disorders such as posttraumatic stress disorder.18 In this study, this was not the case, and it is possible that by developing rituals around injections normalized a procedure that would otherwise have served as a reminder of a distressing event. These results need to be considered in the context of the study’s strengths and limitations. The participants comprise a representative sample of cancer patients with thrombosis and cover a breadth of cancer primaries. As such, the results are likely to be similar to the larger CAT population. This assertion is strengthened by the fact that this was a large patient sample with strong congruence between responses.
Whilst participants were recruited from two settings: a regional cancer center and district general hospital, they were all managed as part of a dedicated service, specializing in the management of CAT. Arguably, the unmet patient needs, as described earlier, may be greater in a more generalist service, and as such, these results could underestimate the challenges of managing CAT. In particular, the patients in this study had a clear lead clinician responsible for the management of the CAT, ie, decision making regarding length of treatment, dosages, and ensuring that an anticoagulant was prescribed. Previous research has identified lack of clinical ownership to be a problem and it is likely that this could contribute further to poor patient experience and distress.21
Many of the patient experiences outlined in this study are likely to be applicable to the CAT population as a whole and will further inform our approaches to managing the challenges of primary thromboprophylaxis in medical and surgical cancer patients.24 However, it must be acknowledged that experiences will also be influenced by cultural diversity and variations in the provision of health care systems across different populations. As such, lessons from this study cannot be considered a panacea of CAT patient experiences across the globe. Consequently, a wider multinational study is underway across a breadth of countries in order to gain a global understanding of the experiences of patients living with CAT.
Conclusion
The diagnosis of CAT is a physically and emotionally distressing phenomenon, frequently experienced in the context of the major life event of a recent cancer diagnosis and ongoing cancer treatment. It is a diagnosis that they have little knowledge or warning of and often receive inadequate support or information at the time of diagnosis. Simple changes to the management of CAT are likely to lead to significant improvements in the physical and psychological outcomes of the CAT. Increased awareness of the risks of CAT, how to recognize them, and targeted support during the diagnostic process are the first priorities for improving the management of this common oncological complication. In summary, this paper highlights that regardless of the strong evidence supporting the diagnosis and treatment of CAT, without an insight into the patient experience of it, we are unlikely to deliver optimal patient-centric treatment.
Acknowledgments
This study was an investigator-initiated study that received funding from Leo Pharma.
Footnotes
Disclosure
SN has received funding for lectures (Pfizer, Leo Pharma, and Bristol Myers Squibb) and as a member of advisory boards (Leo Pharma, Pfizer, Bristol Myers Squibb, Sanofi Aventis, and Boehringer Ingelheim). HP and AN report no conflicts of interest in this work.
Author contributions
SN designed the study protocol and wrote the first draft of the manuscript. HP developed the protocol, conducted interviews, undertook analysis, and contributed to the final draft. AN developed the study protocol, undertook data analysis, and contributed to the final draft.
References
- 1.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158(6):585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 2.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4–I8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 3.Noble S, Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br J Cancer. 2010;102(Suppl 1):S2–S9. doi: 10.1038/sj.bjc.6605599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chew HK, Davies AM, Wun T, Harvey D, Zhou H, White RH. The incidence of venous thromboembolism among patients with primary lung cancer. J Thromb Haemost. 2008;6(4):601–608. doi: 10.1111/j.1538-7836.2008.02908.x. [DOI] [PubMed] [Google Scholar]
- 5.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 6.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 7.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 8.Noble S. The challenges of managing cancer related venous thromboembolism in the palliative care setting. Postgrad Med J. 2007;83(985):671–674. doi: 10.1136/pgmj.2007.061622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S–e494S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farge D, Debourdeau P, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost. 2013;11(1):56–70. doi: 10.1111/jth.12070. [DOI] [PubMed] [Google Scholar]
- 11.Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(17):2189–2204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- 12.Noble SI, Shelley MD, Coles B, Williams SM, Wilcock A, Johnson MJ, Association for Palliative Medicine for Great Britain and Ireland Management of venous thromboembolism in patients with advanced cancer: a systematic review and meta-analysis. Lancet Oncol. 2008;9(6):577–584. doi: 10.1016/S1470-2045(08)70149-9. [DOI] [PubMed] [Google Scholar]
- 13.van Korlaar IM, Vossen CY, Rosendaal FR, et al. The impact of venous thrombosis on quality of life. Thromb Res. 2004;114(1):11–18. doi: 10.1016/j.thromres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Lukas PS, Krummenacher R, Biasiutti FD, Begré S, Znoj H, von Känel R. Association of fatigue and psychological distress with quality of life in patients with a previous venous thromboembolic event. Thromb Haemost. 2009;102(6):1219–1226. doi: 10.1160/TH09-05-0316. [DOI] [PubMed] [Google Scholar]
- 15.Klok FA, Cohn DM, Middeldorp S, et al. Quality of life after pulmonary embolism: validation of the PEmb-QoL Questionnaire. J Thromb Haemost. 2010;8(3):523–532. doi: 10.1111/j.1538-7836.2009.03726.x. [DOI] [PubMed] [Google Scholar]
- 16.Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6(7):1105–1112. doi: 10.1111/j.1538-7836.2008.03002.x. [DOI] [PubMed] [Google Scholar]
- 17.Cohn DM, Nelis EA, Busweiler LA, Kaptein AA, Middeldorp S. Quality of life after pulmonary embolism: the development of the PEmb-QoL questionnaire. J Thromb Haemost. 2009;7(6):1044–1046. doi: 10.1111/j.1538-7836.2009.03341.x. [DOI] [PubMed] [Google Scholar]
- 18.Noble S, Lewis R, Whithers J, Lewis S, Bennett P. Long-term psychological consequences of symptomatic pulmonary embolism: a qualitative study. BMJ Open. 2014;4(4):e004561. doi: 10.1136/bmjopen-2013-004561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noble SI, Finlay IG. Is long-term low-molecular-weight heparin acceptable to palliative care patients in the treatment of cancer related venous thromboembolism? A qualitative study. Palliat Med. 2005;19(3):197–201. doi: 10.1191/0269216305pm1008oa. [DOI] [PubMed] [Google Scholar]
- 20.Mockler A, O’Brien B, Emed J, Ciccotosto G. The experience of patients with cancer who develop venous thromboembolism: an exploratory study. Oncol Nurs Forum. 2012;39(3):E233–E240. doi: 10.1188/12.ONF.E233-E240. [DOI] [PubMed] [Google Scholar]
- 21.Seaman S, Nelson A, Noble S. Cancer-associated thrombosis, low-molecular-weight heparin, and the patient experience: a qualitative study. Patient Prefer Adherence. 2014;8:453–461. doi: 10.2147/PPA.S58595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewins A, Silver C. Using Software in Qualitative Research: A Step-by-Step Guide. Los Angeles; London: SAGE; 2007. [Google Scholar]
- 23.Ritchie J, Spencer L. Qualitative data analysis for applied policy research. In: Bryman A, Burgess R, editors. Analyzing Qualitative Data. New York, NY: Routledge; 1994. [Google Scholar]
- 24.Serra R, de Franciscis S. The importance of extended thromboprophylaxis in patients undergoing major surgery for cancer. Thromb Res. 2014;133(6):965–966. doi: 10.1016/j.thromres.2014.02.016. [DOI] [PubMed] [Google Scholar]