Summary
The kinetochore provides a vital connection between chromosomes and spindle microtubules [1, 2]. Defining the molecular architecture of the core kinetochore components is critical for understanding the mechanisms by which the kinetochore directs chromosome segregation. The KNL1/Mis12 complex/Ndc80 complex (KMN) network acts as the primary microtubule binding interface at kinetochores [3], and provides a platform to recruit regulatory proteins [4]. Recent work found that the inner kinetochore components CENP-C and CENP-T act in parallel to recruit the KMN network to kinetochores [5-8]. However, due to the presence of these dual pathways, it has not been possible to distinguish differences in the nature of kinetochore assembly downstream of CENP-C or CENP-T. Here, we separated these pathways by targeting CENP-C and CENP-T independently to an ectopic chromosomal locus in human cells. Our work reveals that the organization of the KMN network components downstream of CENP-C and CENP-T is distinct. CENP-C recruits the Ndc80 complex through its interactions with KNL1 and the Mis12 complex. In contrast, CENP-T directly interacts with Ndc80, which in turn promotes KNL1/Mis12 complex recruitment through a separate region on CENP-T, resulting in functional relationships for KMN network localization that are inverted relative to the CENP-C pathway. We also find that distinct regulatory paradigms control the assembly of these pathways, with Aurora B kinase promoting KMN network recruitment to CENP-C, and cyclin-dependent kinase (CDK) regulating KMN network recruitment to CENP-T. This work reveals unexpected complexity for the architecture and regulation of the core components of the kinetochore-microtubule interface.
Keywords: Mitosis, Kinetochore, Centromere, Microtubule, Cell Division, Chromosome Segregation
Results
Distinct regions of CENP-T recruit KMN network components
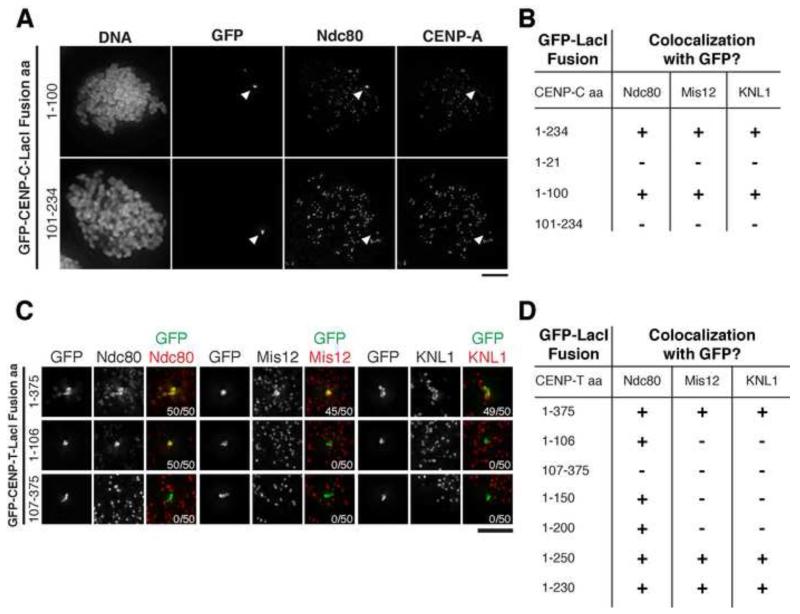
Previous work has analyzed kinetochore assembly primarily at endogenous kinetochores, which contain both CENP-C- and CENP-T-based assembly pathways [5-8]. To circumvent the challenge posed by the presence of these dual pathways at endogenous kinetochores, we targeted CENP-C or CENP-T separately to an ectopic chromosomal locus in human cells. For these experiments, we utilized our established assay in which a lac repressor (LacI) fusion protein targets CENP-C or CENP-T to an integrated lac operon (lacO) array in U2OS cells in the absence of the reciprocal protein [6]. We found that the N-terminal 100 amino acids of CENP-C were necessary and sufficient to recruit all KMN network components (KNL1, Mis12 complex, Ndc80 complex) to the lacO array (Fig. 1A, 1B, S1A), consistent with prior work [6, 9-11]. However, despite previous reports that the N-terminal 21 amino acids of CENP-C were sufficient to interact with the Mis12 complex in vitro [10], we found that CENP-C 1-21 was unable to recruit the KMN network to LacI foci in cells (Fig. 1B, S1A). Direct interactions between the CENP-C N-terminus (residues 1-234) and the entire KMN network can also be reconstituted in vitro (Fig. S2A).
Figure 1. KMN network components display separable recruitment to CENP-T.
A) Immunofluorescence images showing positive (GFP-CENP-C (1-100)-LacI) or negative (GFP-CENP-C (101-234)-LacI) co-localization with anti-Ndc80 in nocodazole-treated cells. Chosen cells lacked overlap between the GFP focus and endogenous kinetochores marked by anti-CENP-A. Images were scaled independently to show the full range of data. Arrowheads indicate position of the GFP focus. B) Summary of immunofluorescence experiments assessing co-localization of KMN components with the indicated CENP-C-LacI fusions. >90% of cells display indicated behavior (N = 50 cells/condition). C) Representative immunofluorescence images showing localization of GFP-CENP-T-LacI foci and KMN components in nocodazole-arrested mitotic cells. Images were scaled independently to show full range of data. Numbers in lower right indicate number of mitotic cells showing co-localization. D) Summary of co-localization of KMN components with CENP-T-LacI fusions. >90% of cells observed display indicated behavior (N = 50 cells/condition). Scale bars, 5 μm. See also Figures S1 and S2.
We next analyzed the requirements for KMN network recruitment downstream of CENP-T. Previous work found that all three KMN network components are recruited to GFP-CENP-T-LacI foci via the N-terminal 375 amino acids of CENP-T (Fig. 1C, 1D, [6]). Although CENP-T binds to the Ndc80 complex directly [5], biochemical experiments cannot recreate robust interactions between CENP-T and the KNL1/Mis12 complex, even with Ndc80 present (Fig. S2B; [5, 8]). In addition, the Mis12 complex and N terminus of CENP-T (aa 76-106) bind to the Ndc80 complex in a mutually exclusive manner, precluding assembly of the KMN network in the canonically defined manner [3, 5, 7, 8, 12-18]. We found that the first 106 amino acids of CENP-T were sufficient to recruit the Ndc80 complex to the lacO array (Fig. 1C, 1D), consistent with previous data [5, 7]. However, neither the Mis12 complex nor KNL1 were recruited by this CENP-T fragment. A reciprocal truncation (CENP-T residues 107-375) was unable to recruit any KMN network components (Fig. 1C, 1D). A series of additional CENP-T truncations (Fig. 1D, S1B) allowed us to refine the minimal functional region required for the recruitment of the complete KMN network to residues 1-230. This analysis suggests that a conserved domain in the vicinity of amino acids 200-230 in CENP-T (Fig. S1C) is important for KNL1/Mis12 complex recruitment. Therefore, although the KMN network is biochemically stable on its own [3, 19, 20], the recruitment of KMN network components to CENP-T foci is separable.
KMN network components display inverted functional relationships downstream of CENP-C and CENP-T
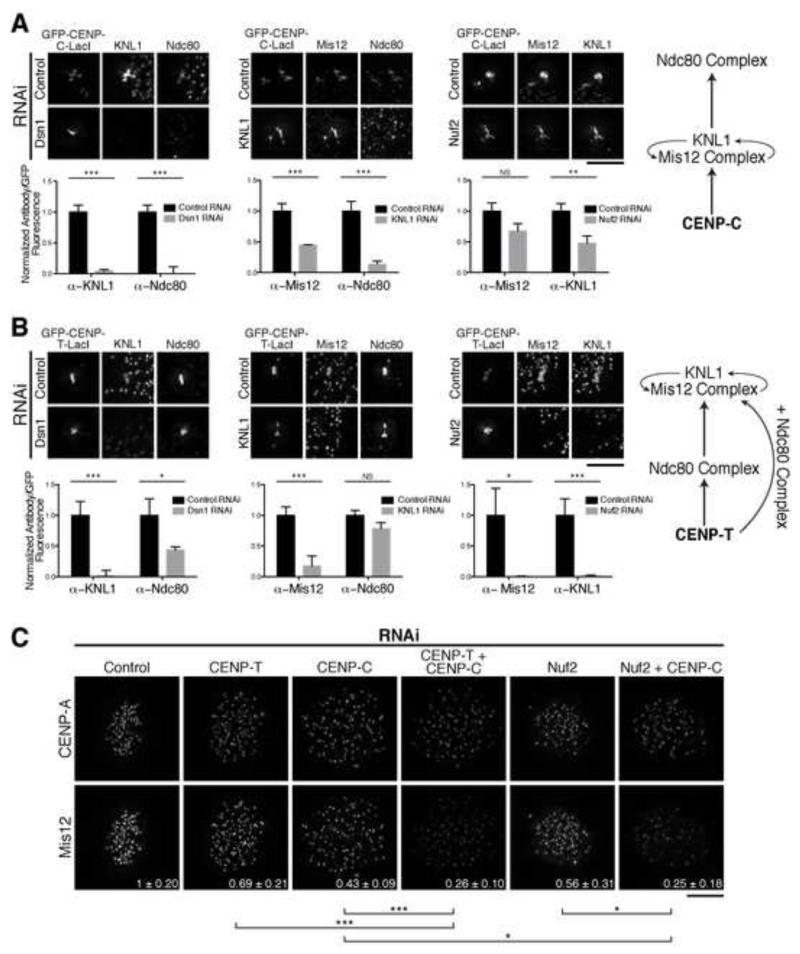
We next sought to dissect the dependency relationships between KMN network components at CENP-C and CENP-T foci. Prior work analyzing the kinetochore assembly hierarchy downstream of CENP-C [9-11, 21] suggested that the Mis12 complex and KNL1 act upstream to recruit the Ndc80 complex [3, 21-23]. To define the relationships between KMN components, we performed RNAi depletions and quantified KMN network recruitment to CENP-T-LacI or CENP-C-LacI foci. Consistent with previous models for KMN network organization, we found that KNL1 and the Mis12 complex are interdependent at both CENP-C and CENP-T foci (Fig. 2A, 2B) [3, 19]. In addition, we found that depletion of KNL1 or the Mis12 complex subunit Dsn1 led to the loss of the Ndc80 complex at CENP-C-LacI foci (Fig. 2A). In contrast, depletion of the Ndc80 complex subunit Nuf2 did not strongly disrupt the recruitment of KNL1 or Mis12 to CENP-C-LacI foci (Fig. 2A). Partial loss of KNL1/Mis12 complex localization following Nuf2 depletion has been described previously [22] and is consistent with a stabilizing role for the Ndc80 complex in KMN network assembly. These dependency relationships agree with previous analyses of the CENP-C pathway, with Ndc80 complex recruitment occurring downstream of KNL1 and Mis12 (Fig. 2A).
Figure 2. The KMN network displays distinct dependency relationships for recruitment downstream of CENP-C and CENP-T.
A and B) Top: Representative immunofluorescence images of GFP-CENP-C (1-100)-LacI (A) and GFP-CENP-T (1-250)-LacI (B) foci following depletion of KMN components. Bottom: Quantification of antibody/GFP intensity ratio at the focus, normalized to control RNAi (+/− SEM). N = 20. Right: Schematic of interdependency of KMN components for the CENP-C (A) and CENP-T (B) pathways determined by RNAi (Fig. 2) and truncation analyses (Fig. 1). For CENP-T, KNL1/Mis12 complex recruitment requires the Ndc80 complex and a second region on CENP-T. C) Representative immunofluorescence images showing anti-CENP-A and anti-Mis12 complex levels in HeLa cells. Quantification of Mis12 is shown in bottom right as fraction of control RNAi +/− standard deviation. N = 10. Student’s t-test (for panels A-C) - NS: not significant, *: p<0.05, **: p<0.01; ***: p<0.001. Scale bars, 5 μm.
In contrast, we found distinct relationships for the KMN network components downstream of CENP-T. KNL1 or Dsn1 depletion resulted in a modest reduction in Ndc80 complex localization (Fig. 2B), suggesting that these proteins are not required for Ndc80 complex recruitment, but may stabilize Ndc80 bound to the CENP-T receptor. Strikingly, depletion of Nuf2 eliminated KNL1 and Mis12 recruitment to CENP-T-LacI foci (Fig. 2B). These data suggest that the Ndc80 complex acts upstream of KNL1 and the Mis12 complex for the CENP-T-based kinetochore assembly pathway (Fig. 2B), such that these functional relationships are inverted relative to the CENP-C-based pathway.
To test whether this organization also exists at endogenous kinetochores, we next conducted RNAi experiments in HeLa cells. Co-depletion of CENP-C and CENP-T significantly reduced KMN network localization relative to the individual depletions (Fig. 2C; [6]), consistent with the presence of dual pathways. As we found that the Ndc80 complex was required upstream of KNL1 and the Mis12 complex at CENP-T foci (Fig. 2B), we predicted that depletion of either Ndc80 or CENP-T should cause a similar failure of KNL1/Mis12 complex recruitment. Indeed, co-depletion of CENP-C and the Ndc80 complex subunit Nuf2 resulted in a severe reduction of Mis12 localization to levels equivalent to those observed when both the CENP-T and CENP-C pathways were disrupted directly (Fig. 2C). These data support a model in which KMN network recruitment downstream of CENP-T is promoted by the Ndc80 complex.
CDK phosphorylation regulates the recruitment of KMN network components through multiple distinct regions within CENP-T
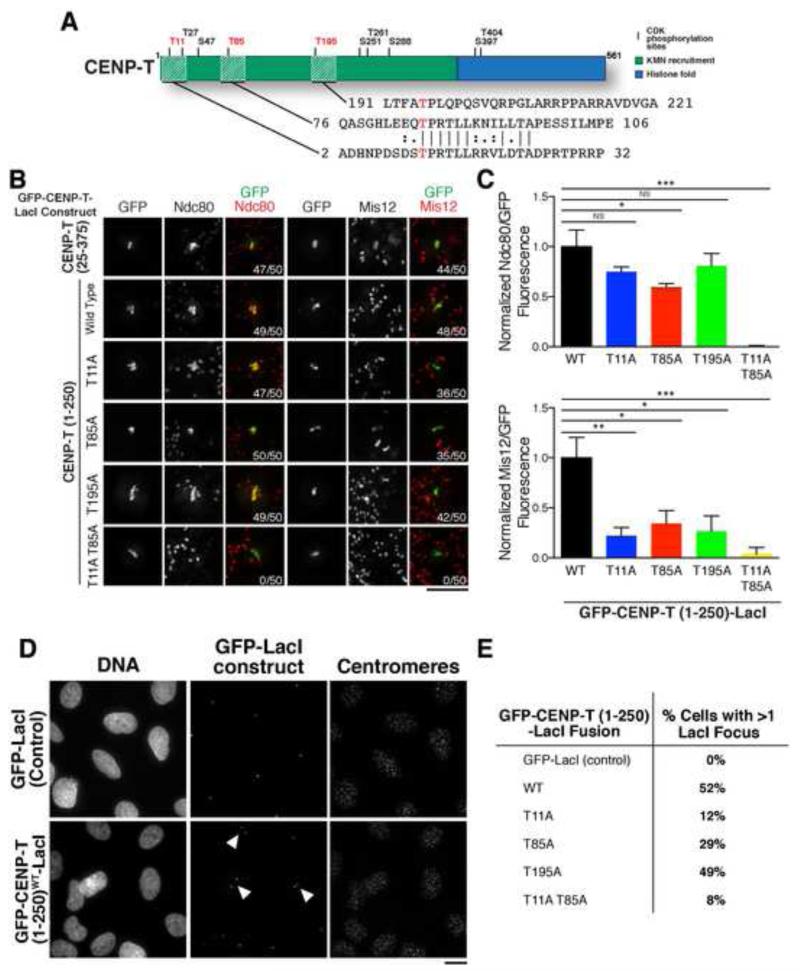
Although CENP-C and CENP-T are present at centromeres throughout the cell cycle, the KMN network assembles prior to mitotic entry and disassembles at mitotic exit [24]. Therefore, we next assessed the regulation of outer kinetochore assembly downstream of CENP-C and CENP-T. Our previous work highlighted the importance of cyclin-dependent kinase (CDK) in promoting the binding and recruitment of the Ndc80 complex to CENP-T [5, 6, 24]. This work identified residues 76-106 in CENP-T as a key Ndc80 complex binding region [5]. Based on sequence homology, there is a similar motif at amino acids 11-25 in CENP-T. Each motif includes a mapped CDK phosphorylation site (residue T11 or T85) (Fig. 3A, [5, 24]). Disrupting these motifs individually through an N-terminal truncation (CENP-T aa 25-375), or using non-phosphorylatable T11A or T85A single mutants, did not prevent KMN network recruitment to the ectopic locus (Fig. 3B, 3C, S3A, S3B). In contrast, a T11A T85A double mutant abrogated recruitment of the KMN network (Fig. 3B, 3C, S3A, S3B). This suggests that both Ndc80 complex binding motifs are functional. However, our data do not distinguish whether a single CENP-T simultaneously recruits two Ndc80 complexes (with incomplete occupancy of these sites), or whether these motifs act together to create a robust binding interface for a single Ndc80 molecule.
Figure 3. Recruitment of KMN network to CENP-T is dependent on CDK phosphorylation.
A) CENP-T schematic indicating its KMN network recruitment [6] and histone fold [25] domains. Indicated residues correspond to CDK phosphorylation sites [5, 24] with those analyzed in this study in red. Sequences corresponding to hatched regions are shown below. Alignment was performed using EMBOSS Water [26]. B) Representative immunofluorescence images showing GFP-CENP-T-LacI foci co-stained for Ndc80 or Mis12 complexes in nocodazole-treated cells. Images were scaled independently to show the full range of data. Numbers in lower right indicate the number of mitotic cells with co-localization between GFP and the indicated KMN component. Wild type CENP-T (1-250) images are duplicated from Fig. S1. Scale bar, 5 μm. C) Quantification of antibody/GFP fluorescence ratio (+/− SEM) at the indicated foci normalized to wild type CENP-T. N = 10 cells/condition. Student’s t-test, NS: not significant, *: p<0.05, **: p<0.01; ***: p<0.001. D) Immunofluorescence images of cells following 72 hr IPTG washout. Cells with >1 GFP focus are marked with arrowheads. Centromeres stained with anti-centromere antibodies (ACA). Scale bar, 15 μm. E) Table showing the frequency of cells with multiple GFP foci following 72 hr IPTG washout. N = 100 cells/condition. See also Figure S3.
We next tested the regulation of KNL1 and Mis12 complex localization downstream of CENP-T. Our truncation analysis identified amino acids 200-230 in CENP-T as critical for KNL1 and Mis12 complex recruitment (Fig. 1D, S1B). Therefore, we generated a phospho-inhibitory mutation in the neighboring CDK phosphorylation site, T195 (Fig. 3A). Although Ndc80 complex localization to CENP-T T195A mutant foci was largely unaffected (Fig. 3C), the levels of KNL1/Mis12 were strongly reduced (Fig. 3B, 3C, S3A, S3B). Despite the importance in vivo of phosphorylation of T11, T85, and T195, phospho-mimetic mutations cannot reconstitute robust interactions between CENP-T and the complete KMN network in vitro (Fig. S2B).
Finally, we tested the effect of altering KMN network recruitment downstream of CENP-T on chromosome segregation. Due to the integration of lacO in the arm of chromosome 1 in these cell lines, ectopic targeting of the CENP-T-LacI fusion creates a dicentric-like chromosome that strongly perturbs chromosome segregation [6, 27]. This behavior results in the accumulation of GFP foci [27], but is suppressed by the addition of IPTG to disrupt the lacO/LacI interaction. Cells expressing GFP-LacI as a control displayed a single focus in each cell, consistent with proper chromosome segregation (Fig. 3D, 3E). In contrast, after removal of IPTG from the growth media for 72 hours, ~50% of cells expressing the wild type or T195A GFP-CENP-T-LacI construct had >1 focus, indicating chromosome mis-segregation (Fig. 3D, 3E). However, CENP-T T11A, T85A, and T11A T85A mutants showed attenuated defects (Fig. 3E), consistent with their reduced ability to recruit the KMN network (Fig. 3C, S3B). Together, these data indicate that CDK regulates the interaction of CENP-T with the KMN network at multiple distinct sites, with functional consequences for chromosome segregation.
Aurora B kinase activity is required for kinetochore assembly downstream of CENP-C
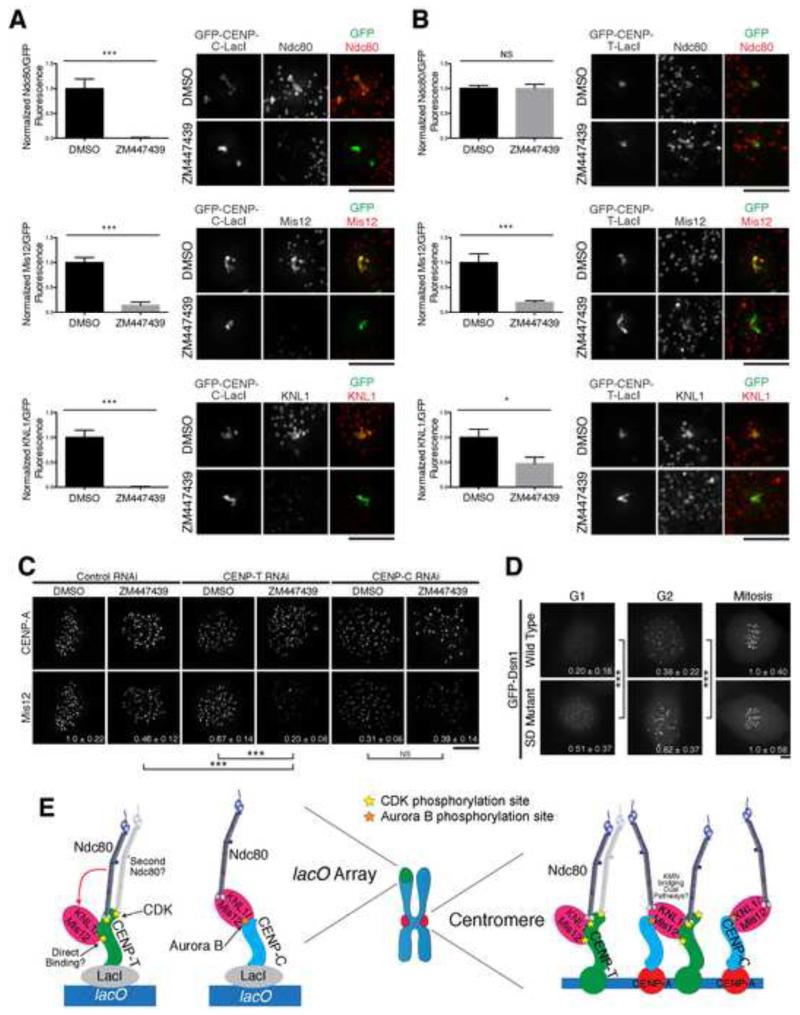
We next analyzed the regulation of the CENP-C pathway. Aurora B kinase, which plays a key role in controlling kinetochore-microtubule attachments [28], has been implicated in kinetochore assembly in Xenopus laevis [29] and budding yeast [30]. Although we previously observed that Aurora B inhibition in HeLa cells resulted in only a modest defect in kinetochore assembly [31], we considered that this defect might be magnified when the CENP-C and CENP-T-based assembly pathways were analyzed separately. Indeed, treatment with the Aurora B inhibitor ZM447439 significantly reduced localization of all KMN network components to CENP-C-LacI foci (Fig. 4A). In contrast, Ndc80 complex recruitment to the CENP-T-LacI focus was unaffected by ZM447439 treatment (Fig. 4B). However, we observed significant loss of KNL1 and Mis12 complex localization to the CENP-T-LacI focus, suggesting that Aurora B phosphorylation may play a role in stabilizing the Mis12/KNL1 interaction, or contribute to KNL1 and Mis12 complex recruitment downstream of CENP-T.
Figure 4. Aurora B kinase regulates KMN network recruitment downstream of CENP-C.
A and B) Left: Quantification of antibody/GFP intensity normalized to DMSO treatment for the indicated conditions (+/− SEM). N=20. Right: Representative immunofluorescence images of GFP-CENP-C (1-100)-LacI (A) and GFP-CENP-T (1-250)-LacI (B) foci after treatment. C) Representative immunofluorescence images showing CENP-A and Mis12 levels in HeLa cells. Quantification of Mis12 at mitotic kinetochores is shown in bottom right as a fraction of control RNAi + DMSO +/− standard deviation. N = 10. All images were scaled relative to their DMSO and RNAi control. D) Representative immunofluorescence images of GFP-Dsn1 wild type and Aurora B phospho-mimetic (S28D S78D S100D S109D) mutant expressing HeLa cells after RNAi depletion of endogenous Dsn1. GFP fluorescence is shown in bottom right as a fraction of mitotic kinetochore fluorescence for each construct +/− standard deviation. N = 90-300 kinetochores/condition from multiple cells. Images were scaled equivalently within each cell line. E) Model for KMN network recruitment at CENP-T or CENP-C foci (left) or endogenous kinetochores (right). It remains unknown whether KNL1/Mis12 interact directly with CENP-T, whether two Ndc80 complexes bind simultaneously to CENP-T, and whether a single KNL1/Mis12 complex can bridge CENP-T and CENP-C at endogenous kinetochores. Stars indicate phosphorylation by the indicated kinase. Student’s t-test (for panels A-D) - NS: not significant, *: p < 0.05, ***: p < 0.001. Scale bars, 5 μm. See also Figure S4.
We next tested the role of Aurora B in regulating endogenous kinetochore assembly. We reasoned that if Aurora B promotes kinetochore assembly downstream of CENP-C, the effect of Aurora B inhibition would be magnified in the absence of the CENP-T-based assembly pathway. Indeed, combining CENP-T RNAi and ZM447439 treatment led to an enhanced reduction in Mis12 complex localization relative to the individual treatments (Fig. 4C). In contrast, combining CENP-C RNAi and ZM447439 treatment did not lead to a further reduction in Mis12 complex localization relative to CENP-C depletion alone (Fig. 4C).
Aurora B kinase directly phosphorylates KNL1, Ndc80, and the Mis12 complex subunit Dsn1 [31]. However, phosphorylation of KNL1 regulates its interactions with PP1 [32] and microtubules [31], and Ndc80 phosphorylation regulates its interactions with microtubules [3, 33]. Therefore, we evaluated the contribution of Aurora B phosphorylation of Dsn1 for promoting Mis12 complex localization. To test this, we analyzed a GFP-Dsn1 mutant in which the mapped Aurora B phosphorylation sites were mutated to aspartic acid (SD) to mimic constitutive phosphorylation [31]. This mutant localized to mitotic kinetochores similarly to wild type Dsn1. However, we found that the GFP-Dsn1 SD mutant displayed enhanced localization to G1 and G2 kinetochores (Fig. 4D). This increased G1 localization was not affected by CENP-T depletion, but was strongly compromised following CENP-C depletion (Fig. S4). In contrast, we previously reported that the corresponding GFP-Dsn1 phospho-inhibitory mutant had minimal effects on its mitotic kinetochores localization [31], suggesting that additional interactions promote mitotic Mis12 complex recruitment. In addition, the KMN network is able to interact with CENP-C in vitro regardless of its phosphorylation state (Fig. S1A; data not shown; [10]). Together, these data suggest Aurora B kinase promotes the recruitment of the KMN network to kinetochores, particularly downstream of CENP-C.
Discussion
The KMN network is organized and regulated differently downstream of CENP-C and CENP-T
Despite the identification of more than 100 different components of the human kinetochore, defining the basic kinetochore architecture remains an ongoing challenge. In particular, it was unclear how kinetochore components assemble downstream of the recently defined CENP-C- and CENP-T-based pathways. Here, we demonstrated that these two pathways are not simply duplications, but rather each pathway displays distinct regulation and functional relationships for KMN network recruitment (Fig. 4E). Recent work from Kim and Yu [34] also identified similar differences in the behavior and regulation of these pathways. When tested separately at the ectopic focus, CENP-T and CENP-C are capable of independently recruiting the entire KMN network. However, it remains to be determined how the two pathways interact when both are present at endogenous kinetochores. For example, the KMN network components may be recruited independently by CENP-C and CENP-T, or a KMN unit may simultaneously interact with both receptors (Fig. 4E). We also cannot rule out the possibility that additional kinetochore components contribute to the recruitment of KNL1 and the Mis12 complex downstream of CENP-T at endogenous kinetochores. In summary, our work has uncovered a complex architecture for the core kinetochore components.
Supplementary Material
Acknowledgements
We thank the members of the Cheeseman lab for their kind support and helpful discussions. This work was supported by a Scholar award to IMC from the Leukemia & Lymphoma Society, a grant from the NIH/National Institute of General Medical Sciences to IMC (GM088313), a Research Scholar Grant to IMC (121776) from the American Cancer Society, and an NSF graduate research fellowship to FR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cheeseman IM, Desai A. Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol. 2008;9:33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- 2.Rago F, Cheeseman IM. Review series: The functions and consequences of force at kinetochores. The Journal of Cell Biology. 2013;200:557–565. doi: 10.1083/jcb.201211113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127:983–997. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 4.London N, Biggins S. Signalling dynamics in the spindle checkpoint response. Nat Rev Mol Cell Biol. 2014;15:736–748. doi: 10.1038/nrm3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishino T, Rago F, Hori T, Tomii K, Cheeseman IM, Fukagawa T. CENP-T provides a structural platform for outer kinetochore assembly. EMBO J. 2013;32:424–436. doi: 10.1038/emboj.2012.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gascoigne KE, Takeuchi K, Suzuki A, Hori T, Fukagawa T, Cheeseman IM. Induced Ectopic Kinetochore Assembly Bypasses the Requirement for CENP-A Nucleosomes. Cell. 2011;145:410–422. doi: 10.1016/j.cell.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malvezzi F, Litos G, Schleiffer A, Heuck A, Mechtler K, Clausen T, Westermann S. A structural basis for kinetochore recruitment of the Ndc80 complex via two distinct centromere receptors. EMBO J. 2013;32:409–423. doi: 10.1038/emboj.2012.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schleiffer A, Maier M, Litos G, Lampert F, Hornung P, Mechtler K, Westermann S. CENP-T proteins are conserved centromere receptors of the Ndc80 complex. Nat. Cell Biol. 2012;14:604–613. doi: 10.1038/ncb2493. [DOI] [PubMed] [Google Scholar]
- 9.Przewloka MR, Venkei Z, Bolanos-Garcia VM, Debski J, Dadlez M, Glover DM. CENP-C is a structural platform for kinetochore assembly. Curr. Biol. 2011;21:399–405. doi: 10.1016/j.cub.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Screpanti E, De Antoni A, Alushin GM, Petrovic A, Melis T, Nogales E, Musacchio A. Direct binding of Cenp-C to the Mis12 complex joins the inner and outer kinetochore. Curr. Biol. 2011;21:391–398. doi: 10.1016/j.cub.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milks KJ, Moree B, Straight AF. Dissection of CENP-C-directed centromere and kinetochore assembly. Mol. Biol. Cell. 2009;20:4246–4255. doi: 10.1091/mbc.E09-05-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciferri C, De Luca J, Monzani S, Ferrari KJ, Ristic D, Wyman C, Stark H, Kilmartin J, Salmon ED, Musacchio A. Architecture of the human ndc80-hec1 complex, a critical constituent of the outer kinetochore. J Biol Chem. 2005;280:29088–29095. doi: 10.1074/jbc.M504070200. [DOI] [PubMed] [Google Scholar]
- 13.Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Reis, Dos G, Maiolica A, Polka J, De Luca JG, De Wulf P, et al. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell. 2008;133:427–439. doi: 10.1016/j.cell.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei RR, Sorger PK, Harrison SC. Molecular organization of the Ndc80 complex, an essential kinetochore component. Proc Natl Acad Sci USA. 2005;102:5363–5367. doi: 10.1073/pnas.0501168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei RR, Schnell JR, Larsen NA, Sorger PK, Chou JJ, Harrison SC. Structure of a central component of the yeast kinetochore: the Spc24p/Spc25p globular domain. Structure. 2006;14:1003–1009. doi: 10.1016/j.str.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Wei RR, Al-Bassam J, Harrison SC. The Ndc80/HEC1 complex is a contact point for kinetochore-microtubule attachment. Nat Struct Mol Biol. 2007;14:54–59. doi: 10.1038/nsmb1186. [DOI] [PubMed] [Google Scholar]
- 17.DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore Microtubule Dynamics and Attachment Stability Are Regulated by Hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 18.Wilson-Kubalek EM, Cheeseman IM, Yoshioka C, Desai A, Milligan RA. Orientation and structure of the Ndc80 complex on the microtubule lattice. The Journal of Cell Biology. 2008;182:1055–1061. doi: 10.1083/jcb.200804170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrovic A, Mosalaganti S, Keller J, Mattiuzzo M, Overlack K, Krenn V, De Antoni A, Wohlgemuth S, Cecatiello V, Pasqualato S, et al. Modular assembly of RWD domains on the Mis12 complex underlies outer kinetochore organization. Mol Cell. 2014;53:591–605. doi: 10.1016/j.molcel.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Petrovic A, Pasqualato S, Dube P, Krenn V, Santaguida S, Cittaro D, Monzani S, Massimiliano L, Keller J, Tarricone A, et al. The MIS12 complex is a protein interaction hub for outer kinetochore assembly. The Journal of Cell Biology. 2010;190:835–852. doi: 10.1083/jcb.201002070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kline SL, Cheeseman IM, Hori T, Fukagawa T, Desai A. The human Mis12 complex is required for kinetochore assembly and proper chromosome segregation. J. Cell Biol. 2006;173:9–17. doi: 10.1083/jcb.200509158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheeseman IM, Niessen S, Anderson S, Hyndman F, Yates JR, Oegema K, Desai A. A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev. 2004;18:2255–2268. doi: 10.1101/gad.1234104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S-T, Rattner JB, Jablonski SA, Yen TJ. Mapping the assembly pathways that specify formation of the trilaminar kinetochore plates in human cells. J. Cell Biol. 2006;175:41–53. doi: 10.1083/jcb.200606020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gascoigne KE, Cheeseman IM. CDK-dependent phosphorylation and nuclear exclusion coordinately control kinetochore assembly state. The Journal of Cell Biology. 2013;201:23–32. doi: 10.1083/jcb.201301006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishino T, Takeuchi K, Gascoigne KE, Suzuki A, Hori T, Oyama T, Morikawa K, Cheeseman IM, Fukagawa T. CENP-T-W-S-X Forms a Unique Centromeric Chromatin Structure with a Histone-like Fold. Cell. 2012;148:487–501. doi: 10.1016/j.cell.2011.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N, Cowley AP, Lopez R. Analysis Tool Web Services from the EMBL-EBI. Nucleic Acids Res. 2013;41:W597–600. doi: 10.1093/nar/gkt376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gascoigne KE, Cheeseman IM. Induced dicentric chromosome formation promotes genomic rearrangements and tumorigenesis. Chromosome Res. 2013;21:407–418. doi: 10.1007/s10577-013-9368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lampson MA, Cheeseman IM. Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 2011;21:133–140. doi: 10.1016/j.tcb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emanuele MJ, Lan W, Jwa M, Miller SA, Chan CSM, Stukenberg PT. Aurora B kinase and protein phosphatase 1 have opposing roles in modulating kinetochore assembly. The Journal of Cell Biology. 2008;181:241–254. doi: 10.1083/jcb.200710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akiyoshi B, Nelson CR, Biggins S. The aurora B kinase promotes inner and outer kinetochore interactions in budding yeast. Genetics. 2013;194:785–789. doi: 10.1534/genetics.113.150839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welburn JPI, Vleugel M, Liu D, Yates JR, III, Lampson MA, Fukagawa T, Cheeseman IM. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol Cell. 2010;38:383–392. doi: 10.1016/j.molcel.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu D, Davydenko O, Lampson MA. Polo-like kinase-1 regulates kinetochore-microtubule dynamics and spindle checkpoint silencing. The Journal of Cell Biology. 2012;198:491–499. doi: 10.1083/jcb.201205090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore Microtubule Dynamics and Attachment Stability Are Regulated by Hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 34.Kim S, Yu H. Multiple assembly mechanisms anchor the KMN spindle checkpoint platform at human mitotic kinetochores. The Journal of Cell Biology. 2015;208:181–196. doi: 10.1083/jcb.201407074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.