Abstract
Purpose
The graft-versus-leukemia (GVL) reaction is an important example of immune-mediated tumor destruction. A coordinated humoral and cellular response accomplishes leukemia cell killing, but the specific targets remain largely uncharacterized. To learn more about the antigens that elicit antibodies during GVL reactions, we analyzed advanced myelodysplasia (MDS) and acute myeloid leukemia (AML) patients who received an autologous, granulocyte-macrophage colony stimulating factor (GM-CSF) secreting tumor cell vaccine early after allogeneic hematopoietic stem cell transplantation (HSCT).
Experimental Design
A combination of tumor-derived cDNA expression library screening, protein microarrays, and antigen-specific ELISAs were employed to characterize sera obtained longitudinally from 15 AML/MDS patients who were vaccinated early after allogeneic HSCT.
Results
A broad, therapy-induced antibody response was uncovered, which primarily targeted intracellular proteins that function in growth, transcription/translation, metabolism, and homeostasis. Unexpectedly, antibodies were also elicited against eight secreted angiogenic cytokines that play critical roles in leukemogenesis. Antibodies to the angiogenic cytokines were evident early after therapy, and in some patients manifested a diversification in reactivity over time. Patients that developed antibodies to multiple angiogenic cytokines showed prolonged remission and survival.
Conclusions
These results reveal a potent humoral response during GVL reactions induced with vaccination early after allogeneic HSCT and raise the possibility that antibodies, in conjunction with NK cells and T lymphocytes, may contribute to immune-mediated control of myeloid leukemias.
Keywords: graft-versus-leukemia, antibodies, angiogenic cytokines, vaccines, AML
Introduction
Allogeneic HSCT is curative treatment for some patients with advanced MDS or AML (1). Disease control with reduced intensity conditioning (RIC) is accomplished principally through a donor-initiated GVL reaction that involves the coordinated interplay of innate and adaptive immune components. Donor T cells detect complexes of host major histocompatibility (MHC) molecules and peptides derived from either leukemia-associated gene products or normal cellular proteins, some of which are polymorphic between donor and host (2). Upon stimulation, the transferred lymphocytes orchestrate an anti-leukemic response that includes both direct cytotoxicity and the triggering of additional cellular and humoral effector pathways (3). The dual recognition of leukemic cells and healthy tissues underlies the close relationship between the therapeutic benefits of GVL and the inflammatory pathology of GVHD (4).
The mechanisms responsible for disease resistance and relapse after allogeneic HSCT remain to be clarified fully, but may involve, at least in some cases, a failure of anti-tumor immunity (3). In this context, recent clinical investigations in the autologous, non-transplant setting have established the ability of active immunotherapy to potentiate endogenous host responses (5), raising the possibility that GVL reactions might be similarly intensified. The period early after allogeneic HSCT may be particularly favorable for active immunotherapy (6). Conditioning regimens compromise mucosal barriers, permitting the release of endogenous microbiota that may activate and mature dendritic cells systemically through engagement of Toll-like and Nod-like receptors (7). Drug-induced lymphopenia also increases the levels of pro-inflammatory cytokines such as IL-7, IL-12, and IL-15 that accelerate the reconstitution of effector T cells relative to FoxP3+ regulatory T cells (Tregs) (8-10), which promotes immune-mediated tumor destruction.
Based upon promising experiments with tumor cell vaccines in murine bone marrow transplant models (11), we undertook a Phase I trial of immunization with irradiated, autologous myeloblasts engineered by adenoviral mediated gene transfer to secrete GM-CSF early after RIC allogeneic HSCT in patients with high risk MDS and refractory AML (12). Eligible patients with relapsed or refractory disease underwent allogeneic HSCT from matched donors using fludarabine and busulfan as the conditioning regimen and tacrolimus and pulse methotrexate as GVHD prophylaxis. Between thirty and forty five days after HSCT, fifteen patients began vaccination with autologous gene-modified cells at a median dose of 1.0 × 107 cells per injection and a mean GM-CSF secretion rate of 52 ng/106 cells/24 hours. Vaccines were administered subcutaneously and intra-dermally at weekly intervals times three and then every other week times three.
Vaccination was well tolerated and did not lead to an exacerbation in frequency or intensity of acute or chronic GVHD. Notwithstanding the concurrent administration of tacrolimus as GVHD prophylaxis, immunization stimulated local infiltrates composed of dendritic cells, granulocytes, macrophages, eosinophils, and lymphocytes. These responses led, likely through priming in regional lymph nodes, to enhanced systemic immunity that was manifested in T cell infiltration into the leukemic bone marrow and T cell mediated delayed-type hypersensitivity reactions to intradermal injections of irradiated, autologous AML cells. Treatment also triggered reductions in the levels of soluble NKG2D ligands, with a corresponding normalization of NKG2D expression on NK cells and CD8+ T lymphocytes. Collectively, these results indicate that vaccination early after allogeneic HSCT augments both innate and adaptive immunity in some patients, thereby intensifying GVL. Moreover, nine of 10 patients who completed the course of six inoculations achieved durable complete remissions.
In the autologous setting, vaccination with irradiated, autologous GM-CSF secreting tumor cells stimulates a coordinated cellular and humoral response (13). Through the screening of tumor-derived cDNA expression libraries with post-vaccination sera, we identified several targets of therapy-induced antibodies, which include intracellular, surface membrane, and secreted proteins (14-19). Functional studies revealed that antibodies directed towards surface and secreted proteins could promote tumor cytotoxicity and block key pathways of tumor progression, underscoring an important contribution of humoral immunity to the therapeutic effects. To determine whether the leukemia cell vaccines early after allogeneic HSCT might similarly elicit a coordinated T and B cell reaction that participates in the GVL reaction, we undertook a detailed characterization of antibody responses in the immunized AML/MDS patients.
Materials and Methods
Clinical protocol
The Phase I trial of vaccination with lethally irradiated, autologous tumor cells engineered by adenoviral mediated gene transfer to secrete GM-CSF in high risk MDS/AML patients receiving a RIC allogeneic HSCT was reported (12). The protocol obtained approval from the Dana-Farber/Harvard Cancer Center Institutional Review Board, Food and Drug Administration, and Recombinant DNA Advisory Committee and was registered at NIH clinicaltrials.gov (NCT00426205).
cDNA expression library screening
The construction of the K008 melanoma-derived cDNA expression library was reported (14). Post-vaccination sera from three patients (9, 12, and 23) were pre-cleared against Escherichia coli and lambda phage lysates and used at a 1:1,000 dilution in TBST (50 mM Tris/138 mM NaCl/2.7 mM KCl/0.05% Tween 20, pH 8.0). Positive plaques were detected with an alkaline phosphatase-conjugated polyclonal goat anti-human pan-IgG antibody (Jackson ImmunoResearch) and 5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium (BCIP/NBT) (Promega). Reactive clones were plaque-purified and the inserts matched to the NCBI Entrez Nucleotide database.
Protein microarray screening
The Invitrogen ProtoArray v5.0 (Cat# PAH052501) that contains approximately 9000 human proteins (expressed as glutathione S-transferase fusion proteins in SF9 insect cells) spotted in duplicate on nitrocellulose-coated glass slides (http://orf.invitrogen.com) was used for screening patient sera (1:500 dilution). After adding an anti-human IgG (1:2000) conjugated to Alexa Fluor 647 dye, the arrays were scanned with a GenePix 4000B Fluorescent Scanner and the data processed using ProtoArray Prospector 2.0 (Invitrogen, Carlsbad, California, USA). Values for each protein were calculated with the Z-factor, which measures the signal to noise ratio.
ELISAs
ELISA plates (Nunc) were coated with 50 μl/well of human proteins in TBS-T (0.1% Tween) overnight at 4° C. The concentrations were: 3 μg/ml for L1CAM (Sino Biological), 1.5 μg/ml for DEL-1 (R&D), 2 μg/ml for angiopoietin-1 (R&D), 0.5 μg/ml for angiopoietin-2 (R&D), 1 μg/ml for vascular endothelial growth factor-A (Peprotech), 3 μg/ml for progranulin (R&D), 0.4 μg/ml for platelet derived growth factor-BB (eBioscience), and 1 μg/ml for hepatocyte growth factor (Sino Biological). Plates were washed and blocked for 1.5 hours at room temperature with 100 μl/well of protein-free blocking buffer (PFB) (0.1% Tween, Pierce, Cat# 37570). Patient sera diluted 1:2000 in PFB-T were added at 50 μl/well in triplicate for 1 hour at 4° C. After washing, 50 μl/well of an HRP-conjugated anti-human IgG (Fab)2 diluted 1:2000 (Southern Biotech, Cat# 6005-05)) was added for 1 hour at room temperature. The plates were washed and 50 μl/well of biotinylated Tyramide (10 μl/ml in amplification diluent concentrate diluted 1:1 with ddH2O, ELAST, PerkinElmer) was added for 30 minutes at room temperature. After washing, wells were incubated with streptavidin-HRP (50 μl/well, concentration of 2 μl/ml) in 1% BSA-PBS-T (0.1% Tween) for 30 min at room temperature. The plate was developed with pNPP substrate (Sigma-Aldrich) and the absorbance measured at 450 nm.
Statistical analysis
The relationship between survival and antibody response in the first sample post-HSCT was determined with a Cox proportional hazards model.
Results
Screening for antibody targets
To explore the development of humoral immunity during GVL reactions associated with vaccination early after allogeneic HSCT, we first sought to define specific gene products that were the targets of high titer antibodies. Post-vaccination sera from three patients who achieved long-term disease control were employed to screen a previously constructed melanoma-derived cDNA expression library (K008) that has proved informative for antigen discovery efforts in several other tumor types (14-19). While the use of the K008 library limits the ability to detect leukemia-specific antigens, it favors the identification of shared tumor antigens that may include proteins commonly involved in transformation. The three screens yielded a total of ninety-nine clones that encoded thirty-six distinct gene products, thirty-four of which are known proteins (Table 1). Most of the targets elicited reactivity in individual patients, perhaps reflecting the application of autologous tumor cell vaccines, which are likely to be antigenically heterogeneous. No obvious property distinguished the four proteins identified in two independent screens with different patient sera.
Table 1.
Targets identified in K008 cDNA expression library with sera from vaccinated AML patients
| Hits | Symbol | Gene name | Function |
|---|---|---|---|
| 13 | BZW2 | Basic leucine zipper and W2 domains 2 | Transcription/Translation |
| 10 | RBPJ | Recombination signal binding protein for immunoglobulin kappa J region | |
| 10 | SPOP | Speckle-type POZ protein | |
| 2 | EIF4G3 | Eukaryotic translation initiation factor 4 gamma, 3 | |
| 2 | ZHX1 | Zinc fingers and homeoboxes 1 | |
| 2 | PNO1 | Partner of NOB1 homolog | |
| 2 | SON | SON DNA binding protein | |
| 1 | ARID4B | AT rich interactive domain 4B (RBP1-like) | |
| 1 | SUPT6H | Suppressor of Ty 6 homolog | |
| 3 | TNKS1 | Tankyrase 1 | Protein homeostasis |
| 2 | KAZN | Kazrin, periplakin interacting protein | |
| 1 | WIPI1 | WD repeat domain, phosphoinositide interacting 1 | |
| 1 | TNKS2 | Tankyrase 2 | |
| 1 | KDM6B | Lysine (K)-specific demethylase 6B | |
| 1 | L1CAM | L1 cell adhesion molecule | Migration/Invasion |
| 1 | IQGAP1 | IQ motif containing GTPase activating protein 1 | |
| 10 | MCCC1 | Methylcrotonoyl-CoA carboxylase 1 (alpha) | Metabolism |
| 2 | AHNAK2 | AHNAK nucleoprotein 2 | |
| 2 | ETNK | Ethanolamine kinase | |
| 2 | NDUFA11 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 11 | |
| 1 | UEVLD | UEV and lactate/malate dehyrogenase domains | |
| 8 | EPRS | Glutamyl-prolyl-tRNA synthetase | Cell cycle |
| 4 | GOLGA4 | Golgi autoantigen, golgin subfamily a, 4 | |
| 3 | NCAPD2 | Non-SMC condensin I complex, subunit D2 | |
| 2 | TACC2 | Transforming, acidic coiled-coil containing protein 2 | |
| 1 | CKAP2 | Cytoskeleton associated protein 2 | |
| 1 | KIF15 | Kinesin family member 15 | |
| 1 | BubR1 | Bub1-related kinase | |
| 2 | PARP2 | Poly (ADP-ribose) polymerase family, member 2 | Others |
| 1 | FLVCR1 | Feline leukemia virus subgroup C cellular receptor 1 | |
| 1 | KBP | KIF1 binding protein | |
| 1 | SILV (gp100) | Premelanosome protein | |
| 1 | TPM3 | Tropomyosin 3 | |
| 1 | DNAJB1 | DnaJ (Hsp40) homolog, subfamily B, member 1 | |
| 1 | CLMN | Calmin | Unknown |
| 1 | INADL | InaD-like |
Red denotes gene products identified in screens with two independent AML patient sera samples.
The antibody targets could be classified into several functional groups, including transcription/translation, protein homeostasis, metabolism, cell cycle regulation, and migration/invasion. Consistent with these fundamental aspects of cell biology, several of the antigens participate in oncogenesis. For example, the recombination signal binding protein for immunoglobulin kappa J region (RBP-jκ) plays a central role in canonical Notch signaling (20), tankyrase 1 and 2 (TNKS1 and TNKS2) contribute to Wnt signaling (21), transforming acidic coiled-coil containing protein 2 (Tacc2) participates in cell cycle regulation (22), and poly (ADP-ribose) polymerase family, member 2 (PARP2) functions in DNA repair and programmed cell death (23). Of particular interest was the identification of L1 cell adhesion molecule L1CAM (CD171), which is overexpressed in a variety of tumors and cooperates with receptor tyrosine kinases and integrins to promote transformation through MAP kinase pathways (24). L1CAM is a cell surface protein, in contrast to the other targets that are intracellular, and may be cleaved into a soluble form that promotes tumor angiogenesis (25). Indeed, anti-L1CAM monoclonal antibodies effectuate tumor destruction in human xenograft models (26), raising the possibility that patient responses to L1CAM might contribute to therapeutic activity.
Bacteriophage-based cDNA expression libraries do not preserve all conformational epitopes and do not capture eukaryotic post-translational modifications. Thus, as a second approach to antigen discovery we employed a commercially available protein microarray that displays approximately 9000 full-length human proteins expressed in SF9 insect cells. Post-vaccination serum from Patient 12, which had recognized the largest number of antigens in the K008 library, was applied to the array and specific binding detected with a fluorescently labeled anti-human IgG. The selection of a stringent Z-factor threshold of 0.8 yielded approximately 500 potential antibody targets, which included human IgG proteins that served as a positive control (Supplemental Table 1). Four of the antigens characterized through the cDNA library (RBP-jκ, cytoskeleton associated protein 2, basic leucine zipper and W2 domains 2, ethanolamine kinase) were also identified with the protein microarray, supporting the specificity of the two screening techniques. Nonetheless, potential differences in the particular antigens represented in the array versus the cDNA library as well as variations in post-translational modifications likely underlie the detection of distinct targets with the two approaches.
The gene products revealed through the microarray, like the cDNA expression library, were frequently linked with fundamental pathways of cell biology. However, we focused our analysis on cell surface or secreted proteins directly linked to tumorigenesis, as antibodies might be able to modulate their activities. From this perspective, developmental endothelial locus-1 (Del-1) and tyrosine kinase with immunoglobulin and epidermal growth factor homology domains 2 (Tie-2) emerged of interest. Del-1 is a secreted protein composed of a phosphatidylserine binding domain and an epidermal growth factor (EGF) domain that contains a canonical arginine-glycine-aspartate (RGD) motif (27). Through engagement with the cognate αvβ3 and αvβ5 integrins expressed on endothelial cells, Del-1 mediates potent angiogenic activity (28). Tie-2 is a receptor tyrosine kinase expressed on endothelial cells that cooperates with vascular endothelial growth factor receptor signaling to promote blood vessel formation (29). Overall, the two screening approaches raised the possibility that vaccination stimulated antibodies against angiogenic factors.
Validating angiogenic cytokines as antibody targets
To confirm the results of the screening studies and to develop a system more amenable to analysis of a larger number of patient samples, we established ELISAs with recombinant proteins. Unexpectedly, post-vaccination sera from the AML/MDS patients displayed high background signals in control wells, whereas this was not observed with samples obtained from solid tumor patients undergoing immunotherapy (not shown). Although the basis for this difference is not clear, several modifications of the assay were identified that improved the signal to noise ratio for the AML/MDS samples. These included the use of a protein-free blocking buffer that contained 0.1% Tween, incubation of patient serum with target antigens at 4°C, the use of an anti-human IgG (Fab)2 fragment conjugated to horseradish peroxidase (HRP) for the secondary reagent, and the inclusion of the Tyramide Signal Amplification (Perkin-Elmer, Inc.) agent to enhance sensitivity. Tyramide is a phenolic compound that is converted by HRP into a reactive intermediate that binds electron rich regions in nearby proteins (primarily tyrosines), thereby intensifying the signal. These alterations collectively enabled the use of very small amounts of AML/MDS serum (dilutions of 1:2000), which minimized non-specific background while retaining sensitivity.
Employing the optimized ELISA conditions, we validated the binding of post-vaccination serum from Patient 12 to recombinant L1CAM and Del-1 (Table 2), confirming the results of the expression library and microarray studies respectively (the significance of the numbers of “+” in the table will be discussed below). Unfortunately, recombinant Tie-2 was not readily available in a form suitable for application in our ELISA, limiting more detailed evaluation of this candidate antigen. Nonetheless, because we previously identified angiopoietin-1 and angiopoietin-2, cognate ligands for Tie-2, as targets for high titer antibodies in vaccinated solid tumor patients (19, 30), we wondered whether this pathway might be generally immunogenic in the context of GM-CSF secreting tumor cell vaccines. Consistent with this idea, post-vaccination sera from Patient 12 recognized both of the Tie-2 ligands (Table 2).
Table 2.
Semi-quantitative analysis of antibody response to angiogenic cytokines in vaccinated AML patients early after allogeneic HSCT
| patient | L1 | DEL-1 | Ang1 | Ang2 | HGF | PDGF | VEGF-A | PGRN |
|---|---|---|---|---|---|---|---|---|
| Pt. 1 | ||||||||
| Pt.4 | + | + | ||||||
| Pt. 8 | + | |||||||
| Pt. 9 | +++ | +++ | ++ | +++ | + | + | ||
| Pt. 11 | + | ++ | + | ++ | + | |||
| Pt. 12 | + | +++ | ++ | + | + | |||
| Pt. 14 | + | ++ | + | + | ||||
| Pt. 16 | ||||||||
| Pt. 17 | ||||||||
| Pt. 19 | ||||||||
| Pt. 20 | + | + | + | |||||
| Pt. 21 | ||||||||
| Pt. 22 | +++ | ++ | + | + | ++ | + | +++ | |
| Pt. 26 | ||||||||
| Pt. 27 | ++ | +++ | +++ | + | + | +++ |
two and one-half to five fold increases
five to seven fold increases
eight and greater fold increases
Given the induction of antibodies to five functionally related proteins, we expanded the analysis to include additional proteins involved in angiogenesis. These included: vascular endothelial growth factor A (VEGF-A) and macrophage migration inhibitory factor (MIF), which we earlier described as antibody targets (19); progranulin (31) and platelet derived growth factor-BB (PDGF-BB) (32), which we identified through other screening studies using sera from melanoma patients; and hepatocyte growth factor (HGF), which was recently implicated in leukemogenesis (33). Among these candidates, post-vaccination sera from Patient 12 recognized VEGF-A (Table 2). To determine whether other vaccinated AML/MDS patients developed antibodies to the panel of angiogenic cytokines, we examined sera obtained approximately one month after the last vaccination in the entire cohort of fifteen patients (Table 2). No reactivity to MIF was detected in any patient; the basis for this difference compared to solid tumors remains to be clarified. However, nine patients showed responses to at least one of the antigens, and five patients harbored antibodies to five or more factors.
Longitudinal analysis of antibody development
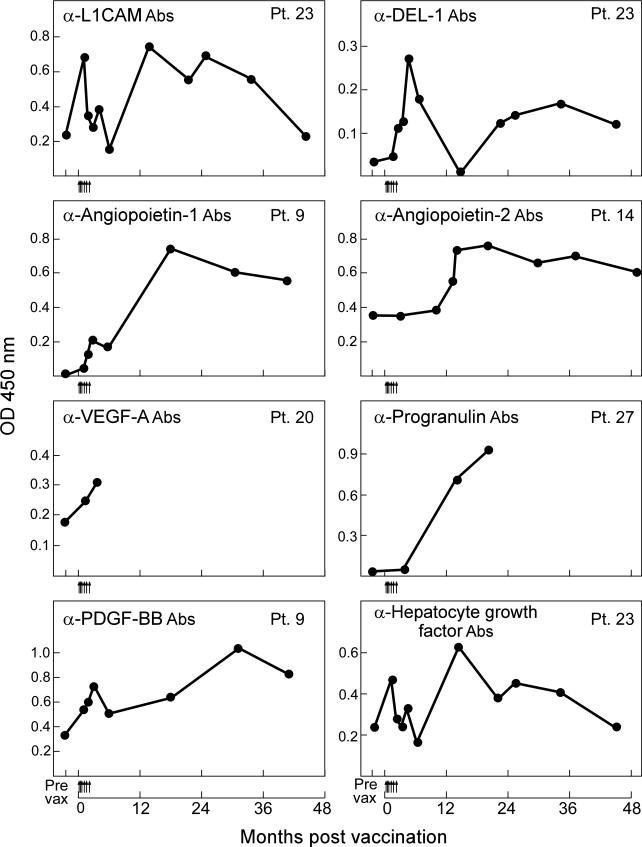
To learn more about the kinetics of antibody development, we performed a time-course analysis for each antigen in all of the immunized patients. Examples of reactivity to individual angiogenic cytokines are presented in Figure 1. Considerable heterogeneity was evident in the patterns of responses. For example, Patient 9 rapidly generated antibodies to angiopoietin-1 and PDGF-BB upon vaccination, whereas these were sustained for several years. In contrast, Patient 27 developed antibodies to progranulin shortly after the completion of vaccination, while Patient 14 mounted antibodies to angiopoietin-2 approximately one year after completing immunization; in both cases these were then sustained. Patient 23 rapidly developed antibodies to L1CAM, Del-1, and HGF upon initiating vaccination, but titers then decreased before a second, more sustained increase. Interestingly, the varying levels of antibodies were temporally associated with changes in clinical status. Patient 23 entered a complete remission with the HSCT that was maintained only briefly during vaccination, when antibodies were first elicited; this was then followed by a relapse, when the antibodies decreased, and then a second remission upon withdrawal of immunosuppression, when the antibody titers increased again. The precise mechanisms that account for the diversity in tempo and persistence of antibodies across the patient cohort remain to be clarified, but the findings in Patient 23 suggest disease burden as one potential factor.
Figure 1. Autologous, GM-CSF secreting leukemia cell vaccines early after allogeneic HSCT stimulate antibodies to angiogenic cytokines.
Representative examples are shown for longitudinal studies of responses to L1CAM, Del-1, angiopoietin-1, angiopoietin-2, VEGF-A, progranulin, PDGF-BB, and hepatocyte growth factor. Sera samples obtained after allogeneic HSCT were diluted 1:2000 and tested for reactivity against recombinant His-tagged angiogenic proteins or a 6xHis control. A horseradish peroxidase conjugated anti-human IgG (Fab)2 was used to detect the patient antibodies, and Tyramide was added to amplify the signal. Absorbance was measured at 450 nm. Arrows along the abscissa denote individual vaccinations. Similar results were observed in at least three independent assays.
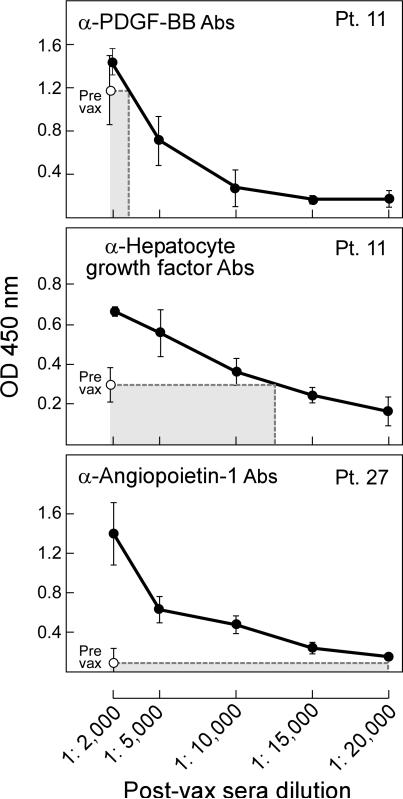
To quantify the magnitude of increase in antibody response, we compared serial dilutions of the samples with highest reactivity for each antigen with baseline levels in the pre-vaccination sera. Representative studies that illustrate differing levels of antibody induction are shown in Figure 2. In Patient 11, the peak antibodies to PDGF-BB display a modest elevation relative to pre-vaccine levels (two to three fold), whereas the antibodies to HGF manifest a greater augmentation (six fold). An even larger increase in antibodies to angiopoietin-1 (ten fold) was evident for Patient 27. Based on these examples, we classified treatment response as “+” for two and one-half to five fold increases, “++” for five to seven fold increases, and “+++” for eight and greater fold increases. Table 2 summarizes the results of the semi-quantitative analysis for all antigens and patients. Patients that mounted antibody responses to multiple angiogenic cytokines also generated the strongest increases in antibody titers, often against several targets. In contrast, patients that generated antibodies to only a few or one of the antigens showed only modest increases in antibody titers. Together, these results reveal heterogeneity in the breadth and intensity of response to angiogenic cytokines.
Figure 2. Vaccination early after allogeneic HSCT increases antibodies to angiogenic cytokines.
Representative examples are shown for comparisons of peak post-vaccination responses with pre-vaccination levels, illustrating from top to bottom modest (“+”), moderate (“++”), or strong (“+++”) responses. Examples of reactivity to PDGF, hepatocyte growth factor, and angiopoietin-1 are presented for patients 11 and 27. See Table 2 for a complete analysis of antibody induction for each angiogenic cytokine and patient. Post-vaccination sera samples showing the highest reactivity to an individual cytokine were serially diluted and compared with pre-vaccination sera samples. A horseradish peroxidase conjugated anti-human IgG (Fab)2 was used to detect the patient antibodies, and Tyramide was added to amplify the signal. Absorbance was measured at 450 nm. Similar results were observed in at least three independent assays.
Clinical associations of early antibody responses
To understand the potential clinical associations of the humoral reactions, we assessed the development of antibodies to individual antigens as a function of clinical course. For this analysis, we considered the time to antibody response as the first sera sample after the initiation of vaccination in which antibody titers had increased at least two and one-half fold above pre-treatment levels. Although this time-point may not reflect the peak titer, we were interested in determining the point at which immune responsiveness first became evident.
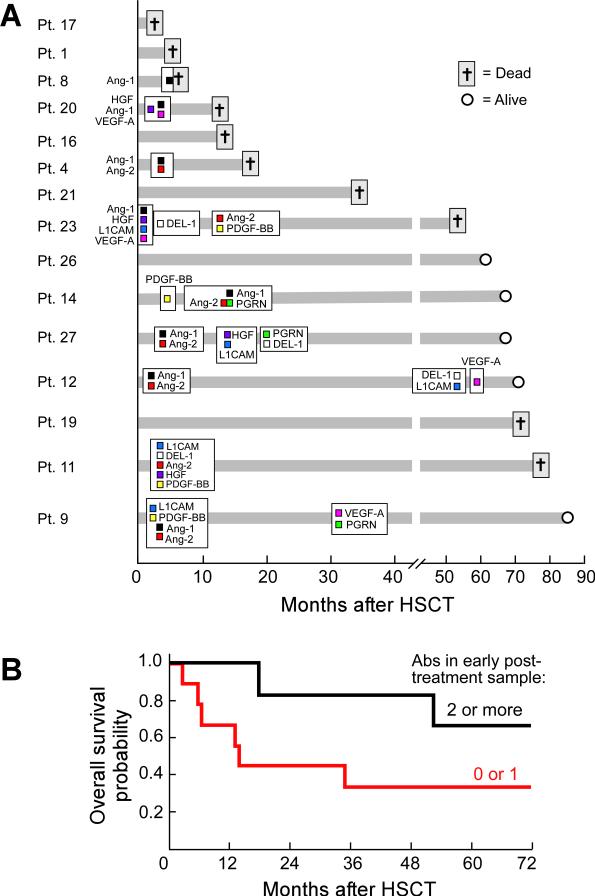
Figure 3A displays the longitudinal courses for the fifteen vaccinated patients. Of the six patients who succumbed to disease within the first two years after HSCT, only three mounted antibodies to at least one antigen, and these were only of modest intensity (Table 2). In contrast, all six of the patients who developed antibodies to multiple cytokines, which were often of high intensity, achieved prolonged survival. Nonetheless, not all of the long-term survivors showed reactivity to these gene products, suggesting that additional important targets may remain to be discovered. For those patients who developed multiple antibodies, a diversification of reactivity was evident over time, but generally occurred within two years of HSCT and vaccination. However, two patients displayed much later responses that were associated with significant clinical events. Patient 12 mounted antibodies to L1CAM, Del-1, and VEGF-A approximately five years post-HSCT, when a Human Papilloma Virus (HPV) associated cutaneous squamous cell carcinoma was noted (and effectively treated with excision). Patient 9 generated antibodies to VEGF-A and progranulin approximately two and one-half years after HSCT, and these were correlated with a mild exacerbation of chronic GVHD in the oral mucosa (treated with local measures only). Nonetheless, there was no clear association between antibody response and chronic GVHD across the entire vaccinated cohort. Patient 27 mounted antibodies in the absence of chronic GVHD, whereas Patients 9, 11, 12, 14, and 23 generated antibodies to multiple targets prior to chronic GVHD. Patients 19, 20, and 26 developed chronic GVHD but no antibody response.
Figure 3.
The timing and breadth of antibody response to vaccination early after allogeneic HSCT may be associated with clinical outcome. (A) Longitudinal analysis of initial antibody response to angiogenic cytokines as a function of clinical course. The time of first antibody development is shown as months after allogeneic HSCT. Survival at most recent follow-up is indicated. Ang-1, angiopoietin-1; HGF, hepatocyte growth factor; VEGF-A, vascular endothelial growth factor-A; Ang-2, angiopoietin-2; L1CAM, L1 cell adhesion molecule; DEL-1, developmental endothelial locus-1; PDGF-BB, platelet derived growth factor-BB; PGRN, progranulin. (B) Likelihood of patient survival depending on numbers of antibodies detected in first post-vaccination sample. N=9 for two or more antibodies, n=6 for zero or one antibody. 5-year overall survival: 67% vs. 33%, respectively, p=0.13.
Because the early development of antibodies appeared to be linked with a subsequent diversification of reactivity, we wondered whether the initial antibody response might also be associated with clinical outcome. To address this issue, we analyzed the presence of antibodies in the first post-vaccination sample available, which ranged from 1.1 to 3.9 months after allogeneic-HSCT. The median follow-up time among survivors in the study is currently 69 months (range 62 to 85). As shown in Figure 3B, patients with antibodies to two or more angiogenic cytokines at an early time point showed improved survival compared to subjects with zero or one antibody. Although the magnitude of the difference appears substantive, it does not reach statistical significance due to the limited size of the patient cohort (5-year overall survival: 67% vs. 33%, respectively, p=0.13). This potential association will be tested more rigorously through a recently initiated randomized trial comparing vaccination to placebo in a larger but similar patient population.
Discussion
These experiments were undertaken in an effort to learn more about the immune mechanisms involved in GVL reactions associated with irradiated, autologous GM-CSF secreting myeloblast vaccinations early after RIC allogeneic HSCT. Our prior studies had demonstrated that immunization elicited local dendritic cell infiltrates and systemic NK and T cell responses (12), but potential roles for antibodies and B cells had not been explored. In the non-transplant setting, GM-CSF secreting tumor cell vaccines engender a coordinated humoral and cellular response that effectuates tumor destruction. Similarly, intra-tumoral infiltrates composed of B and T follicular helper cells in association with CD8+ T lymphocytes are closely linked with favorable clinical outcomes in colorectal carcinoma patients (34). Here we reveal a diverse and potent humoral response during the GVL reaction triggered with autologous leukemia cell vaccines early after allogeneic HSCT.
The development of antibodies was evident within a few months after HSCT, when calcineurin inhibitors were still being applied as GVHD prophylaxis. This unexpected finding may reflect the favorable immunologic milieu created during the initial phases of immune reconstitution (6-10). The ability to generate an early antibody response may also characterize patients who are more likely to achieve a diversified reaction over time, yielding higher levels of anti-leukemic immunity. Indeed, the delineation of defined antigens linked with potent GVL reactions is an important goal. The application of high-throughput genomic technologies has accelerated the identification of mutated, tumor-specific antigens recognized by high affinity cytotoxic T cells that have escaped central deletion in the thymus (35). However, the key attributes of the targets for humoral reactions remain to be clarified (5). Following cell death, antibodies specific for intracellular antigens might form immune complexes that can be opsonized by dendritic cells for cross-presentation, thereby eliciting CD4+ and CD8+ T cell responses. Additionally, antibodies directed against tumor cell surface or secreted proteins may promote Fc receptor dependent cytotoxicity or blockade of major oncogenic functions respectively.
The selection of a melanoma cDNA expression library and protein microarray for antigen discovery biased our search towards gene products that might be shared among tumor types. The utility of this strategy was suggested through our earlier report that one of the AML patients immunized early after allogeneic HSCT generated antibodies to protein disulfide isomerase, a vaccine target originally uncovered in a renal cell carcinoma system (36). In accordance with this finding, the screening efforts with the AML/MDS sera yielded a large number of proteins involved in fundamental aspects of cell biology. Most targets were intracellular in location, but L1CAM, Del-1, and Tie-2 were of particular interest due to their surface expression/secretion profiles and functional inter-relatedness.
Angiogenesis is considered a hallmark of cancer in solid malignancies (37), but its role in leukemogenesis is less well established (38). Histopathologic examination of AML and MDS patient bone marrows reveals increased microvessel density (39, 40) while non-invasive imaging suggests that the degree of vascularity might have prognostic importance (41). Furthermore, the circulating levels of several angiogenic factors are frequently elevated in leukemic patients (38). A cross-talk of bone marrow endothelial cells and stem cells is increasingly being recognized to regulate normal and malignant hematopoiesis (42). In this regard, endothelial cells promote the survival of leukemic progenitors through contact dependent and soluble factors, whereas leukemic cells provide angiogenic proteins that support endothelial cell growth/remodeling and new blood vessel formation (43).
Given the potential contribution of angiogenesis to AML/MDS pathogenesis, we built upon the results of the screening studies to expand the interrogation of antibody reactions to a larger panel of angiogenic cytokines. Although responses to Tie-2 could not be analyzed in detail, the cognate ligands angiopoietin-1 and angiopoietin-2 evoked reactivity in some patients, consistent with their immunogenicity in vaccinated solid tumor patients (19). Underscoring the common targeting of angiogenesis with GM-CSF secreting vaccines, we also detected antibodies to VEGF-A, progranulin, and PDGF-BB, which had been identified through other screens performed in immunized melanoma patients. Moreover, HGF, an angiogenic growth factor that also contributes to AML through cell autonomous effects on leukemic blasts (33), proved to be another antibody target. The induction of humoral reactions to as many as seven angiogenic cytokines in long-term responding AML/MDS patients emphasizes the potential importance of this oncogenic pathway for GVL responses. Indeed, it is tempting to speculate that high levels of angiogenic proteins, which may derive from either autologous myeloblasts or bone marrow stromal elements retained within the vaccine inoculum, might be rendered immunogenic through GM-CSF stimulated dendritic cell antigen presentation combined with allo-reactivity. While the long-term responding patients also generated anti-leukemic T cells, as evidenced in delayed-type hypersensitivity reactions to irradiated, autologous leukemia cells and T cell infiltrates in bone marrow (12), further studies are required to determine whether these T cells recognize peptides derived from the angiogenic cytokines.
Therapeutic strategies that target individual angiogenic factors have shown only modest activity against solid tumors thus far, and clinical trials of anti-VEGF-A monoclonal antibodies or small molecule VEGFR inhibitors in AML have similarly revealed limited anti-tumor effects to date (44, 45). Combinatorial approaches might be more effective, however, and experiments in murine models suggest that integrating a vascular disrupting agent with VEGF-A blockade accomplishes impressive therapeutic effects against human leukemia xenografts (46). In this context, humoral responses against multiple angiogenic cytokines might broadly interfere with angiogenesis and potentially block the immunosuppressive activities of these factors (47), thereby intensifying GVL effects.
Overall, our investigations have revealed a broad and potent antibody response during GVL reactions induced with leukemia cell vaccinations early after allogeneic HSCT. Six of nine long-term surviving patients developed antibodies to multiple angiogenic cytokines, highlighting the importance of angiogenesis in leukemia, transplantation, and tumor immunity. Our results extend prior work characterizing humoral reactions after allogeneic HSCT and donor lymphocyte infusion (2, 48-50), and collectively these experiments delineate important roles for antibodies and B cells in GVL and GVHD. Lastly, we have recently initiated a randomized Phase II trial comparing this autologous tumor cell vaccination strategy with placebo in a similar MDS/AML patient population; this work should provide additional insights into the precise contributions of vaccination and RIC allogeneic HSCT for antibody induction and leukemia control.
Supplementary Material
Statement of translational relevance.
The identification of antigens associated with immune-mediated tumor destruction contributes to an improved understanding of the mechanisms underlying protective tumor immunity. Towards this end, we performed a detailed analysis of the targets of high titer antibodies during graft-versus-leukemia reactions in patients with advanced myelodysplasia or acute myeloid leukemia who received an autologous, granulocyte-macrophage colony stimulating factor secreting tumor cell vaccine early after allogeneic hematopoietic stem cell transplantation. Our work revealed the evolution of a potent humoral reaction that unexpectedly included antibodies against multiple angiogenic cytokines. The antibodies were detectable early after immunotherapy and manifested a diversification of reactivity over time in patients who achieved prolonged survival. These results highlight a key role for antibodies during graft-versus-leukemia reactions and raise the possibility that angiogenic cytokines might be effectively targeted with immunotherapy.
Acknowledgements
We thank the staff of the Dana-Farber Cancer Institute Cell Manipulation Core Facility for help with the patient samples, Dr. Gayane Badalian-Very for fruitful discussions on ELISA techniques, and Luis Peña and Youjin Lee for help with the assays.
Grant Support
This work was supported with NCI R01CA143083, P01CA78378, the Leukemia & Lymphoma Society, Stand Up to Cancer-Cancer Research Institute Cancer Immunology Translational Cancer Research Grant, and the Research Foundation for the Treatment of Ovarian Cancer. Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
Footnotes
Authors’ Contributions
Conception and design: M. Piesche and G. Dranoff
Development of methodology: M. Piesche, Y. Nakazaki, M. Nehil, N. Yaghi, D. Kolodin, J. Weiser, P. Altevogt, H. Kiefel, G. Dranoff
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): V.T. Ho, E.P. Alyea, J.H. Antin, C. Cutler, J. Koreth, C. Canning, J. Ritz, R.J. Soiffer
Analysis and interpretation of data (statistical analysis, biostatistics, computational analysis): H. Kim
Writing, revision, and/or review of the manuscript: M. Piesche, V.T. Ho, H. Kim, Y. Nakazaki, M. Nehil, N. Yaghi, D. Kolodin, J. Weiser, P. Altevogt, H. Kiefel, E.P. Alyea, J.H. Antin, C. Cutler, J. Koreth, C. Canning, J. Ritz, R.J. Soiffer, and G. Dranoff
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases: H. Kim, J. Ritz
Study supervision: G. Dranoff
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest to disclose.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Ofran Y, Ritz J. Targets of tumor immunity after allogeneic hematopoietic stem cell transplantation. Clin Cancer Res. 2008;14:4997–9. doi: 10.1158/1078-0432.CCR-08-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu CJ, Ritz J. Induction of tumor immunity following allogeneic stem cell transplantation. Adv Immunol. 2006;90:133–73. doi: 10.1016/S0065-2776(06)90004-2. [DOI] [PubMed] [Google Scholar]
- 4.Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–8. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rapoport AP, Stadtmauer EA, Aqui N, Badros A, Cotte J, Chrisley L, et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat Med. 2005;11:1230–7. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 7.Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–12. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alpdogan O, Eng JM, Muriglan SJ, Willis LM, Hubbard VM, Tjoe KH, et al. Interleukin-15 enhances immune reconstitution after allogeneic bone marrow transplantation. Blood. 2005;105:865–73. doi: 10.1182/blood-2003-09-3344. [DOI] [PubMed] [Google Scholar]
- 10.Mirmonsef P, Tan G, Zhou G, Morino T, Noonan K, Borrello I, et al. Escape from suppression: tumor-specific effector cells outcompete regulatory T cells following stem-cell transplantation. Blood. 2008;111:2112–21. doi: 10.1182/blood-2007-06-096586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teshima T, Mach N, Hill GR, Pan L, Gillessen S, Dranoff G, et al. Tumor cell vaccine elicits potent antitumor immunity after allogeneic T- cell-depleted bone marrow transplantation. Cancer Res. 2001;61:162–71. [PubMed] [Google Scholar]
- 12.Ho VT, Vanneman M, Kim H, Sasada T, Kang YJ, Pasek M, et al. Biologic activity of irradiated, autologous, GM-CSF-secreting leukemia cell vaccines early after allogeneic stem cell transplantation. Proc Natl Acad Sci U S A. 2009;106:15825–30. doi: 10.1073/pnas.0908358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodi FS, Dranoff G. Combinatorial cancer immunotherapy. Adv Immunol. 2006;90:337–60. doi: 10.1016/S0065-2776(06)90009-1. [DOI] [PubMed] [Google Scholar]
- 14.Hodi FS, Schmollinger JC, Soiffer RJ, Salgia R, Lynch T, Ritz J, et al. ATP6S1 elicits potent humoral responses associated with immune mediated tumor destruction. Proc Natl Acad Sci USA. 2002;99:6919–24. doi: 10.1073/pnas.102025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mollick JA, Hodi FS, Soiffer RJ, Nadler LM, Dranoff G. MUC1-like tandem repeat proteins are broadly immunogenic in cancer patients. Cancer Immunity. 2003;3:3–20. [PubMed] [Google Scholar]
- 16.Schmollinger JC, Vonderheide RH, Hoar KM, Maecker B, Schultze JL, Hodi FS, et al. Melanoma inhibitor of apoptosis protein (ML-IAP) is a target for immune-mediated tumor destruction. Proc Natl Acad Sci USA. 2003;100:3398–403. doi: 10.1073/pnas.0530311100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jinushi M, Hodi FS, Dranoff G. Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proc Natl Acad Sci U S A. 2006;103:9190–5. doi: 10.1073/pnas.0603503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sittler T, Zhou J, Park J, Yuen NK, Sarantopoulos S, Mollick J, et al. Concerted potent humoral immune responses to autoantigens are associated with tumor destruction and favorable clinical outcomes without autoimmunity. Clin Cancer Res. 2008;14:3896–905. doi: 10.1158/1078-0432.CCR-07-4782. [DOI] [PubMed] [Google Scholar]
- 19.Schoenfeld J, Jinushi M, Nakazaki Y, Wiener D, Park J, Soiffer R, et al. Active immunotherapy induces antibody responses that target tumor angiogenesis. Cancer Res. 2010;70:10150–60. doi: 10.1158/0008-5472.CAN-10-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagao H, Setoguchi T, Kitamoto S, Ishidou Y, Nagano S, Yokouchi M, et al. RBPJ is a novel target for rhabdomyosarcoma therapy. PLoS One. 2012;7:e39268. doi: 10.1371/journal.pone.0039268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polakis P. Wnt signaling in cancer. Cold Spring Harbor perspectives in biology. 2012:4. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takayama K, Horie-Inoue K, Suzuki T, Urano T, Ikeda K, Fujimura T, et al. TACC2 is an androgen-responsive cell cycle regulator promoting androgen-mediated and castration-resistant growth of prostate cancer. Mol Endocrinol. 2012;26:748–61. doi: 10.1210/me.2011-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riffell JL, Lord CJ, Ashworth A. Tankyrase-targeted therapeutics: expanding opportunities in the PARP family. Nature reviews Drug discovery. 2012;11:923–36. doi: 10.1038/nrd3868. [DOI] [PubMed] [Google Scholar]
- 24.Raveh S, Gavert N, Ben-Ze'ev A. L1 cell adhesion molecule (L1CAM) in invasive tumors. Cancer Lett. 2009;282:137–45. doi: 10.1016/j.canlet.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Friedli A, Fischer E, Novak-Hofer I, Cohrs S, Ballmer-Hofer K, Schubiger PA, et al. The soluble form of the cancer-associated L1 cell adhesion molecule is a pro-angiogenic factor. The international journal of biochemistry & cell biology. 2009;41:1572–80. doi: 10.1016/j.biocel.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Arlt MJ, Novak-Hofer I, Gast D, Gschwend V, Moldenhauer G, Grunberg J, et al. Efficient inhibition of intra-peritoneal tumor growth and dissemination of human ovarian carcinoma cells in nude mice by anti-L1-cell adhesion molecule monoclonal antibody treatment. Cancer Res. 2006;66:936–43. doi: 10.1158/0008-5472.CAN-05-1818. [DOI] [PubMed] [Google Scholar]
- 27.Hanayama R, Tanaka M, Miwa K, Nagata S. Expression of developmental endothelial locus-1 in a subset of macrophages for engulfment of apoptotic cells. J Immunol. 2004;172:3876–82. doi: 10.4049/jimmunol.172.6.3876. [DOI] [PubMed] [Google Scholar]
- 28.Aoka Y, Johnson FL, Penta K, Hirata Ki K, Hidai C, Schatzman R, et al. The embryonic angiogenic factor Del1 accelerates tumor growth by enhancing vascular formation. Microvascular research. 2002;64:148–61. doi: 10.1006/mvre.2002.2414. [DOI] [PubMed] [Google Scholar]
- 29.Martin V, Liu D, Fueyo J, Gomez-Manzano C. Tie2: a journey from normal angiogenesis to cancer and beyond. Histol Histopathol. 2008;23:773–80. doi: 10.14670/HH-23.773. [DOI] [PubMed] [Google Scholar]
- 30.Schoenfeld JD, Dranoff G. Anti-angiogenesis immunotherapy. Human vaccines. 2011;7:976–81. doi: 10.4161/hv.7.9.16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toh H, Cao M, Daniels E, Bateman A. Expression of the growth factor progranulin in endothelial cells influences growth and development of blood vessels: a novel mouse model. PLoS One. 2013;8:e64989. doi: 10.1371/journal.pone.0064989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. The International journal of developmental biology. 2011;55:261–8. doi: 10.1387/ijdb.103167dr. [DOI] [PubMed] [Google Scholar]
- 33.Kentsis A, Reed C, Rice KL, Sanda T, Rodig SJ, Tholouli E, et al. Autocrine activation of the MET receptor tyrosine kinase in acute myeloid leukemia. Nat Med. 2012;18:1118–22. doi: 10.1038/nm.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–95. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Hacohen N, Fritsch EF, Carter TA, Lander ES, Wu CJ. Getting personal with neoantigen-based therapeutic cancer vaccines. Cancer Immunol Res. 2013;1:11–5. doi: 10.1158/2326-6066.CIR-13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fonseca C, Soiffer R, Ho V, Vanneman M, Jinushi M, Ritz J, et al. Protein disulfide isomerases are antibody targets during immune-mediated tumor destruction. Blood. 2009;113:1681–8. doi: 10.1182/blood-2007-09-114157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carmeliet P, Jain R. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 38.Reikvam H, Hatfield KJ, Fredly H, Nepstad I, Mosevoll KA, Bruserud O. The angioregulatory cytokine network in human acute myeloid leukemia - from leukemogenesis via remission induction to stem cell transplantation. European cytokine network. 2012;23:140–53. doi: 10.1684/ecn.2012.0322. [DOI] [PubMed] [Google Scholar]
- 39.Padro T, Ruiz S, Bieker R, Burger H, Steins M, Kienast J, et al. Increased angiogenesis in the bone marrow of patients with acute myeloid leukemia. Blood. 2000;95:2637–44. [PubMed] [Google Scholar]
- 40.Hussong JW, Rodgers GM, Shami PJ. Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood. 2000;95:309–13. [PubMed] [Google Scholar]
- 41.Shih TT, Hou HA, Liu CY, Chen BB, Tang JL, Chen HY, et al. Bone marrow angiogenesis magnetic resonance imaging in patients with acute myeloid leukemia: peak enhancement ratio is an independent predictor for overall survival. Blood. 2009;113:3161–7. doi: 10.1182/blood-2008-08-173104. [DOI] [PubMed] [Google Scholar]
- 42.Trujillo A, McGee C, Cogle CR. Angiogenesis in acute myeloid leukemia and opportunities for novel therapies. Journal of oncology. 2012;2012:128608. doi: 10.1155/2012/128608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reikvam H, Hatfield KJ, Lassalle P, Kittang AO, Ersvaer E, Bruserud O. Targeting the angiopoietin (Ang)/Tie-2 pathway in the crosstalk between acute myeloid leukaemia and endothelial cells: studies of Tie-2 blocking antibodies, exogenous Ang-2 and inhibition of constitutive agonistic Ang-1 release. Expert opinion on investigational drugs. 2010;19:169–83. doi: 10.1517/13543780903485659. [DOI] [PubMed] [Google Scholar]
- 44.Karp JE, Gojo I, Pili R, Gocke CD, Greer J, Guo C, et al. Targeting vascular endothelial growth factor for relapsed and refractory adult acute myelogenous leukemias: therapy with sequential 1-beta-d-arabinofuranosylcytosine, mitoxantrone, and bevacizumab. Clin Cancer Res. 2004;10:3577–85. doi: 10.1158/1078-0432.CCR-03-0627. [DOI] [PubMed] [Google Scholar]
- 45.Fiedler W, Serve H, Dohner H, Schwittay M, Ottmann OG, O'Farrell AM, et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105:986–93. doi: 10.1182/blood-2004-05-1846. [DOI] [PubMed] [Google Scholar]
- 46.Madlambayan GJ, Meacham AM, Hosaka K, Mir S, Jorgensen M, Scott EW, et al. Leukemia regression by vascular disruption and antiangiogenic therapy. Blood. 2010;116:1539–47. doi: 10.1182/blood-2009-06-230474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive Strategies that are Mediated by Tumor Cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu C, Yang X-F, McLaughlin S, Neuberg D, Canning C, Stein B, et al. Detection of a potent humoral response associated with immune-induced remission of chronic myelogenous leukemia. J Clin Invest. 2000;106:705–14. doi: 10.1172/JCI10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bellucci R, Wu CJ, Chiaretti S, Weller E, Davies FE, Alyea EP, et al. Complete response to donor lymphocyte infusion in multiple myeloma is associated with antibody responses to highly expressed antigens. Blood. 2004;103:656–63. doi: 10.1182/blood-2003-07-2559. [DOI] [PubMed] [Google Scholar]
- 50.Sarantopoulos S, Stevenson KE, Kim HT, Washel WS, Bhuiya NS, Cutler CS, et al. Recovery of B-cell homeostasis after rituximab in chronic graft-versus-host disease. Blood. 2011;117:2275–83. doi: 10.1182/blood-2010-10-307819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.