Abstract
Background
Binge alcohol drinking is a particularly risky pattern of alcohol consumption that often precedes alcohol dependence and addiction. The transition from binge alcohol drinking to alcohol addiction likely involves mechanisms of synaptic plasticity and learning in the brain. The mitogen-activated protein kinase (MAPK) signaling cascades have been shown to be involved in learning and memory, as well as the response to drugs of abuse, but their role in binge alcohol drinking remains unclear. The present experiments were designed to determine the effects of acute alcohol on extracellular signaling related kinases (ERK1/2) expression and activity, and to determine whether ERK1/2 activity functionally regulates binge-like alcohol drinking.
Methods
Adult male C57BL/6J mice were injected with ethanol (3.0 mg/kg, IP) 10, 30 or 90 minutes prior to brain tissue collection. Next, mice that were brought to freely consume unsweetened ethanol in a binge-like access procedure were pretreated with the MEK1/2 inhibitor SL327 or the p38 MAP kinase inhibitor SB239063.
Results
Acute ethanol increased pERK1/2 immunoreactivity relative to vehicle in brain regions known to be involved in drug reward and addiction, including the central amygdala and prefrontal cortex. However, ethanol decreased pERK1/2 immunoreactivity relative to vehicle in the nucleus accumbens core. SB239063 pretreatment significantly decreased ethanol consumption only at doses that also produced nonspecific locomotor effects. SL327 pretreatment significantly increased ethanol, but not sucrose, consumption without inducing generalized locomotor effects.
Conclusions
These findings indicate that ERK1/2MAPK signaling regulates binge-like alcohol drinking. Since alcohol increased pERK1/2 immunoreactivity relative to vehicle in brain regions known to regulate drug self-administration, SL327 may have blocked this direct pharmacological effect of alcohol and thereby inhibited the termination of binge-like drinking.
Keywords: binge drinking, ERK1/2, MAP kinase, amygdala, nucleus accumbens, alcohol
INTRODUCTION
Binge drinking is a particularly dangerous pattern of alcohol consumption that is strongly linked to increased risk of lifetime alcohol dependence (Courtney and Polich, 2009), with approximately 92% of excessive drinkers in the United States engaging binge drinking (Town et al., 2006). The National Institute of Alcohol Abuse and Alcoholism has defined a binge drinking episode as a level of alcohol consumption that brings blood alcohol concentration above 0.08 gram percent (NIAAA, 2004), which corresponds to the consumption of five drinks in two hours for males and four drinks in two hours for females. Successful development of rodent models of binge-like drinking (Rhodes et al., 2005, Thiele et al., 2014) has facilitated investigation of the determinants of this behavior; however, the neurobiological mechanisms remain to be fully characterized.
The transition from binge drinking to alcohol dependence occurs through gradual changes in brain structure and function over time, and likely involves alterations in synaptic plasticity (Kauer and Malenka, 2007). The identification of molecular targets of alcohol that play a role in synaptic plasticity is therefore of use to elucidate the ways in which binge alcohol exposure may modify brain function, and may also suggest new pharmacotherapies for alcoholism. The mitogen-activated protein kinase (MAPK) family of intracellular signaling molecules has been identified as a critical regulator of synaptic plasticity and learning in the brain (Thomas and Huganir, 2004). Three signaling cascades comprise the MAPK family: c-Jun N-terminal (JNK), p38 protein kinases, and extracellular signal-regulated kinases (ERK1/2) (Boulton and Cobb, 1991, Robinson and Cobb, 1997, Krishna and Narang, 2008). All three MAPKs translocate to the nucleus upon phosphorylation, where they alter gene transcription (Chen et al., 1992, Roux and Blenis, 2004).
Of the three molecules, ERK1/2 has a well-established role in regulation of behavior, including fear learning (Maldonado et al., 2014), memory retention (Languille et al., 2009) and parental behaviors (Kuroda et al., 2007). ERK1/2 is expressed in brain regions that have been shown to be functionally involved in drug self-administration and dependence, including the prefrontal cortex, nucleus accumbens, amygdala and bed nucleus of the stria terminalis (Ortiz et al., 1995, Valjent et al., 2004). A wealth of literature has suggested that many drugs of abuse increase the active (phosphorylated) form of ERK1/2 in these brain regions, including cocaine (Lu et al., 2006), nicotine (Brunzell et al., 2003), morphine (Ortiz et al., 1995, Haghparast et al., 2014), d-amphetamine (Choe et al., 2002), and Δ9-tetrahydrocannabinol (Valjent et al., 2001).
Emerging evidence indicates that ERK1/2 signaling is involved in the biochemical and behavioral effects of alcohol. Several studies have shown that alcohol, or alcohol-paired cues, alter pERK1/2 in rodent brain tissue and cell culture (Bachtell et al., 2002, Schroeder et al., 2008, Pandey et al., 2008, Ibba et al., 2009, Ku et al., 2007, Hendrickson et al., 1998, Sanna et al., 2002, Tsuji et al., 2003, Chandler and Sutton, 2005, Kalluri and Ticku, 2002). Work in our lab has revealed that inhibition of ERK1/2 activation increases operant alcohol self-administration suggesting that ERK1/2 activity may underlie the positive reinforcing effects of alcohol (Faccidomo et al., 2009). However, the role of ERK1/2 signaling in binge alcohol drinking has not been investigated.
To address this question, we first determined the effects of acute alcohol injection on ERK1/2 expression and phosphorylation in the mouse brain using an immunohistochemical approach. By utilizing experimenter-administered ethanol, we were able to eliminate variability in ethanol dose. Use of immunohistochemistry allowed the brain response to alcohol to be resolved at the level of discrete brain regions and sub-nuclei. We also sought to investigate the role of ERK1/2 activation in binge-like alcohol drinking. The systemically active MEK1/2 inhibitor SL327 was used to inhibit downstream ERK1/2 activation. In addition, the p38 inhibitor SB239063 was also tested in order to determine the selectivity of ERK1/2 MAPK signaling in alcohol drinking behavior.
MATERIALS AND METHODS
Animals
Adult male C57BL/6J mice (Jackson Laboratories, Bar Harbor, Maine) were housed either four to a cage (immunohistochemistry experiments) or individually housed (behavioral experiments) in standard Plexiglass cages with a small PVC pipe for environmental enrichment. Food and water were available ad libitum in the home-cage except where noted. The colony room was maintained on a 12-h light/dark cycle at 21°C, and all testing occurred during the dark cycle. All procedures were carried out in accordance with the NIH Guide to Care and Use of Laboratory Animals (National Research Council, 1996) and approved by the Internal Review Board of the University of North Carolina, Chapel Hill.
Drugs
Ethanol for injection (20% v/v) was prepared by diluting 95% ethanol in 0.9% saline. All ethanol drinking solutions (v/v) were prepared by diluting 95% ethanol (Pharmco Products Inc., Brookfield, CT, USA) with tap water. The MEK1/2 inhibitor SL327 and the p38 inhibitor SB239063 (Tocris Bioscience; Ellisville, MO, USA) were freshly suspended in 45% β-cyclodextrin on the morning of each drug testing day.
Acute ethanol time course
Mice were weighed, and habituated to handling and intraperitoneal injection (saline, 0.1ml/10g), for three days prior to the start of the experiment. On the day of the experiment, mice received ethanol (0 or 3 g/kg, IP) and after 10, 30, or 90 minutes were sacrificed and brains were rapidly removed and prepared for immunohistochemistry as described below. The dosage of ethanol (3 g/kg, IP) is sub-hypnotic and was chosen to be within the range of doses (approximately half-maximal) consumed by C57BL/6J mice in the drinking studies reported here.
Tissue Preparation and Immunohistochemical Analysis
After ethanol injection, mice were anesthetized with pentobarbital and transcardially perfused with ice cold phosphate buffered saline (100mM) followed by 4% formaldehyde (pH 7.4). Brains were removed and post fixed for 48 hours at 4°C, rinsed with phosphate buffer. The fixed brains were stored in phosphate buffer at 4°C until sectioning. Coronal brain sections (40μm) were cut on a vibratome (VT1000S, Leica Microsystems, Bannockburn, IL) and stored in cryoprotectant at -20°C until processing for immunohistochemistry.
Sections were washed in PBS prior to free-floating immunohistochemical processing. Following pre-blocking, sections were agitated with either pERK1/2 (phospho-p44/42 MAPK, Thr202/Tyr204) or total ERK1/2 (tERK1/2; p44/42 MAPK) antibody (Cell Signaling Technologies, Danvers, MA), each at a dilution of 1:400. Sections were washed and visualized with DAKO labeled polymer-HRP anti-rabbit secondary antibody (full strength) followed by DAKO DAB+ chromagen (DAKOCytomation, Carpinteria, CA). Sections were counterstained with toluidine blue and coverslipped with Cytoseal. Sections were viewed at 20-40X magnification under brightfield illumination (Olympus CX41 light microscope, Olympus America, Center Valley, PA). Images were acquired using a digital camera (Regita OEM fast, QImaging, Burnaby, BC) and immunoreactivity was quantified using Bioquant Life Science software (R&M Biometric, Nashville, TN). Cell counts and pixel measurements were divided by the area of the region and expressed as cell counts/mm2 or pixels/mm2.
Anatomical Coordinates
Anatomical coordinates were taken from the Mouse Brain in Stereotaxic coordinates (Paxinos and Watson, 2001). Analyses of the nucleus accumbens and proximal brain regions were evaluated in coronal sections ranging from 0.86 to 1.34 mm anterior to Bregma. The amygdala and proximal brain regions were evaluated in coronal section ranging from -1.58 to -2.18 mm posterior to Bregma.
Initiation of Binge Drinking
All mice were allowed seven days to acclimate to our colony facility before experimental manipulations began. During this time, mice received vehicle (45% w/v β-cyclodextrin, i.p.) habituation injections. Beginning on day 7, home-cage water bottles were removed and replaced with a drinking tube (10mL with double-ball bearing affixed to the wire cage lid with a medium binder clip) containing 20% (v/v) unsweetened ethanol (ethanol experiments) or 1% sucrose (SL327 control experiments). In a variation of the drinking-in-the-dark procedure (Rhodes et al., 2005), mice had access to the solution for two hours. The procedure was repeated on day eight, and on day nine the ethanol access period was extended to a four-hour session. Ethanol volumes were recorded immediately after ethanol tubes were placed on the cage and at one, two and four hour time points. On the tenth day, the animals did not have access to ethanol, and a pattern of every-other-day four-hour binge ethanol access was maintained for the remainder of the experiments.
Drug Testing
Beginning on experimental day 17, animals were weighed and injected i.p. with either SB239063 (0, 1, 10, 30 mg/kg) or SL327 (0, 10, 30 mg/kg) 30 minutes prior to the drinking session. Animals in each experiment were dosed in a Latin-Square design with four days between each injection, such that one ethanol drinking day without drug pretreatment occurred between each drug testing day. Ethanol consumption was measured at one, two and four-hour intervals. In the sucrose control experiments, mice were pretreated with the lowest effective dose of SL327 (10 mg/kg).
Locomotor Testing
Mice were pretreated with either vehicle, 30 mg/kg SB239063 or 30 mg/kg SL327 30 minutes prior to a one-hour (SB139063) or four-hour (SL327) locomotor activity test (n=6/dose). Open field activity was measured in Plexiglas activity monitor chambers (27.9 cm2; ENV-510, Med Associates) with two sets of 16 pulse-modulated infrared photobeams located on opposite walls to record X–Y ambulatory movements. Distance traveled (in meters) throughout the session was quantified by assessing the mouse’s position in the open field every 100 miliseconds. Data from each chamber were collected by a computer.
Blood Alcohol Concentration
Blood alcohol levels were determined as previously described (Faccidomo et al., 2009). Mice were pretreated with vehicle, 30 mg/kg SB239063 or 30 mg/kg SL327 30 minutes prior to a 2 g/kg i.p. 20% (v/v) ethanol injection (n=6/dose) and blood alcohol concentration was determined from tail blood samples collected at 10, 60, 120, 180 and 240 minutes post-injection. Immediately after the final non-drug four-hour drinking session, mice were rapidly decapitated and heart blood samples collected to determine blood ethanol concentration following a binge drinking session.
Data Analysis
For the immunohistochemistry experiments, each treatment group consisted of 8 animals. Measurements were taken from the left and right side of each section and 2-4 sections were analyzed for each brain region. Measurements from individual brain regions for each animal were averaged to produce a single value for a given region in each animal. As sections at different time points were stained separately, data were analyzed separately at each time point using Student’s t-test to compare saline vs. ethanol treated tissue (Graphpad Software, San Diego, CA).
In the behavioral experiments, data were expressed as grams of ethanol consumed or milliliters of sucrose solution consumed (respectively) per kilogram of body weight. One mouse was excluded from the SL327 data set because intake on the testing day was a significant outlier using Grubb’s test (p <0.05). Dose effects on ethanol consumption were determined using one-way repeated measures ANOVA with Student-Newman-Keuls test for post-hoc analysis where appropriate, whereas effects on sucrose consumption were analyzed via single-tailed t-test. Blood ethanol clearance data were analyzed via two-way repeated measures ANOVA (dose × time). Locomotor data were computed in 20 minute bins and analyzed via two-way repeated measures ANOVA (dose × time). Locomotor data in the SL327 experiment were further analyzed for potential anxiolytic effects in an open field test. Thigmotaxis was evaluated by comparing distance (cm) traveled in the center zone (inner 25% of the area) to distance traveled in the periphery (outer 75% of the area) as previously described (e.g.,(Hodge et al., 2002, Kash et al., 1999). Data were then analyzed in a dose × zone two-way repeated measures ANOVA. Alpha was set at 0.05 for all comparisons.
RESULTS
Distribution of tERK1/2 and pERK1/2 in Mouse Brain
tERK1/2 and pERK1/2 immunoreactivties were detected in numerous brain regions (Table 1). The highest densities of tERK1/2 immunoreactivity were observed in the prefrontal cortex, nucleus accumbens (core and shell), bed stria terminalis, and the central amygdala. Moderate levels of immunostaining were observed in the cingulate, insular, and piriform cortices, as well as in the basolateral amygdala. The cytological pattern of tERK1/2 immunoreactivity showed localization primarily at the plasma membrane (Fig 1). Analysis of tERK1/2 immunoreactivity showed no effect of ethanol treatment on tERK1/2 abundance in the core or shell of the nucleus accumbens, the central or basolateral amygdala, the bed stria terminalis, or the paraventricular nucleus of the thalamus at either 10 or 90 minutes (Table 1).
Table 1. Effects of acute ethanol on tERK1/2 immunoreactivity.
| 10 minutes | 90 minutes | |||
|---|---|---|---|---|
|
| ||||
| Brain region | Saline | Ethanol | Saline | Ethanol |
| Nucleus Accumbens | ||||
| Core | 33814 ± 6981 | 36793 ± 25932 | 18723 ± 4245 | 11893 ± 2225 |
| Shell | 41225 ± 13501 | 38562 ± 24186 | 23204 ± 6744 | 18096 ± 3868 |
| Amygdala | ||||
| Central | 82024 ± 27424 | 61740 ± 47661 | 22577 ± 3680 | 11480 ± 4376 |
| Basolateral | 20716 ± 3431 | 13448 ± 2405 | 7705 ± 881 | 5039 ± 1993 |
| Bed stria terminalis | 749627 ± 372334 | 406361 ± 235395 | 47070 ± 9680 | 46737 ± 10890 |
| PVN Thalamus | 117252 ± 37405 | 126097 ±78730 | 75072 ± 21063 | 30114 ± 9255 |
Mean number (± SEM) Mean (± SEM) immunoreactivity of the tERK1/2 positive area at 10 and 90 minutes following 3.0 g/kg ethanol treatment expressed as pixels/mm2.
Significantly different from saline control at the given time point (p<0.05; Student’s t-test).
Figure 1. tERK1/2 and pERK1/2 immunoreactivity.
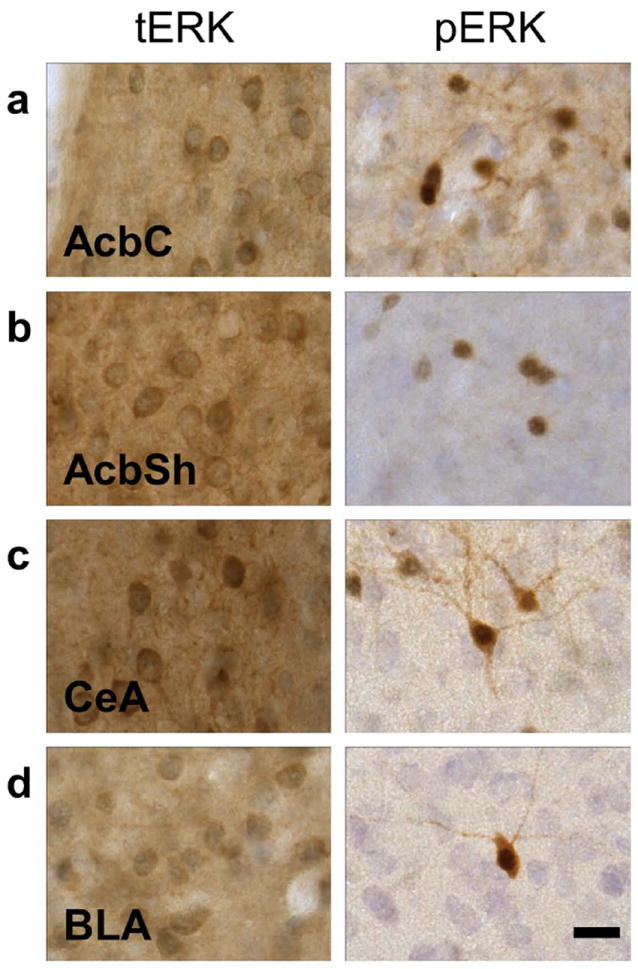
Representative photomicrographs (100X) of the cytological patterns of ERK1/2 immunoreactivity in the nucleus accumbens (a) core and (b) shell, (c) central amygdala, and (d) basolateral amygdala (scale bar, 10 microns).
The highest levels of pERK1/2 immunoreactivity were observed in the prefrontal cortex, nucleus accumbens (core and shell), paraventricular nucleus (PVN) of the thalamus and the central amygdala, with lower levels of immunostaining seen in the cingulate, insular, and piriform cortices, and the basolateral amygdala. pERK1/2 immunoreactivity generally produced a heavy, punctuate pattern of staining, suggestive of nuclear localization, with lighter, diffuse staining within the cell body and projections (Fig 1).
Effect of Acute Ethanol on pERK1/2 Immunoreactivity
Nucleus Accumbens
The effects of acute ethanol (3g/kg) administration on pERK1/2 immunoreactivity in nucleus accumbens were sub-regionally specific. In the core of the nucleus accumbens, acute ethanol reduced pERK1/2 immunoreactivity relative to vehicle (cell counts) in a rapid and time dependent manner (Fig 2a). pERK1/2 immunoreactivity was 86% lower in the nucleus accumbens core at 10 minutes [t(12) = 3.39; p<0.01] and 45% 30 minutes [t(12) = 2.42; p <0.5] following ethanol treatment as compared to vehicle controls (Table 2). Representative photomicrographs illustrating the cytological pattern of pERK1/2 immunoreactivity in the nucleus accumbens core are shown in Figure 2b. In contrast, acute ethanol failed to alter pERK1/2 immunoreactivity in the shell of the nucleus accumbens (Fig 2c-d;p>0.05).
Figure 2. Effects of acute ethanol on ERK1/2 phosphorylation in the nucleus accumbens as a function of time.
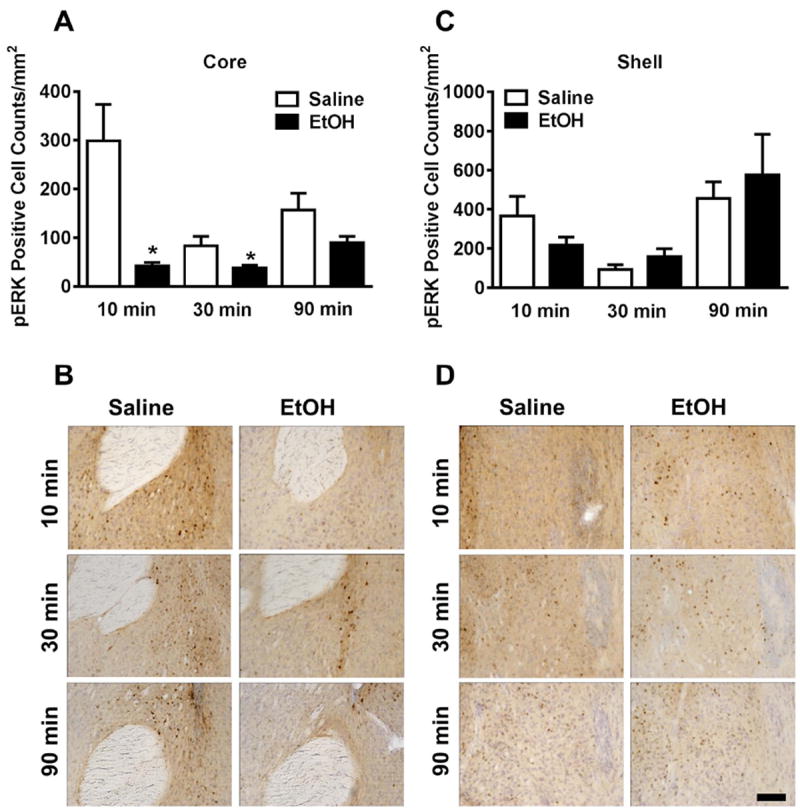
(A) Mean (± SEM) immunoreactivity of the pERK1/2 positive area in the nucleus accumbens core following 3.0 g/kg ethanol treatment expressed as relative change versus saline control. (B) Representative photomicrographs (20X) of the cytological pattern of pERK1/2 immunoreactivity in the core. (C) Mean (± SEM) immunoreactivity of the pERK1/2 positive area in the nucleus accumbens shell following 3.0 g/kg ethanol treatment expressed as relative change versus saline control. (D) Representative photomicrographs (20X) of the cytological pattern of pERK1/2 immunoreactivity in the shell (scale bar, 50 microns).
*Significantly different from saline control at the given time point (p<0.05; Student’s t-test).
Table 2. Effects of acute ethanol on pERK-positive cells counts over time.
| 10 minutes | 30 minutes | 90 minutes | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Brain region | Saline | Ethanol | Saline | Ethanol | Saline | Ethanol |
| Nucleus Accumbens | ||||||
| Core | 298.5 ± 75.30 | 42.13 ± 7.182* | 82.67 ± 20.22 | 37.35 ± 6.211* | 156.8 ± 34.71 | 89.58 ± 13.23 |
| Shell | 366.3 ± 100.5 | 218.2 ± 39.3 | 91.84 ± 25.58 | 158.3 ± 40.16 | 456.0 ± 84.43 | 574.6 ± 209.5 |
| Amygdala | ||||||
| Basolateral | 146.3 ± 19.82 | 136.9 ± 30.49 | 28.05 ± 9.167 | 42.79 ± 10.47 | 9.394 ± 2.630 | 44.57 ± 15.49 |
| Central | 652.9 ± 128.8 | 1188 ± 108.3* | 276.2 ± 59.35 | 708.3 ± 104.5* | 167.0 ± 55.46 | 240.7 ± 68.06 |
| Prefrontal Cortex | 282.0 ± 57.38 | 466.9 ± 26.67* | 109.5 ± 64.56 | 446.9 ± 39.31* | 127.3 ± 41.57 | 136.2 ± 33.47 |
| Cingulate Cortex | 109.0 ± 16.23 | 143.7 ± 19.71 | 54.02 ± 24.45 | 172.2 ± 19.66* | 39.69 ± 12.12 | 49.17 ± 15.42 |
| Insular Cortex | 125.5 ± 10.74 | 135.4 ± 11.33 | 29.42 ± 17.29 | 64.67 ± 11.95 | 36.46 ± 11.27 | 42.75 ± 12.40 |
| Piriform Cortex | 80.85 ± 18.21 | 245.7 ± 43.06* | 109.1 ± 34.26 | 78.47 ± 25.55 | 11.61 ± 5.962 | 14.46 ± 8.162 |
| PVN Thalamus | 248.0 ± 26.62 | 437.3 ± 64.46* | 87.19 ± 50.12 | 187.2 ± 53.07 | 42.02 ± 12.41 | 175.9 ± 41.05* |
| Bed stria terminalis | 249.1 ± 62.44 | 731.9 ± 128.7 | 245.2 ± 109.4 | 649.9 ± 153.8 | 20.31 ± 6.484 | 213.5 ± 98.19 |
Mean number (± SEM) of pERK1/2 positive cells following saline or 3.0 g/kg ethanol treatment expressed as counts/mm2.
Significantly different from saline control at the given time point (p<0.05; Student’s t-test).
Amygdala
Acute ethanol (3g/kg) administration selectively increased pERK1/2 immunoreactivity in the central but not basolateral nuclei of the amygdala as compared to vehicle controls. In the central amygdala, the ethanol-induced increase in ERK1/2 phosphorylation was rapid, with a 82% increase in pERK1/2 immunoreactivity observed 10 minutes after ethanol treatment [t(14) = 3.19, p <0.0] and increasing to 156% 30 minutes after ethanol treatment [t(13) = 3.45, p <0.01; Fig 3a. In contrast, ethanol effects were not observed in the basolateral amygdala, although there was an insignificant trend for increases in pERK1/2 immunoreactivity at 90 minutes (p = 0.06) after ethanol treatment (Fig 3c). Representative photomicrographs illustrating the cytological pattern of pERK1/2 immunoreactivity in the central amygdala and basolateral amygdala are shown in Figure 3, panels b and d.
Figure 3. Effects of acute ethanol on ERK1/2 phosphorylation in the amygdala as a function of time.
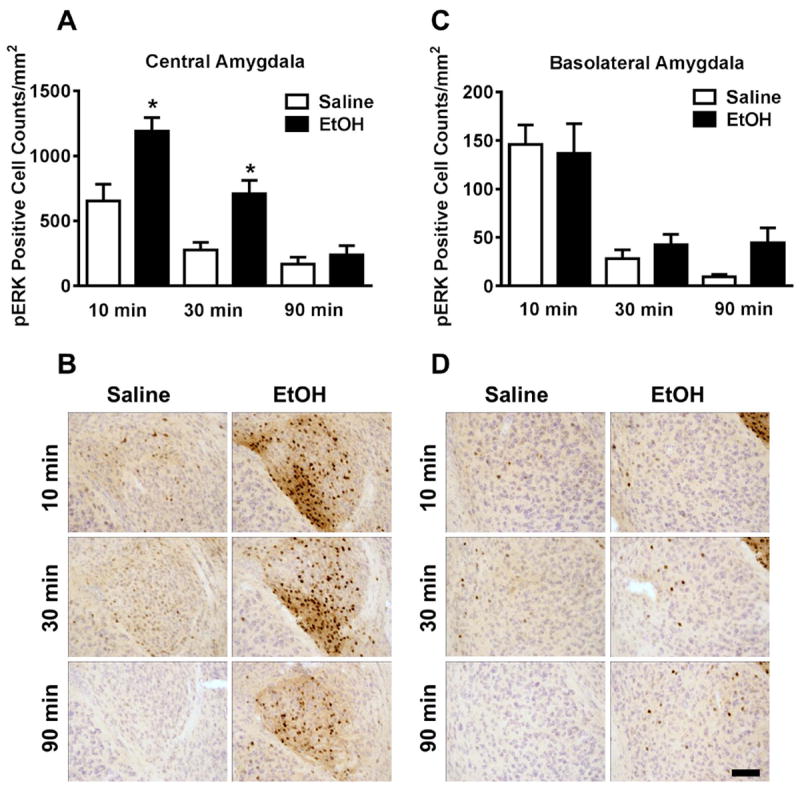
(A) Mean (± SEM) immunoreactivity of the pERK1/2 positive area in the central amygdala following 3.0 g/kg ethanol treatment expressed as relative change versus saline control. (B) Representative photomicrographs of the cytological pattern of pERK1/2 immunoreactivity in the central amygdala. (C) Mean (± SEM) immunoreactivity of the pERK1/2 positive area in the basolateral amygdala following 3.0 g/kg ethanol treatment expressed as relative change versus saline control. (D) Representative photomicrographs of the cytological pattern of pERK1/2 immunoreactivity in the basolateral amygdala (scale bar, 50 microns). *Significantly different from saline control at the given time point (p<0.05; Student’s t-test).
Other Regions
Other brain regions showed time-dependent increases in pERK1/2 immunoreactivity following acute ethanol treatment, including the bed stria terminalis and the PVN of the thalamus. In the PVN thalamus, pERK1/2 immunoreactivity was significantly elevated at 10 [t(13) = 2.85, p <0.05] and 90 [t(11) = 2.91, p <0.05] minutes after ethanol treatment (Table 2) compared to vehicle controls. The greatest effects of acute ethanol treatment were seen in the bed stria terminalis. Acute ethanol significantly increased pERK1/2 immunoreactivity in the region at the 10 minute time point [t(10) = 2.97, p< 0.05) and pERK1/2 positive cell counts were increased by 194% (Table 2). Acute ethanol treatment also increased pERK1/2 immunoreactivity in the medial prefrontal, cingulate, and piriform regions of the cortex in a time dependent manner as compared to controls (Table 4). Ethanol significantly increased pERK1/2 immunoreactivity in the medial prefrontal cortex at 10 [t(10) = 3.23, p<0.01] and 30 minutes [t(12) = 4.71, p<0.001] and cingulate cortex at 30 minutes [t(13) = 3.81, p<0.01]. In the piriform cortex, ethanol significantly increased pERK1/2 immunoreactivity at the 10 minute time point [t(13) = 3.35, p<0.01]. No significant increases in p-ERK1/2 immunoreactivity or pERK1/2-positive cell counts were observed in the insular cortex.
Effects of Inhibition of MAPK Siganling on Ethanol Drinking
SL327 Effects
SL327 pretreatment did not alter ethanol consumption during the first hour of drinking (Figure 4A). However, over the total four-hour drinking session SL327 significantly increased ethanol consumption [main effect of Dose, F (2) = 4.26, p <0.05 Figure 4B]. Student-Newman-Keuls test revealed that both 10 mg/kg and 30 mg/kg SL327 significantly increased ethanol consumption compared to vehicle, p <0.05.
Figure 4. Systemic ERK1/2 inhibition increases binge-like ethanol intake.
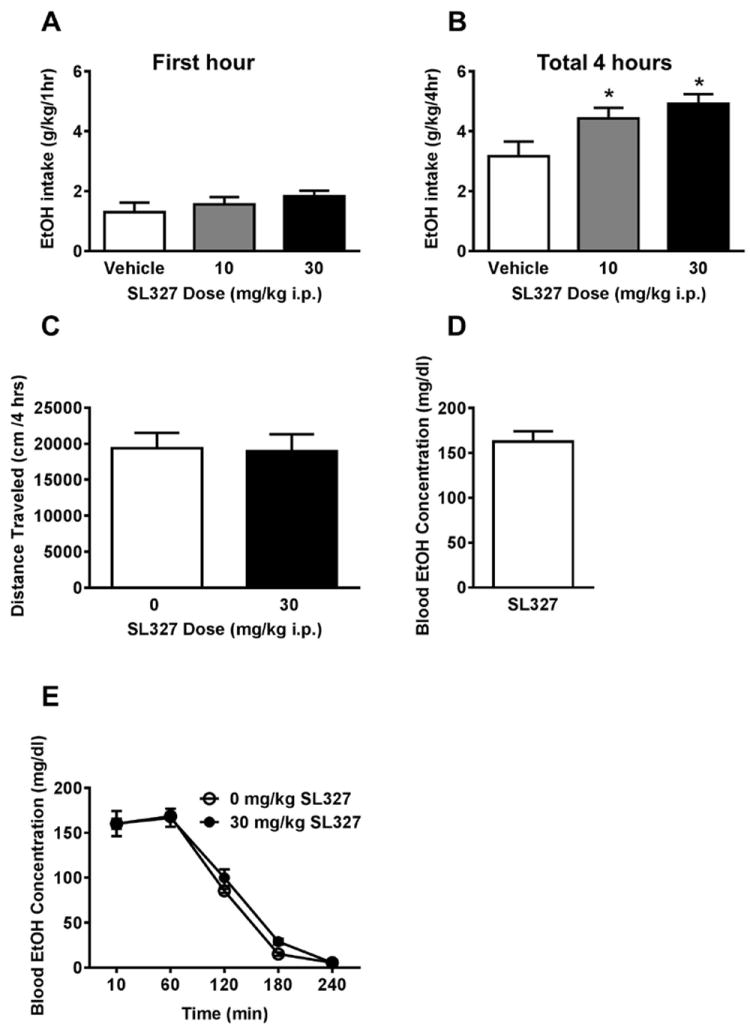
(A) No effect of pretreatment with the MEK1/2 inhibitor SL327 on ethanol intake emerged during the first hour of drinking. (B) Over the total four-hour drinking session, SL327 significantly increased ethanol drinking at the 10 and 30 mg/kg doses. (C) Mice achieved a binge-level of alcohol consumption during the 4-hour testing period. (D) SL327 pretreatment did not affect total distance traveled in a four-hour locomotor activity test. (E) SL327 pretreatment did not alter blood ethanol metabolism following an acute 2g/kg i.p. ethanol injection. *Significantly different than vehicle at the given dose (p<0.05; Student-Newman-Keuls)
To test for nonselective locomotor effects, mice were pretreated with 0 or 30 mg/kg SL327 before a four-hour locomotor activity test. Pretreatment with 30 mg/kg SL327 did not alter total distance traveled during the four-hour locomotor activity test (p >0.05, Figure 4C).
Blood samples collected following the last four-hour binge on a non-drug treatment day revealed an average blood ethanol concentration of approximately 160 mg/dL (Figure 4D). This value exceeds the NIAAA criteria for a binge-drinking session (80 mg/dL) (NIAAA, 2004). SL327 (30mg/kg) failed to alter blood ethanol concentration after a 2g/kg challenge injection, indicating a lack of effect on blood ethanol metabolism. (p >0.05, Figure 4E).
SB239063 Effects
SB239063 pretreatment significantly reduced ethanol consumption during the first hour of drinking [main effect of Dose, F (3) = 10.14, p <0.001]. Student-Newman-Keuls test indicated that the 30 mg/kg dose was significantly decreased compared to vehicle in the first hour of drinking, p <0.05 (Figure 5A). By the end of the drinking session, the dose effect was no longer present with all doses consuming roughly equivalent amounts of ethanol at the four-hour time point (p >0.05, Figure 5B).
Figure 5. Systemic p38 inhibition non-selectively decreases binge ethanol intake.
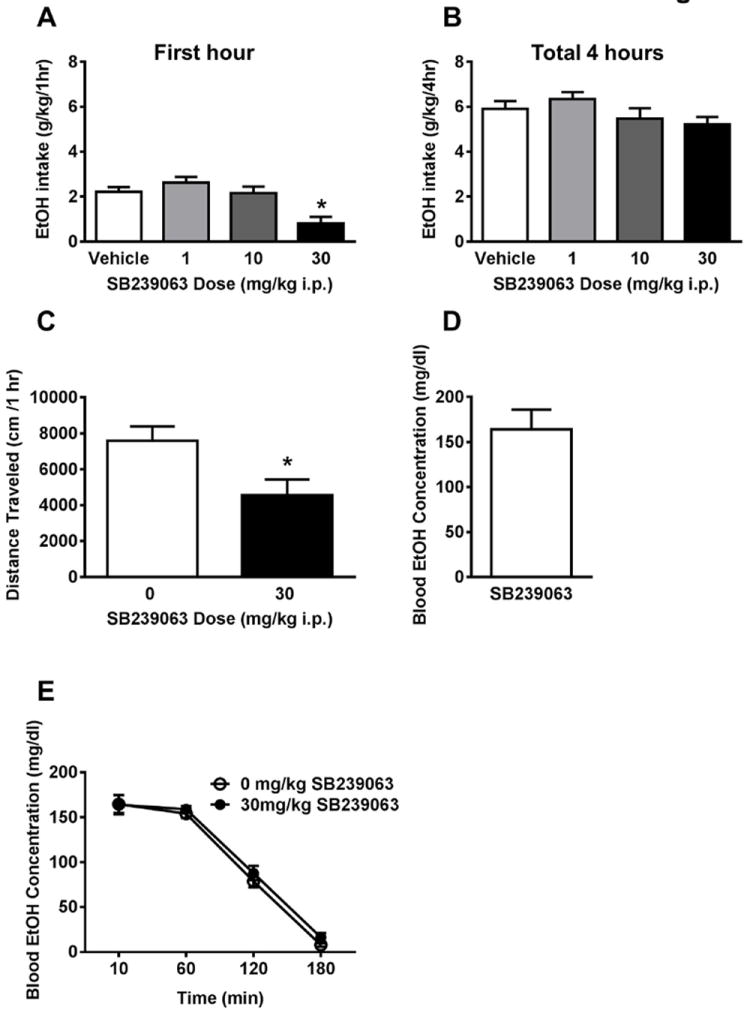
(A) Pretreatment with the p38 inhibitor SB239063 (30 mg/kg) reduced ethanol intake during the first hour of ethanol drinking. (B) The effect was no longer present by the end of the four-hour drinking session. (C) Mice achieved a binge-level of alcohol consumption during the 4-hour testing period. (D) SB239063 pretreatment reduced total distance traveled in a one-hour locomotor activity test. (E) SB239063 pretreatment did not alter blood ethanol metabolism following an acute 2g/kg i.p. ethanol injection. *Significantly different than vehicle at the given dose (p<0.05; Student-Newman-Keuls)
During a four-hour locomotor activity test, SB239063 pretreatment significantly decreased total distance traveled [t (11) = 5.61, p <0.001 Figure 5C]. SB239063 pretreatment failed to alter blood ethanol concentration after a 2g/kg i.p. challenge injection (p >0.05, Figure 5D).
Selectivity of SL327 Effects
To establish the selectivity of the SL327 effect for ethanol consumption, an additional drinking experiment was conducted using the non-drug reinforcer sucrose. The lowest effective dose of SL327 (10 mg/kg) was selected for comparison with vehicle. SL327 pretreatment did not affect sucrose consumption during the first hour (Figure 6A) or over the entire four hour drinking period (p >0.05, Figure 6B).
Figure 6. Effects of SL327 are selective for ethanol reinforcement.
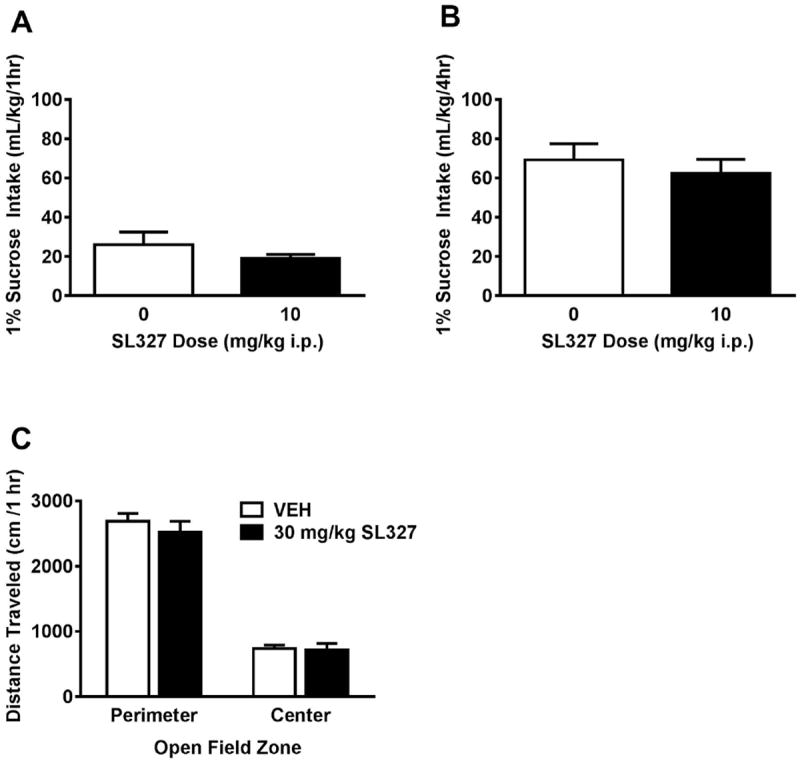
Mice were given access to a 1% sucrose solution on the same schedule as the ethanol consumption. No effect of SL327 on sucrose consumption emerged over the (A) one-hour or (B) four-hour access period. (C) SL327 pretreatment did not alter time spent in the center vs. perimeter of the open field during locomotor activity testing. *Significantly different than vehicle at the given dose (p<0.05; Student-Newman-Keuls)
To evaluate potential anxiolytic effects of SL327, locomotor data were analyzed for time spent in the center vs. perimeter of the chamber. A main effect of zone emerged, such that mice spent more time in the perimeter of the chamber than in the center zone [F(1,10) = 504.0, p <0.0001]. No significant effect of SL327 pretreatment emerged in either zone (p >0.05, Figure 6C).
DISCUSSION
The neurobiological mechanisms that regulate binge alcohol drinking remain to be fully elucidated. Long-term adaptations in molecular brain functions driven by synaptic plasticity have been hypothesized to play an essential role in the establishment of pathological drug use (Kauer and Malenka, 2007). ERK1/2 signaling is required for synaptic plasticity and learning and memory (Thomas and Huganir, 2004), and therefore is of interest as a potential target of alcohol’s activity in the brain. The present experiments were designed to test the effects of alcohol on brain ERK1/2 expression and phosphorylation and determine a functional role for ERK1/2 activity in the regulation of binge-like alcohol drinking behavior.
Previous studies have shown that ERK1/2 activity plays a significant role in the pathophysiology of alcoholism (Aroor and Shukla, 2004), including acute and chronic responses to ethanol as well as behavioral pathologies associated with abuse such as self-administration, withdrawal, and relapse (Faccidomo et al., 2009, Schroeder et al., 2008, Sanna et al., 2002, Pandey et al., 2008, Chandler and Sutton, 2005, Kalluri and Ticku, 2002). In an effort to further explore the effects of ethanol on ERK1/2 activation, we examined brain regional effects of acute ethanol exposure on ERK1/2 phosphorylation in vivo. Results showed that acute IP administration of ethanol (3 g/kg) produced global increases in ERK1/2 phosphorylation across most of the brain regions examined without altering total ERK1/2 immunoreactivity. At 10 and 30 minutes post-injection, ethanol (3g/kg) increased pERK1/2 immunoreactivity in the central amygdala and several sub-regions of the cortex. At 90 minutes after ethanol treatment, pERK1/2 immunoreactivity was elevated only in the paraventricular nuclei of the thalamus. Only the nucleus accumbens core displayed a reduction in pERK1/2 immunoreactivity, at 10 and 30 minutes post-injection. These results indicate that ethanol alters ERK1/2 phosphorylation in a time-and brain region-dependent manner in the mammalian brain.
Current literature suggests that acute ethanol exposure can both increase and decrease ERK1/2 phosphorylation. In the majority of the brain regions examined in this study, pERK1/2 immunoreactivity was elevated at one or more time points after acute ethanol challenge. These results complement earlier findings showing ethanol-induced increases in ERK1/2 phosphorylation in vivo. In mice, acute ethanol exposure (2.4g/kg) increased pERK1/2 immunoreactivity in the Edinger-Westfal (EW) nucleus, 15 minutes after treatment (Bachtell et al., 2002). In rats, low dose acute ethanol treatment (1g/kg; 15 and 60 min) produced increases in pERK1/2 immunoreactivity in the nucleus accumbens, the bed stria terminalis, and the amygdala (Pandey et al., 2008, Ibba et al., 2009). Additionally, acute ethanol exposure has also been shown to increase pERK1/2 levels in mouse hippocampal neurons (100 mM; 1, 3, or 6 hours of exposure) (Ku et al., 2007). Ethanol-induced increases in ERK1/2 phosphorylation have been associated with increases in phosphorylation of down-stream effectors, such as Elk-1 and CREB, in the amygdala, and ethanol-induced increases in c-fos expression, another down-stream effector of ERK1/2, can be blocked by the MEK/ERK-signaling pathway inhibitor SL327 (Bachtell et al., 2002). These data suggest that ethanol-induced increases in pERK1/2 levels are associated with increased ERK1/2 activity.
There is also evidence that acute ethanol treatment may decrease ERK1/2 phosphorylation in the brain. Although we only found decreases in pERK1/2 immunoreactivity in the nucleus accumbens core, acute ethanol treatment has been shown to reduce ERK1/2 phosphorylation in other brain regions. In mice, acute exposure to ethanol (3.5 g/kg; 10 minutes) decreased pERK1/2 levels in the cerebral cortex (Kalluri and Ticku, 2002). In rats, acute ethanol treatment (3.5 g/kg, 60 minutes) decreased pERK1/2 levels in the cerebral cortex, as well as in the hippocampus and cerebellum (Chandler and Sutton, 2005). Acute ethanol exposure (100-150 mM; 10-60 minutes) also reduced pERK1/2 levels in cultured cortical neurons (Kalluri and Ticku, 2003), NG108-15 cells (Constantinescu, 2004), and SH-SY5Y cells (Seiler et al., 2001). Given that we found both increases and decreases in ERK1/2 phosphorylation following acute ethanol treatment, it seems likely that the disparate findings in the literature are partly due to brain regional, ethanol dose and/or time-dependent differences in response to ethanol. Additional experiments testing lower dose ranges (1-2 g/kg) would be useful in reconciling these disparate findings in the literature. However, it should be noted that the studies that found decreases in pERK1/2 immunoreactivity in the cortex, hippocampus, and cerebellum used western blotting methods to measure pERK1/2 changes in large regions that are comprised of distinct subregions. By using immunohistochemical methods, the present study provides novel information regarding ethanol effects on ERK1/2 phosphorylation in specific subnuclei.
It is important to note that restraint stress has been shown to produce rapid (< 1 min) increases in ERK1/2 phosphorylation in the brain that dissipate by 30 min (Meller et al., 2003). Thus, handling and injection stress could contribute to changes in pERK1/2 levels following acute drug injection in mice. Indeed, results suggested that the number of pERK1/2 positive cells decreased over time in the saline injected control groups (Table 1). For this reason, all ethanol data were compared to parallel saline controls at each time point (10 – 90 min), which counterbalances any potential effects of injection stress across the measurement time points. Thus, it is not likely that injection stress significantly contributes to the observed increases in pERK1/2 immunoreactivity in the ethanol challenged animals. Alternatively, the effects of injection may be important to the decreases in pERK1/2 immunoreactivity seen in the nucleus accumbens core following ethanol treatment. The relative decreases in ERK1/2 phosphorylation observed in ethanol treated animals could be interpreted as ethanol inhibition of injection-induced ERK1/2 phosphorylation. However, this does not fully account for the ethanol effect, as the pERK1/2 positive cell counts are significantly lower at 10 and 30 minutes (p<0.05), relative to the 90 minute time point, indicating a recovery of baseline pERK1/2 abundance in the ethanol treated animals. It is possible that ethanol injection is significantly more stressful than saline injection in these conditions, leading to an exaggerated response of pERK1/2 to ethanol. It will be interesting to determine in future experiments if self-administered ethanol, which should not produce acute stress effects, results in similar changes in ERK1/2 phosphorylation.
We also sought to determine the extent to which MAPK signaling was functionally involved in binge-like alcohol drinking behavior. Inhibition of ERK1/2 phosphorylation with the MEK1/2 inhibitor SL327 significantly increased home-cage alcohol drinking over the four-hour access period without affecting open field locomotor activity. In contrast, the p38 inhibitor SB239063 significantly decreased alcohol drinking during the first hour of intake at a dose that also decreased locomotor activity. These findings indicate that a reduction in ERK1/2 activity stimulates increased alcohol drinking under these conditions. This effect appears to be selective for the alcohol solution, since SL327 pretreatment did not alter sucrose consumption in the same access procedure.
Of the MAPK signaling pathways, the p38 signaling cascade has received relatively little investigation in the addiction literature. While there has been some evidence for p38 in morphine conditioned place preference (Zhang et al., 2012) and reinstatement of cocaine preference (Bruchas et al., 2011), other studies have failed to find an effect of acute cocaine injection on p38 expression or phosphorylation (Zhang et al., 2004, Valjent et al., 2000). In the present study, the p38 inhibitor SB239063 reduced ethanol drinking only at a dose that also induced nonspecific suppression of open-field locomotor activity. These findings do not support a functional role for p38 signaling in alcohol drinking.
In contrast, the MEK1/2 inhibitor SL327 selectively increased alcohol but not sucrose drinking without affecting locomotor behavior. This finding is consistent with previous work in our lab, which has demonstrated that pretreatment with SL327 increases operant self-administration of alcohol but not sucrose in mice (Faccidomo et al., 2009). Increased self-administration under either access condition can occur due to alterations in the motivation to consume the alcohol or blockade of the pharmacological effects of alcohol. Our previous work used a progressive ratio schedule of reinforcement to determine if SL327 affected motivation to self-administer ethanol, and found that SL327 was without effect in this condition. Therefore, it is unlikely that SL327 is increasing the motivation to consume alcohol in these animals.
Results of the present study indicate that alcohol exposure rapidly increases ERK1/2 phosphorylation in specific brain regions. Thus, administration of the MEK1/2 inhibitor, which prevents ERK1/2 phosphorylation, prior to binge drinking may have antagonized the pharmacological activity of ethanol on ERK1/2. This would be expected to increase the amount of alcohol consumed in order to achieve an equivalent pharmacological effect. Similar results have been observed with cocaine and opiate self-administration. Antagonism of the primary pharmacological target of these substances (dopamine D1/D2 receptors and μ opioid receptors, respectively) increased drug self-administration (Woods et al., 1975, Koob et al., 1987). This observation may account for the increased alcohol consumption exhibited by mice pretreated with the MEK1/2 inhibitor SL327. The precise pharmacological effect of ERK1/2 activation by alcohol is not clear from the present experiments. However, the appearance of the SL327 effect during the later stages of testing suggests that SL327 may have altered the termination of alcohol drinking, such that animals treated with SL327 continued drinking for longer during the session than vehicle treated animals. Previous studies have identified pharmacological manipulations that selectively alter termination of alcohol self-administration, including the GABA-A agonist muscimol (Hodge et al., 1995) and the dopamine D2-like antagonist raclopride (Hodge et al., 1994, Samson et al., 1993). SL327 may act similarly to extend the period of alcohol consumption by inhibiting the pharmacological effect of alcohol on ERK1/2 activation. This interpretation points to ERK1/2 activation as a potential cue for satiety or subjective intoxication. However, ERK1/2 inhibition in the amygdala increases the discriminative stimulus effects of a low dose of alcohol (Besheer et al., 2012) and is without effect in accumbens, which suggests the potential importance of other brain regions detected in this study such as the prefrontal cortex. Further evaluation of ERK1/2 activity in specific brain regions will clarify its potential role as a termination signal in alcohol drinking.
Alternatively, SL327 effects may be considered in the context of stress-induced alterations of alcohol drinking. Acute and chronic stress has been shown to alter alcohol consumption (Alegria et al., 2010, Lu and Richardson, 2014), and intraperitoneal injection of drug or vehicle prior to drinking may have been stressful in these experiments. SL327 has been shown to alleviate the sensitizing effect of a stressor on ethanol withdrawal-induced anxiety (Knapp et al., 2011). Thus, an alternative explanation for our findings is that injection stress reduced alcohol drinking in the vehicle condition whereas SL327 administration blocked this effect. However, pretreatment with SL327 failed to affect thigmotaxis in an open-field test suggesting lack of an anxiolytic-like effect under these conditions. Future experiments to directly investigate the contributions of ERK1/2 signaling to stress-induced alterations in alcohol drinking would clarify the findings presented here and could potentially point to an alternative mechanism for ERK1/2’s effects on alcohol drinking.
Immunohistochemistry experiments revealed that acute ethanol injection (3 g/kg) rapidly increases ERK1/2 phosphorylation in several brain regions including the nucleus accumbens, amygdala and prefrontal cortex. Each of these brain regions has been shown to regulate alcohol self-administration (Hodge et al., 1996, Hodge et al., 1992, Schroeder et al., 2003) but use of systemically administered inhibitor in this study makes it difficult to identify which, if any, of these regions mediate the SL327-induced increase in alcohol consumption. Future experiments utilizing site-specific microinjection strategies would clarify the relative contributions of ERK1/2 signaling in these nuclei to binge alcohol drinking.
In conclusion, our findings indicate that acute ethanol injection facilitates a general increase in ERK1/2 phosphorylation throughout many brain regions known to be involved in drug reward and addiction, such as the central and basal lateral amygdala, prefrontal cortex, and bed nucleus of the stria terminals. In contrast, ethanol injection resulted in decreases in ERK1/2 phosphorylation in the nucleus accumbens core. Systemic administration of the MEK1/2 inhibitor SL327 increases binge-like alcohol consumption in mice, suggesting that ERK1/2 activation regulates the termination of binge-like drinking.
Acknowledgments
This work supported by NIAAA grants R37AA014983 and P60AA11605 to CW Hodge.
References
- ALEGRIA AA, HASIN DS, NUNES EV, LIU SM, DAVIES C, GRANT BF, BLANCO C. Comorbidity of generalized anxiety disorder and substance use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. The Journal of clinical psychiatry. 2010;71:1187–95. doi: 10.4088/JCP.09m05328gry. quiz 1252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AROOR AR, SHUKLA SD. MAP kinase signaling in diverse effects of ethanol. Life sciences. 2004;74:2339–64. doi: 10.1016/j.lfs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- BACHTELL RK, TSIVKOVSKAIA NO, RYABININ AE. Alcohol-induced c-Fos expression in the Edinger-Westphal nucleus: pharmacological and signal transduction mechanisms. The Journal of pharmacology and experimental therapeutics. 2002;302:516–24. doi: 10.1124/jpet.102.036046. [DOI] [PubMed] [Google Scholar]
- BESHEER J, FISHER KR, CANNADY R, GRONDIN JJ, HODGE CW. Intra-amygdala inhibition of ERK(1/2) potentiates the discriminative stimulus effects of alcohol. Behavioural brain research. 2012;228:398–405. doi: 10.1016/j.bbr.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOULTON TG, COBB MH. Identification of multiple extracellular signal-regulated kinases (ERKs) with antipeptide antibodies. Cell regulation. 1991;2:357–71. doi: 10.1091/mbc.2.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUCHAS MR, SCHINDLER AG, SHANKAR H, MESSINGER DI, MIYATAKE M, LAND BB, LEMOS JC, HAGAN CE, NEUMAIER JF, QUINTANA A, PALMITER RD, CHAVKIN C. Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71:498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUNZELL DH, RUSSELL DS, PICCIOTTO MR. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. Journal of neurochemistry. 2003;84:1431–41. doi: 10.1046/j.1471-4159.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- CHANDLER LJ, SUTTON G. Acute ethanol inhibits extracellular signal-regulated kinase, protein kinase B, and adenosine 3’:5’-cyclic monophosphate response element binding protein activity in an age- and brain region-specific manner. Alcoholism, clinical and experimental research. 2005;29:672–82. doi: 10.1097/01.alc.0000158935.53360.5f. [DOI] [PubMed] [Google Scholar]
- CHEN RH, SARNECKI C, BLENIS J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Molecular and cellular biology. 1992;12:915–27. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOE ES, CHUNG KT, MAO L, WANG JQ. Amphetamine increases phosphorylation of extracellular signal-regulated kinase and transcription factors in the rat striatum via group I metabotropic glutamate receptors. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;27:565–75. doi: 10.1016/S0893-133X(02)00341-X. [DOI] [PubMed] [Google Scholar]
- COURTNEY KE, POLICH J. Binge drinking in young adults: Data, definitions, and determinants. Psychological bulletin. 2009;135:142–56. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FACCIDOMO S, BESHEER J, STANFORD PC, HODGE CW. Increased operant responding for ethanol in male C57BL/6J mice: specific regulation by the ERK1/2, but not JNK, MAP kinase pathway. Psychopharmacology. 2009;204:135–47. doi: 10.1007/s00213-008-1444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGHPARAST A, FATAHI Z, ALAMDARY SZ, REISI Z, KHODAGHOLI F. Changes in the levels of p-ERK, p-CREB, and c-fos in rat mesocorticolimbic dopaminergic system after morphine-induced conditioned place preference: the role of acute and subchronic stress. Cellular and molecular neurobiology. 2014;34:277–88. doi: 10.1007/s10571-013-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENDRICKSON RJ, CAHILL PA, MCKILLOP IH, SITZMANN JV, REDMOND EM. Ethanol inhibits mitogen activated protein kinase activity and growth of vascular smooth muscle cells in vitro. European journal of pharmacology. 1998;362:251–9. doi: 10.1016/s0014-2999(98)00771-7. [DOI] [PubMed] [Google Scholar]
- HODGE CW, CHAPPELLE AM, SAMSON HH. GABAergic transmission in the nucleus accumbens is involved in the termination of ethanol self-administration in rats. Alcoholism, clinical and experimental research. 1995;19:1486–93. doi: 10.1111/j.1530-0277.1995.tb01012.x. [DOI] [PubMed] [Google Scholar]
- HODGE CW, CHAPPELLE AM, SAMSON HH. Dopamine receptors in the medial prefrontal cortex influence ethanol and sucrose-reinforced responding. Alcoholism, clinical and experimental research. 1996;20:1631–8. doi: 10.1111/j.1530-0277.1996.tb01709.x. [DOI] [PubMed] [Google Scholar]
- HODGE CW, RABER J, MCMAHON T, WALTER H, SANCHEZ-PEREZ AM, OLIVE MF, MEHMERT K, MORROW AL, MESSING RO. Decreased anxiety-like behavior, reduced stress hormones, and neurosteroid supersensitivity in mice lacking protein kinase Cepsilon. The Journal of clinical investigation. 2002;110:1003–10. doi: 10.1172/JCI15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGE CW, SAMSON HH, HARAGUCHI M. Microinjections of dopamine agonists in the nucleus accumbens increase ethanol-reinforced responding. Pharmacology, biochemistry, and behavior. 1992;43:249–54. doi: 10.1016/0091-3057(92)90665-3. [DOI] [PubMed] [Google Scholar]
- HODGE CW, SAMSON HH, TOLLIVER GA, HARAGUCHI M. Effects of intraaccumbens injections of dopamine agonists and antagonists on sucrose and sucrose-ethanol reinforced responding. Pharmacology, biochemistry, and behavior. 1994;48:141–50. doi: 10.1016/0091-3057(94)90510-x. [DOI] [PubMed] [Google Scholar]
- IBBA F, VINCI S, SPIGA S, PEANA AT, ASSARETTI AR, SPINA L, LONGONI R, ACQUAS E. Ethanol-induced extracellular signal regulated kinase: role of dopamine D1 receptors. Alcoholism, clinical and experimental research. 2009;33:858–67. doi: 10.1111/j.1530-0277.2009.00907.x. [DOI] [PubMed] [Google Scholar]
- KALLURI HS, TICKU MK. Ethanol-mediated inhibition of mitogen-activated protein kinase phosphorylation in mouse brain. European journal of pharmacology. 2002;439:53–8. doi: 10.1016/s0014-2999(01)01599-0. [DOI] [PubMed] [Google Scholar]
- KALLURI HS, TICKU MK. Regulation of ERK phosphorylation by ethanol in fetal cortical neurons. Neurochem Res. 2003;28:765–9. doi: 10.1023/a:1022822119560. [DOI] [PubMed] [Google Scholar]
- KASH SF, TECOTT LH, HODGE C, BAEKKESKOV S. Increased anxiety and altered responses to anxiolytics in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1698–703. doi: 10.1073/pnas.96.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUER JA, MALENKA RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–58. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- KNAPP DJ, WHITMAN BA, WILLS TA, ANGEL RA, OVERSTREET DH, CRISWELL HE, MING Z, BREESE GR. Cytokine involvement in stress may depend on corticotrophin releasing factor to sensitize ethanol withdrawal anxiety. Brain, behavior, and immunity. 2011;25(Suppl 1):S146–54. doi: 10.1016/j.bbi.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOOB GF, LE HT, CREESE I. The D1 dopamine receptor antagonist SCH 23390 increases cocaine self-administration in the rat. Neuroscience letters. 1987;79:315–20. doi: 10.1016/0304-3940(87)90451-4. [DOI] [PubMed] [Google Scholar]
- KRISHNA M, NARANG H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cellular and molecular life sciences : CMLS. 2008;65:3525–44. doi: 10.1007/s00018-008-8170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KU BM, LEE YK, JEONG JY, MUN J, HAN JY, ROH GS, KIM HJ, CHO GJ, CHOI WS, YI GS, KANG SS. Ethanol-induced oxidative stress is mediated by p38 MAPK pathway in mouse hippocampal cells. Neuroscience letters. 2007;419:64–7. doi: 10.1016/j.neulet.2007.03.049. [DOI] [PubMed] [Google Scholar]
- KURODA KO, MEANEY MJ, UETANI N, FORTIN Y, PONTON A, KATO T. ERK-FosB signaling in dorsal MPOA neurons plays a major role in the initiation of parental behavior in mice. Molecular and cellular neurosciences. 2007;36:121–31. doi: 10.1016/j.mcn.2007.05.010. [DOI] [PubMed] [Google Scholar]
- LANGUILLE S, DAVIS S, RICHER P, ALCACER C, LAROCHE S, HARS B. Extracellular signal-regulated kinase activation is required for consolidation and reconsolidation of memory at an early stage of ontogenesis. The European journal of neuroscience. 2009;30:1923–30. doi: 10.1111/j.1460-9568.2009.06971.x. [DOI] [PubMed] [Google Scholar]
- LU L, KOYA E, ZHAI H, HOPE BT, SHAHAM Y. Role of ERK in cocaine addiction. Trends in neurosciences. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- LU YL, RICHARDSON HN. Alcohol, stress hormones, and the prefrontal cortex: A proposed pathway to the dark side of addiction. Neuroscience. 2014;277C:139–151. doi: 10.1016/j.neuroscience.2014.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALDONADO NM, ESPEJO PJ, MARTIJENA ID, MOLINA VA. Activation of ERK2 in basolateral amygdala underlies the promoting influence of stress on fear memory and anxiety: influence of midazolam pretreatment. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2014;24:262–70. doi: 10.1016/j.euroneuro.2013.10.005. [DOI] [PubMed] [Google Scholar]
- MELLER E, SHEN C, NIKOLAO TA, JENSEN C, TSIMBERG Y, CHEN J, GRUEN RJ. Region-specific effects of acute and repeated restraint stress on the phosphorylation of mitogen-activated protein kinases. Brain Res. 2003;979:57–64. doi: 10.1016/s0006-8993(03)02866-x. [DOI] [PubMed] [Google Scholar]
- NIAAA. NIAAA council approves definition of binge drinking. NIAAA Newsletter. 2004;3:3. [Google Scholar]
- ORTIZ J, HARRIS HW, GUITART X, TERWILLIGER RZ, HAYCOCK JW, NESTLER EJ. Extracellular signal-regulated protein kinases (ERKs) and ERK kinase (MEK) in brain: regional distribution and regulation by chronic morphine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:1285–97. doi: 10.1523/JNEUROSCI.15-02-01285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANDEY SC, ZHANG H, UGALE R, PRAKASH A, XU T, MISRA K. Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:2589–600. doi: 10.1523/JNEUROSCI.4752-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHODES JS, BEST K, BELKNAP JK, FINN DA, CRABBE JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology & behavior. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- ROBINSON MJ, COBB MH. Mitogen-activated protein kinase pathways. Current opinion in cell biology. 1997;9:180–6. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- ROUX PP, BLENIS J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiology and molecular biology reviews : MMBR. 2004;68:320–44. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMSON HH, HODGE CW, TOLLIVER GA, HARAGUCHI M. Effect of dopamine agonists and antagonists on ethanol-reinforced behavior: the involvement of the nucleus accumbens. Brain research bulletin. 1993;30:133–41. doi: 10.1016/0361-9230(93)90049-h. [DOI] [PubMed] [Google Scholar]
- SANNA PP, SIMPSON C, LUTJENS R, KOOB G. ERK regulation in chronic ethanol exposure and withdrawal. Brain research. 2002;948:186–91. doi: 10.1016/s0006-8993(02)03191-8. [DOI] [PubMed] [Google Scholar]
- SCHROEDER JP, OLIVE F, KOENIG H, HODGE CW. Intra-amygdala infusion of the NPY Y1 receptor antagonist BIBP 3226 attenuates operant ethanol self-administration. Alcoholism, clinical and experimental research. 2003;27:1884–91. doi: 10.1097/01.ALC.0000098875.95923.69. [DOI] [PubMed] [Google Scholar]
- SCHROEDER JP, SPANOS M, STEVENSON JR, BESHEER J, SALLING M, HODGE CW. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–54. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEILER AE, ROSS BN, RUBIN R. Inhibition of insulin-like growth factor-1 receptor and IRS-2 signaling by ethanol in SH-SY5Y neuroblastoma cells. J Neurochem. 2001;76:573–81. doi: 10.1046/j.1471-4159.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- THIELE TE, CRABBE JC, BOEHM SL., 2ND “Drinking in the Dark” (DID): a simple mouse model of binge-like alcohol intake. Current protocols in neuroscience / editorial board, Jacqueline N Crawley … [et al] 2014;68:9 49 1–9 49 12. doi: 10.1002/0471142301.ns0949s68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS GM, HUGANIR RL. MAPK cascade signalling and synaptic plasticity. Nature reviews Neuroscience. 2004;5:173–83. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- TOWN M, NAIMI TS, MOKDAD AH, BREWER RD. Health care access among U.S. adults who drink alcohol excessively: missed opportunities for prevention. Preventing chronic disease. 2006;3:A53. [PMC free article] [PubMed] [Google Scholar]
- TSUJI R, GUIZZETTI M, COSTA LG. In vivo ethanol decreases phosphorylated MAPK and p70S6 kinase in the developing rat brain. Neuroreport. 2003;14:1395–9. doi: 10.1097/01.wnr.0000071763.92388.41. [DOI] [PubMed] [Google Scholar]
- VALJENT E, CORVOL JC, PAGES C, BESSON MJ, MALDONADO R, CABOCHE J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:8701–9. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALJENT E, PAGES C, HERVE D, GIRAULT JA, CABOCHE J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. The European journal of neuroscience. 2004;19:1826–36. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- VALJENT E, PAGES C, ROGARD M, BESSON MJ, MALDONADO R, CABOCHE J. Delta 9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. The European journal of neuroscience. 2001;14:342–52. doi: 10.1046/j.0953-816x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- WOODS JH, DOWNS DA, CARNEY J. Behavioral functions of narcotic antagonists: response-drug contingencies. Federation proceedings. 1975;34:1777–84. [PubMed] [Google Scholar]
- ZHANG L, LOU D, JIAO H, ZHANG D, WANG X, XIA Y, ZHANG J, XU M. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:3344–54. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG XQ, CUI Y, CHEN Y, NA XD, CHEN FY, WEI XH, LI YY, LIU XG, XIN WJ. Activation of p38 signaling in the microglia in the nucleus accumbens contributes to the acquisition and maintenance of morphine-induced conditioned place preference. Brain, behavior, and immunity. 2012;26:318–25. doi: 10.1016/j.bbi.2011.09.017. [DOI] [PubMed] [Google Scholar]


