Abstract
Objectives
While 3 tesla (T) breast magnetic resonance imaging has increased in use over the past decade, there is little data comparing its use for assessing ductal carcinoma in situ (DCIS) versus 1.5T. We sought to compare the accuracies of DCIS extent of disease measures on pre-operative 3T versus 1.5T MRI.
Methods
This institutional review board-approved prospective study included 20 patients with ductal carcinoma in situ diagnosed by core needle biopsy (CNB) who underwent pre-operative breast MRI at both 3T (resolution=0.5×0.5×1.3 mm) and 1.5T (0.85×0.85×1.6 mm). All patients provided informed consent, and the study was HIPPA compliant. Lesion sizes and imaging characteristics (morphologic and kinetic enhancement) were recorded for the 3T and 1.5T examinations. Lesion size measures at both field strengths were correlated to final pathology, and imaging characteristics also were compared.
Results
Of the initial cohort of 20 patients with CNB-diagnosed DCIS, 19 underwent definitive surgery. Median DCIS sizes of these 19 patients were 6 mm (range: 0–67 mm) on 3T, 13 mm (0–60 mm) on 1.5T, and 6 mm (0–55 mm) on surgical pathology. Size correlation between MRI and pathology was higher for 3T (Spearman’s ρ=0.66, p=0.002) than 1.5T (ρ=0.36, p=0.13). In 10 women in which a residual area of suspicious enhancement was identified on both field strengths, there was agreement of morphologic description (NME vs. mass) in nine, and no significant difference in dynamic contrast enhanced kinetics at 3T compared to 1.5T.
Conclusions
Pre-operative breast MRI at 3T provided higher correlation with final pathology size of DCIS lesions compared to 1.5T, and may be more accurate for assessment of disease extent prior to definitive surgery.
Keywords: Ductal Carcinoma in Situ, pre-operative, Breast MRI, 3 tesla
Introduction
The use of 3 tesla (T) MRI systems has increased for dynamic contrast-enhanced (DCE) breast imaging over the past decade. The primary benefit of imaging at 3T over 1.5T is increased signal-to-noise ratio, which can allow higher spatial resolution 1. In addition, 3T MRI potentially could improve the conspicuity or contrast resolution of enhancing lesions compared to that seen at 1.5T, due to differential effects of higher field strength on T1 relaxation times of non-enhancing compared to gadolinium-enhancing tissue 2. This concept is supported by several studies showing a greater degree of enhancement for a given dose of gadolinium-based contrast with higher field strengths 3–5.
Accurate pre-operative determination of breast cancer extent can be a valuable guide to surgical planning. Multiple studies have shown that MRI is the most sensitive means of assessing the extent of malignancy, including the presence of multifocal and multicentric disease, in women newly diagnosed with breast cancer 6. This benefit may be particularly important for the pre-invasive malignancy ductal carcinoma in situ (DCIS) since positive surgical margins are a predictor of disease recurrence and pre-operative underestimation of DCIS extent by mammography has been found to occur in one quarter of women 7. Although breast MRI was initially thought to be less useful for evaluating DCIS than invasive breast cancer, it has subsequently been shown to have both a higher sensitivity for detection at screening 8 and correlation to final pathologic size 9, 10 of DCIS lesions. Thus, breast MRI used in conjunction with mammography may help guide clinical management of DCIS 11, 12. However, challenges remain assessing DCIS extent at 1.5T, perhaps because DCIS is more likely than invasive cancer to present on MRI as poorly defined non-mass enhancement (NME) 13–15. Accordingly, the improved spatial and contrast resolution offered by 3T imaging may be particularly useful for the evaluation of ductal carcinoma in situ (DCIS).
There are few studies to date examining the overall accuracy of breast MRI performed at 3T compared to 1.5T 16, 17 and only one prospective study that includes intra-individual comparisons 18. In their initial experience with DCE breast MRI performed at 1.5T and 3T in the same patients, Kuhl and colleagues found higher image quality scores and higher diagnostic confidence at 3T compared to 1.5T 18. Only three of the 37 women in their study had pure DCIS, and lesion sizes were not compared between field strengths. The purpose of this study was to compare the accuracies of extent of disease measures of DCIS at 3T versus 1.5T MRI, and to assess differences in imaging features between field strengths.
Methods
This Health Insurance Portability and Accountability Act (HIPAA)-compliant, Institutional Review Board-approved, prospective study was performed over a two-year period from June 2010 through May 2012. All patients provided written informed consent prior to participating in the trial.
Patient Population
Sixty-nine patients were diagnosed with pure DCIS (defined as DCIS with no foci of invasion) by core needle biopsy (CNB) and underwent a standard pre-operative 3T clinical breast MRI within 6 weeks of DCIS diagnosis during the study interval. Four patients were not approached due to barriers (language and/or cognitive) to obtaining informed consent. An additional six patients were ineligible because they underwent an additional biopsy after the 3T breast MRI was performed and before 1.5T imaging could be performed. Of the remaining 59 eligible patients, 20 consented to participate and underwent an additional pre-operative breast MRI at 1.5T within 4 weeks following 3T imaging. Thus, the final cohort included 20 consecutive patients who sequentially underwent both clinical 3T and research 1.5T breast MRI after CNB-diagnosis of pure DCIS and prior to surgical treatment.
Image-guided Biopsy Technique
Diagnosis of DCIS was made prior to MRI and surgery by image-guided CNB. CNB was performed with either a 9-gauge vacuum-assisted breast biopsy device (Eviva, Hologic, Bedford, MA, USA) for lesions biopsied under stereotactic guidance or a spring loaded 14-gauge device (Achieve, CareFusion, San Diego, CA, USA) for lesions biopsied under sonographic guidance.
MRI Acquisition
The MRI protocols followed guidelines established by the American College of Radiology breast MRI accreditation program 19. Both 3T and 1.5T MRI protocols included a 3D T1-weighted fast gradient echo-based DCE series with one pre-and three sequential post-gadolinium contrast-enhanced sequences. For both field strengths, DCE sequences were acquired with comparable scan durations of approximately 3 minutes each. A summary of the DCE-MRI technical parameters at both field strengths is provided in Table 1.
Table 1.
Technical parameters for the 3 tesla (T) and 1.5T imaging protocols utilized in this study. Note that the 3T protocol achieves 41% greater in plane spatial resolution than the 1.5T protocol in a similar overall scan time.
| Parameter | 3 tesla | 1.5 tesla |
| Plane | Axial | Axial |
| Breast coil type | 16 channel | 8 channel |
| Mode | 3D | 3D |
| Sequence type | Fast gradient echo | Fast gradient echo |
| Fat Suppression | SPAIR | SPIR |
| Parallel Imaging Factor | 2.7 R/L, 2.0 S/I | 1.5 R/L |
| TR (msec) | 5.9 | 5.6 |
| TE (msec) | 3 | 3 |
| Flip angle (degrees) | 10 | 10 |
| Field of View (cm2) | 22 A/P × 33 R/L | 36 A/P × 36 R/L |
| Matrix | 440 × 660 | 420 × 420 |
| In-plane voxel size (mm) | 0.5 | 0.85 |
| Slice thickness (mm) | 1.3 → 0.65 recon | 1.6 |
| Scan time (min) | 2:51 | 2:53 |
Abbreviations: SPAIR = Spectral Attenuated Inversion Recovery, SPIR = Spectral Presaturation with Inversion Recovery, R = right, L = left, A = anterior, P= posterior, recon = reconstructed
3T MRI was performed on a Philips Achieva Tx system (Philips Medical Systems, Best, the Netherlands) with a 16-channel breast coil (Mammotrak, Philips Healthcare) with the following DCE-MRI parameters: TR/TE=5.9/3 msec, flip angle=10°, spatial resolution = 0.5 × 0.5 × 1.3 mm. Post-contrast sequences were acquired with k space centered at 120, 300, and 480 seconds after contrast injection. 1.5T MRIs were performed on a GE LX system (GE Medical Systems, Waukesha, WI) with a Sentinelle 8-channel breast coil (Hologic, Bedford, MA) with the following DCE-MRI parameters: TR/TE=5.6/3 msec, flip angle=10°, spatial resolution = 0.85 × 0.85 × 1.6 mm. Post-contrast sequences were acquired with k space centered at 90, 270, and 450 seconds. Gadolinium contrast-material dose and delivery was the same for both protocols: 0.1 mmol/kg at 2 cc/sec followed by a 15 cc saline flush. Gadodiamide (Omniscan, GE Healthcare) was used for all examinations from June 2010 to November 2010 (6 of 20 cases) and Gadoteridol (ProHance, Bracco Diagnostics, Monroe Township, NJ) was used from November 2010 to May 2012 (14 of 20 cases). The same contrast agent was used at 3T and 1.5T for each study patient.
Breast MRI Interpretation
Each 1.5T and 3T breast MRI was prospectively interpreted by one of seven fellowship-trained radiologists specializing in breast imaging. Images were reviewed on a picture archiving and communication system (PACS) (Centricity, GE Healthcare, Fairfield, CT) with the assistance of commercially available computer-aided evaluation software (CADstream, Merge Healthcare, Chicago, IL). MRI interpretation was performed in conjunction with the clinical history and correlative breast mammograms and ultrasound, when available. For each patient, the 3T examination was interpreted prior to the 1.5T MRI, and a different radiologist interpreted each 1.5T study (blinded to the corresponding 3T images and report). All examinations were interpreted prior to surgery, and thus the radiologists were unaware of the final surgical extent of the DCIS lesion in question. Index lesion morphologic descriptors (NME or mass) and maximum sizes (defined as the largest value in anterior-posterior, cranial-caudal, or transverse dimension) were described at the time of radiologist interpretation using the first post-contrast series (including axial acquired as well as coronal and sagittal multi-planar reformats) in the DCE MR examination. In cases where no residual suspicious enhancement was described at the site of known DCIS, a maximum lesion size of 0 mm was assigned.
Kinetic enhancement data also were assessed using in-house software developed with ImageJ (NIH open source software). Semi-quantitative DCE kinetic parameters were calculated for whole tumor regions of interest (ROIs) based on the signal enhancement ratio (SER) method as previously described 20. The initial phase kinetic parameters characterized at the first post-contrast time-point were as follows: 1) peak enhancement (PE) (defined as the greatest signal intensity [SI] increase on the initial post-contrast MR sequence compared to the pre-contrast sequence for the 3 × 3 × 3 volume of voxels with maximum SI change within the lesion), 2) percentage of the lesion with medium enhancement (SI increase = 50–100% from pre-contrast), 3) percentage of the lesion with rapid enhancement (SI increase > 100% from pre-contrast). Delayed-phase kinetics characterizing the change in SI from the initial post-contrast series to the final post-contrast series were calculated on a voxel-by-voxel basis for all voxels demonstrating at least medium initial enhancement. The following delayed phase kinetic features were recorded: 1) percentage of the lesion with persistent delayed enhancement (SI increase > 10% between initial and final post-contrast acquisitions), 2) percentage of the lesion with plateau (change in SI ≤ 10%), and 3) percentage of the lesion with washout (SI decrease > 10%).
Clinical Data
Patient features, including age at diagnosis, modality guiding CNB, and times between diagnosis of the index DCIS lesion, MR examinations, and final surgical excision were recorded. Pathologic assessment of maximum lesion sizes, nuclear grade, and presence of invasive disease were obtained from the final surgical pathology reports.
Statistical Analysis
Differences in DCIS lesion sizes determined by pre-operative MRI and final surgical pathology were evaluated by Bland-Altman analysis. The accuracies of 3T and 1.5T MRI for measuring size of DCIS as determined by pathology were further assessed and compared by calculating Spearman correlation coefficients (ρ) and by Wilcoxon signed-rank test. MRI morphology types at 3T and 1.5T were compared by Fisher’s Exact test and differences in MRI kinetic features at 3T and 1.5T were evaluated by Wilcoxon signed-rank test. All computations were performed using statistical software (SAS version 9.3, SAS Institute Inc., Cary, NC), and p values less than 0.05 were considered statistically significant for all comparisons.
Results
The mean patient age was 54.9 (range 40–74) years. Seventeen women (85%) underwent stereotactic CNB and three (15%) underwent ultrasound-guided CNB for the pre-operative diagnosis of DCIS. All 20 patients subsequently underwent pre-operative MRI at both 3T and 1.5T; 19 patients underwent surgical resection at our institution, and one patient was lost to follow-up (no final surgical pathology available). Mean biopsy, MRI, and surgery times were as follows: between lesion biopsy and clinical 3T MRI = 17.1 days (range 4–34), between 3T and 1.5T MRIs = nine days (range 1–24), and between CNB and surgical resection = 50.5 days (range 20–93). Of the 19 cases with final surgical pathology available, 12 lesions were high nuclear grade, five were intermediate nuclear grade, and two had no residual disease (Table 2). Two of the 19 cases had foci (<2 mm) of invasive disease on final surgical excision, and both were associated with high nuclear grade DCIS lesions.
Table 2.
Summary of 3 tesla (T) MRI, 1.5T MRI, final pathology sizes, and final nuclear grade for all ductal carcinoma in situ (DCIS) lesions.
| DCIS Lesion | MRI | Surgical Excision | ||
|---|---|---|---|---|
| 3T Size (mm) | 1.5T Size (mm) | Pathologic Size (mm) | Nuclear Grade | |
| 1 | 5 | 5 | 6 | Intermediate |
| 2 | 0 | 0 | 6 | Intermediate |
| 3 | 7 | 4 | 4 | Intermediate |
| 4 | 59 | 0 | 50 | High |
| 5 | 0 | 0 | 8 | High |
| 6 | 0 | 0 | 2 | High |
| 7 | 0 | 20 | 0 | - |
| 8 | 24 | 28 | 15 | High |
| 9 | 0 | 0 | 0 | - |
| 10 | 67 | 60 | 55 | Intermediate |
| 11 | 0 | 30 | 3 | High |
| 12 | 0 | 0 | 11 | High# |
| 13 | 24 | 23 | 6 | High# |
| 14 | 7 | 6 | 6 | High |
| 15 | 41 | 43 | 6 | High |
| 16 | 49 | 48 | 45 | High |
| 17 | 57 | 53 | Unknown | Unknown |
| 18 | 24 | 20 | 28 | High |
| 19 | 0 | 23 | 15 | Intermediate |
| 20 | 0 | 0 | 2 | High |
Abbreviations: DCIS: ductal carcinoma in situ; T: tesla
focus of invasive disease <2mm in size was present on surgical excision specimen
3T vs. 1.5T MRI DCIS Size Comparisons
The median radiologist-assessed maximum DCIS size was 6 mm (range 0–67 mm) on 3T and 13 mm (range 0–60 mm) on 1.5T. The median pathologist-assessed maximum size for DCIS lesions was 6 mm (range 0–55 mm) on final surgical excision (Table 3). In the three cases in which a lesion was identified on 1.5T but no lesion was identified on 3T, one patient had 20 mm of NME at 1.5T and no residual DCIS on surgical excision, one had 30 mm of NME at 1.5T and 3 mm of high nuclear grade DCIS, and one patient had 23 mm of NME at 1.5T and 15 mm of intermediate nuclear grade DCIS. In the single case in which a lesion was visible on 3T but not identified on 1.5T, the patient had 59 mm of NME on 3T and 50 mm of high nuclear grade DCIS on surgical excision, Figure 1. Of the six cases in which no finding was described on both the 3T or 1.5T exams, the median final pathology size was 4 mm (range= 0–11 mm, Table 1).
Table 3.
Correlations of 3 tesla (T) and 1.5 T ductal carcinoma in situ sizes with final surgical pathology size.
| Maximum Size (mm) | Correlation to Pathology | |||
|---|---|---|---|---|
| Median | Mean | Range | ρ (p)* | |
| 3T MRI (n=20) | 6.0 | 18.2 | 0–67 | 0.66 (0.002)* |
| 1.5T MRI (n=20) | 13.0 | 18.2 | 0–60 | 0.36 (0.13) |
| Pathology (n=19) | 6 | 14.1 | 0–55 | |
Abbreviations: mm: millimeters, T: tesla, ρ = Spearman’s correlation coefficient (rho)
Indicates statistically significant difference
P values calculated by Wilcoxon signed-rank test
Fig. 1.
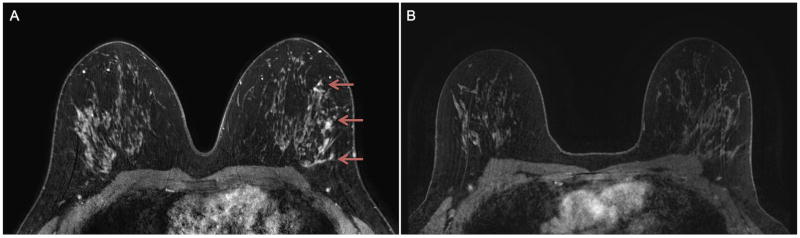
Same-patient initial post-contrast T1-weighted with fat suppression pre-operative MR images at A) 3 tesla (T) and B) 1.5T in a woman diagnosed with high nuclear grade ductal carcinoma in situ (DCIS) by core needle biopsy. Assessed DCIS lesion size at 3T was 59 mm (non-mass enhancement, arrows), while at 1.5T no lesion was described (size = 0 mm). 3T MRI extent better correlated with the final pathology size of 50 mm of high nuclear grade DCIS.
Bland-Altman plots of the 19 lesions that underwent surgery indicated MRI sizes more closely matched pathologic extents for 3T compared to 1.5T, illustrated by narrower 95% limits of agreement (Figure 2). Size correlation between MRI and pathology was higher for 3T (mean difference 7.5 mm, range 0–35 mm; ρ=0.66, p=0.002) than for 1.5T (mean difference 11.5 mm, range 0–50 mm; ρ=0.36, p=0.13, Table 3).
Fig. 2.
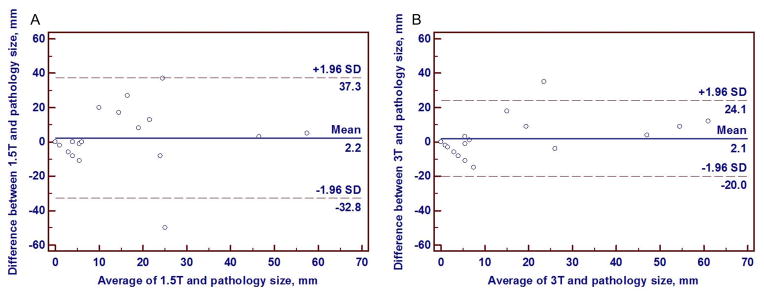
Bland-Altman plots of DCIS displaying the differences in lesion sizes versus pathology sizes on 1.5 tesla (T) breast MRI (A) and 3T breast MRI (B). Note that the limits of agreement with pathology size are narrower for 3T size (B) than 1.5T size (A), indicating greater agreement of 3T DCIS lesion sizes with final pathology sizes.
3T vs. 1.5T MR Imaging Features of DCIS
As a secondary aim, we further assessed the agreement of morphological and kinetic features for the subset of 10 lesions in which an area of enhancement suspicious for residual DCIS was described at both 3T and 1.5T. Of those 10 lesions, there were no significant differences in morphology (p=1.0), as nine were given the same morphologic descriptor (7 NME, 2 mass) on both field strengths (Table 4). In the single discordant case, the finding was described as a mass on 3T MRI and as NME on 1.5T MRI. Overall, there were no statistically significant differences (p>0.05 for all comparisons) in initial or delayed phase kinetic features at 1.5T compared to 3T (Table 4).
Table 4.
Imaging features of the 10 DCIS lesions described on both 3 tesla (T) and 1.5 T protocols.
| Imaging Feature | 3T MRI | 1.5T MRI | P value |
|---|---|---|---|
|
| |||
| Morphology | 1.0 | ||
| Mass | N=3 (30%) | N=2 (20%) | |
| Non-mass enhancement | N=7 (70%) | N=8 (80%) | |
|
| |||
| Initial enhancement | |||
| Mean peak enhancement (%) | 173.8 | 118.2 | 0.08 |
| Mean percent medium (%) | 66.7 | 80.2 | 0.12 |
| Mean percent rapid (%) | 33.3 | 19.8 | 0.12 |
|
| |||
| Delayed enhancement | |||
| Mean percent persistent (%) | 54.6 | 62.8 | 0.29 |
| Mean percent plateau (%) | 23.2 | 21.0 | 0.05 |
| Mean percent washout (%) | 22.2 | 16.1 | 0.22 |
Abbreviations: T: tesla
P values were determined by Wilcoxon signed-rank test except morphology comparison, which was determined by Fisher’s Exact test.
Conclusions
In this prospective study, the first comparing same-patient MRI features of newly diagnosed pure DCIS at both 3T and 1.5T field strengths, we found that maximum lesion size on 3T MRI more highly correlated with final pathology size than maximum lesion size on 1.5T examinations. This suggests pre-operative evaluation of extent of disease for DCIS is more accurate when performing breast MRI at higher field strength. We observed no significant differences in morphological and kinetic features between the field strengths despite higher spatial resolution and a hypothesized increase in contrast resolution at 3T.
Defining the optimal imaging and management algorithm of newly diagnosed DCIS remains an area of active research that has been identified to be of high priority for investigation by the National Institutes of Health 21. There is longstanding controversy regarding the ultimate benefit of treating all patients with newly diagnosed DCIS, and it is estimated that less than 50% of all lesions would affect a woman’s life if left untreated 22. Accordingly, it has been suggested that small DCIS lesions of lower biologic activity may require less treatment 23. However, because current risk-stratification methods do not accurately identify which DCIS lesions will remain indolent, uniform treatment of all lesions including complete surgical resection with clear margins remains the prevailing treatment approach. Thus, improved pre-operative determination of DCIS extent of disease with imaging could aid in treatment decision-making and potentially the evolution of optimal DCIS management 24.
Mammography is limited for the determination of the full extent of DCIS since it typically detects only calcified portions of the process. A study by Dillon and colleagues showed considerable underestimation of DCIS size (defined as imaging-to-pathology discrepancy of greater than 1 cm) with mammography in 40% of patients who had positive surgical margins compared to only 14% of patients with negative margins 7. Over the past decade due to an increased emphasis on spatial resolution, it has become clear that breast MRI is superior to mammography for both DCIS detection 8, 11, 25 and correlation with pathologic size 9, 10.
Imaging at 3T offers several theoretical advantages over 1.5T, including potential for higher spatial, temporal, and contrast resolution. Because kinetic features have not been found to be as useful as morphologic features for diagnostic breast MRI, particularly for DCIS 12, 13, 15, MRI protocols at our institution emphasize spatial resolution over temporal resolution. Accordingly, we hypothesized that MRI performed at 3T field strength with higher spatial resolution than achievable at 1.5T could incrementally improve assessment of DCIS extent of disease through improved morphologic depiction or conspicuity. Our finding of greater correlation of 3T size with pathology size supports this hypothesis. It is important to note that across institutions not all 3T protocols emphasize spatial resolution, which may limit the improved depiction of size found in our study 2.
As secondary analyses, we compared morphological and kinetic features for the 10 cases in which an area of residual suspicious enhancement was identified on MRI at the site of known cancer for both field strengths. We found that there was morphological agreement (mass vs. NME) in 90% of cases (9/10) between field strengths. This suggests that the effect of higher spatial resolution may have less of an impact on qualitative morphologic assessment than on accurate size depiction. In addition, we observed no significant differences in initial and delayed phase kinetic enhancement features between field strengths. It should be noted, however, that because only 10 lesions were compared between field strengths, this study was underpowered to identify differences in kinetic features and validation of our findings in larger cohorts is warranted.
Our study had several additional limitations. All MR examinations were performed after CNB. While this reflects the normal clinical care pathway for patients in the pre-operative setting, assessment of some of the lesions was likely impacted by post-biopsy changes such as hematoma formation and in some cases removal of the majority of the DCIS lesion. It is possible that this affected 3T assessments to a greater extent than at 1.5T since these exams were universally performed closer to the CNB. Due to a desire to not delay the normal treatment pathway for patients, we could not account for differences in background enhancement related to differences in menstrual cycle phase in pre-menopausal women, which could have impacted size measurements of non-mass enhancement lesions. Another potential limitation of the study is that the center of k space for the first post-contrast T1-weighted series was later for the 3T studies when compared to 1.5T (120 sec vs. 90 sec). Because it is known that DCIS lesions can reach peak enhancement later than invasive tumors, it is possible that this difference in timing could have contributed to the improved determination of lesion extent observed at 3T. Finally, this study was designed to determine the effect of using 3T MRI for DCIS characterization in the pre-operative setting compared to 1.5T. As a result, the effect on specific clinical outcomes (e.g. rate of negative margins or local recurrence) was not assessed.
In summary, this prospective study of women with newly diagnosed DCIS demonstrated that pre-operative MRI at 3T emphasizing greater spatial resolution provided higher correlation with final pathology size of DCIS lesions compared to 1.5T. This suggests that 3T MRI may be more accurate for assessment of extent of DCIS prior to surgery and could thereby improve treatment outcomes. Further investigation of the use of 3T MRI to pre-operatively characterize newly diagnosed DCIS is warranted given potential patient management advantages.
Highlights.
We compared sizes of known ductal carcinoma in situ (DCIS) on pre-operative breast MRI at 3 tesla (T) and 1.5 T with final pathology sizes.
DCIS sizes on 3T MRI correlated better with pathologic sizes than 1.5T MRI.
Imaging features of DCIS, including morphology and kinetics, were similar at 3T and 1.5T MRI.
Acknowledgments
Funding support: This study was supported by a Research Grant from Phillips Healthcare (Koninklijke Philips Electronics NV Research Grant) and a 2014–2016 RSNA Research Scholar Grant.
This study was supported by a Research Grant from Philips Healthcare (Koninklijke Philips Electronics NV Research Grant), a 2014–2016 Radiological Society of North America (RSNA) Research Scholar Grant, and a National Institutes of Health (NIH) R01 grant (R01CA151326).
Role of Funding Sources
Grant funding from Philips Healthcare provided support to perform additional MRI scans on patients in this trial as well as salary support for W.B.D. Grant funding from RSNA provided salary support for H.R. Grant funding from NIH provided salary support for S.C.P.
Footnotes
Previous presentations: This study was presented at the 2013 annual RSNA meeting in Chicago, IL, USA.
Conflict of Interest Statement
The authors of this manuscript declare relationships with the following companies: 1) GE Healthcare, grant support (DeMartini) and consultant (Lehman), 2) Philips Healthcare, grant support (DeMartini and Partridge), and 3) Bayer Healthcare, consultant (Lehman)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Soher BJ, Dale BM, Merkle EM. A review of MR physics: 3T versus 1. 5T. Magnetic resonance imaging clinics of North America. 2007;15(3):277–90. v. doi: 10.1016/j.mric.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Rahbar H, Partridge SC, DeMartini WB, Thursten B, Lehman CD. Clinical and technical considerations for high quality breast MRI at 3 Tesla. Journal of magnetic resonance imaging : JMRI. 2013;37(4):778–90. doi: 10.1002/jmri.23834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araoz PA, Glockner JF, McGee KP, et al. 3 Tesla MR imaging provides improved contrast in first-pass myocardial perfusion imaging over a range of gadolinium doses. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2005;7(3):559–64. doi: 10.1081/jcmr-200060622. [DOI] [PubMed] [Google Scholar]
- 4.Elster AD. How much contrast is enough? Dependence of enhancement on field strength and MR pulse sequence European radiology. 1997;7 (Suppl 5):276–80. doi: 10.1007/pl00006908. [DOI] [PubMed] [Google Scholar]
- 5.Rinck PA, Muller RN. Field strength and dose dependence of contrast enhancement by gadolinium-based MR contrast agents. European radiology. 1999;9(5):998–1004. doi: 10.1007/s003300050781. [DOI] [PubMed] [Google Scholar]
- 6.Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(19):3248–58. doi: 10.1200/JCO.2007.15.2108. [DOI] [PubMed] [Google Scholar]
- 7.Dillon MF, Mc Dermott EW, O’Doherty A, Quinn CM, Hill AD, O’Higgins N. Factors affecting successful breast conservation for ductal carcinoma in situ. Annals of surgical oncology. 2007;14(5):1618–28. doi: 10.1245/s10434-006-9246-y. [DOI] [PubMed] [Google Scholar]
- 8.Kuhl CK, Schrading S, Bieling HB, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007;370(9586):485–92. doi: 10.1016/S0140-6736(07)61232-X. [DOI] [PubMed] [Google Scholar]
- 9.Esserman LJ, Kumar AS, Herrera AF, et al. Magnetic resonance imaging captures the biology of ductal carcinoma in situ. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(28):4603–10. doi: 10.1200/JCO.2005.04.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcotte-Bloch C, Balu-Maestro C, Chamorey E, et al. MRI for the size assessment of pure ductal carcinoma in situ (DCIS): a prospective study of 33 patients. European journal of radiology. 2011;77(3):462–7. doi: 10.1016/j.ejrad.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Hwang ES, Kinkel K, Esserman LJ, Lu Y, Weidner N, Hylton NM. Magnetic resonance imaging in patients diagnosed with ductal carcinoma-in-situ: value in the diagnosis of residual disease, occult invasion, and multicentricity. Annals of surgical oncology. 2003;10(4):381–8. doi: 10.1245/aso.2003.03.085. [DOI] [PubMed] [Google Scholar]
- 12.Lehman CD. Magnetic resonance imaging in the evaluation of ductal carcinoma in situ. Journal of the National Cancer Institute Monographs. 2010;2010(41):150–1. doi: 10.1093/jncimonographs/lgq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansen SA, Newstead GM, Abe H, Shimauchi A, Schmidt RA, Karczmar GS. Pure ductal carcinoma in situ: kinetic and morphologic MR characteristics compared with mammographic appearance and nuclear grade. Radiology. 2007;245(3):684–91. doi: 10.1148/radiol.2453062061. [DOI] [PubMed] [Google Scholar]
- 14.Kim JA, Son EJ, Youk JH, et al. MRI findings of pure ductal carcinoma in situ: kinetic characteristics compared according to lesion type and histopathologic factors. AJR American journal of roentgenology. 2011;196(6):1450–6. doi: 10.2214/AJR.10.5027. [DOI] [PubMed] [Google Scholar]
- 15.Rosen EL, Smith-Foley SA, DeMartini WB, Eby PR, Peacock S, Lehman CD. BI-RADS MRI enhancement characteristics of ductal carcinoma in situ. The breast journal. 2007;13(6):545–50. doi: 10.1111/j.1524-4741.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 16.Elsamaloty H, Elzawawi MS, Mohammad S, Herial N. Increasing accuracy of detection of breast cancer with 3-T MRI. AJR American journal of roentgenology. 2009;192(4):1142–8. doi: 10.2214/AJR.08.1226. [DOI] [PubMed] [Google Scholar]
- 17.Lourenco AP, Donegan L, Khalil H, Mainiero MB. Improving outcomes of screening breast MRI with practice evolution: Initial clinical experience with 3T compared to 1.5T. Journal of magnetic resonance imaging : JMRI. 2013 doi: 10.1002/jmri.24198. [DOI] [PubMed] [Google Scholar]
- 18.Kuhl CK, Jost P, Morakkabati N, Zivanovic O, Schild HH, Gieseke J. Contrast-enhanced MR imaging of the breast at 3.0 and 1. 5 T in the same patients: initial experience. Radiology. 2006;239(3):666–76. doi: 10.1148/radiol.2392050509. [DOI] [PubMed] [Google Scholar]
- 19.American College of Radiology. [Accessed: July 14, 2014];Breast MRI Accreditation Program. Available from: http://www.acr.org/Quality-Safety/Accreditation/BreastMRI.
- 20.Partridge SC, Vanantwerp RK, Doot RK, et al. Association between serial dynamic contrast-enhanced MRI and dynamic 18F-FDG PET measures in patients undergoing neoadjuvant chemotherapy for locally advanced breast cancer. Journal of magnetic resonance imaging : JMRI. 2010;32(5):1124–31. doi: 10.1002/jmri.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Journal of the National Cancer Institute Monographs; Proceedings of the National Institutes of Health State-of-the-Science Conference, Diagnosis and Management of Ductal Carcinoma In Situ; September 2009; 2010. pp. 111–222. [PubMed] [Google Scholar]
- 22.Allred DC. Ductal carcinoma in situ: terminology, classification, and natural history. Journal of the National Cancer Institute Monographs. 2010;2010(41):134–8. doi: 10.1093/jncimonographs/lgq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCormick B. Radiation Therapy for Duct Carcinoma in Situ: Who Needs Radiation Therapy, Who Doesn’t? Hematology/oncology clinics of North America. 2013;27(4):673–86. doi: 10.1016/j.hoc.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Rahbar H, Partridge SC, Demartini WB, et al. In vivo assessment of ductal carcinoma in situ grade: a model incorporating dynamic contrast-enhanced and diffusion-weighted breast MR imaging parameters. Radiology. 2012;263(2):374–82. doi: 10.1148/radiol.12111368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baur A, Bahrs SD, Speck S, et al. Breast MRI of pure ductal carcinoma in situ: Sensitivity of diagnosis and influence of lesion characteristics. European journal of radiology. 2013 doi: 10.1016/j.ejrad.2013.05.002. [DOI] [PubMed] [Google Scholar]


