Abstract
Background
Little is known regarding mechanisms regulating ethanol self-administration during adolescence or if the mechanisms differ from adults. One of the best models of abuse liability is operant self-administration. Therefore, we characterized operant sweetened ethanol self-administration behavior in adolescent and adult rats.
Methods
Adolescent (36 days old at first ethanol exposure) and adult male Long-Evans rats were first trained to self-administer 10% sucrose (10S) in an appetitive/consummatory operant model for 1 week, and then the drinking solution was switched to 10% sucrose plus 10% ethanol (10S10E) for 2 weeks. Next, rats were switched to a fixed ratio 2 schedule, and this was followed by one session using a progressive ratio schedule of reinforcement. Lastly, rats were tested for cue-induced reinstatement of lever pressing behavior under extinction conditions after 13 days of abstinence. Blood ethanol concentration (BEC) of sweetened ethanol (self-administered or intragastric (IG) administration of 1 g/kg) was determined via gas chromatography. Control rats drank only 10S.
Results
Consumption of sweetened ethanol was not different between adolescents and adults under any schedule tested, reaching 1 g/kg in 20 min in the appetitive/consummatory model. Appetitive behavior directed at sweetened ethanol was less focused in adolescents vs. adults. No age differences were found in motivation for sweetened ethanol. Cue-induced reinstatement of ethanol-seeking behavior after abstinence also did not differ by age. In control groups, no age difference was found in appetitive behavior or the amount of sucrose consumed, although adults exhibited greater cue-induced reinstatement. BEC after self-administration or IG administration of sweetened ethanol was higher in adults than adolescents.
Conclusions
Consumption and motivation for sweetened ethanol is similar in adolescents and adults, although adolescents are more vulnerable to the effects of ethanol consumption on appetitive behavior. The IG results suggest larger volume of distribution and higher first pass metabolism of sweetened ethanol in adolescents vs. adults, which may limit the reinforcing effects of ethanol in some adolescents. Overall, we have begun to establish an operant sweetened ethanol self-administration model in adolescent rats.
Keywords: operant, juvenile, relapse, development, periadolescent
Introduction
Alcohol is among the most widely abused drug during adolescence and young adulthood. Epidemiological studies show rates of heavy consumption and binge drinking exceed any other time in life, and rates of alcohol abuse and dependence increase the younger a person starts drinking (SAMHSA, 2011). However, the mechanisms that contribute to alcohol drinking during adolescence are still largely unknown. Therefore, research is needed using relevant animal models of adolescent alcohol self-administration. The focus of this study is voluntary alcohol drinking that starts during early adolescence; thus, we choose postnatal day (P) 36 as the first day our male adolescent rats had the opportunity to self-administer sweetened ethanol, which should correspond roughly to just before puberty starts and early adolescence in male rats (Schneider, 2008; Spear, 2000; McCutcheon and Marinelli, 2009; Lewis et al., 2002).
Most, but not all, non-operant rodent models appear to mirror some aspects of epidemiological human data, with higher ethanol intake in early adolescence compared with adulthood in males (Doremus et al., 2005; Vetter et al., 2007; Walker et al., 2008; Tambour et al., 2008; Garcia-Burgos et al., 2009; Bell et al., 2011; Broadwater et al., 2011); however, some studies show no age difference (Schramm-Sapyta et al., 2010) or less intake (Siegmund et al., 2005) in adolescents compared to adults, and results can depend on genetic background (Moore et al., 2010; Dhaher et al., 2012). To our knowledge, direct comparisons between adolescents and adults using alcohol and operant models have not been performed, even though operant models are thought to be among the most behaviorally relevant measure of drug and alcohol abuse liability (Schuster and Thompson, 1969; Meisch, 1982; Ator and Griffiths, 2003). Thus, our first aim was to characterize operant self-administration of sweetened ethanol in adolescents, and compare with adult male rats using multiple schedules of access and reinforcement, including a progressive ratio (PR) test of the motivation to drink, and a test of cue-induced reinstatement of ethanol-seeking after abstinence. In addition, we also examined operant self-administration of a sucrose-only solution as a control for inherent age differences in operant behavior.
We compared operant self-administration of sweetened alcohol between adolescents and adults using a model with the following advantages: 1) relatively quick acquisition of drinking behavior to promote stable operant self-administration during the short period of adolescence in rodents, 2) separation of appetitive and consummatory phases of behavior (Samson et al., 1998), and 3) use of a sweetened ethanol solution to model the fact that a large proportion of adolescents drinking alcohol consume flavored or sweetened solutions, e.g. wine coolers or malt beverages (Johnston et al., 2013). We also determined blood ethanol concentrations (BEC) in adolescent rats during operant sweetened ethanol self-administration, and in ethanol-naïve rats after intragastric (IG) administration of sweetened ethanol at a dose comparable to the average dose our self-administering rats were achieving.
Methods
Animals
Male Long-Evans rats (Charles River, Raleigh, NC) arrived at postnatal day (P) 22 (45-67 g; shipped with lactating dam) or P60-65 (290-301 g). Rats were housed by age in groups of two (25°C; 12 h light/12 h dark; lights on 0700 h), and handled daily for at least one week before testing. Food and water were available ad libitum in home cages, except as noted below for brief food or water deprivation. All procedures complied with the NIH Guide for Care and Use of Laboratory Animals (7th Ed., 1998) and were approved by the IACUC of the University of Texas at Austin.
Drugs
Drinking solutions (10% sucrose (w/v) (10S) or 10% sucrose + 10% ethanol (v/v) (10S10E)) were made from 95% ethanol (AAPER, Shelbyville, KY), ultra-pure sucrose (MP Biomedicals, Solon, OH), and distilled water.
Equipment
Self-administration was conducted in standard rat operant conditioning chambers housed in sound-attenuating cubicles with the exterior doors removed (MedAssociates, St Albans, VT). Each chamber was equipped with one retractable lever (6 cm above the grid floor; 4.5 cm-wide; calibrated to depress at approximately 10.5 g). After the appropriate lever response requirement occurred, a retractable ball-point drinking spout (Ancare Corp., Bellmore, NY) attached to a 50 ml conical vial entered the chamber adjacent to the lever (6 cm above the grid floor). The metal grid floor bars were connected to the metal spout of the drinking bottle through a lickometer circuit. An interior chamber light and sound-attenuating fan were activated at the start of each session, and lever presses activated a cue light 4 cm above the lever for 0.1 sec. Operant chamber components and data collection were controlled via MedAssociates software (Med PC IV). Consumption of drinking solutions was monitored by the lickometer circuit, and by weighing the bottles before and after sessions accounting for spillage and evaporation.
Operant behavioral testing
Operant sessions occurred across four phases (Fig. 1), and rats were separated into two drinking groups (10S and 10S10E) after the initial training phase: 1) initial training of all rats on 10S; 2) appetitive/consummatory model; 3) fixed ratio (FR) and progressive ratio (PR) testing; and 4) cue-induced reinstatement of seeking behavior after forced abstinence.
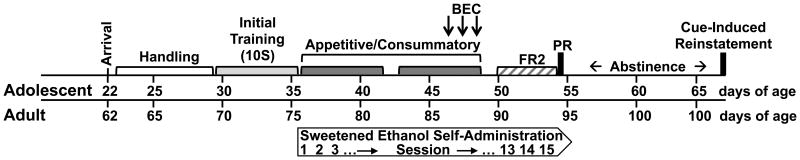
Figure 1.
Timeline of operant sweetened ethanol self-administration in adolescent and adult rats. Adolescents arrived in the lab at postnatal day (P) 22, adults ∼P62. All rats were initially trained on a 10% sucrose solution (10S). Sweetened ethanol (10% sucrose + 10% ethanol, 10S10E) self-administration began at P36 for adolescents, ∼P76 for adults. Rats were tested on appetitive/consummatory, fixed ratio two (FR2), progressive ratio (PR), and cue-induced reinstatement (extinction responses after 13 days of abstinence) models of operant self-administration. A subset of rats had blood ethanol concentration (BEC) measured after operant self-administration. Control rats drank only 10S throughout the experiment.
Initial training on 10S and the transition to the appetitive/consummatory model
Training and testing procedures were similar to our previous study with adult rats (Carrillo et al., 2008). Operant sessions (20 min) occurred once a day (at the same time 1000-1500 h), five days per week (except as noted below). Rats were water deprived (22 h/d, water given after the session) during the first 3 days of training. Initial training was a habituation session (40 min) with the lever and drinking spout inserted into the chamber for the entire session; lever presses had no consequence, and only the 10S solution was freely available to drink. Approximately 24 h later, all rats were trained to lever press for access to the 10S solution on a fixed ratio one (FR1) schedule of reinforcement, with each lever press yielding 10 sec access to the drinking spout. For most rats (4 adolescents were excluded, see statistics section), a reliable lever-pressing response for 10S occurred within 1-2 days using one 40 min session per day; once established, rats were given water ad libitum for the remainder of the experiment. Subsequently, all rats had four sessions of 10S self-administration, during which rats were gradually habituated to a 5 min wait period which preceded access to the lever and drinking solution, and the lever response requirement was progressively increased to four (RR4; response requirement of 4). The appetitive phase of testing consisted of a 5 min wait period, and measures of latency to press the lever the first time and time to complete the lever response requirement. When the response requirement was completed, the lever retracted, the drinking spout extended into the chamber, and rats had access to the drinking solution for 20 min, i.e. the consummatory phase. After the seventh training session on 10S, the ethanol groups were switched to the 10S10E drinking solution, while the sucrose controls remained drinking 10S throughout the study. Appetitive/consummatory sessions continued for two weeks (5 min wait period, RR4, 20 min drink period), excluding weekends. Adolescent rats were at P36 at initial ethanol exposure.
Fixed and progressive ratio (PR)
The next phase consisted of a transition from the appetitive/consummatory model to testing the motivation to drink using a PR schedule of reinforcement. We implemented a FR2 schedule of reinforcement for 4 days, where every two lever presses resulted in 10 sec access to the drinking solution (20 min sessions). The next day the PR session had a lever press requirement that increased progressively within the session, for a maximum of 60 min. The progression of response requirements on the PR schedule started with two lever presses and was similar to Walker and Koob (2007).
Cue-induced reinstatement of ethanol-seeking after abstinence
This consisted of a single test of ethanol (or sucrose)-seeking behavior by measuring operant lever press responding after 13 days of forced abstinence in their home cage. A 20 min test occurred under extinction conditions where the rats could see and smell the drinking solutions but they could not drink, and lever presses only resulted in illumination of the cue light.
Blood ethanol concentration (BEC) after operant self-administration and intragastric (IG) administration
In a subset of rats self-administering the 10S10E solution, we sampled blood at 12 min (allowed 5 min to drink), 22 min (allowed 15 min to drink), or 35 min (allowed 20 min to drink) after the start of the operant session on days 8-10 of the appetitive/consummatory phase (Fig. 1). Blood was collected from the saphenous vein while under isoflurane anesthesia. The ethanol content in 10 μl of blood was determined via gas chromatography using published procedures (Carrillo et al., 2008).
For the operant self-administration experiment, BEC was analyzed using an age (between subjects factor) X sampling time (12, 22, or 35 min after start of drinking; between subjects factor) ANOVA, and a Pearson correlation analysis between ethanol dose consumed and BEC for each age group separately. For the IG study, we administered a bolus dose (1.0 g/kg) of sweetened ethanol to ethanol-naïve adolescent (P44-46, 210-230 g) and adult (approximate P84-92, 430-440 g) rats after overnight food restriction. Blood samples were collected between 10-120 min from saphenous veins (3-6 samples per rat). The BEC was determined via gas chromatography (same as above). Peak BEC and time of peak BEC were estimated by visual inspection of the BEC time courses at 10, 20, 30, 60, 90, 120 min post-administration, and the slope of the linear portion of the ethanol elimination time course was determined. Target sample times are indicated on Fig. 7A, but not all samples were taken at the exact target time. For presentation and statistical analysis the sample times were binned around the target time.
Figure 7.
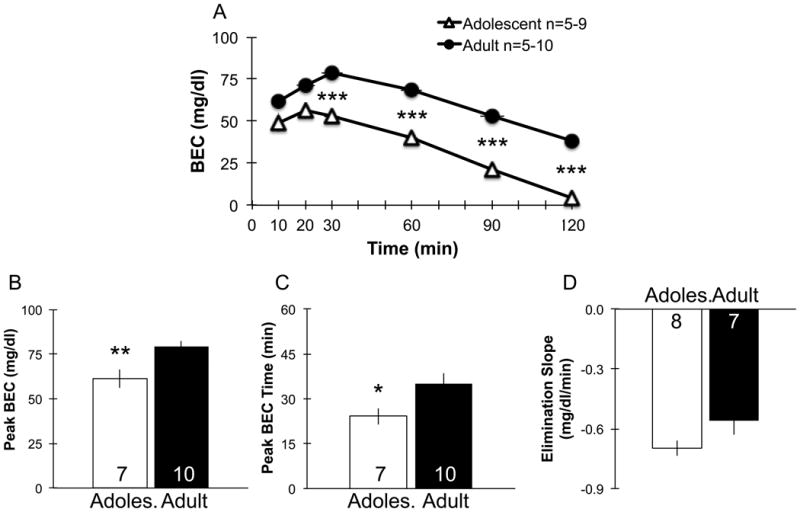
Blood ethanol concentration (BEC) after intragastric (IG) administration of sweetened ethanol (10S10E) in ethanol-naïve adolescents and adults. Adolescents had a significantly lower BEC at 30, 60, 90 and 120 min post-administration compared with adults (*** denotes p<0.001; A). Adolescents exhibited significantly lower estimated peak BEC (** p<0.01; B), and estimated peak BEC occurred at a significantly sooner time point (* p<0.05; C), compared to adults. The linear portion of the ethanol elimination time course was not significantly different in adolescents compared with adults (D). Adoles. = Adolescent. Group numbers at each time point range from 5-9 for adolescents, and 5-10 for adults for A; group numbers are displayed inside bars for B-D. Bars and points represent mean ± SEM. For A, vertical error bars are obscured by the size of the symbols, and horizontal error bars indicate ± SEM of sampling time.
Statistics
Eighty-nine rats were included (Table 1). Four adolescents did not establish stable self-administration and were excluded; one drank 10S, and three drinking 10S10E did not consume on average ≥ 0.3 g/kg ethanol for the last five appetitive/consummatory sessions.
Table 1. N values for different experimental phases.
| Phase | Final Drinking Solution | ||||
|---|---|---|---|---|---|
| 10S10E Age |
10S Age |
||||
| Adolescent | Adult | Adolescent | Adult | ||
| 1 | Acquisition of operant behavior | 33* | 22* | 9* | 6* |
| 2 | Appetitive/Consummatory model | 33 | 22 | 9 | 6 |
| 3 | BEC after operant self-administration | 26 | 16 | -- | -- |
| 4 | PR schedule of reinforcement | 8 | 6 | 9 | 6 |
| 5 | Cue-induced reinstatement of ethanol- or sucrose-seeking behavior after abstinence | 8 | 6 | 9 | 6 |
| 6 | BEC after IG administration of sweetened ethanol | 9 | 10 | -- | -- |
-
-Phases 1-5 used rats from an initial pool of 33 adolescents and 22 adults for 10S10E, and 9 adolescents and 6 adults for 10S
-
-* All rats were trained on 10S during phase 1 Acquisition of operant behavior
-
-10S10E=10% sucrose + 10% ethanol solution; 10S=10% sucrose solution; BEC=Blood ethanol concentration; PR=Progressive ratio; IG=Intragastric
Behavioral analyses
Robust differences in behavior exist between rats drinking a sweetened ethanol solution (10S10E) compared with a sucrose only solution (10S), therefore our a priori analyses of possible age differences in drinking behavior between adolescents and adults occurred in 10S10E and 10S drinking groups separately; the exception to this was analysis of the cue-induced reinstatement behavior in which drinking group was included as a factor. Self-administration parameters were analyzed individually for each drinking group using two-way mixed-measures analyses of variance (ANOVA), with age (adolescent and adult) as the between-subjects factor, and session as a within-subjects repeated measure. We compared acquisition of the operant response between age groups on the last session of FR1 and on the last session of training on the appetitive/consummatory model (all rats drinking 10S; independent samples t-tests). During this acquisition of operant behavior phase, the number of subjects for the t-tests are variable due to technical issues on the last FR1 session (12 adolescents and 8 adults did not have consumption measured; broken lickometer for one adolescent), and during the last training day four adolescents failed to complete the lever response requirement. Parameters measured during the FR2 sessions and PR test were compared using independent samples two-tailed t-tests. For the cue-induced reinstatement test, lever pressing was analyzed using an age (between subjects factor) × drinking solution (10S10E and 10S; between subjects factor) ANOVA.
BEC analyses
For the operant self-administration experiment, BEC was analyzed using an age (between subjects factor) X sampling time (12, 22, or 35 min after start of drinking; between subjects factor) ANOVA, and a Pearson correlation analysis between ethanol dose consumed and BEC for each age group separately. For the IG experiment, not every rat had blood samples taken at each of the six time points. Therefore, we compared age groups at each time point separately using independent samples t-tests (n=5-10/time point/age group). Similarly, for some individual rats we were not able to confidently determine peak BEC or time of peak BEC. For all analyses, follow-up post hoc comparisons with Bonferroni's correction were used as appropriate, and results were significant if p<0.05.
Results
Acquisition of operant behavior
Most adolescents (except 4) and all adults acquired operant self-administration behavior using 10S as the reinforcer. We use the time it takes a rat to go from pressing the lever to drink from the spout as a measure of associating an operant response with a reinforcer, and adolescents and adults did not differ (2.6 ± 0.3 vs. 2.0 ± 0.6 sec with n=28-41; t67=0.9, ns). Following acquisition of operant responding on a FR1 schedule, rats began training on the appetitive/consummatory model (no water deprivation). On the last training day, adolescents exhibited a significantly longer latency to first press (42 ± 21 vs. 12 ± 7 sec with n=28-38; t64=2.6, p<0.05) and longer times to complete the lever response requirement compared to adults (122 ± 22 vs. 15 ± 4 sec with n=28-38; t64=2.8, p<0.05), however, the amount drank (5.6 ± 0.7 vs. 4.7 ± 0.5 g/kg sucrose with n=28-38; t64=1.7, ns) and the time between pressing and licking did not differ by age on the last training day (4.2 ± 1.4 vs. 1.8 ± 0.2 sec with n=28-38; t64=1.7, ns).
Appetitive/consummatory model
Adolescents displayed differential appetitive behavior directed towards sweetened ethanol compared to adults (Fig. 2). Adolescents had longer latencies to first lever press (main effect of age F1,52=10.1, p<0.01; Fig. 2A), and took longer to complete the lever response requirement compared with adults (main effect of age F1,52=9.5, p<0.01; Fig. 2C). In the control sucrose groups, no significant age differences occurred in either of these measures of appetitive behavior (latency, main effect of age F1,13=2.0, ns; Fig. 2B; or time to complete presses, main effect of age F1,13=4.6, ns; Fig. 2D).
Figure 2.
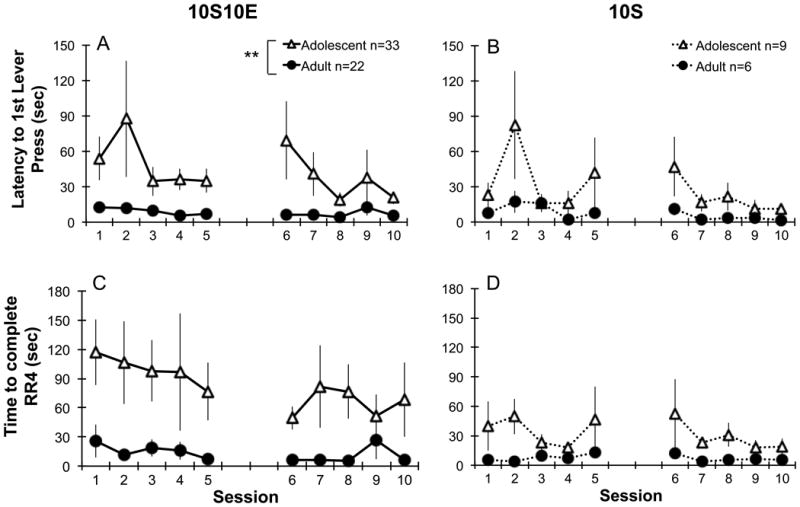
Appetitive behavior in adolescents and adults during operant self-administration of sweetened ethanol (left, 10S10E: A, C) or sucrose only solutions (right, 10S: B, D) using an appetitive/consummatory model. The latency to first lever press (A) and time to complete the response requirement of four presses (RR4; C) were significantly longer in adolescents compared with adults during operant self-administration of sweetened ethanol (** p<0.01). During sucrose only self-administration, adolescents and adults did not differ in latency to the first lever press or time to complete the response requirement (B, D). The gap between sessions 5 and 6 denote a weekend with no operant sessions taking place. 10S10E = 10% sucrose +10% ethanol, 10S = 10% sucrose. Points represent mean ± SEM.
The consumption of sweetened ethanol did not differ between adolescents and adults (Fig. 3A). The ethanol dose consumed increased from 0.3 g/kg to 1.0 g/kg by the 5th session, and there was no effect of age (F1,52=0.7, ns; Fig. 3A). Sweetened ethanol intake significantly increased over sessions (F9,468=13.1, p<0.001), with posthoc comparisons confirming session 1 was different than the other 9 sessions (p<0.001). Control sucrose intake (g/kg) was stable (no effects of session or interactions, ns) across the 10 sessions with no effect of age (F1,13=0.1, ns; Fig. 3B).
Figure 3.
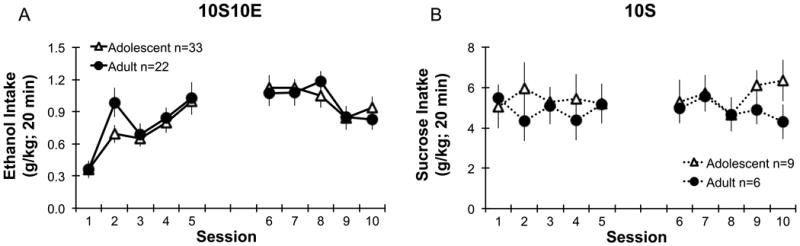
Amount of ethanol or sucrose consumed in adolescents and adults during operant self-administration of sweetened ethanol (left, 10S10E: A) or sucrose only solutions (right, 10S: B) using an appetitive/consummatory model. Adolescents and adults did not differ in ethanol (A) or sucrose (B) intake (g/kg in 20 min). The gap between sessions 5 and 6 denote a weekend with no operant sessions taking place. 10S10E = 10% sucrose +10% ethanol, 10S = 10% sucrose. Points represent mean ± SEM.
Blood ethanol concentration (BEC) after operant self-administration
In a subset of rats, we took blood samples at three different times after the beginning of the drink period in the appetitive/consummatory model (Fig. 1 indicates timing of the sampling days). Figure 4A indicates that overall consumption in adolescent and adult rats was similar among the different groups of rats (F1,36=0.1 for main effect of age, ns; F2,36=0.8 for main effect of sample time, ns). However, BECs were significantly higher in adults compared to adolescents (Fig. 4B; main effect of age, F1,36=4.7, p<0.05). There was a significant correlation between BEC and ethanol dose consumed in both age groups (Fig. 4C, adolescents, F1,24=20.4, p<0.05; adults, F1,14=14.7, p<0.05). Importantly, a large number of adolescent rats had little or no ethanol in their blood after drinking (11 out of 26 rats with BECs < 5 mg/dl) while all of the adult rats showed BECs > 5 mg/dl.
Figure 4.
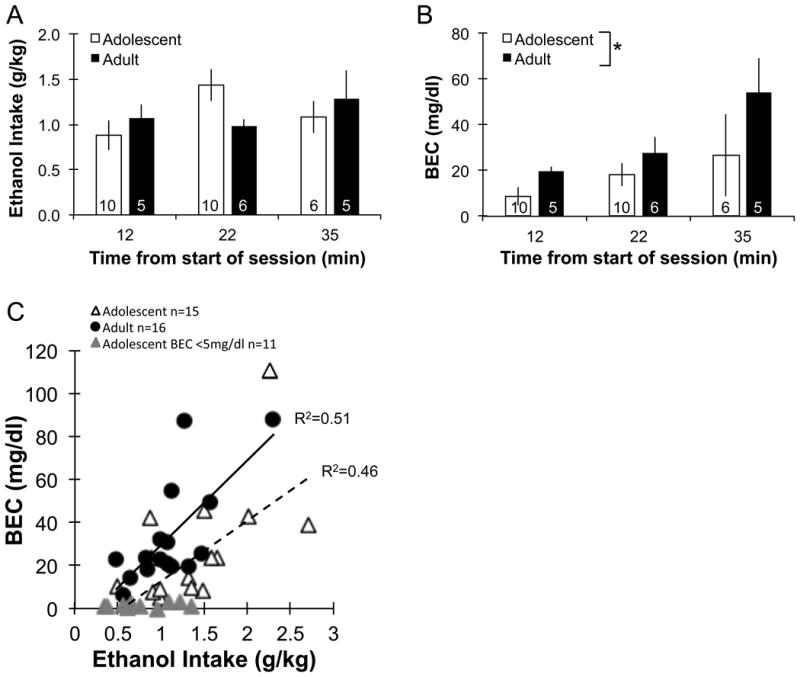
Blood ethanol concentration in adolescents and adults during operant self-administration using an appetitive/consummatory model. Sweetened ethanol intake (g/kg) did not differ between adolescents and adults (A); rats were removed from the operant chamber after 5, 15 or 20 min of self-administration. Blood ethanol concentrations (BEC) sampled at 12, 22 or 35 min after start of drinking were significantly higher in the adults compared to the adolescents (B; * p<0.05). BEC significantly correlated to the amount of ethanol consumed (g/kg) in both age groups (C; adolescent R2 = 0.46, p<0.05; adult R2 = 0.51, p<0.05). However, a large number of adolescents had little or no ethanol in their blood after drinking (11 out of 26 rats with blood ethanol concentrations < 5 mg/dl; grey triangles). In contrast all of the adult rats showed blood ethanol concentrations > 5 mg/dl. Group numbers are displayed inside bars for A and B. Bars represent mean ± SEM.
PR schedule of reinforcement
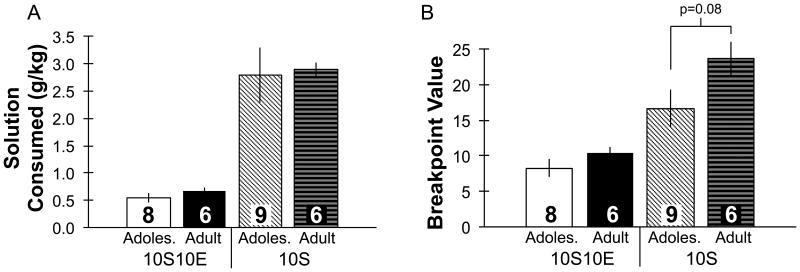
No age differences occurred in the motivation to consume sweetened ethanol using a PR schedule of reinforcement (Fig. 5). Prior to transitioning to PR testing, no differences occurred between groups during FR2 testing (ethanol intake (g/kg) on the last FR2 day: adolescents 0.6 ± 0.1, adults 0.7 ± 0.1; sucrose intake (g/kg) on the last FR2 day: adolescents 3.4 ± 0.8, adults 3.8 ± 0.3). During PR testing, adolescents and adults consumed a similar amount of ethanol (t12 = -0.9, ns; Fig. 5A), and reached a similar breakpoint value (t12 = -1.2, ns; Fig. 5B). In the control 10S groups, no age difference occurred in the amount of sucrose consumed (t13=-0.2, ns; Fig. 5A), however, there was a trend for adults to exhibit increased breakpoint values (t13=-1.9, p=0.08; Fig. 5B).
Figure 5.
Operant self-administration of sweetened ethanol or sucrose only solutions in adolescents and adults using a progressive ratio model. Adolescent and adults did not differ in the amount of ethanol or sucrose consumed (g/kg; A) or in the breakpoint values (B) obtained (for 10S groups adults trended toward higher breakpoint values compared to adolescents, p=0.08); values for intake and breakpoint were greater in sucrose compared with ethanol groups regardless of age. 10S10E = 10% sucrose +10% ethanol, 10S = 10% sucrose, Adoles. = Adolescent. Group numbers are displayed inside bars. Bars represent mean ± SEM.
Cue-induced reinstatement of ethanol- or sucrose-seeking behavior after abstinence
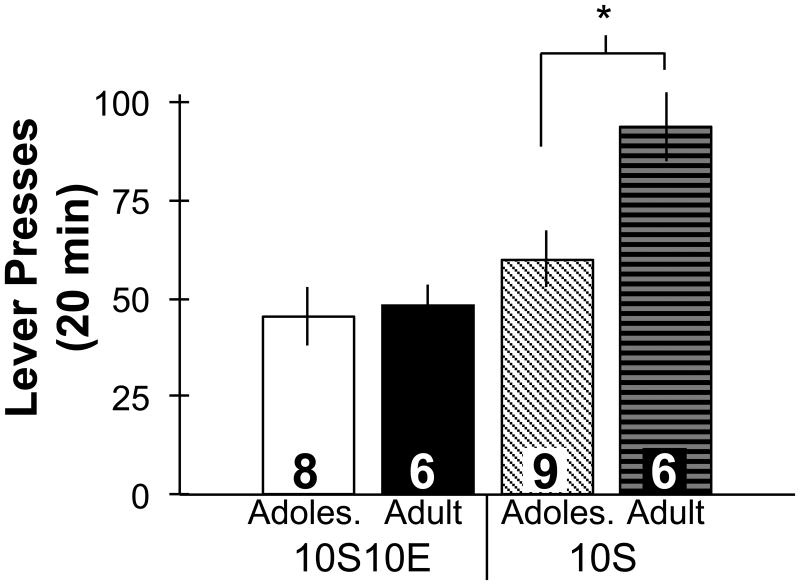
Cue-induced reinstatement of ethanol-seeking behavior did not differ by age, but adults seeking sucrose exhibited more robust reinstatement of behavior compared to adolescents (Fig. 6). Analysis of lever presses revealed a significant interaction between age and drinking solution (F1,25=4.1, p=0.05). Posthoc comparisons confirmed that in ethanol-experienced groups no age difference occurred in the number of lever presses (t12=-0.3, ns), while the adult-onset sucrose group exhibited a significantly greater number of lever presses compared to the adolescent-onset sucrose group (t13=-2.9, p<0.05).
Figure 6.
Cue-induced reinstatement of seeking behavior in adolescents and adults after 13 days of abstinence from operant self-administration of sweetened ethanol or sucrose only solutions. The number of lever presses in 20 min did not differ between adolescent and adult ethanol-experienced groups (10S10E). The adult-onset sucrose group exhibited significantly greater number of lever presses compared to the adolescent-onset sucrose group (* p<0.05; 10S). 10S10E = 10% sucrose +10% ethanol, 10S = 10% sucrose, Adoles. = Adolescent. Group numbers are displayed inside bars. Bars represent mean ± SEM.
Blood ethanol concentration (BEC) after intragastric (IG) administration of sweetened ethanol
Adolescents had a significantly lower BEC at 30, 60, 90 and 120 min post-administration compared with adults (t =-4.6 to -8.0 d.f.=8 to 16, p<0.05 for 30-120 min time points, Fig. 7A). Also, adolescents exhibited significantly lower estimated peak BECs compared with adults (t17=-3.2, p<0.01; Fig. 7B). The time at which the peak BEC occurred was significantly shorter compared to adults (t15=-2.3, p<0.05; Fig. 7C). The linear portion of the ethanol elimination time course was slightly steeper in adolescents, but this did not reach statistical significance (t13=-1.8, p=0.10; Fig. 7D).
Discussion
We have begun to establish an operant model of sweetened ethanol self-administration in adolescent rats. Utilizing appetitive/consummatory, fixed ratio, and progressive ratio schedules of reinforcement, we found that adolescents do not differ from adults in sweetened ethanol intake or motivation to consume sweetened ethanol. Although consumption is not different, adolescents display some differential appetitive behavior directed towards sweetened ethanol compared to adults. Also, cue-induced reinstatement of sweetened ethanol-seeking behavior after 13 days of abstinence was not different between adolescents and adults. Our results do suggest the pharmacokinetics of ethanol combined with a sweet vehicle are different in adolescents compared to adults. Adolescents exhibited lower BEC after operant self-administration, and following intragastric administration several parameters suggest adolescents have increased volume of distribution and higher first pass metabolism of sweetened ethanol. Lastly, our control groups show that general age differences in sucrose intake do not occur, although motivation to consume and seek sucrose was higher in adults compared with adolescents.
The present results suggest that appetitive behavior directed towards sweetened ethanol self-administration is less focused in adolescents compared to adults. In rats directing operant behavior towards obtaining sweetened ethanol, adolescents exhibited significantly longer latencies to first lever press and took longer to complete the response requirement of four, compared to adults. These appetitive differences occurred in the absence of ethanol in the body because lever pressing occurred before consumption. Similar age differences in appetitive behavior were found prior to the introduction of ethanol to the drinking solution during training on the sucrose only solution. In contrast, appetitive behavior between adolescents and adults was not statistically different during operant self-administration of sucrose alone after the first week of training. Together these data suggest that experience with ethanol consumption is one factor that contributed to the appetitive differences between adolescents and adults. Previous work has shown that ethanol alters performance of various cognitive tasks in adolescents to a greater degree compared with adults. With ethanol on board, adolescents exhibit less discrimination of ethanol-associated cues using a Pavlovian conditioned approach task (Anderson and Spear, 2014), and acquisition and subsequent retention of spatial memory is more potently inhibited by acute exposure to ethanol in adolescent rats, compared with adults (Markwiese et al., 1998; Rajendran and Spear, 2004). Furthermore, there is evidence that long-term alcohol use during human adolescence is associated with impaired attention during protracted abstinence (Tapert et al., 2002). It must be noted that adolescent rats are significantly smaller in body size compared to adults. Thus, we cannot rule out body size influencing our appetitive results regardless of motivational state. That being said, we believe our results do inform us of age-specific appetitive differences because the levers were calibrated to be very easy to depress and our measure of learning (time between pressing and licking) did not differ by age group on the last training day. Overall, our data suggest adolescents may display inherent age differences in appetitive behavior relative to adults that could be exaggerated by ethanol consumption.
Other factors that may have influenced appetitive behavior for sweetened ethanol in adolescents include sucrose consumption during training and testing or hormonal changes that occur during adolescence. Some evidence suggests that sucrose consumption during early adolescence leads to reduced consumption of ethanol or sweet substances in adulthood (Vendruscolo et al., 2010). Furthermore, sucrose consumption during adolescence has been shown to impair spatial learning and memory, and transiently increase fasting blood glucose, compared to adults (Kendig et al., 2013). To the best of our knowledge, we are unaware of data on the effect of ethanol on blood glucose levels in adolescents. We cannot exclude the possibility of an interaction between early sucrose consumption and behavior directed towards sweetened ethanol in our study, but since total sucrose consumption in the present study was less than the Kendig et al. (2013) study, it is unlikely that such an interaction would occur. Moreover, our data shows improvement in instrumental, appetitive performance in adolescents with continued sucrose consumption, and no effect in adults. With regard to hormonal changes that occur during adolescence having an influence on our age-specific behavior results, we can only speculate. Varlinskaya et al. (2013) suggested that the relationship between pubertal hormones and age-specific behavior is still unclear, and that fluctuations in hormones during puberty only modestly influence alcohol-associated behavior. On this basis we speculate that hormonal changes are not likely to have played a major role in the differences in appetitive behavior between adolescents and adults in our study.
In contrast to our findings with appetitive behavior, adolescents and adults consume the same amount of sweetened ethanol in our operant model. Our operant self-administration results counter most, but not all, studies deploying two bottle choice procedures that show increased ethanol intake in young adolescents compared to adults. Several reports used procedures most similar to our operant testing, i.e. limited access to sweetened ethanol and group housing, and showed that adolescents drink significantly more ethanol compared to adults (Walker et al., 2008; Ristuccia and Spear, 2008; Broadwater et al., 2011). Other reports used various procedures and show either higher ethanol intake in adolescents (Doremus et al., 2005; Vetter et al., 2007; Tambour et al., 2008; Garcia-Burgos et al., 2009; Bell et al., 2011); no age difference (Schramm-Sapyta et al., 2010; Schindler et al., 2014) or less intake in adolescents (Siegmund et al., 2005). A major methodological difference between the majority of these studies and the present study is the age of the adolescents at the start of ethanol self-administration; in most two bottle choice studies ethanol self-administration begins at <P30, which is about a week before we started and roughly 10 days before puberty begins in male rats (Schneider, 2008; Lewis et al., 2002). Also, having the rats work for ethanol in a context outside their home cage may reduce any age differences. Lastly, most two bottle choice studies comparing adolescents and adults utilize a strain of rats (Sprague-Dawley) which do not readily self-administer unsweetened ethanol in an operant setting and do not show preference for ethanol (e.g. Doremus et al., 2005). Important for the validity of our model, our adolescents drank similar amounts of sweetened ethanol as the only other report of adolescents in an operant setting (Gilpin et al., 2012).
The short period of adolescence (approximately 20 days in males (Spear, 2000; Schneider, 2008; Lewis et al., 2002)) necessitated the use of a sweetened ethanol solution to ensure quick acquisition and maintenance of pharmacologically relevant amounts of operant ethanol self-administration. Other methods such as the traditional sucrose fading method require at least four weeks to establish stable levels of non-sweetened ethanol intake (Samson, 1986), and therefore, it is not possible to use the sucrose-fading method in adolescent rats to measure operant self-administration during adolescence. It is also possible to train adult rats to consume unsweetened ethanol without exposure to a sweet solution (Simms et al., 2010). However, this method also requires a four-week induction period, which would not allow operant self-administration during adolescence. Furthermore, our rat model using sweetened ethanol has face validity compared with human adolescents that consume flavored or sweetened alcohol beverages (Johnston et al., 2013).
To our knowledge we are the first to compare the motivation to consume and seek sweetened ethanol in adolescents and adults using operant procedures. Adolescents and adults exhibited similar motivation to drink sweetened ethanol, as measured by breakpoint lever press values during the PR test. Furthermore, we analyzed cumulative lever presses (data not shown) and only observed the expected body weight-dependent pattern of the larger sized adults pressing the lever at a higher rate early in the session to ingest a larger volume of fluid, compared to adolescents. Following a two-week period of forced abstinence, rats that were drinking sweetened ethanol as adolescents or adults displayed similar cue-induced reinstatement of lever pressing behavior directed towards sweetened ethanol. Together, our results suggest that the reinforcing efficacy of sweetened ethanol, and relapse to cue-induced ethanol-seeking behavior, is not different based on the age that sweetened ethanol self-administration begins.
Our results suggest the pharmacokinetics of a moderate oral dose of sweetened ethanol are different in adolescent compared with adult male Long-Evans rats. Two separate experiments support this conclusion. First, adolescents exhibited decreased BEC compared to adults at three different sampling time points after operant self-administration of sweetened ethanol. Interestingly, in a large subset of adolescents (11 of 26) we observed extremely low or undetectable blood ethanol (< 5 mg/dl), even though these rats drank > 0.3 g/kg ethanol on sampling day. In a second experiment, using intragastric administration of 1 g/kg sweetened ethanol in ethanol-naïve rats, we observed decreased peak BEC, reduced time to reach peak BEC, and almost complete elimination of ethanol after two hours in adolescents compared with adults. Together, our blood ethanol data are consistent with a larger volume of distribution of ethanol in adolescents due, in part, to the ratio of total body water to fat being higher in smaller rats (Von Dreele, 1988). Our data also suggest that adolescent rats may have higher first-pass metabolism of ethanol after oral administration compared with adults, and this is consistent with the previous finding of a higher ratio of alcohol dehydrogenase activity to liver size in smaller rats (Bloom et al., 1982).
Other studies administering similar doses (1-2 g/kg) of unsweetened ethanol via the intraperitoneal route also support our results of decreased BEC (Bloom et al., 1982; Willey et al., 2012), reduced time to reach peak BEC (Little et al., 1996), and increased elimination of ethanol in adolescents compared with adults (Little et al., 1996). Since the present study is the first to directly compare adolescents and adults in an operant setting, more research is needed to have a clear understanding of how potential age differences in blood ethanol time course might influence operant ethanol self-administration data. Importantly, the majority of our adolescents did display detectable BEC after operant self-administration, suggesting that these rats did experience the central pharmacological effects of ethanol during the course of the operant self-administration sessions.
Previous studies have shown that the presence of sucrose in a solution containing ethanol reduces BEC at several time points after consumption compared with ethanol alone in adults (Matthews et al., 2001; Roberts et al., 1999). It is possible that the presence of sucrose in our study altered BEC in adolescents more than adults. However, the study by Walker and Ehlers (2009) also reported decreased BEC and an increased ethanol elimination rate in adolescent compared with adult Wistar rats that received moderate doses of unsweetened ethanol (0.75 and 1.5 g/kg ethanol, IG). Therefore, it seems likely that the presence of sucrose in our study was not a major factor in the difference in BEC between adolescents and adults, but further work is necessary to confirm this possibility.
Age differences in natural reward sensitivity or reinforcement by sweet substances, such as sucrose, is relatively unexplored. We showed that, except for initial age differences in appetitive behavior during training, consumption of a liquid 10 % sucrose solution does not differ between adolescents and adults when normalized to body weight (g/kg). However, we found that motivation to consume and seek this sucrose solution was reduced in adolescents compared with adults using a progressive ratio schedule of reinforcement and a cue-induced reinstatement of seeking behavior model. To our knowledge, only a few other studies have directly compared adolescent and adult sucrose self-administration. When corrected for body weight, adolescents consume more sucrose compared to adult rats using two bottle choice procedures (Vaidya et al., 2004; Wilmouth and Spear, 2009) or operant self-administration of pellets (Li and Frantz, 2010).
In conclusion, we have begun to establish an operant model of sweetened ethanol self-administration in adolescent male Long-Evans rats, and we directly compared various aspects of adolescent self-administration behavior to adults. Our findings suggest adolescents and adults do not differ with respect to motivational drive for or consumption of sweetened ethanol, but adolescents may be less focused on the performance of appetitive behavior required to obtain the sweetened ethanol. Also, adolescents appear to have larger volume of distribution and higher first pass metabolism of ethanol compared with adults, and this may contribute to the significant subset of adolescent rats that had minimal BECs after operant self-administration. Further adjustments to the model may be needed to optimize the number of adolescents that achieve significant BECs after operant self-administration relative to adults. Overall, we provide the initial steps in the development of a novel, voluntary animal model of alcohol abuse in adolescent rats. Unlike involuntary models of ethanol administraion (i.e. intraperitoneal, IG, and vapor), operant models may be the most behaviorally relevant measure of alcohol abuse liability (Schuster and Thompson, 1969; Meisch, 1982; Ator and Griffiths, 2003). Thus, the data we present with our current model may help in the identification of neural mechanisms that underlie self-administration of ethanol in adolescents and whether these differ from adults.
Acknowledgments
Funding provided by NIH-NIAAA (T32 AA00747 to JMD, and R37 AA11852 to RAG). Authors thank Wonbin Song, Priscila Cevallos, and Christine Park for technical assistance, and Drs. Christine Duvauchelle and Saloman Stavchansky for discussion of the results.
References
- Anderson RI, Spear LP. Age differences in ethanol discrimination: acquisition and ethanol dose generalization curves following multiple training conditions in adolescent and adult rats. Alcohol Clin Exp Res. 2014;38:186–194. doi: 10.1111/acer.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend. 2003;70:S55–72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Smith RJ, Toalston JE, Franklin KM, McBride WJ. Modeling binge-like ethanol drinking by peri-adolescent and adult P rats. Pharmacol Biochem Behav. 2011;100:90–97. doi: 10.1016/j.pbb.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom F, Lad P, Pittman Q, Rogers J. Blood alcohol levels in rats: non-uniform yields from intraperitoneal doses based on body weight. Br J Pharmacol. 1982;75:251–254. doi: 10.1111/j.1476-5381.1982.tb08780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Chronic intermittent ethanol exposure in early adolescent and adult male rats: effects on tolerance, social behavior, and ethanol intake. Alcohol Clin Exp Res. 2011;35:1392–1403. doi: 10.1111/j.1530-0277.2011.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo J, Howard EC, Moten M, Houck BD, Czachowski CL, Gonzales RA. A 3-day exposure to 10% ethanol with 10% sucrose successfully initiates ethanol self-administration. Alcohol. 2008;42:171–178. doi: 10.1016/j.alcohol.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaher R, McConnell KK, Rodd ZA, McBride WJ, Bell RL. Daily patterns of ethanol drinking in adolescent and adult, male and female, high alcohol drinking (HAD) replicate lines of rats. Pharmacol Biochem Behav. 2012;102:540–548. doi: 10.1016/j.pbb.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Garcia-Burgos D, Gonzalez F, Manrique T, Gallo M. Patterns of ethanol intake in preadolescent, adolescent, and adult Wistar rats under acquisition, maintenance, and relapse-like conditions. Alcohol Clin Exp Res. 2009;33:722–728. doi: 10.1111/j.1530-0277.2008.00889.x. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PloS One. 2012;7:e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on drug use: Overview of key findings, 1975-2012: Volume 1 & 2. Ann Arbor: Institute for Social Research, The University of Michigan; 2013. [Google Scholar]
- Kendig MD, Boakes RA, Rooney KB, Corbit LH. Chronic restricted access to 10% sucrose solution in adolescent and young adult rats impairs spatial memory and alters sensitivity to outcome devaluation. Physiol Behav. 2013;120:164–172. doi: 10.1016/j.physbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Lewis EM, Barnett JF, Jr, Freshwater L, Hoberman AM, Christian MS. Sexual maturation data for Crl Sprague-Dawley rats: criteria and confounding factors. Drug Chem Toxicol. 2002;25:437–458. doi: 10.1081/dct-120014794. [DOI] [PubMed] [Google Scholar]
- Li C, Frantz KJ. Time-dependent increases in cue-induced reinstatement of sucrose seeking after sucrose self-administration in adolescence. Behav Brain Res. 2010;213:109–112. doi: 10.1016/j.bbr.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- Matthews DB, Overstreet DH, Rezvani AH, Devaud LL, Morrow AL. Effects of sweetened ethanol solutions on ethanol self-administration and blood levels. Pharmacol Biochem Behav. 2001;68:13–21. doi: 10.1016/s0091-3057(00)00458-5. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Marinelli M. Age Matters. Eur J Neurosci. 2009;29:997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisch RA. Animal studies of alcohol intake. Br J Psychiatry. 1982;141:113–120. doi: 10.1192/bjp.141.2.113. [DOI] [PubMed] [Google Scholar]
- Moore EM, Mariani JN, Linsenbardt DN, Melon LC, Boehm SL., 2nd Adolescent C57BL/6J (but not DBA/2J) mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood. Alcohol Clin Exp Res. 2010;34:734–742. doi: 10.1111/j.1530-0277.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran P, Spear LP. The effects of ethanol on spatial and nonspatial memory in adolescent and adult rats studied using an appetitive paradigm. Ann N Y Acad Sci. 2004;1021:441–444. doi: 10.1196/annals.1308.060. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Adolescent and adult heart rate responses to self-administered ethanol. Alcohol Clin Exp Res. 2008;32:1807–1815. doi: 10.1111/j.1530-0277.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Koob GF. Operant self-administration of sweetened versus unsweetened ethanol: effects on blood alcohol levels. Alcohol Clin Exp Res. 1999;23:1151–1157. [PubMed] [Google Scholar]
- SAMHSA. (NSDUH Series H-41).Substance Abuse and Mental Health Services Administration, Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. 2011 HHS Publication No. (SMA) 11-4658. [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Slawecki CJ, Sharpe AL, Chappell A. Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcohol Clin Exp Res. 1998;22:1783–1787. [PubMed] [Google Scholar]
- Schindler AG, Tsutsui KT, Clark JJ. Chronic Alcohol Intake During Adolescence, but not Adulthood, Promotes Persistent Deficits in Risk-Based Decision Making. Alcohol Clin Exp Res. 2014;38:1622–1629. doi: 10.1111/acer.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol. 2008;13:253–263. doi: 10.1111/j.1369-1600.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, DiFeliceantonio AG, Foscue E, Glowacz S, Haseeb N, Wang N, Zhou C, Kuhn CM. Aversive effects of ethanol in adolescent versus adult rats: potential causes and implication for future drinking. Alcohol Clin Exp Res. 2010;34:2061–2069. doi: 10.1111/j.1530-0277.2010.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster CR, Thompson T. Self administration of and behavioral dependence on drugs. Annu Rev Pharmacol. 1969;9:483–502. doi: 10.1146/annurev.pa.09.040169.002411. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Simms JA, Bito-Onon JJ, Chatterjee S, Bartlett SE. Long-Evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology. 2010;35(7):1453–1463. doi: 10.1038/npp.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Tambour S, Brown LL, Crabbe JC. Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcohol Clin Exp Res. 2008;32:2100–2106. doi: 10.1111/j.1530-0277.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Baratta MV, Abrantes AM, Brown SA. Attention dysfunction predicts substance involvement in community youths. J Am Acad Child Adolesc Psychiatry. 2002;41:680–686. doi: 10.1097/00004583-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Vaidya JG, Grippo AJ, Johnson AK, Watson D. A comparative developmental study of impulsivity in rats and humans: the role of reward sensitivity. Ann N Y Acad Sci. 2004;1021:395–398. doi: 10.1196/annals.1308.051. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Vetter-O'Hagen CS, Spear L. Puberty and gonadal hormones: role in adolesent-typical behavioral alterations. Horm Behav. 2013;64:343–349. doi: 10.1016/j.yhbeh.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Gueye AB, Vendruscolo JC, Clemens KJ, Mormede P, Darnaudery M, Cador M. Reduced alcohol drinking in adult rats exposed to sucrose during adolescence. Neuropharmacology. 2010;59:388–394. doi: 10.1016/j.neuropharm.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Dreele MM. Age-related changes in body fluid volumes in young spontaneously hypertensive rats. Am J Physiol. 1988;255:953–956. doi: 10.1152/ajprenal.1988.255.5.F953. [DOI] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Appetitive motivational experience during adolescence results in enhanced alcohol consumption during adulthood. Behav Neurosci. 2009;123:926–935. doi: 10.1037/a0016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Walker JL, Ehlers CL. Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol. 2008;42:83–89. doi: 10.1016/j.alcohol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey AR, Anderson RI, Morales M, Ramirez RL, Spear LP. Effects of ethanol administration on corticosterone levels in adolescent and adult rats. Alcohol. 2012;46:29–36. doi: 10.1016/j.alcohol.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Hedonic sensitivity in adolescent and adult rats: taste reactivity and voluntary sucrose consumption. Pharmacol Biochem Behav. 2009;92:566–573. doi: 10.1016/j.pbb.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]