Summary
Intense noise damages the cochlear organ of Corti, particularly the outer hair cells (OHCs)[1], however this epithelium is not innervated by nociceptors of somatosensory ganglia, which detect damage elsewhere in the body. The only sensory neurons innervating the organ of Corti originate from the spiral ganglion, roughly 95% of which innervate exclusively inner hair cells (IHCs)[2-4]. Upon sound stimulation, IHCs release glutamate to activate AMPA-type receptors on these myelinated type-I neurons, which carry the neuronal signals to the cochlear nucleus. The remaining spiral ganglion cells (type-IIs) are unmyelinated and contact OHCs[2-4]. Their function is unknown. Using immunoreactivity to cFos, we documented neuronal activation in the brainstem of Vglut3−/− mice, in which the canonical auditory pathway (activation of type-I afferents by glutamate released from inner hair cells) is silenced[5, 6]. In these deaf mice, we found responses to noxious noise, that damages hair cells, but not to innocuous noise, in neurons of the cochlear nucleus, but not in the vestibular or trigeminal nuclei. This response originates in the cochlea and not in other areas also stimulated by intense noise (middle ear and vestibule) as it was absent in CD1 mice with selective cochlear degeneration but normal vestibular and somatosensory function. These data imply the existence of an alternative neuronal pathway from cochlea to brainstem that is activated by tissue-damaging noise and does not require glutamate release from IHCs. This detection of noise-induced tissue damage, possibly by type-II cochlear afferents, represents a novel form of sensation we term auditory nociception.
Results and Discussion
A mouse model lacking the canonical sensory pathway from cochlea to brain
Although cochlear hair cells are specialized for detecting sound-induced vibration, intense and persistent noise will damage and ultimately destroy them [1]. Throughout most of the body, nociceptors of the dorsal root and trigeminal ganglia detect tissue damage (or the physical stimuli causing it) of this sort. However, somatosensory neurons do not innervate the organ of Corti, raising the question of whether its damage goes undetected, or whether the cochlea has an alternative, nociceptor-like mechanism. To address these questions, we sought an animal model in which the known form of communication from cochlea to brain, i.e. the activation of myelinated type-I sensory neurons by glutamate released from inner hair cells, has been silenced.
IHCs express only one isoform of the vesicular glutamate transporter (VGLUT3) that loads glutamate into presynaptic vesicles [5, 6]. IHCs lacking VGLUT3 do not release glutamate and thus fail to activate type-I afferents, which comprise 95% of the cochlea sensory fibers. As expected, electrophysiology in Vglut3−/− mice shows no sign of cochlear nerve responses to sound, even though hair cells retain normal mechanoelectric transduction [5, 6]. In addition Vglut3−/− mice do not show startle responses to noise at levels up 125 dB. Hence, in Vglut3−/− mice the canonical auditory pathway is completely silenced [6].
We confirmed that VGLUT3 expression was abolished in Vglut3−/− mice (Fig S1A-H). Consistent with previous reports [5-7], we did not detect VGLUT3 in OHCs (Fig S1A-H). We also confirmed the loss of spiral ganglion neurons in Vglut3−/− mice, thought to arise from lack of synaptic stimulation [6]. Interestingly, this neuronal loss was selective for type-I cells (Fig S1I-K). The survival of type-IIs in Vglut3−/− mice is consistent with the lack of VGLUT3 immunoreactivity in OHCs and with the lack of expression of AMPA-type glutamate receptors in adult type-II afferent terminals [8]. These observations suggest that transmission at the OHC/type-II synapse is very different from that at the IHC/type-I synapse. Thus, in Vglut3−/− mice, the IHC/type-I pathway from cochlea to brain is silenced. Any response of these mice to sound would imply an alternative mechanism of auditory sensing, perhaps involving the type-II innervation of OHCs.
Noise avoidance requires VGLUT3
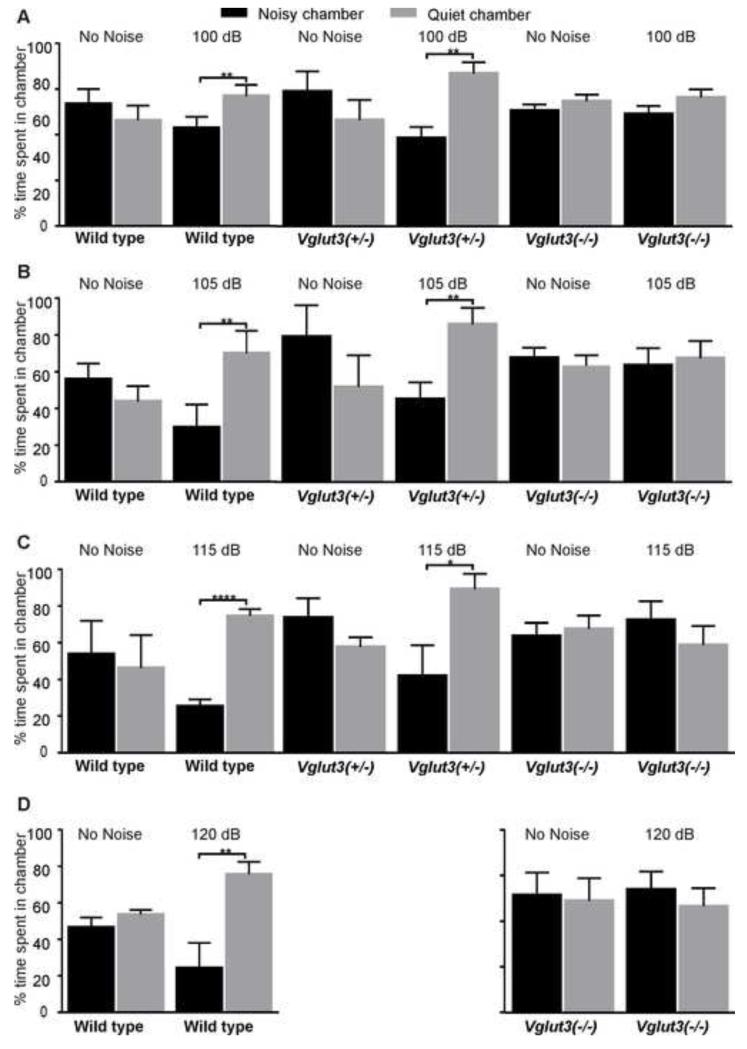
We first tested whether Vglut3−/− mice display any nocifensive behavior in response to intense noise. We developed an assay in which freely move between two interconnected compartments: one exposed to noise and another with attenuated sound levels. Measuring the time spent in the noisy vs attenuated environments (Fig 1) revealed noise-avoidance behavior in mice expressing VGLUT3 (wildtype and Vglut3+/− heterozygotes), whereas Vglut3−/− mice showed no preference. These results further demonstrate that Vglut3−/− mice do not respond behaviorally to sound and suggest that noise avoidance requires the canonical auditory pathway involving glutamatergic activation of type-I neurons.
Figure 1. VGLUT3 is required for noise avoidance behavior.
(A-D) Behavioral noise avoidance assays measuring the preference of an animal for a noisy vs. quiet environment. We place animals in a chamber with two interconnected compartments, and every 4 min present octave-band noise (8-16 kHz) lasting 2 min to one compartment and measure the time spent in the noisy (100, 105, 115, or 120 dB SPL) vs. quiet (attenuated by ~25 dB) environments. To prevent spatial bias, we alternate the source of noise between the two compartments. Each pair of columns represents the average time spent in the noisy compartment (black columns) and the quiet compartment (grey columns). We also measure the % time spent in each chamber during the intercalating 2 min silent periods, which demonstrates no compartment preference in the absence of noise (columns under “No Noise” labels). Mice expressing VGLUT3 (wild types and/or Vglut3+/− mice), but not mice lacking VGLUT3 (Vglut3−/−), display avoidance to noise at 100 dB SPL (A), 105 dB SPL (B), 115 dB SPL (C), and 120 dB SPL (D) (***p<l0.0001,**p<l0.001; Student’s t-test, n=4 for each genotype and sound exposure level). All error bars represent the standard deviation.
Noxious noise activates neurons in cochlear nuclei through a VGLUT3-independent pathway
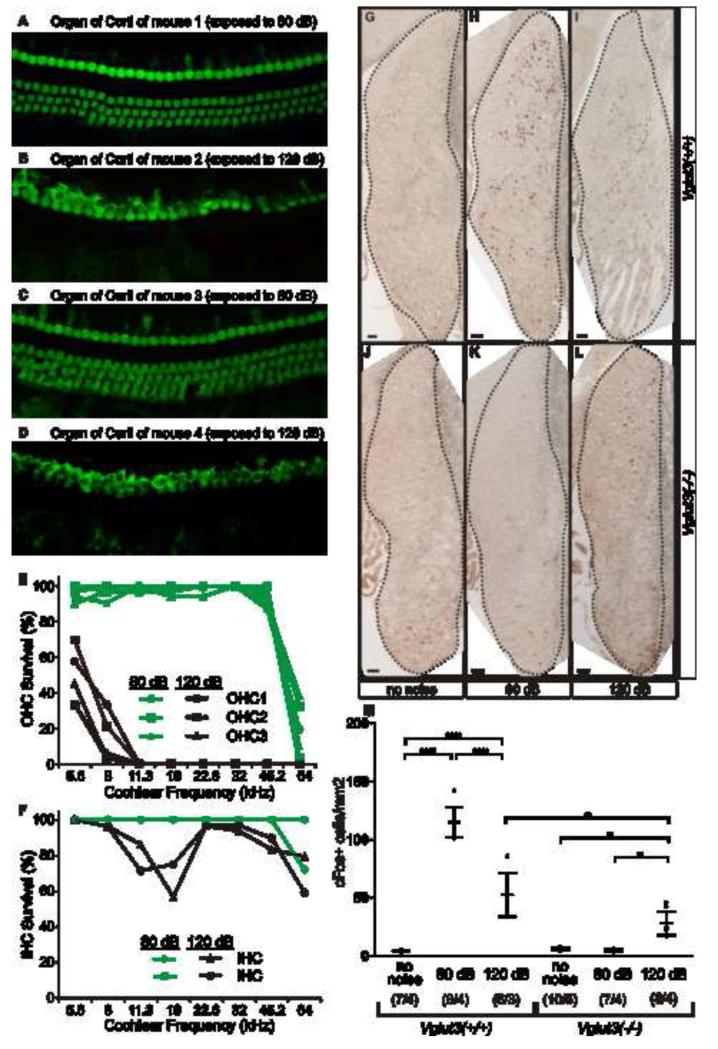
The brief (2 min) and intermittent (every 4 min) exposures up to 120 dB used in the noise-avoidance assay are probably not sufficiently traumatic to cause hair cell damage [9]. In the somatosensory system, Aδ nociceptors trigger nocifensive avoidance, but it is the slower C-fiber nociceptors that cause the slow and lasting pain resulting from tissue damage. In order to determine whether the cochlea has a system for detecting tissue-damaging noise, we exposed animals for 1 hr to octave-band noise (8-16 kHz) at 120 dB SPL or 80 dB SPL and confirmed that the 80 dB exposure was innocuous (did not kill hair cells), whereas the 120 dB exposure was extremely noxious, destroying all most OHCs and some IHCs (Fig 2A-F). We then assessed the effects of the exposure on neuronal activity in the brainstem using immunoreactivity to the immediate-early gene c-fos, an indicator of neuronal activity [10]. Although in our experience spiral ganglion neurons themselves do not upregulate c-Fos in response to sound, many of their postsynaptic targets in the cochlear nucleus (CN) do so [11] (Fig S2). In wild type mice, exposure to 80 dB triggered abundant neuronal activity in CN, as did exposure to 120 dB, albeit at a lower level (Fig 2G-I,M). Lower neuronal activity following high-level exposure has been previously described [11] and presumably arises from the reduction in cochlear nerve response during the exposure, due to the accumulating damage to the sensory cells. In Vglut3−/− mice, exposure to the innocuous 80 dB noise did not trigger neuronal activity (Fig 2J,K,M). However, the noxious 120 dB noise did trigger neuronal activity in the CN of Vglut3−/− mice (Fig 2L,M), suggesting a novel form of nociception that detects tissue-damaging auditory stimuli and communicates that information to the brain via a signaling mechanism that does not require glutamatergic activation of type-Is (the canonical auditory pathway).
Figure 2. Tissue-damaging noise activates neurons in the cochlear nucleus (CN) via a VGLUT3-independent pathway.
(A-D) Noise-induced hair cell loss is documented with myosin VIIa labeling. Here, we show the middle of the cochlear spiral (22.6 kHz), from 2 wild type mice 2 wks after 1 hr exposure to octave-band noise (8-16 kHz) at (A,C) 80 dB SPL or (B,D) 120 dB SPL. (E,F) The 120 dB exposure destroys OHCs throughout much of the cochlea (E) and destroys a significant number of IHCs in the region of the noise band (F). By contrast, hair cell loss is minimal after 80 dB: the loss at the extreme base is likely the age-related degeneration characteristic of C57BL/6 [40]. (G-M) Immunohistochemistry with an antibody to c-Fos on coronal sections of the cochlear nucleus (CN) following (G,J) no noise, (H,K) 80 dB SPL or (I,L) 120 dB SPL exposures to same noise band shown in A-F. In wild type mice, 80 or 120 dB SPL triggers c-Fos expression in neurons throughout the CN. InVglut3−/− mice, stimulation with innocuous (80 dB SPL) noise triggers no c-Fos immunoreactivity (density of c-Fos+ neurons is indistinguishable from that in unexposed controls), while the noxious (120 dB SPL) noise triggers cFos expression. (M) Average densities of c-Fos expressing cells reveal significant increases in CN activity (compared to baseline) in wild types following 80 dB SPL and, to a lesser extent 120 dB SPL. However, in Vglut3−/− mice only the noxious (120 dB SPL) noise triggered CN activity. Error bars represent the standard deviation. The sample size (# of CN / # of animals) is indicated for each group in parentheses. Pair wise comparisons were calculated with Type III F-test [F (2,22) = 72.56] where P values are <l0.0001 (****), and <l0.01 (**). Scale bars are 50 μm.
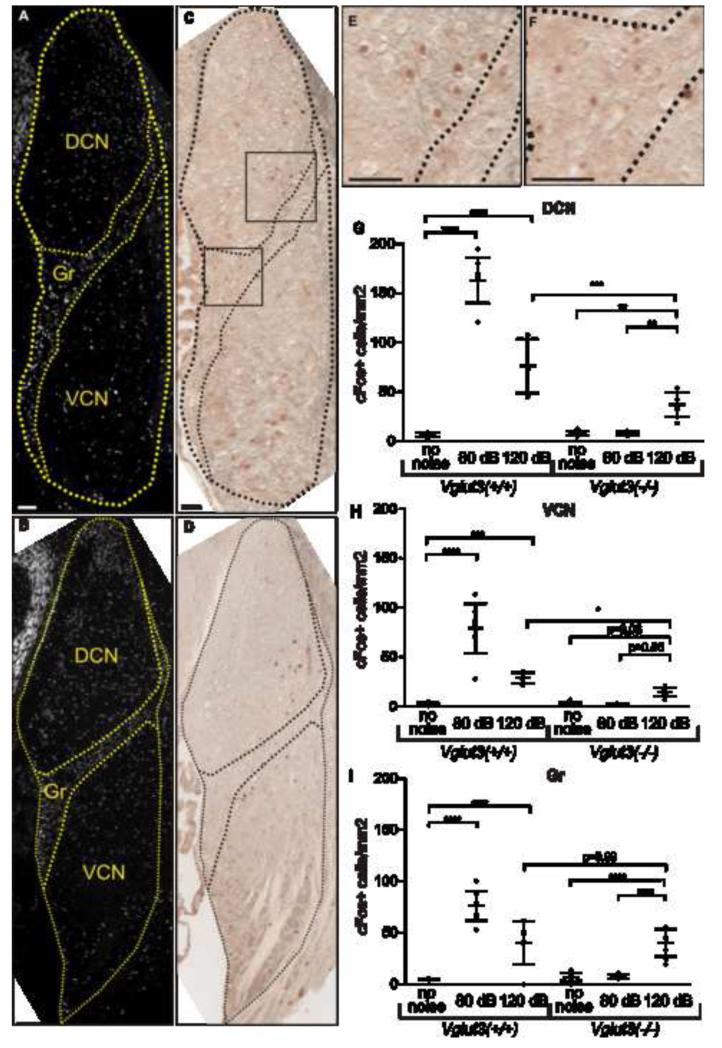
In wild type mice, the number of CN neurons activated by 120 dB was roughly double that in Vglut3−/− mice (52.6 vs 28.1; p=0.0017; Fig 2G). This implies that, in wild types, 120 dB activates CN neurons through both VGLUT3-dependent and VGLUT3-independent pathways. Examining the activation patterns in the three major subdivisions of CN, dorsal (DCN), ventral (VCN) and granule cell regions (Gr) (Fig 3), shows that this difference occurs in DCN (76.4 vs 37.3 cells/mm2; p=0.0007; Fig 3G) and VCN (28.8 vs 14.5 cells/mm2; p=0.039; Fig 3H), but not in Gr (40.3 vs 40.2 cells/mm2; p=0.99; Fig 3I). Thus noxious noise activates neurons in Gr exclusively via a VGLUT3-independent pathway. Correspondingly, whereas both type-I and -II afferents project to DCN and VCN [12, 13], type-I central projections do not reach Gr, and only type-IIs directly innervate this subdivision [12-14]. Hence, while the VGLUT3-independent noxious noise activation of DCN and VCN is consistent with either type-I or type-II innervation, the activation of Gr is more in keeping with type-II innervation. Interestingly, type-IIs immunoreact with antibodies directed against markers of somatosensory nociceptors [15](Fig S3), and may be molecularly equipped to detect tissue-damaging, noxious stimuli.
Figure 3. Tissue-damaging noise activates neurons in CN granule cell region primarily through a VGLUT3-independent pathway.
(A,B) DAPI fluorescence of coronal sections of Vglut3−/− CN (A) and Vglut3+/+CN (B) exposed to 120 dB SPL reveals its three subdivisions: dorsal cochlear nucleus (DCN), ventral cochlear nucleus (VCN), and granule cell region (Gr). (C, D) c-Fos immunoreactivity within the same coronal section reveals positive cells throughout the three CN subdivisions. Areas of panel (C) magnified in (E,F) demonstrate positive neurons within the DCN and Gr areas of Vglut3−/− CN. (G-I) Densities of c-Fos-expressing neurons in each CN subdivision: DCN (G), VCN (H), and Gr (I). Wild type mice display significant increases of neuronal activity in all three subdivisions (G-I) following exposure to 80 dB SPL and 120 dB SPL. Vglut3−/− mice display significant increases of neuronal activity following exposure to 120 dB SPL in the DCN (G) and Gr (I), while increases in VCN approached significance (p=0.06; H). However, in the granule cell region, which is not innervated by type-I afferents, neuronal activity induced by noxious (120 dB SPL) noise is VGLUT3 independent (indistinguishable between wild type and Vglut3−/− mice). Error bars represent the standard deviation. The sample size in G-I is the same as in Fig 2M. Pair wise comparisons were calculated for each CN area with Type III F-test [DCN (F) (2, 22) = 76.95]; [VCN (F) (2, 22) = 40.01]; [Gr (F) (2, 22) = 44.85]. P values are <l0.0001 (****), <l0.001 (***), and <l0.01 (**). Scale bars are 50 μm.
Tissue-damaging noise is detected by the cochlea
The CN activation we observed after noxious noise could be mediated via somatosensory or saccular, rather than cochlear, afferents. The tympanic membrane is extremely sensitive to touch. It is innervated by nociceptors of the trigeminal ganglion [16], which could also be stimulated by intense noise, and some trigeminal neurons project indirectly to the CN [17]. Similarly, saccular afferents can respond to intense low-frequency sound [18], and some saccular afferents also project to the CN [19, 20]. We investigated the possible contribution of somatosensory and vestibular pathways in two ways.
First, if noxious noise were activating vestibular or somatosensory afferents, it should also activate their primary targets in brainstem. However, while exposure of both wild type and Vglut3−/− mice to noxious noise (120 dB for 1 hr) triggered cFos expression in CN neurons, such exposure did not activate neurons of trigeminal (Sp5 and Pr5) or vestibular (VeNu) nuclei, where most of the vestibular and trigeminal afferents terminate (Fig S4).
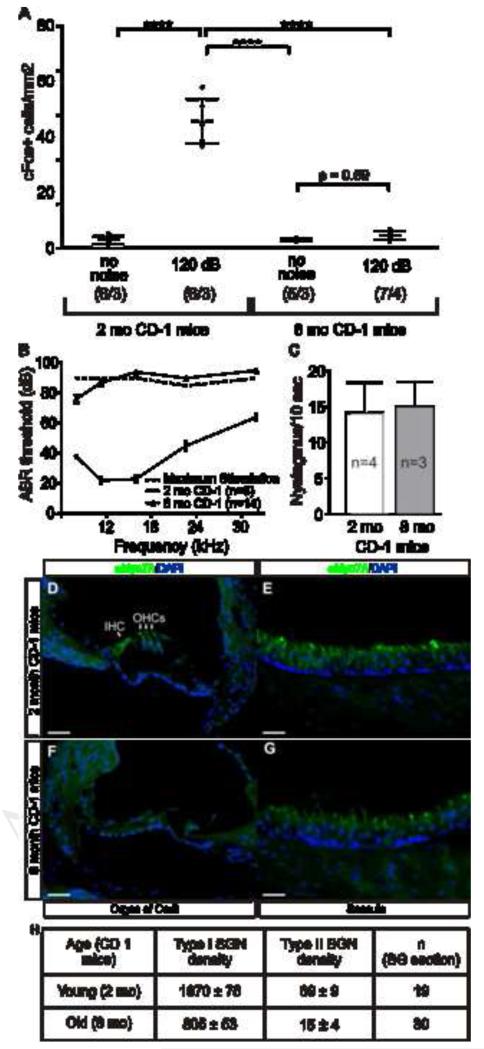
Second, if noxious noise were activating vestibular or somatosensory pathways, mice lacking a functional cochlea, but retaining normal somatosensory and vestibular responses, should continue to show CN activation after 120 dB noise. CD1 mice show a progressive sensorineural hearing loss useful for addressing this question. Though normal-hearing at 3 months, by 8 months CD1 mice display massive loss of cochlear hair cells and sound-evoked electrical responses (Fig 4B,D,F) with partial loss of spiral ganglion neurons (Fig 4h). However, they do not lose saccular hair cells or vestibular function (Fig 4C,E,G); and pain thresholds (mechanical and thermal) do not change between 3 and 12 months of age [21]. In young CD1 mice, with normal cochlear function, tissue-damaging noise activated CN neurons (Fig 4A). However, in aged CD1 mice, with normal somatosensory and vestibular function but degenerated cochleae, tissue-damaging noise failed to activate CN neurons (Fig 4A). Hence, the VGLUT3-independent pathway that detects tissue-damaging auditory stimuli requires cochlear integrity. Together the evidence suggests that activation of CN neurons by noxious noise is not mediated by somatosensory or vestibular afferents.
Figure 4. CN activation following tissue-damaging noise requires an intact cochlea.
(A) Tissue-damaging noise activates CN neurons in young (2 months) but not middle-age (8 months) CD1 mice, which have (B) severe cochlear dysfunction due to (D,F) a massive loss of hair cells but (c) normal vestibular function and (E,G) appearance as well as normal somatosensory pain thresholds [21]. Pair wise comparisons were calculated with Type III F-test [F (1,11) = 90.72] where P values are <l0.0001 (****); the sample size (# of CN/ # of animals) is indicated for each group in parentheses. (B) Average ABR thresholds of young (n = 6) and middle-age (n = 14) CD1 mice. The dotted line represents the maximum sound tested at each frequency, so values shown above it represent non-responding ears. (C) Nystagmus, assessed as the number of saccades during 10 seconds after rotation at 250 r.p.m., reveals no difference (p = 0.68; Student’s t-test) in vestibulo-ocular reflex between young and middle-age CD1 mice. (D-G) Immunohistochemistry for Myosin VIIA and nuclear counterstain with DAPI to (D, E) young and (F, G) middle-age CD1 inner ear reveals loss of (F) cochlear but not (G) saccular hair cells in aged CD1 mice. Scale bars are 50 μm. (H) Neuronal counts show that middle-age CD1 mice have a reduced number of both type-I (−59%) and type-II (−78%) cochlear afferents. Counts were made on sections from 2 young and 6 old CD1 mice.
The VGLUT3-independent response to noxious noise appears to be mediated by cochlear afferents, i.e. either type-Is innervating IHCs or type-IIs innervating OHCs. All activity in type-Is evoked by IHC depolarization is abolished by the AMPA receptor blocker NBQX or by elimination of the vesicular glutamate transporter VGLUT3 [6]. Hence, all known activity in the type-I pathway is glutamatergic in origin. Type-II fibers are poorly understood, however, several lines of evidence suggest that synaptic transmission is fundamentally different. Although recording from neonatal type-IIs show responses to OHC depolarization that are blocked by NBQX [22], mature type-II fibers show no response to non-noxious sound [23-25], they do not display AMPA-type glutamate receptors (GluR2/3) [8] or the glutamatergic postsynaptic marker PSD95 [26] in their terminals, and they do not show the dramatic swelling of postsynaptic terminals seen in type-Is after either cochlear glutamate perfusion or acoustic overstimulation [27, 28].
After 120 dB noise, cochlear afferents may not be responding to neurotransmitter release, but to other signals released during cellular damage. Given that OHCs are much more vulnerable than IHCs (Fig 2A-F)[9], type-IIs, whose processes branch out and extend under the OHCs, are best positioned to detect this damage [3]. Furthermore, type-IIs can be activated by extracellular ATP [22], a pain-producing chemical that is released by damaged cells [29], including OHCs [30]. Mechanical rupture and ablation of individual OHCs causes a robust activation (depolarization and burst of action potentials) of type-IIs [31].
This activation of type-IIs by tissue damage is not inconsistent with their reported synaptic activation from early postnatal OHCs [22, 32]. Each form of neuronal activation (by neurotransmitter release or by tissue damage) may occur in different circumstances. Most importantly, type-II, but not type-I, central axons directly innervate the granule cell regions, the only CN subdivision in which activation by noxious noise was unaffected by elimination of the VGLUT3-dependent (i.e., type-I) pathway (Fig. 3I). The reduction in DCN and VCN activation with elimination of the VGLUT3-dependent pathway (Fig. 3G,H) is also consistent with the anatomical data, since both type-I and -II neurons project to these regions [14, 33, 34]. However, although several lines of evidence suggest type-IIs as mediators of the VGLUT3-independent detection of tissue-damaging noise, type-Is could be involved if they were also activated in the absence of VGLUT3 by 120 dB noise, perhaps by the less pronounced damage of IHCs or even by diffusible signals from the more distant, damaged OHCs.
Irrespective of the neurons involved (type-II or -I) or the signaling mechanism used (released chemicals from damaged hair cells such as ATP, protons and potassium; or synaptic stimulation from hair cells via an unknown form neurotransmission) our results reveal a novel form of communication between cochlea and brain that detects tissue-damaging noise in the absence of VGLUT3 and hence differs from the canonical auditory pathway. This represents a novel sensory modality, which we term auditory nociception. Nociception is the detection by the nervous system, such as neurons of somatosensory ganglia, of stimuli that are harmful, actually or potentially tissue-damaging [35]. Nociception often triggers the sensation of pain, although pain may occur without nociception (e.g., neuropathic pain). Nociception may also elicit autonomic responses such as pallor, diaphoresis, tachycardia, hypertension, lightheadedness, nausea and fainting [36]. Finally, nociceptors also respond to noxious stimulation by releasing neuropeptides in the damaged periphery (the axon-reflex), leading to neurogenic inflammation [37]. It remains to be discovered what physiological reaction or perception is triggered by the auditory nociception we have observed. Obvious possibilities are the unpleasantness of intense noise, or a protective efferent response to it. It is interesting that a majority (86%) of patients affected with hyperacusis report a sensation of earache (76%) or headache (10%) in response to noise (http://hyperacusisresearch.org/). The auditory nociceptive system could transmit and/or trigger this auditory pain in analogy to the role somatosensory nociceptors play in neuropathic pain, hyperalgesia and allodynia. However, as mentioned above, auditory nociception need not cause pain and may instead elicit an axon reflex, an autonomic reaction or an efferent response. In this regard, it is interesting that the sensory terminals of cochlear type-IIs branch to contact numerous cells and contain synaptic-like vesicles [8], the same features that permit axon-reflex/neurogenic inflammation in somatosensory nociceptors [37].
We show here that auditory nociception on its own does not trigger avoidance behavior, which requires VGLUT3 and hence glutamate release from hair cells. This is not unexpected given what we know about somatosensory nociception. First, among somatosensory neurons, myelinated Aδ nociceptors mediate the “fast pain” that elicits the nocifensive avoidance, whereas the more numerous, unmyelinated C-fiber nociceptors mediate the slow and lasting pain often resulting from tissue damage. The form of auditory nociception described here is more akin to C-fiber nociception. In this regard, it is worth noting that cochlear type-II afferents and somatosensory C-fiber nociceptors share anatomical features (pseudounipolar with small diameter and unmyelinated [3, 4, 12], and with vesicles in their highly branched peripheral terminals [8, 38]), physiological properties (slow conducting and with ~10× higher electrical thresholds [39]) and protein expression (e.g. peripherin [15] and perhaps also ASIC2α and TRPV1; Fig S3).
Second, it is also important to note that pain only triggers avoidance if it can be attributed to an external source, such as a burning or puncturing object. Pains caused by internal sources, such as a headache or a middle ear ache, do not trigger avoidance. In this regard, even if the auditory nociceptive system here described mediated the sensation of auditory pain and contributed to noise avoidance in wild type animals, in the absence of VGLUT3 and hence of normal hearing this pain may not be attributable to noise and be perceived more like an earache or headache (curiously, the common sensations reported by patients with hyperacusis). Future studies aimed at eliminating the VGLUT3-independent auditory sensing here described (perhaps through ablation or inactivation of type-IIs) could resolve the issue of whether this form of auditory nociception contributes to nocifensive behaviors.
Supplementary Material
Acknowledgments
We thank Claus-Peter Richter for auditory-testing and Jody Ciolino (Robert H. Lurie Biostatistics Facility) for statistics. Funded by F31DC012013 (ENF), R01DC00188 (MCL), and R01NS044363, R21DC006089, and N00014-14-1-0709 from the Office of Naval Research (JG-A).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
JG-A, AD, and MCL initiated the study. ENF, JG-A, AD, TM, MCL, AKH, FGM and GC performed experiments. ENF and JG-A analyzed results. RS and RE provided the Vglut3 KOs. JG-A, ENF and MCL wrote the manuscript.
References
- 1.Liberman MC, Kiang NY. Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity. Acta Otolaryngol Suppl. 1978;358:1–63. [PubMed] [Google Scholar]
- 2.Dannhof BJ, Bruns V. The innervation of the organ of Corti in the rat. Hearing research. 1993;66:8–22. doi: 10.1016/0378-5955(93)90255-y. [DOI] [PubMed] [Google Scholar]
- 3.Spoendlin H. Innervation densities of the cochlea. Acta Otolaryngol. 1972;73:235–248. doi: 10.3109/00016487209138937. [DOI] [PubMed] [Google Scholar]
- 4.Kiang NY, Rho JM, Northrop CC, Liberman MC, Ryugo DK. Hair-cell innervation by spiral ganglion cells in adult cats. Science. 1982;217:175–177. doi: 10.1126/science.7089553. [DOI] [PubMed] [Google Scholar]
- 5.Ruel J, Emery S, Nouvian R, Bersot T, Amilhon B, Van Rybroek JM, Rebillard G, Lenoir M, Eybalin M, Delprat B, et al. Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. Am J Hum Genet. 2008;83:278–292. doi: 10.1016/j.ajhg.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, et al. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–275. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akil O, Seal RP, Burke K, Wang C, Alemi A, During M, Edwards RH, Lustig LR. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron. 2012;75:283–293. doi: 10.1016/j.neuron.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiers FA, Nadol JB, Jr., Liberman MC. Reciprocal synapses between outer hair cells and their afferent terminals: evidence for a local neural network in the mammalian cochlea. Journal of the Association for Research in Otolaryngology: JARO. 2008;9:477–489. doi: 10.1007/s10162-008-0135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. Journal of the Association for Research in Otolaryngology: JARO. 2002;3:248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annual review of neuroscience. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 11.Brown MC, Liu TS. Fos-like immunoreactivity in central auditory neurons of the mouse. The Journal of comparative neurology. 1995;357:85–97. doi: 10.1002/cne.903570109. [DOI] [PubMed] [Google Scholar]
- 12.Brown MC, Berglund AM, Kiang NY, Ryugo DK. Central trajectories of type II spiral ganglion neurons. The Journal of comparative neurology. 1988;278:581–590. doi: 10.1002/cne.902780409. [DOI] [PubMed] [Google Scholar]
- 13.Brown MC, Ledwith JV., 3rd Projections of thin (type-II) and thick (type-I) auditory-nerve fibers into the cochlear nucleus of the mouse. Hearing research. 1990;49:105–118. doi: 10.1016/0378-5955(90)90098-a. [DOI] [PubMed] [Google Scholar]
- 14.Berglund AM, Benson TE, Brown MC. Synapses from labeled type II axons in the mouse cochlear nucleus. Hearing research. 1996;94:31–46. doi: 10.1016/0378-5955(95)00231-6. [DOI] [PubMed] [Google Scholar]
- 15.Hafidi A. Peripherin-like immunoreactivity in type II spiral ganglion cell body and projections. Brain research. 1998;805:181–190. doi: 10.1016/s0006-8993(98)00448-x. [DOI] [PubMed] [Google Scholar]
- 16.Saunders RL, Weider D. Tympanic membrane sensation. Brain. 1985;108(Pt 2):387–404. doi: 10.1093/brain/108.2.387. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Shore S. Projections from the trigeminal nuclear complex to the cochlear nuclei: a retrograde and anterograde tracing study in the guinea pig. Journal of neuroscience research. 2004;78:901–907. doi: 10.1002/jnr.20343. [DOI] [PubMed] [Google Scholar]
- 18.Jones GP, Lukashkina VA, Russell IJ, Lukashkin AN. The vestibular system mediates sensation of low-frequency sounds in mice. Journal of the Association for Research in Otolaryngology: JARO. 2010;11:725–732. doi: 10.1007/s10162-010-0230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burian M, Gstoettner W. Projection of primary vestibular afferent fibres to the cochlear nucleus in the guinea pig. Neuroscience letters. 1988;84:13–17. doi: 10.1016/0304-3940(88)90329-1. [DOI] [PubMed] [Google Scholar]
- 20.Kevetter GA, Perachio AA. Projections from the sacculus to the cochlear nuclei in the Mongolian gerbil. Brain Behav Evol. 1989;34:193–200. doi: 10.1159/000116505. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz-Medina J, Baulies A, Bura SA, Valverde O. Paclitaxel-induced neuropathic pain is age dependent and devolves on glial response. European journal of pain. 2013;17:75–85. doi: 10.1002/j.1532-2149.2012.00172.x. [DOI] [PubMed] [Google Scholar]
- 22.Weisz C, Glowatzki E, Fuchs P. The postsynaptic function of type II cochlear afferents. Nature. 2009;461:1126–1129. doi: 10.1038/nature08487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown MC. Antidromic responses of single units from the spiral ganglion. Journal of neurophysiology. 1994;71:1835–1847. doi: 10.1152/jn.1994.71.5.1835. [DOI] [PubMed] [Google Scholar]
- 24.Robertson D. Horseradish peroxidase injection of physiologically characterized afferent and efferent neurones in the guinea pig spiral ganglion. Hearing research. 1984;15:113–121. doi: 10.1016/0378-5955(84)90042-x. [DOI] [PubMed] [Google Scholar]
- 25.Robertson D, Sellick PM, Patuzzi R. The continuing search for outer hair cell afferents in the guinea pig spiral ganglion. Hearing research. 1999;136:151–158. doi: 10.1016/s0378-5955(99)00120-3. [DOI] [PubMed] [Google Scholar]
- 26.Yuan Y, Shi F, Yin Y, Tong M, Lang H, Polley DB, Liberman MC, Edge AS. Ouabain-induced cochlear nerve degeneration: synaptic loss and plasticity in a mouse model of auditory neuropathy. Journal of the Association for Research in Otolaryngology: JARO. 2014;15:31–43. doi: 10.1007/s10162-013-0419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pujol R, Lenoir M, Robertson D, Eybalin M, Johnstone BM. Kainic acid selectively alters auditory dendrites connected with cochlear inner hair cells. Hearing research. 1985;18:145–151. doi: 10.1016/0378-5955(85)90006-1. [DOI] [PubMed] [Google Scholar]
- 28.Robertson D. Functional significance of dendritic swelling after loud sounds in the guinea pig cochlea. Hearing research. 1983;9:263–278. doi: 10.1016/0378-5955(83)90031-x. [DOI] [PubMed] [Google Scholar]
- 29.Cook SP, McCleskey EW. Cell damage excites nociceptors through release of cytosolic ATP. Pain. 2002;95:41–47. doi: 10.1016/s0304-3959(01)00372-4. [DOI] [PubMed] [Google Scholar]
- 30.Gale JE, Piazza V, Ciubotaru CD, Mammano F. A mechanism for sensing noise damage in the inner ear. Current biology: CB. 2004;14:526–529. doi: 10.1016/j.cub.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Liu C, Glowatzki E, Fuchs P. Purinergic Modulation of Type II Cochlear Afferents: Sensing Trauma in the Ear? Assoc. Res. Otolaryngol. Abs. 2014;37:419. [Google Scholar]
- 32.Weisz CJ, Lehar M, Hiel H, Glowatzki E, Fuchs PA. Synaptic transfer from outer hair cells to type II afferent fibers in the rat cochlea. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:9528–9536. doi: 10.1523/JNEUROSCI.6194-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berglund AM, Brown MC. Central trajectories of type II spiral ganglion cells from various cochlear regions in mice. Hearing research. 1994;75:121–130. doi: 10.1016/0378-5955(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 34.Benson TE, Brown MC. Postsynaptic targets of type II auditory nerve fibers in the cochlear nucleus. Journal of the Association for Research in Otolaryngology: JARO. 2004;5:111–125. doi: 10.1007/s10162-003-4012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loeser JD, Treede RD. The Kyoto protocol of IASP Basic Pain Terminology. Pain. 2008;137:473–477. doi: 10.1016/j.pain.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 36.Feinstein B, Langton JN, Jameson RM, Schiller F. Experiments on pain referred from deep somatic tissues. The Journal of bone and joint surgery. American volume. 1954;36:981–997. [PubMed] [Google Scholar]
- 37.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 38.Nadol JB., Jr. Reciprocal synapses at the base of outer hair cells in the organ of corti of man. The Annals of otology, rhinology, and laryngology. 1981;90:12–17. doi: 10.1177/000348948109000104. [DOI] [PubMed] [Google Scholar]
- 39.Brown MC. Anatomical and Physiological Studies of Type I and Type II Spiral Ganglion Neurons. In: Merchán MA, Juiz JM, Godfrey DM, Mugnaini E, editors. The Mammalian Cochlear Nuclei Organization and Function. Vol. 239. Plenum Press; New York: 1993. pp. 43–54. [Google Scholar]
- 40.Spongr VP, Flood DG, Frisina RD, Salvi RJ. Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life spans. The Journal of the Acoustical Society of America. 1997;101:3546–3553. doi: 10.1121/1.418315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.