Abstract
Purpose
To investigate whether longitudinal functional PET imaging of mammary tumors using the radiopharmaceuticals [18F]FDG (to measure glucose uptake), [18F]FES (to measure estrogen receptor (ER) levels), or [18F]FFNP (to measure progesterone receptor (PgR) levels) is predictive of response to estrogen deprivation therapy.
Experimental Design
[18F]FDG, [18F]FES and [18F]FFNP uptake in endocrine-sensitive and -resistant mammary tumors was quantified serially by PET before ovariectomy or estrogen withdrawal in mice, and on days 3 and 4 after estrogen deprivation therapy. Specificity of [18F]FFNP uptake in ERα+ mammary tumors was determined by competition assay using unlabeled ligands for PgR or glucocorticoid receptor (GR). PgR expression was also assayed by immunohistochemistry (IHC).
Results
The levels of [18F]FES and [18F]FDG tumor uptake remained unchanged in endocrine-sensitive tumors after estrogen deprivation therapy compared to those at pre-treatment. In contrast, estrogen deprivation therapy led to a reduction in PgR expression and [18F]FFNP uptake in endocrine-sensitive tumors, but not in endocrine-resistant tumors, as early as 3 days post-treatment; the changes in PgR levels were confirmed by IHC. Unlabeled PgR ligand R5020 but not GR ligand dexamethasone blocked [18F]FFNP tumor uptake, indicating that [18F]FFNP bound specifically to PgR. Therefore, a reduction in FFNP tumor to muscle ratio in mammary tumors predicts sensitivity to estrogen deprivation therapy.
Conclusions
Monitoring the acute changes in ERα activity by measuring [18F]FFNP uptake in mammary tumors predicts tumor response to estrogen deprivation therapy. Longitudinal noninvasive PET imaging using [18F]FFNP is a robust and effective approach to predict tumor responsiveness to endocrine treatment.
Introduction
Breast cancer is the second most deadly cancer for women in the United States. About 80% of all newly diagnosed breast cancers are classified as estrogen receptor-α+ (ERα+) (1). ERα, together with progesterone receptor (PgR) and HER2, are part of the standardized clinicopathological evaluation of breast cancer. Since they provide important information to guide treatment decisions, accurate and reproducible assessment of the levels of these biomarkers is critical. ERα, PgR and HER2 are routinely assayed using immunohistochemistry (IHC). HER2 amplification is also frequently detected by fluorescence in situ hybridization. However, discordance rates ranging from 9% to 39% were observed for PgR, depending on the techniques used to obtain biopsy specimens (2). This discrepancy can be partly explained by tumor heterogeneity leading to sampling error during biopsy (3). Therefore, noninvasive functional imaging of the whole lesion using positron emission tomography (PET) may provide a more complete molecular characterization of the tumor in its native setting. In addition, PET imaging is also an effective approach to monitor advanced metastatic disease during antitumor therapy when repeated biopsies may not be possible.
[18F]Fluoroestradiol ([18F]FES) and [18F]fluoro furanyl norprogesterone ([18F]FFNP) are well validated noninvasive molecular imaging radiopharmaceuticals for ERα and PgR, respectively (4, 5). [18F]FES is an estradiol analog that binds to ERα with high affinity and selectivity (6). Differences in FES uptake in multiple tumor sites within the same patient have demonstrated heterogeneity of metastases and highlight the value of using functional PET imaging to monitor changes in tumor characteristics with disease progression (7). In addition, tumor FES uptake before endocrine treatment is correlated with subsequent clinical response to therapy (8-10). Finally, blockade of tumor FES uptake after the initiation of tamoxifen or fulvestrant treatment is indicative of the pharmacodynamic effectiveness of the dosing schedule (11). Together, these approaches underscore the utilities of functional FES-PET.
Although pre-treatment ERα expression, as measured by IHC or FES-PET, is predictive for response to endocrine therapies, about 40-60% of ERα+ or FES-PET+ patients do not derive long-term benefits from endocrine treatment (8, 12). Since PgR expression is directly upregulated by activation of ERα signaling, we hypothesize that monitoring PgR expression before and during therapy might provide insights into the functional status of ERα activation and thus the susceptibility of the tumor cells to respond to antiestrogens or estrogen deprivation therapies. [18F]FFNP binds PgR with high affinity, and targets PgR-rich, but not PgR-poor, organs with high selectivity (13). In clinical studies, the ratio of FFNP uptake in the primary breast cancer lesion over the contralateral normal breast is closely correlated with results from PgR IHC (5). Therefore, FFNP-PET is an approach well suited for following the rapid changes of PgR levels in vivo without the need for repeated biopsies.
Using a preclinical model of ERα+ breast cancer, we previously demonstrated that antitumor treatment with the ERα antagonist fulvestrant reduced tumor FFNP uptake in fulvestrant-sensitive tumors compared to untreated tumors (14). Interestingly, no difference in tumor FFNP uptake was observed between fulvestrant-resistant mammary tumors and untreated tumors, suggesting that the difference in FFNP tumor uptake may differentiate between endocrine responsive and non-responsive breast cancers (14). As opposed to our previous study that focused on post-treatment FFNP-PET, our goal in this study was to test the hypothesis that serial FFNP-PET imaging, before and after initiation of estrogen deprivation treatment, is feasible and effective in monitoring the acute functional changes of ERα signaling and predicting the ultimate response of the tumor. In addition, this study was also designed to directly compare the efficacy of FFNP-PET with FES-PET and FDG-PET as imaging biomarkers predictive of the success of endocrine treatment. Since neoadjuvant endocrine treatment of breast cancer is increasingly being implemented to assess tumor responsiveness before surgery and to guide treatment strategies post-surgery, our findings that FFNP-PET is more effective than FES-PET and FDGPET in predicting endocrine responsiveness provide strong support for noninvasive functional imaging as an informative imaging modality.
Materials and Methods
Cell cultures
The maintenance of the ERα+ PgR+ STAT1−/− mammary tumor cell lines SSM2 and SSM3 has been described (15). MCF7 breast cancer cells from Benita Katzenellenbogen were maintained in Improved MEM Zinc++ Option with phenol red supplemented with 5% heat-inactivated bovine calf serum and 5 nM estradiol.
Mice
Six to 8-week-old wildtype (WT) 129S6/SvEv mice and athymic nude-Foxn1nu mice were purchased from Taconic Farms and the National Cancer Institute, respectively, and maintained on an estrogen-free diet (Harlan Teklad). 1.2 × 106 of SSM2 or SSM3 tumor cells were transplanted into the right thoracic mammary fat pads of WT mice. Nude mice were ovariectomized, fed drinking water supplemented with 10 μg/ml estradiol for 7 days, and subsequently transplanted with 5 × 106 MCF7 cells. Tumors were measured with calipers at 2 perpendicular diameters every 3-4 days and the average diameters were used for analyses. Tumor-bearing mice were ovariectomized when the SSM2 or SSM3 tumors measured about 5 mm in diameter. All animal experiments were carried out according to the guidelines of the American Association for Laboratory Animal Science under an approved protocol by the Animal Studies Committees and performed in AAALAC-accredited specific pathogen-free facilities at Washington University School of Medicine in St. Louis.
Radiopharmaceuticals, biodistribution assay and small-animal μPET/CT
[18F]FDG was provided by the Cyclotron Facility at Washington University School of Medicine in St. Louis. [18F]FES and [18F]FFNP were synthesized using optimized methods as described previously (5, 14, 16, 17). The specific activities for both [18F]FES and [18F]FFNP averaged 7700 ± 3000 Ci/mmol (average ± SEM). Mice were injected intravenously with 11.1 MBq (300 μCi) of [18F]FDG, 5.55 MBq (150 μCi) of [18F]FES, or 1.11 MBq (30 μCi) of [18F]FFNP. These injected doses correspond to approximately 20 or 4 pmol for the [18F]FES and [18F]FFNP agents, respectively, and at a tumor uptake of 5%ID/g, the tumor concentrations would be 1 or 0.2 pmol/g, which is far below the receptor levels in the tumors (ca. 30 pmol/g) (14).
To examine whether [18F]FFNP selectively binds to PgR, unlabeled ligand competitors were used to block [18F]FFNP uptake in the SSM3 tumors. Promegestone (R5020; PerkinElmer) has high affinity for the progesterone receptor (PgR) (relative binding affinity [RBA] = 100), but does not bind the glucocorticoid receptor (GR) at significant levels (RBA = 2.6) (18). Dexamethasone (Dex; MP Biomedicals) has high affinity for GR (RBA = 100), but does not have any appreciable affinity for PgR (RBA = 0.21) (19). The IC50 of Dex for GR in rat lung is 0.1 μM whereas the IC50 of R5020 for PgR in sheep uterus is 0.05 μM (19). Blocking doses were determined based on the IC50’s and published results (20, 21). 0.5 μmol of Dex or 0.055 μmol of R5020 was injected into SSM3-bearing mice immediately prior to the injection of [18F]FFNP. For the in vivo tissue biodistribution assays, organs were harvested at 1 hour post-injection and radioactivity was measured. The percentage of injected dose per gram (% ID/g) of tissue was analyzed. For small-animal μPET/CT studies, tumor-bearing mice were imaged before surgery (either sham-operation or ovariectomy; Day 0), and 3 and 4 days post-surgery (Days 3 and 4). Since glucose levels are known to affect [18F]FDG uptake, tumor-bearing mice were fasted overnight the day before FDG imaging. For the MCF7 tumor study, estradiol was withdrawn from drinking water for 6 days starting on Day 0. PET and CT images were acquired and analyzed as described previously (14). Briefly, mice anesthetized with 1.5-2.0% isoflurane were scanned in the supine position in a small-animal μPET/CT scanner (Inveon and Focus-220, Siemens Preclinical Solutions) 1 hour following the injection of radiotracer. Images were analyzed using Inveon Research Workplace 3.0. μPET and CT images are co-registered by visual alignment in all three planes (transverse, sagittal, coronal). Regions of interest were manually drawn around the non-necrotic areas of the tumor on the co-registered μPET/CT images. Activity in triceps muscle was used as nontarget tissue uptake. Activity measurements (Bq/cm3) were divided by the decay-corrected injected dose (Bq) and multiplied by 100 to obtain a tissue uptake index expressed as percentage injected dose per gram (% ID/g) of tissue. Tumor to muscle (T:M) ratio was calculated as the ratio of % ID/g of tumor to that of muscle.
Immunohistochemistry
Four-micron sections of formalin-fixed paraffin-embedded mammary tumors were deparaffinized, rehydrated, heated in citrate buffer (pH 6), and stained using antibodies against PgR (Dako; A0089; 1:100). Positive signal was developed according to instructions from the EnVision HRP System (Dako) or MACH 4 (Biocare) followed by DAB chromogen.
Statistical analyses
All numerical results are presented as mean±standard error of mean (s.e.m.). Unpaired t test was used to determine the statistical significance between control and experimental groups whereas paired t test was implemented to compare across different time points within the same group of animals. All tests are two-sided and a p value ≤ 0.05 was considered significant.
Results
Kinetics of [18F]FES and [18F]FFNP Uptake in Endocrine-Sensitive Mammary Tumors Following Ovarian Ablation
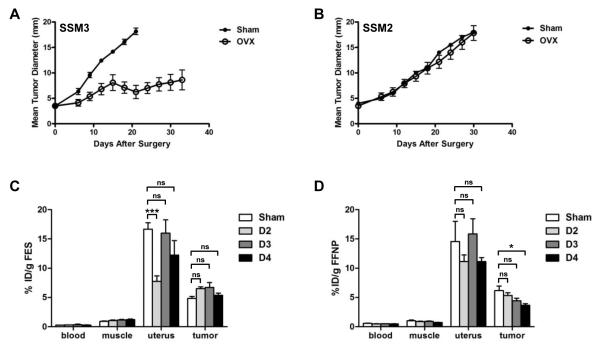
A preclinical model of ERα+ breast cancer that faithfully recapitulates the pathophysiological and developmental characteristics of human ERα+ breast cancer was utilized in this study (15, 22). One of the advantages of this preclinical model is the availability of paired ERα+ PgR+ endocrine-sensitive SSM3 and endocrine-resistant SSM2 mammary tumor cell lines, since potential imaging biomarkers can be examined and compared in the two tumor systems (14, 15) (Fig. 1A and 1B). Ovariectomy (OVX) was carried out as the treatment strategy to eliminate estradiol production by the ovaries (Fig. 1A and 1B). Since the contribution of extragonadal aromatase activity in the mouse is negligible due to the lack of extragonadal-specific promoters in the mouse aromatase gene loci (23), OVX mirrors ovarian suppression plus aromatase inhibition (AI) for premenopausal women or AI alone for postmenopausal women. Using this treatment protocol, we observed that the growth of the SSM3 mammary tumors was blunted by estrogen-deprivation therapy (Fig. 1A). In contrast, progression of the SSM2 mammary tumors remained unaffected (Fig. 1B).
Figure 1. Response of the ERα+ STAT1−/− mammary tumors to estrogen deprivation therapy, and in vivo tissue biodistribution of [18F]FES and [18F]FFNP, following therapy.
(A and B) Mice bearing transplanted SSM3 (A) or SSM2 (B) mammary tumors were sham-operated (solid circles, n = 5) or ovariectomized (OVX; open circles, n = 5) when tumors were measured around 4 mm in diameter. Tumor growth was followed for 35 days after the surgery. (C and D) SSM3-bearing mice were sham-operated (Sham; n = 5) or OVX for 2, 3, or 4 days (D2, D3, or D4, respectively; n = 5 per time point). The indicated organs were harvested. The biodistribution of [18F]FES (C) and [18F]FFNP (D) was determined in the harvested tissues 1 hr post-FES or FFNP injection, respectively. The percentage of injected dose per gram (% ID/g) of tissue was analyzed. * p<0.05, *** p<0.001. ns = not significant.
We first sought to determine whether an acute reduction in [18F]FES uptake could be observed in hormone-dependent SSM3 mammary tumors after estrogen-deprivation therapy. A tissue biodistribution study was carried out on sham-operated SSM3-bearing mice (Sham) and on tumor-bearing mice that were ovariectomized for 2, 3 or 4 days (D2, D3 or D4, respectively) (Fig. 1C). Tumor, uterus, muscle and blood were harvested from these mice, and radioactivity was measured in the indicated organs. [18F]FES uptake in the SSM3 tumors did not change significantly after ovarian ablation (Fig. 1C). Interestingly, [18F]FES uptake in the uterus was transiently reduced on Day 2 post-OVX and returned to basal levels on Days 3 and 4 post-OVX, mimicking a short term transient regulation of uterine ERα by E2 previously noted by others (24).
In contrast to FES-PET, [18F]FFNP uptake in the SSM3 tumors was reduced gradually over the course of 4 days following OVX (Fig. 1D). By day 4 post-OVX, tumor FFNP uptake was decreased by 40% compared to that in the tumors of sham-operated mice (p = 0.01). Taken together, these results indicate that [18F]FFNP uptake, but not [18F]FES uptake, in tumors is decreased acutely by depletion of ovarian hormones.
[18F]FFNP Uptake in SSM3 Tumors is Specific to PgR
Because [18F]FFNP binds the glucocorticoid receptor (GR) with modest affinity (18), we examined whether [18F]FFNP uptake in the SSM3 tumors was specific to PgR. The unlabeled forms of the PgR-selective ligand R5020 or GR-selective ligand dexamethasone (Dex) were used to compete with the in vivo binding of [18F]FFNP. Results from the competition assay indicated that [18F]FFNP uptake in PgR-rich organs, such as tumors and uterus, was blocked only by the PgR-selective ligand R5020, not by the GR-selective ligand Dex (Fig. 2). In contrast, [18F]FFNP uptake in the PgR-negative liver, which is due to metabolism and clearance, was not affected by either blocking agent (Fig. 2). These findings therefore demonstrate that [18F]FFNP binds specifically to PgR in the SSM3 mammary tumors.
Figure 2. [18F]FFNP uptake in the SSM3 tumors and uteri was specific to PgR.
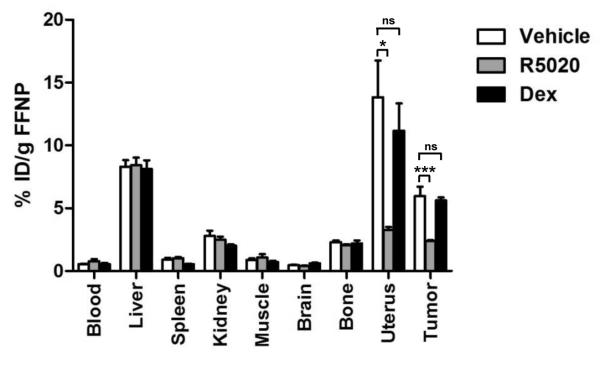
The unlabeled forms of the PgR-selective ligand R5020 or glucocorticoid receptor (GR)-selective ligand dexamethasone (Dex) were injected into SSM3 tumor-bearing mice immediately before the injection of [18F]FFNP (5 mice per group). The indicated organs were harvested. The in vivo tissue biodistribution of [18F]FFNP was determined in the harvested tissues 1 hr post-FFNP injection. Representative results from 3 independent experiments are shown. * p<0.05, *** p<0.001. ns = not significant.
Longitudinal FFNP-μPET/CT Imaging of Endocrine-Sensitive Mammary Tumors
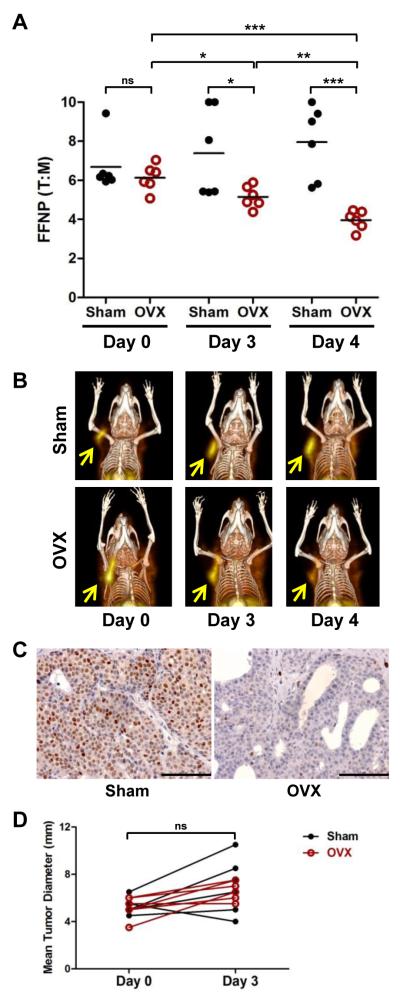
Having demonstrated the specificity and kinetics of [18F]FFNP binding following OVX by biodistribution, we next evaluated the feasibility of noninvasive functional imaging using [18F]FFNP in following the functional changes of ERα signaling in breast tumor cells over the course of endocrine therapy. SSM3 tumor-bearing mice were assigned to either sham-operation or OVX groups to standardize tumor size (Fig. 3). Longitudinal FFNP-μPET/CT imaging was performed on the two cohorts of mice before surgery (day 0), and 3 and 4 days after surgery; these time intervals were chosen based on the kinetic study presented in Fig. 1D. Tumor to muscle (T:M) ratio of [18F]FFNP uptake did not differ significantly between sham and OVX groups on day 0 before the surgery (Fig. 3A). However, T:M ratios in the OVX mice were significantly lower than those in the sham mice on days 3 and 4 after the surgery (p = 0.04 and p = 0.0005, respectively) (Fig. 3A and 3B). Consistent with the biodistribution data presented in Fig. 1D, the T:M ratio in the OVX group was steadily decreased by 15% from the basal level on day 0 to day 3 post-OVX (p = 0.01), and by another 23% from day 3 to day 4 post-OVX (p = 0.009). The overall reduction in T:M ratio was 35% on day 4 post-OVX, compared to the baseline measurement on day 0 (p = 0.0002). Results obtained from immunohistochemical analysis also indicated that the number of PgR+ tumor cells in OVX SSM3 tumors was significantly diminished compared to the SSM3 tumors in sham-operated mice (Fig. 3C). Importantly, tumor size in sham and OVX mice, as measured by caliper, did not differ on either days 3 or 4 post-surgery, indicating that the acute reduction in PgR expression in the OVX tumors occurs before a decrease in tumor size can be detected (Fig. 3D). Taken together, these data clearly demonstrate that a reduction in PgR expression in endocrine-responsive mammary tumor cells following downregulation of ERα signaling can be measured serially and noninvasively using [18F]FFNP.
Figure 3. Longitudinal [18F]FFNP μPET/CT imaging of SSM3 tumor-bearing mice.
(A) Mice bearing SSM3 tumors were scanned 1 hr after [18F]FFNP injection. % ID/g of tumor and muscle were calculated. Ratio of % ID/g in tumor to % ID/g in muscle (i.e., tumor to muscle ratio [T:M]) from Sham (n = 6) and OVX mice before surgery (Day 0; n = 6) and on Days 3 and 4 post-surgery (n = 6) were graphed. * p<0.05, ** p<0.01, *** p<0.001. ns = not significant. (B) 3D maximum intensity projection (MIP) from co-registered μPET and CT images of representative mice bearing SSM3 mammary tumors. Serial images from the same mouse in each group are shown. Arrows indicate tumors. (C) Representative PgR IHC images of SSM3 tumors on Day 4 following surgery. (D) Mean tumor diameter of SSM3 tumors before surgery (Day 0) and after surgery (Day 3). No significant difference in tumor size was observed between the two cohorts of mice. Representative results of two independent studies are shown.
Longitudinal FFNP-μPET/CT Imaging of Endocrine-Resistant Mammary Tumors
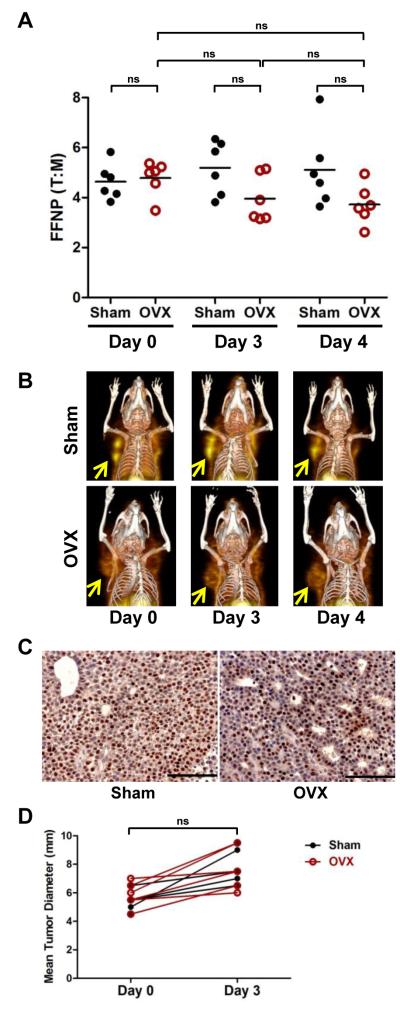
Since the ERα+ PR+ mammary tumor cell line SSM2 is nonresponsive to ovarian hormone-depletion (Fig. 1B), we hypothesized that PgR expression and thus [18F]FFNP uptake would not be affected acutely by OVX. Indeed, FFNP T:M ratios of sham and OVX mice were not significantly different on days 0, 3, and 4 post-surgery (Fig. 4A and 4B). In addition, there was also no significant change in the T:M ratios in the OVX tumors as a group when the same tumors were monitored longitudinally from day 0 to days 3 or 4. Results from IHC analysis also indicated that PgR expression remained elevated in the OVX SSM2 tumors compared to sham SSM2 tumors, confirming the FFNP-PET imaging findings (Fig. 4C). No significant difference in tumor sizes was observed between sham and OVX groups (Fig. 4D). Together, acute reduction in tumor [18F]FFNP uptake and PgR expression is associated with downregulation of ERα signaling in endocrine-sensitive tumor (SSM3) whereas the lack of reduction in FFNP uptake and PgR expression is associated with endocrine-resistant tumor (SSM2).
Figure 4. Longitudinal [18F]FFNP μPET/CT imaging of SSM2 tumor-bearing mice.
(A) Mice bearing SSM2 tumors were scanned 1 hr after [18F]FFNP injection. % ID/g of tumor and muscle were calculated. Ratio of % ID/g in tumor to % ID/g in muscle (i.e., tumor to muscle ratio [T:M]) from Sham (n = 6) and OVX mice before surgery (Day 0; n = 6) and on Days 3 and 4 post-surgery (n = 6) were graphed. ns = not significant. (B) 3D maximum intensity projection (MIP) from co-registered μPET and CT images of representative mice bearing SSM2 mammary tumors. Serial images from the same mouse in each group are shown. Arrows indicate tumors. (C) Representative PgR IHC images of SSM2 tumors on Day 4 following surgery. (D) Mean tumor diameter of SSM2 tumors before surgery (Day 0) and after surgery (Day 3). No significant difference in tumor size was observed between the two cohorts of mice. Representative results of two independent studies are shown.
Longitudinal FDG-μPET/CT Imaging of Endocrine-Sensitive and -Resistant Mammary Tumors
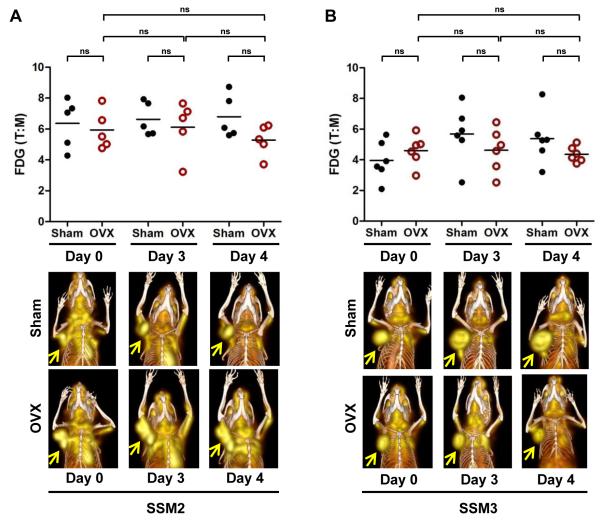
[18F]FDG is the most frequently used radiopharmaceutical to evaluate clinical response to therapies. We next examined whether FDG-PET would be suitable to monitor tumor response in the preclinical neoadjuvant setting of estrogen-deprivation therapy. As shown in Fig. 5A, we did not observe any changes in [18F]FDG uptake in the OVX-resistant SSM2 tumors following OVX. The T:M ratio of [18F]FDG uptake remained unchanged on days 3 and 4 after OVX compared to the basal level before OVX. There was also no statistically significant difference in the FDG T:M ratio between sham and OVX tumors at all 3 time points.
Figure 5. Longitudinal [18F]FDG μPET/CT imaging of SSM2 (A) and SSM3 (B) mammary tumors.
(top panels) Mice bearing SSM2 (A) or SSM3 (B) tumors were scanned 1 hr after [18F]FDG injection. % ID/g of tumor and muscle were calculated. Tumor to muscle ratio (T:M) of FDG uptake in SSM2 (n = 5 per group) (A) and SSM3 (n = 6 per group) (B) tumors from Sham and OVX mice before surgery (Day 0) and on Days 3 and 4 post-surgery. ns = not significant. (bottom panels) 3D maximum intensity projection (MIP) from co-registered μPET and CT images of representative mice bearing SSM2 (A) and SSM3 (B) mammary tumors. Serial images from the same mouse in each group are shown. Arrows indicate tumors. Representative results of two independent studies are shown.
In contrast to the SSM2 tumors, since SSM3 tumor growth was blunted by OVX, we expected to observe a decrease in [18F]FDG uptake in the SSM3 tumors following OVX. To our surprise, the T:M ratio of [18F]FDG uptake was indistinguishable between the sham and OVX groups at all time-points examined (Fig. 5B). There was also no difference in the T:M ratio between days 0, 3 and 4 in the OVX cohort. Within this time frame examined, the SSM3 tumors did not show significant tumor progression, as measured by caliper (Fig. 3D). Therefore, levels of [18F]FDG uptake remained unchanged in both endocrine-sensitive and -resistant mammary tumors.
Longitudinal FFNP-μPET/CT Imaging of Endocrine-Sensitive MCF7 Mammary Tumors
To extend and validate our findings, we tested whether the endocrine-sensitive ERα+ MCF7 human breast tumors displayed a similar FFNP binding profile following estrogen depletion. Since the MCF7 xenograft tumors grew at a slower pace than the SSM3 tumors, we monitored [18F]FFNP uptake after 6 days of estrogen withdrawal (Fig. 6; -E2 group). Consistent with the SSM3 results, [18F]FFNP uptake in the MCF7 tumors was significantly reduced in the absence of estrogen on Day 6 compared to the baseline levels on Day 0 (Fig. 6; p = 0.0003). [18F]FFNP uptake was also lower in the estrogen-depleted group (-E2) compared to the estrogen-supplemented group (+E2) on Day 6 (p = 0.0008). Taken together, rapid changes in [18F]FFNP uptake following estrogen deprivation were also observed in the prototypical ERα+ MCF7 breast cancer xenograft model.
Figure 6. Longitudinal [18F]FFNP μPET/CT imaging of MCF7-bearing mice.
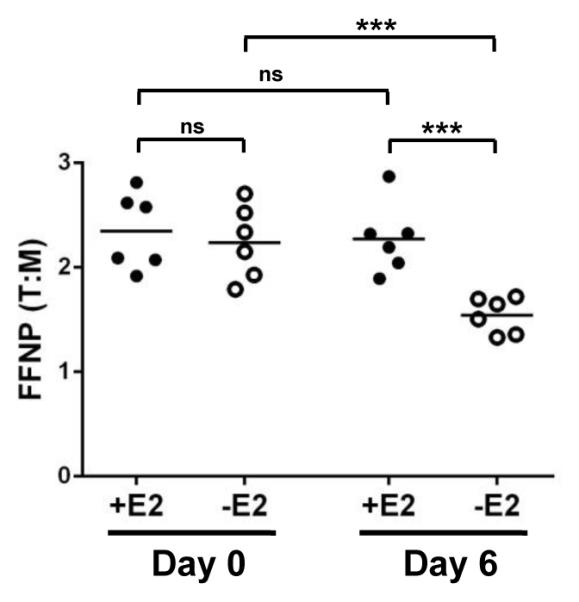
Mice bearing MCF7 tumors were scanned 1 hr after [18F]FFNP injection. Tumor to muscle ratio (T:M) of FFNP uptake in MCF7 human breast tumors before estrogen (E2) deprivation (Day 0) and 6 days after E2 deprivation (Day 6, -E group, n = 6). Estrogen was supplemented in the drinking water of control mice (Day 6, +E group, n = 6). Representative of two independent experiments is shown. *** p<0.001. ns = not significant.
Discussion
In this study, we demonstrated that serial FFNP-PET imaging of ERα+ mammary tumors before estrogen-deprivation therapy and shortly after treatment was an effective approach to inform treatment response. A significant drop in [18F]FFNP uptake levels in tumors post-treatment compared to pre-treatment level predicted a positive response to estrogen deprivation therapy. In contrast, a lack of reduction in [18F]FFNP uptake in tumors predicted resistance to therapy. As [18F]FFNP binds PgR with high affinity and selectivity, a decrease in [18F]FFNP binding in endocrine-sensitive tumors reflects a reduction in PgR expression level post-treatment, as confirmed by immunohistochemical analysis. Since PgR is a direct target of ERα signaling, PgR expression and thus [18F]FFNP tumor uptake are surrogate markers of the activation state of ERα signaling in tumor cells. These findings are consistent with clinical data that show a strong correlation between downregulation of ERα-regulated gene expression and inhibition of tumor cell proliferation by aromatase inhibition (25). Since a decrease in tumor cell proliferation has been shown to be a good prognostic marker for endocrine therapies (26-28), our preclinical study is a proof-of-principle to further support that monitoring the biological and molecular consequences of ERα signaling is highly informative in predicting therapeutic response.
Our findings also indicated that FFNP-PET was more sensitive than FES-PET in monitoring the physiological response of ERα signaling blockade. A reduction in [18F]FFNP binding in the endocrine-sensitive SSM3 tumors was observed as early as 3 days after the initiation of estrogen deprivation therapy. This drop in [18F]FFNP uptake in the tumors continued on day 4 post-OVX. Although it is possible that the levels of [18F]FES uptake in endocrine-sensitive mammary tumors might change at later time points following OVX, a delay in identifying endocrine-resistant patients could potentially lead to a delay in the initiation of alternative effective treatments. In addition, monitoring a decrease in ER levels by uptake of [18F]FES in response to estrogen deprivation is not a direct measure of ER function in the same way as is the measure of decreasing levels of PgR by [18F]FFNP.
FFNP-PET is also an early, more robust biomarker than changes in tumor size or FDG-PET in predicting endocrine response. Our previous study demonstrated that a change in [18F]FDG uptake in the SSM3 tumors was detected 1 week after fulvestrant therapy (14). Together with the current findings, these results suggest that a decrease in [18F]FFNP tumor uptake precedes that in [18F]FDG tumor uptake. Therefore, monitoring acute changes downstream of ERα signaling provides a more rapid assessment than measuring glucose metabolism in the tumors following estrogen deprivation therapy.
In contrast to our current study, FDG-PET was demonstrated to be effective in predicting therapeutic response of the partial agonist tamoxifen or of low-dose estradiol therapy (9, 10, 29). We have previously demonstrated that tamoxifen or low-dose estradiol causes metabolic flare in tumors such that an increase in tumor glucose metabolism and [18F]FDG uptake 24 hours after estradiol administration is associated with a positive therapeutic response (9, 10, 29). It will be interesting to test whether FFNP-PET can also be used as an imaging biomarker in these settings where metabolic flare is observed, and whether it provides a signal that is more rapid and robust than that of FDG-PET, as it is shown in this current study with estrogen deprivation.
Quantification of tumor cell proliferation by immunohistochemical analysis of Ki67 antigen has also been proven to be an effective biomarker in predicting outcome of endocrine therapies (26). Inhibition of tumor cell proliferation by antiestrogens or estrogen deprivation is closely correlated with better prognosis (28, 30). Since there is a strong concordance between Ki67 proliferation index and [18F]FLT uptake in breast cancers (31), future studies assessing the effectiveness of FLT-PET as an imaging biomarker for endocrine treatments will be needed.
In conclusion, we demonstrated that serial FFNP-PET imaging was more effective than FDG-PET and FES-PET in predicting response to estrogen deprivation therapy in preclinical models of ERα+ breast cancer. In the context of our present study with estrogen deprivation therapy and previous results with fulvestrant, one may extrapolate this biomarker approach to additional endocrine interventions, such as toremifene, which is also antagonistic in the breast. Measurement of acute changes in PgR expression by noninvasive FFNP-PET may be beneficial in a neoadjuvant setting so that a more aggressive treatment course can be implemented after surgery if the primary tumor is predicted to be endocrine-nonresponsive.
Translational Relevance.
Although endocrine therapies are the standard-of-care for patients with estrogen receptor-alpha-positive (ERα+) breast cancer, about half of the patients do not benefit from these treatments due to intrinsic or acquired resistance. Therefore, biomarkers that predict tumor responsiveness to endocrine therapies are highly valuable for identifying patients who will require alternative treatments. Noninvasive functional PET imaging offers a unique opportunity to serially follow the molecular changes in tumors in response to therapies. Using preclinical models of ERα+ breast cancer, we demonstrated that a significant reduction in binding of [18F]FFNP to progesterone receptor predicts a positive tumor response to estrogen-deprivation therapy. By contrast, [18F]FES or [18F]FDG uptake by tumors remained unchanged, suggesting that ERα expression and glucose metabolism were not acutely affected by estrogen deprivation. Thus, measurement of ERα function by longitudinal FFNP-PET imaging provides a more robust biomarker for response to endocrine-deprivation therapies than measurement of ERα level by FES-PET.
Acknowledgements
We thank B. Katzenellenbogen (University of Illinois) for supplying MCF7 cells and for a critical review of the manuscript. The authors are also grateful to A. Klaas, M. Morris, L. Strong and A. Stroncek (Washington University) for their excellent technical assistance. This work was supported by grants from the National Institutes of Health (U01CA141541 to S.R.C. and R01CA25836 to J.A.K.).
References Cited
- 1.Maxmen A. The hard facts. Nature. 2012;485:S50–1. doi: 10.1038/485S50a. [DOI] [PubMed] [Google Scholar]
- 2.Arnedos M, Nerurkar A, Osin P, A’Hern R, Smith IE, Dowsett M. Discordance between core needle biopsy (CNB) and excisional biopsy (EB) for estrogen receptor (ER), progesterone receptor (PgR) and HER2 status in early breast cancer (EBC) Ann Oncol. 2009;20:1948–52. doi: 10.1093/annonc/mdp234. [DOI] [PubMed] [Google Scholar]
- 3.Allred DC, Wu Y, Mao S, Nagtegaal ID, Lee S, Perou CM, et al. Ductal carcinoma in situ and the emergence of diversity during breast cancer evolution. Clin Cancer Res. 2008;14:370–8. doi: 10.1158/1078-0432.CCR-07-1127. [DOI] [PubMed] [Google Scholar]
- 4.Sundararajan L, Linden HM, Link JM, Krohn KA, Mankoff DA. 18F-Fluoroestradiol. Semin Nucl Med. 2007;37:470–6. doi: 10.1053/j.semnuclmed.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Dehdashti F, Laforest R, Gao F, Aft RL, Dence CS, Zhou D, et al. Assessment of progesterone receptors in breast carcinoma by PET with 21-18F-fluoro-16alpha,17alpha-[(R)-(1′-alpha-furylmethylidene)dioxy]-19-norpregn- 4-ene-3,20-dione. J Nucl Med. 2012;53:363–70. doi: 10.2967/jnumed.111.098319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoo J, Dence CS, Sharp TL, Katzenellenbogen JA, Welch MJ. Synthesis of an estrogen receptor beta-selective radioligand: 5-[18F]fluoro-(2R,3S)-2,3-bis(4-hydroxyphenyl)pentanenitrile and comparison of in vivo distribution with 16alpha-[18F]fluoro-17beta-estradiol. Journal of medicinal chemistry. 2005;48:6366–78. doi: 10.1021/jm050121f. [DOI] [PubMed] [Google Scholar]
- 7.Kurland BF, Peterson LM, Lee JH, Linden HM, Schubert EK, Dunnwald LK, et al. Between-patient and within-patient (site-to-site) variability in estrogen receptor binding, measured in vivo by 18F-fluoroestradiol PET. J Nucl Med. 2011;52:1541–9. doi: 10.2967/jnumed.111.091439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linden HM, Stekhova SA, Link JM, Gralow JR, Livingston RB, Ellis GK, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol. 2006;24:2793–9. doi: 10.1200/JCO.2005.04.3810. [DOI] [PubMed] [Google Scholar]
- 9.Dehdashti F, Mortimer JE, Trinkaus K, Naughton MJ, Ellis M, Katzenellenbogen JA, et al. PET-based estradiol challenge as a predictive biomarker of response to endocrine therapy in women with estrogen-receptor-positive breast cancer. Breast cancer research and treatment. 2009;113:509–17. doi: 10.1007/s10549-008-9953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;19:2797–803. doi: 10.1200/JCO.2001.19.11.2797. [DOI] [PubMed] [Google Scholar]
- 11.Linden HM, Kurland BF, Peterson LM, Schubert EK, Gralow JR, Specht JM, et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin Cancer Res. 2011;17:4799–805. doi: 10.1158/1078-0432.CCR-10-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma CX, Sanchez CG, Ellis MJ. Predicting endocrine therapy responsiveness in breast cancer. Oncology (Williston Park) 2009;23:133–42. [PubMed] [Google Scholar]
- 13.Buckman BO, Bonasera TA, Kirschbaum KS, Welch MJ, Katzenellenbogen JA. Fluorine-18-labeled progestin 16 alpha, 17 alpha-dioxolanes: development of high-affinity ligands for the progesterone receptor with high in vivo target site selectivity. Journal of medicinal chemistry. 1995;38:328–37. doi: 10.1021/jm00002a014. [DOI] [PubMed] [Google Scholar]
- 14.Fowler AM, Chan SR, Sharp TL, Fettig NM, Zhou D, Dence CS, et al. MicroPET imaging of steroid hormone receptors predicts tumor response to endocrine therapy using a preclinical model of breast cancer. J Nucl Med. 2012;53:1–8. doi: 10.2967/jnumed.112.103465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan SR, Vermi W, Luo J, Lucini L, Rickert C, Fowler AM, et al. STAT1-deficient mice spontaneously develop estrogen receptor a-positive luminal mammary carcinomas. Breast Cancer Res. 2012;14:R16. doi: 10.1186/bcr3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim JL, Zheng L, Berridge MS, Tewson TJ. The use of 3-methoxymethyl-16 beta, 17 beta-epiestriol-O-cyclic sulfone as the precursor in the synthesis of F-18 16 alpha-fluoroestradiol. Nuclear medicine and biology. 1996;23:911–5. doi: 10.1016/s0969-8051(96)00126-6. [DOI] [PubMed] [Google Scholar]
- 17.Zhou D, Lin M, Yasui N, Al-Qahtani MH, Dence CS, Schwarz S, et al. Optimization of the preparation of fluorine-18-labeled steroid receptor ligands 16alpha-[(18) F]fluoroestradiol (FES), [(18) F]fluoro furanyl norprogesterone (FFNP), and 16beta-[(18) F]fluoro-5alpha-dihydrotestosterone (FDHT) as radiopharmaceuticals. J Labelled Comp Radiopharm. 2014;57:371–7. doi: 10.1002/jlcr.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH, Zhou HB, Dence CS, Carlson KE, Welch MJ, Katzenellenbogen JA. Development of [F-18]fluorine-substituted Tanaproget as a progesterone receptor imaging agent for positron emission tomography. Bioconjugate chemistry. 2010;21:1096–104. doi: 10.1021/bc1001054. [DOI] [PubMed] [Google Scholar]
- 19.Issar M, Sahasranaman S, Buchwald P, Hochhaus G. Differences in the glucocorticoid to progesterone receptor selectivity of inhaled glucocorticoids. Eur Respir J. 2006;27:511–6. doi: 10.1183/09031936.06.00060005. [DOI] [PubMed] [Google Scholar]
- 20.Pomper MG, Kochanny MJ, Thieme AM, Carlson KE, VanBrocklin HF, Mathias CJ, et al. Fluorine-substituted corticosteroids: synthesis and evaluation as potential receptor-based imaging agents for positron emission tomography of the brain. Int J Rad Appl Instrum B. 1992;19:461–80. doi: 10.1016/0883-2897(92)90161-q. [DOI] [PubMed] [Google Scholar]
- 21.Zhou D, Sharp TL, Fettig NM, Lee H, Lewis JS, Katzenellenbogen JA, et al. Evaluation of a bromine-76-labeled progestin 16alpha,17alpha-dioxolane for breast tumor imaging and radiotherapy: in vivo biodistribution and metabolic stability studies. Nuclear medicine and biology. 2008;35:655–63. doi: 10.1016/j.nucmedbio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan SR, Rickert CG, Vermi W, Sheehan KC, Arthur C, Allen JA, et al. Dysregulated STAT1-SOCS1 control of JAK2 promotes mammary luminal progenitor cell survival and drives ERalpha tumorigenesis. Cell Death Differ. 2014;21:234–46. doi: 10.1038/cdd.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao H, Pearson EK, Brooks DC, Coon JSt, Chen D, Demura M, et al. A humanized pattern of aromatase expression is associated with mammary hyperplasia in mice. Endocrinology. 2012;153:2701–13. doi: 10.1210/en.2011-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nephew KP, Long X, Osborne E, Burke KA, Ahluwalia A, Bigsby RM. Effect of estradiol on estrogen receptor expression in rat uterine cell types. Biol Reprod. 2000;62:168–77. doi: 10.1095/biolreprod62.1.168. [DOI] [PubMed] [Google Scholar]
- 25.Gao Q, Patani N, Dunbier AK, Ghazoui Z, Zvelebil M, Martin LA, et al. Effect of Aromatase Inhibition on Functional Gene Modules in Estrogen Receptor-Positive Breast Cancer and Their Relationship with Antiproliferative Response. Clin Cancer Res. 2014;20:2485–94. doi: 10.1158/1078-0432.CCR-13-2602. [DOI] [PubMed] [Google Scholar]
- 26.Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. Journal of the National Cancer Institute. 2011;103:1656–64. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype--ACOSOG Z1031. J Clin Oncol. 2011;29:2342–9. doi: 10.1200/JCO.2010.31.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, Griffith C, et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res. 2005;11:951s–8s. [PubMed] [Google Scholar]
- 29.Ellis MJ, Gao F, Dehdashti F, Jeffe DB, Marcom PK, Carey LA, et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. Jama. 2009;302:774–80. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellis MJ, Tao Y, Luo J, A’Hern R, Evans DB, Bhatnagar AS, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. Journal of the National Cancer Institute. 2008;100:1380–8. doi: 10.1093/jnci/djn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chalkidou A, Landau DB, Odell EW, Cornelius VR, O’Doherty MJ, Marsden PK. Correlation between Ki-67 immunohistochemistry and 18F-fluorothymidine uptake in patients with cancer: A systematic review and meta-analysis. Eur J Cancer. 2012;48:3499–513. doi: 10.1016/j.ejca.2012.05.001. [DOI] [PubMed] [Google Scholar]