Abstract
Background
Activation of the kynurenine pathway of tryptophan metabolism results in increased production of potentially depressogenic tryptophan catabolites and a reduction in tryptophan availability for serotonin synthesis. Since alcohol consumption affects tryptophan metabolism and disposition, we determined serum levels of kynurenine, tryptophan and the kynurenine/tryptophan ratio (KT ratio) in alcohol-use disorder (AUD) patients and compared their levels considering abstinence duration, AUD severity and comorbid depression.
Methods
The study sample included 169 AUD inpatients from eight alcohol treatment facilities in Kathmandu, Nepal. The Composite International Diagnostic Interview was administered to generate the AUD diagnosis. The Alcohol Use Disorder Identification Test (AUDIT) captured AUD severity and patterns of alcohol use. The Hopkins Symptom Checklist-25 was used to reveal current depressive symptoms. Serum kynurenine and tryptophan levels were determined by high-performance liquid chromatography and tryptophan degradation was measured by KT ratio (kynurenine/tryptophan × 103).
Results
Patients with above average AUDIT scores had higher mean serum levels of kynurenine (2.1μM±0.7 vs 1.8 μM ±0.6, p= 0.006) and KT ratios (48.6±17.6 vs 40.4±14.3, p=0.002) than those with below average scores. Patients with current depressive symptoms had higher mean tryptophan concentrations (49.9 μM ±13 vs 45.7 μM±14.1, p= 0.047) and lower KT ratios (41.4 μM ±14 vs 47.5 μM ±17.6, p=0.028) compared to patients whose reported depressive symptoms were below the standard cut-off. Higher tryptophan levels and lower KT ratios in the depressed group was specific to patients with longer abstinence and higher AUD severity.
Conclusions
Depression-related deregulation in tryptophan metabolism was found to depend on length of abstinence and on AUD severity. Together, results suggest that in AUD populations, peripheral tryptophan metabolism is subject to interactions between AUD severity and depressive symptoms.
Keywords: alcohol, depression, comorbidity, tryptophan metabolism, kynurenine pathway
1. Introduction
The frequent comorbidity between alcohol-use disorders (AUD) and major depression (MD) is well established (Lynskey, 1998, Kessler et al., 1994, Sullivan et al., 2005). Still, the biological nature of the relationship between these chronic and burdensome disorders remains unclear. Some of the biological pathways involved in the development and progression of depressive disorders are also affected by alcohol. Specifically, serotonin (5-HT) bioavailability, widely believed to be key in the pathogenesis of depressive disorders (Coppen, 1967, Maes and Meltzer, 1995), is amenable to alcohol consumption (LeMarquand et al., 1994). Indeed, both acute and chronic alcohol intake have a profound effect on the metabolism of tryptophan, which is the essential amino acid precursor for 5-HT synthesis (Badawy, 2002, Badawy et al., 2009). These intersecting pathways underscore the need to include alcohol measures when studying the mechanisms of depression, and the need to examine the biological processes of depression among people with AUD.
A small proportion of circulatory tryptophan is used for 5-HT synthesis, while most is metabolized through the kynurenine pathway producing kynurenine and biologically active metabolites, including quinolinic acid and anthranilic acid which can be neurotoxic (Maes et al., 2007). The systemic and central nervous system depletion of tryptophan and the neurodegeneration associated with increased production of the neurotoxic tryptophan catabolites have been suggested as possible mechanisms for depression (Maes et al., 2011b, Capuron et al., 2003). Indeed, reduced concentrations of plasma and cerebral spinal fluid tryptophan, and a reduced ratio of tryptophan to other amino acids that share the same transporter for entry into the brain, have long been correlated with depression (DeMyer et al., 1981, Cowen et al., 1989).
Recent research has focused on immune activation of the tryptophan degrading enzyme indoleamine 2, 3-dioxygenase (IDO). The serum kynurenine to tryptophan ratio (KT ratio) reliably reflects IDO activity, which is subject to activation by inflammatory cytokines (Schrocksnadel et al., 2006, Raison et al., 2010). This ratio as a measure of tryptophan degradation has been shown to be increased in depression (Maes et al., 2011b, Myint et al., 2007). Owing to heterogeneity and diagnostic variability of affective disorders, contrary results have also been reported, which suggest that altered IDO activity may only characterize subsets of depressed populations (Maes et al., 2011a, Dunjic-Kostic et al., 2013). Another rate-limiting enzyme of the kynurenine pathway of tryptophan catabolism is tryptophan 2, 3-dioxygenase (TDO) in the liver. Like IDO, it results in the same downstream catabolites (Stone et al., 2012). Interestingly, alcohol-induced liver damage can result in altered TDO activity, leading to a disturbance in tryptophan metabolism and altered levels of circulating tryptophan and kynurenine. In the context of alcohol misuse, factors such as duration of and time since alcohol intake have been shown to determine the nature of tryptophan dysregulation (Badawy et al., 2009, Friedman et al., 1988).
Previous studies have found that an imbalance in the KT ratio can last for several months after abstinence from alcohol. Recently, Gleissenthall et al. reported that, among 54 AUD patients, those abstinent from alcohol for 11 weeks had higher levels of kynurenine and KT ratios compared to patients in their 4th week of abstinence (Gleissenthall et al., 2014). The authors interpreted this finding as a result of increased TDO activity, caused by elevated glucocorticoid levels in response to stress during the transition from short-to-medium term abstention from alcohol (Sinha et al., 2009). This is plausible, since TDO activity is influenced by stress-induced cortisol (Oxenkrug, 2010), whereas IDO activation is mostly immune-mediated (Muller and Schwarz, 2007). Gleissenthall et al. study also reported marked reduction in depressive symptoms alongside an increase in serum kynurenine and KT ratio levels. When tryptophan metabolism is biased towards kynurenine production, reduced bioavailability of circulatory tryptophan should result in 5-HT deficiency in the brain (Fernstrom, 1983) and lead to depression in a dose-dependent manner (Branchey et al., 1984b, Branchey et al., 1984a). The counter-intuitive finding in the Gleissenthall et al. study warrants closer inspection with a larger sample stratified by depression status.
We have previously shown in patients with AUD that MD-associated immune changes are attenuated by drinking severity (Neupane et al., 2014). Given the strong influence of alcohol use on the neurobiological processes associated with depression, it is useful and necessary to examine these processes in the context of AUD. Thus, we examined the associations of AUD severity, depressive symptoms, and length of abstinence with serum levels of kynurenine, tryptophan and KT ratio among Nepalese AUD patients in treatment. We also aimed to determine the relationships between length of abstinence as well as AUD severity and serum levels of kynurenine, tryptophan and KT ratios in AUD patients with and without depressive symptoms.
2. Materials and Methods
2.1 Participants and procedure
Consecutively admitted patients in eight different alcohol treatment institutions in the Kathmandu and Lalitpur districts of central Nepal were screened for eligibility. The setting, enrolment procedure and participant characteristics have previously been described in detail (Neupane and Bramness, 2014). Briefly, we interviewed 198 consenting patients who had alcohol as the primary substance of concern. Altogether, 188 participants met the Fourth version of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria for alcohol-use disorder. The current analysis is based on 169 AUD subjects with a complete set of psychometric evaluation data and laboratory measures of serum tryptophan and kynurenine levels. Of these 146 (86%) had alcohol dependence and the remaining 23 (14%) had alcohol abuse only. We excluded patients undergoing complicated withdrawal (n=3), intoxication (n=2) or presenting psychotic features (n=1) at the time of screening. Also excluded from further analysis were those with incomplete interviews (n=3), refusal or absence of blood samples (n=11) and dropout (n=5).
Consent was obtained from participants separately for interview and collection of blood samples. Literate patients provided written informed consent, while a witness confirmed informed consent and a thumb print from illiterate participants. Because we expected a level of illiteracy among our participants, all questionnaires were administered by the first author, a Nepali clinician certified to administer the Composite International Diagnostic Interview (CIDI). Blood sampling and interviewing was conducted on site in private rooms designated by the participating institution. Institutional stays in a controlled abstinence environment for 10 days was required prior to the interview.
The Norwegian Committee for Medical Research Ethics (region South-East) and the Ethics Committee of the National Health Research Council of Nepal approved the study protocol.
2.2 Psychometric evaluation
Initially, participants were administered the CIDI (World Health Organization, 1997) Nepalese version 2.1 to generate the DSM-IV diagnosis of AUD. Additionally, a translated version of the Alcohol Use Disorder Identification Test (AUDIT) (Saunders et al., 1993) was used to assess AUD severity. The most recent alcohol consumption episode was also documented to calculate the abstinence duration in days. Since satisfying an AUD diagnosis was required for inclusion in the analysis, a majority of the sample would score well above the cut-offs used for harmful and hazardous alcohol use (average of 8). Therefore, the sample's mean AUDIT score was used to construct a dichotomous AUD severity variable. Other measures of AUD severity included typical past year drinking frequency (defined as consuming any alcohol on 4 or more vs. 3 or fewer days a week), and binge drinking (defined as drinking 6 or more units per drinking episode).
Depression measures were defined in terms of major depression diagnosis (trait feature) and depression symptomatology (state feature). Major depression diagnoses (lifetime and past year) were based on the CIDI. More recent (2 weeks) depressive symptoms, excluding distress in the first week post-admission, were captured with the Hopkins Symptom Checklist-25 (HSCL-25) (Derogatis et al., 1974). The HSCL-25 includes 10 items in the anxiety and 15 items in the depression section; each item represents symptoms that are scored from 1 (none) through 2 (a little), 3 (quite a bit) to 4 (extremely). Caseness for depression and anxiety symptoms was set at the conventional standard cutoff score of 1.75 average scores on the respective scales. Both the CIDI and HSCL-25 have shown utility as diagnostic/screening instruments in comparable populations (van Ommeren et al., 1999, Wittchen, 1994, Shrestha et al., 1998).
2.3 Blood sample collection and serum separation
After screening or following a completed interview, depending on the patient's preference, 8 ml of venous blood was collected in a serum separating BD vacutainer® gel tube on site from all consenting patients. The clotted blood sample was spun within 2.5 hours at 1300 × g for 12 minutes at the recruitment hospital site using a swing-out centrifuge. The separated serum was stored in a polypropylene tube at -70 °C until transported while frozen to Norway for analysis.
2.4 Kynurenine & tryptophan assays
Serum tryptophan and kynurenine levels were determined by high-performance liquid chromatography (Widner et al., 1997). In brief, an aliquot of 200 μL serum was precipitated with 125 μl 25% (w/v) trichloroacetic acid, mixed and centrifuged. For separation, reversed phase Kinetex cartridges (2.6 μ C18 100A columns) from Phenomenex (Torrance, CA, USA) were used. Kynurenine was detected with an ultraviolet absorption detector and tryptophan was detected using a fluorescence detector. Instrumental analysis was performed on Agilent Infinity 1290 (Agilent Technologies, CA, USA). The ratio of kynurenine to tryptophan concentrations × 103 (KT ratio) was calculated and used as a measure of tryptophan degradation.
2.5 Statistical analysis
To address our first aim we compared kynurenine, tryptophan and KT ratio levels between patients according to length of abstinence, AUD severity and presence of depressive symptoms. For sensitivity analysis, we constructed two variables measuring abstinence: a dichotomy at 30 days of abstinence and the quartiles of abstinence duration. To address the second aim, we divided the sample according to the HSCL cut-off indicating depressive symptoms, and compared kynurenine, tryptophan and KT ratio levels across the dichotomized measures of length of abstinence and AUD severity. Serum tryptophan and kynurenine levels and the KT ratio were tested for normality of distribution using the Kolmogorov-Smirnov test, in which only tryptophan complied. Between-group comparisons were performed using the Student's t-tests on normally distributed variables and using the Mann Whitney U-test for variables not normally distributed. Relationships between continuous variables were assessed with the Spearman's rank order correlation coefficient ρ (rho) and dichotomous variables were compared between groups with χ2- tests of independence. The significance level for all tests was set at p < 0.05. Regression analysis was used to control for effects of the confounding factors of depression on tryptophan metabolism. Crude p-values and the effects of accounting for multiple comparisons are given wherever appropriate. All statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS Statistics) version 22.0 (SPSS Inc., IL, USA).
3. Results
3.1 Sample characteristics
Table 1 shows the demographic and clinical characteristics of the study participants according to recent depressive symptoms as measured by the HSCL-25 depression subscale. Recent depression was more common among younger participants, those with a parental history of drinking problems and those with a previous major depressive episode. Apart from these differences, recent depression in the sample was uniformly distributed across demographic and alcohol-use characteristics (Table 1).
Table 1. Demographic and clinical characteristics of participants by recent depressive symptoms (HSCL-25 mean cutoff >1.75 in depression subscale), given as N (%).
| Characteristics | All patients N= 169 |
No depression N=118 |
Depression N=51 |
P-value*,# |
|---|---|---|---|---|
| Gender: Male | 153 (90.5) | 110 (93.2) | 43 (84.3) | 0.087 |
| Age (years): mean (SD) | 35.6 (10.2) | 36.8 (10.3) | 32.8 (9.5) | 0.018 |
| Residence (urban vs rural) | 118 (69.8) | 81 (68.7) | 37 (72.5) | 0.612 |
| Marital status (marital and cohabitating vs others) | 106 (62.7) | 75 (63.6) | 31 (60.8) | 0.732 |
| Occupation (stable) | 101 (60.1) | 74 (63.2) | 27 (52.9) | 0.210 |
| Education (high vs low) | 122 (72.2) | 86 (72.9) | 36 (70.6) | 0.760 |
| Caste (High vs low) | 97 (57.4) | 71 (60.2) | 26 (51.0) | 0.267 |
| Parental drinking (yes vs no) | 65 (38.5) | 39 (33.1) | 26 (51.0) | 0.028 |
| Institution (rehabilitation centre vs hospital) | 146 (86.4) | 100 (84.7) | 46 (90.2) | 0.343 |
| Life time major depression | 74 (44.0) | 35 (29.9) | 39 (76.5) | <0.001 |
| Recent anxiety | 34 (20.6) | 10 (8.8) | 24 (47.1) | <0.001 |
| Current smokers | 113(66.9) | 80 (67.8) | 33 (64.7) | 0.695 |
| History of illicit substance use | 51 (30.2) | 31 (26.3) | 20 (39.2) | 0.092 |
| Alcohol dependence (vs alcohol abuse only) | 146 (86.4) | 98 (83.4) | 48 (92.3) | 0.222 |
| Main drink (locally produced beverage vs bottled) | 87 (51.5) | 64 (54.2) | 23 (45.1) | 0.275 |
| High risk drinker (AUDIT score >20) | 136 (81.9) | 94 (80.3) | 42 (85.7) | 0.412 |
| Drinking ≥6 units on one occasion (vs non-bingers) | 119 (71.3) | 84 (71.8) | 35 (70.0) | 0.814 |
| Daily alcohol use (ethanol units) | 12.4 (5.7) | 11.9 (5.4) | 14.4 (6.1) | 0.033 |
| Drinking days per week (4 or more) | 123 (73.7) | 83 (70.9) | 40 (80.0) | 0.224 |
| Abstinence duration (days): mean (SD) | 34.8 (32.8) | 35.6 (32.9) | 32.7 (31.6) | 0.675 |
HSCL-25: Hopkins Symptom Checklist- 25,
P-values were calculated by χ2- tests for dichomotmous variables and Mann Whitney-U test for age, daily alcohol use and abstinence duration. Percentages apply for within recent-depression categories.
P values less than 0.01 were deemed significant after the Bonferroni adjustment.
3.2 Tryptophan, kynurenine and KT ratio by demographics and alcohol-use features
Serum levels of kynurenine, tryptophan and the KT ratio did not vary according to gender or other demographics (data not shown), with the exception of the serum KT ratio which did increase with age (ρ =0.17, p=0.026). Alcohol-abuse- and alcohol-dependent subgroups did not have statistically different levels of tryptophan (48.2 ±14.0 vs 46.7 ±14.0; p =0.639), kynurenine (1.9 μM ±0.5 vs 2.1 μM ±0.7; p=0.205) or KT ratios (40.3 ±13.0 vs 46.6 ±17.3; p =0.098). Duration of abstinence was associated with both tryptophan (ρ =0.20, p=0.011) and kynurenine levels (ρ =0.16, p=0.037), but not with KT ratios (ρ =-0.05, p=0.553). Daily amount of alcohol use was associated with KT ratios only (ρ =0.21, p=0.019). Stratified analysis showed that the main difference in tryptophan and kynurenine levels was in patients in the second (8-19 days) and the last quartile (53+ days) of abstinence duration (P =0.004 and P =0.049, respectively), whereas, none of the parameters varied across different quartile ranges of daily drinking units.
Patients who scored higher than average on the AUDIT had higher kynurenine levels and KT ratios (Table 2). Similarly, kynurenine levels were significantly higher among patients who reported binge drinking (2.1 μM ±0.7) compared to those who did not (1.8 μM ±0.5; p =0.013), as were KT ratios (47.6 ±17.4 vs 39.0 ±12.2; p =0.002). In comparison to patients who drank less frequently, KT ratios were significantly higher in patients who drank alcohol 4 or more days a week (47.2±17.1 vs 39.5±13.2; p =0.006). The KT ratio, but not tryptophan or kynurenine separately, was positively associated with the AUDIT score (ρ = 0.19, p =0.013) and daily drinking units (ρ =0.20, p =0.019). Subgroup analysis showed higher kynurenine and KT ratios among people who scored above the mean sample AUDIT score compared with those who scored below it only within the non-depressed stratum (p =0.026 and p <0.001, respectively) (Fig 1, second panel).
Table 2.
Serum levels of tryptophan, kynurenine and the kynurenine to tryptophan ratio according to recent depression status (HSCL-25 mean cutoff >1.75 in depression subscale) and alcohol-use severity dichotomized at mean score on AUDIT scale. Values in the parentheses are standard deviation
| AUDIT score <28 (n= 65) |
AUDIT score ≥28 (n= 101) |
P-value* | No depression N=118 |
Depression N=51 |
P-value* | |
|---|---|---|---|---|---|---|
| Tryptophan (μM) | 47.6 (13.7) | 46.6 (14.1) | 0.639 | 45.7 (14.1) | 49.9 (13.0) | 0.047 |
| Kynurenine (μM) | 1.8 (0.6) | 2.1 (0.7) | 0.006 | 2.0 (0.7) | 2.0 (0.6) | 0.641 |
| Kynurenine/tryptophan X 103 | 40.4 (14.3) | 48.6 (17.6) | 0.002 | 47.5 (17.6) | 41.4 (14.0) | 0.028 |
HSCL-25: Hopkins Symptom Checklist- 25, AUDIT: Alcohol-Use Disorder Identification Test.
P-values were calculated by Student's t-test (tryptophan) and Mann Whitney U-test for the other variables. There were three missing data points for AUDIT score. Results are based on complete case analysis.
Fig 1.
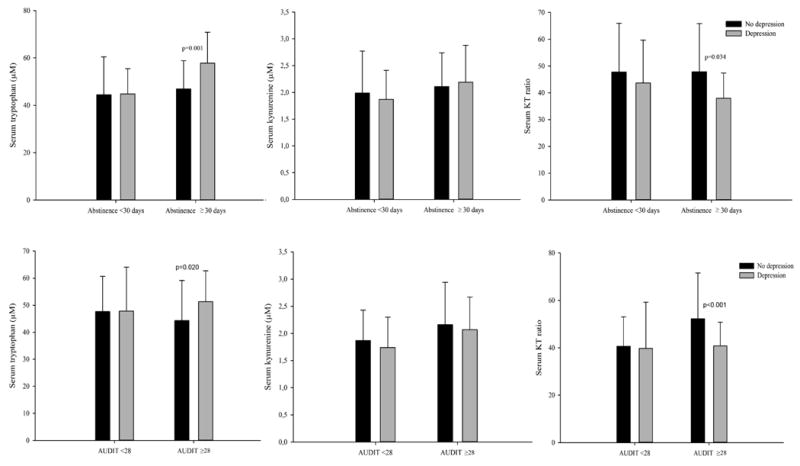
Serum levels of tryptophan, kynurenine and kynurenine/tryptophan × 103 (KT ratio), according to abstinence duration dichotomized at 30 days (first panel) and alcohol-use disorder (AUD) severity dichotomized at average Alcohol-Use Disorder Identification Test (AUDIT) score of 28 (second panel), by AUD patients with and without recent depressed state assessed by using Hopkins Symptom Checklist- 25 (HSCL-25) depression scores. A mean score of 1.75 or above for both genders was defined as recent depression. Significant p values are given.
3.3 Kynurenine, tryptophan and KT ratio in depression
Although there was a high concordance between a history of major depression and currently experiencing depressive symptoms (table 1), kynurenine and tryptophan levels, and KT ratios were only associated with the latter. Patients with depressive symptoms had higher tryptophan levels and lower KT ratios than patients without depressive symptoms (Table 2). These differences remained after controlling for the amount of alcohol consumed and parental history of drinking problems. Age attenuated the significant differences in the whole sample, without affecting the shorter abstinence and low AUD severity strata where differences were observed, as shown in fig 1.
Depression scores had small but statistically significant, positive correlations with serum tryptophan level (ρ =0.17, p =0.026), but not with serum kynurenine level (ρ =0.01, p =0.950). We observed a non-significant negative correlation between depression score and KT ratios (ρ =-0.15, p =0.052). A priori defined sensitivity analysis showed no difference in tryptophan, kynurenine or KT ratio levels between depressed and non-depressed groups in patients with abstinence shorter than 30 days. In patients with over 30 days of abstinence, however, the depressed group had higher tryptophan level and lower KT ratio compared to the non-depressed group (Fig 1, first panel). Patients with less severe AUD, that is, those who were below the sample mean AUDIT score, showed no difference in tryptophan, kynurenine or KT ratio levels between depressed and non-depressed groups. Conversely, in the high AUD severity stratum, depression was associated with higher tryptophan levels and lower KT ratios. Fig. 1, second panel, illustrates these associations according to AUDIT scores. Similar results were obtained for other measures of AUD severity: drinking frequency, drinking intensity, and binge drinking.
4. Discussion
This study of treatment-seeking AUD patients in Nepal showed that tryptophan degradation was related both to AUD severity and to state depression. The first major finding is higher kynurenine levels and KT ratios in patients with greater AUD severity compared to less severe AUD, particularly those with no current depressive symptoms. Secondly, AUD patients with depressive symptoms had higher tryptophan concentrations and lower KT ratios compared to patients without depressive symptoms, particularly among those with longer abstention and higher AUD severity.
Previous studies both using animal models and human AUD subjects have demonstrated altered tryptophan metabolism at various stages of alcohol intake, withdrawal, and sobriety (Badawy, 2002, LeMarquand et al., 1994). Acute alcohol intake activates TDO, while chronic alcohol use inhibits TDO activity. During withdrawal from heavy alcohol use, TDO activity is again increased (Badawy, 2002, Badawy et al., 2009, Branchey et al., 1984b). Our data indicates that both tryptophan and kynurenine concentrations may increase after a month of abstinence, possibly because of increased dietary support and improved intestinal absorption during recovery. A non-depressed sample of AUD individuals was found to have increased tryptophan degradation following oral tryptophan challenge after four weeks of sobriety, and not upon treatment admission (Friedman et al., 1988). Still, there is a paucity of literature on the impact of AUD severity on plasma tryptophan and kynurenine concentrations and central 5-HT turnover. This study has shown that the duration of alcohol abstinence, AUD severity and pattern of alcohol use is associated with alcohol-related changes in tryptophan metabolism. The observation of higher kynurenine concentrations and KT ratios among AUD patients with greater AUD severity indicates IDO and/or TDO activation. The fact that we did not observe a significantly lower level of serum tryptophan across levels of AUD severity suggests other mechanisms of tryptophan turnover are involved. Dietary protein intake is relevant to our patient setting. A low protein diet may contribute to low plasma tryptophan levels (Markus, 2008), and it is possible that an improvement in dietary protein intake while in treatment sustains tryptophan levels even while depletion is occurring via an activated IDO/TDO kynurenine pathway (Martinez et al., 2014). Another consideration is our sample characteristics; a treatment population may, in itself, represent a special group according to the local social context, and their chances of being in treatment may affect the observed differences (Neupane et al., 2013). This may partly explain unexpected findings. Heterogeneity associated with clinical samples, and lack of validation studies on applied instruments in AUD populations warrant careful interpretation. More research is needed in clinical and non-clinical settings, including measures of alternative mechanisms of tryptophan metabolism, such as cortisol levels for TDO activity, and dietary protein intake.
Although our findings of higher tryptophan levels and lower tryptophan degradation index among the depressed group are unexpected relative to most of the literature, they are consistent with recent findings from Quak et al who also reported lower KT ratios in depression alongside raised inflammatory markers (Quak et al., 2014, Neupane et al., 2014). Inflammatory cytokines can induce IDO activation, and therefore their potential effects on the serotonin system via tryptophan degradation remains an active area of research (O’Connor et al., 2009, Hughes et al., 2012, Dantzer et atl., 2011). Our findings may still be in line with the monoamine hypothesis since increased circulatory levels of tryptophan may also represent reduced uptake in the brain, as tryptophan has to compete with other amino acids (mainly leucine, isoleucine, valine, tyrosine and phenylalanine) for CNS uptake (Markus, 2008), which would result in depleted brain serotonin levels. Furthermore, a higher KT ratio can indicate not only central IDO activity but also peripheral hepatic activation of TDO. We found that the difference in KT ratios between depressed and non-depressed patients was solely due to tryptophan concentrations; the serum kynurenine level was not different between the two groups. It is possible that the dietary improvement during recovery rebalances serum tryptophan levels relatively earlier in the depressed group. This remains to be tested. Another potential explanation for our findings is that studies of depression which show a relationship with tryptophan metabolism are often limited by diagnostic perturbations. For example, Maes and colleagues found increased IDO activity in somatization rather than clinical depression or when depression was assessed using a symptom checklist (Maes et al., 2011a). This may contribute to observed differences in tryptophan metabolism between depressive disorders and depression symptomatology.
The serotonin - kynurenine hypothesis of depression suggests that depleted serum tryptophan and increased neurotoxic tryptophan catabolites along the kynurenine pathway both contribute to depression symptomatology (Lapin, 1973, Maes et al., 2011b). However, recent data from populations with major depression have cast doubt on the relationship between activation of the kynurenine pathway and depression disorders, and suggest possibilities of alternative physiological pathways for depression, including the varying roles of downstream metabolites of tryptophan catabolism. Contrary to our findings, Hughes and colleagues (Hughes et al., 2012) found that KT ratios were higher in depressed individuals than healthy controls without any difference in kynurenine levels, suggesting that the kynurenine pathway was not activated in their clinical population with major depression. A more recent investigation (Quak et al., 2014) showed a slightly reduced KT ratio in their current MD group, refuting the positive associations between tryptophan degradation and depression. Nonetheless, when anti-depressant use was accounted for, the latter effect was removed. Inadequate control for alcohol use and AUD in these samples is likely to explain the contradictory findings. Our findings, in conjunction with the frequent comorbidity between alcohol problems and depression, suggest these results would vary according to AUD severity.
A higher KT ratio among older individuals in our data indicates increased tryptophan degradation with age as a result of TDO/IDO activity, which is in line with the literature showing immune activation in older age (Frick et al., 2004). Increased tryptophan degradation could be a possible contributor to the depression risk in the elderly (Oxenkrug, 2010). In contrast, we observed depression to be more common among younger participants and the KT ratio was not higher in the depressed group. This may be explained by the clinical nature of our sample, in that depression is often more common among younger, heavy alcohol users. Being in treatment for AUD may have its own, independent effect on tryptophan metabolism and depressive symptoms, such as one mediated by physical activity and diet.
While we met our primary aim of comparing the levels of tryptophan and kynurenine and KT ratios between AUD patients with and without depression, we cannot comment on the causal pathways of alcohol use, tryptophan catabolism and depression. Longitudinal designs and healthy controls are required to rigorously test the effect of changes in tryptophan metabolism in AUD and depression. Such studies should consider other psychiatric disorders that are frequently comorbid with AUD, such as personality disorders and bipolar affective disorders, as potential sources of confounding. Future research should consider applying biomarkers for the objective verification of self-reported alcohol consumption. Although some differences in the parameters of tryptophan metabolism observed across comparison groups in our study were subtle and some confounders not completely accounted for, depression and alcohol use seem to interact in their effect on the peripheral tryptophan metabolism. This issue has high clinical relevance.
Acknowledgments
We thank the study participants and participating institutions for their co-operation. We are also thankful to Dr. Mark van Ommeren, World Health Organization for the psychometric instruments and to Innland Hospital Trust, Norway for funding the laboratory analysis. Finally, Dr. Thomas E. Gundersen and Ms. Siv Kaland at the Vitas Analytical Services, Oslo, are acknowledged for prompt laboratory analysis.
This study was funded by the Research Council of Norway and University of Oslo, Norway
References
- Badawy AA. Tryptophan metabolism in alcoholism. Nutrition research reviews. 2002;15:123–152. doi: 10.1079/NRR200133. [DOI] [PubMed] [Google Scholar]
- Badawy AA, Doughrty DM, Marsh-Richard DM, Steptoe A. Activation of liver tryptophan pyrrolase mediates the decrease in tryptophan availability to the brain after acute alcohol consumption by normal subjects. Alcohol Alcohol. 2009;44:267–271. doi: 10.1093/alcalc/agp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchey L, Branchey M, Shaw S, Lieber CS. Depression, suicide, and aggression in alcoholics and their relationship to plasma amino acids. Psychiatry Res. 1984a;12:219–226. doi: 10.1016/0165-1781(84)90027-1. [DOI] [PubMed] [Google Scholar]
- Branchey L, Branchey M, Shaw S, Lieber CS. Relationship between changes in plasma amino acids and depression in alcoholic patients. Am J Psychiatry. 1984b;141:1212–1215. doi: 10.1176/ajp.141.10.1212. [DOI] [PubMed] [Google Scholar]
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biological psychiatry. 2003;54:906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- Coppen A. The biochemistry of affective disorders. The British journal of psychiatry : the journal of mental science. 1967;113:1237–1264. doi: 10.1192/bjp.113.504.1237. [DOI] [PubMed] [Google Scholar]
- Cowen PJ, Parry-Billings M, Newsholme EA. Decreased plasma tryptophan levels in major depression. Journal of affective disorders. 1989;16:27–31. doi: 10.1016/0165-0327(89)90051-7. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMyer MK, Shea PA, Hendrie HC, Yoshimura NN. Plasma tryptophan and five other amino acids in depressed and normal subjects. Arch Gen Psychiatry. 1981;38:642–646. doi: 10.1001/archpsyc.1981.01780310042003. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behavioral science. 1974;19:1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- Dunjic-Kostic B, Ivkovic M, Radonjic NV, Petronijevic ND, Pantovic M, Damjanovic A, Poznanovic ST, Jovanovic A, Nikolic T, Jasovic-Gasic M. Melancholic and atypical major depression--connection between cytokines, psychopathology and treatment. Progress in neuro-psychopharmacology & biological psychiatry. 2013;43:1–6. doi: 10.1016/j.pnpbp.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Fernstrom JD. Role of precursor availability in control of monoamine biosynthesis in brain. Physiological reviews. 1983;63:484–546. doi: 10.1152/physrev.1983.63.2.484. [DOI] [PubMed] [Google Scholar]
- Frick B, Schroecksnadel K, Neurauter G, Leblhuber F, Fuchs D. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clinical biochemistry. 2004;37:684–687. doi: 10.1016/j.clinbiochem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Friedman MJ, Krstulovic AM, Severinghaus JM, Brown SJ. Altered conversion of tryptophan to kynurenine in newly abstinent alcoholics. Biological psychiatry. 1988;23:89–93. doi: 10.1016/0006-3223(88)90109-6. [DOI] [PubMed] [Google Scholar]
- Gleissenthall GV, Geisler S, Malik P, Kemmler G, Benicke H, Fuchs D, Mechtcheriakov S. Tryptophan metabolism in post-withdrawal alcohol-dependent patients. Alcohol Alcohol. 2014;49:251–255. doi: 10.1093/alcalc/agu011. [DOI] [PubMed] [Google Scholar]
- Hughes MM, Carballedo A, McLoughlin DM, Amico F, Harkin A, Frodl T, Connor TJ. Tryptophan depletion in depressed patients occurs independent of kynurenine pathway activation. Brain, behavior, and immunity. 2012;26:979–987. doi: 10.1016/j.bbi.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Lapin IP. Kynurenines as probable participants of depression. Pharmakopsychiatrie, Neuro-Psychopharmakologie. 1973;6:273–279. doi: 10.1055/s-0028-1094391. [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: clinical evidence. Biological psychiatry. 1994;36:326–337. doi: 10.1016/0006-3223(94)90630-0. [DOI] [PubMed] [Google Scholar]
- Lynskey MT. The comorbidity of alcohol dependence and affective disorders: treatment implications. Drug Alcohol Depend. 1998;52:201–209. doi: 10.1016/s0376-8716(98)00095-7. [DOI] [PubMed] [Google Scholar]
- Maes M, Galecki P, Verkerk R, Rief W. Somatization, but not depression, is characterized by disorders in the tryptophan catabolite (TRYCAT) pathway, indicating increased indoleamine 2,3-dioxygenase and lowered kynurenine aminotransferase activity. Neuro endocrinology letters. 2011a;32:264–273. [PubMed] [Google Scholar]
- Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011b;35:702–721. doi: 10.1016/j.pnpbp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer H. The serotonin hypothesis of major depression. Psychopharmacology: The fourth generation of progress. 1995;10:933–934. [Google Scholar]
- Maes M, Mihaylova I, Ruyter MD, Kubera M, Bosmans E. The immune effects of TRYCATs (tryptophan catabolites along the IDO pathway): relevance for depression -and other conditions characterized by tryptophan depletion induced by inflammation. Neuro endocrinology letters. 2007;28:826–831. [PubMed] [Google Scholar]
- Markus CR. Dietary amino acids and brain serotonin function; implications for stress-related affective changes. Neuromolecular medicine. 2008;10:247–258. doi: 10.1007/s12017-008-8039-9. [DOI] [PubMed] [Google Scholar]
- Martinez P, Tsai AC, Muzoora C, Kembabazi A, Weiser SD, Huang Y, Haber JE, Martin JN, Bang Berg DR, Hunt PW. Reversal of the kynurenin pathway of tryptophan catabolism march improve depression in ART-treated HIV-infected Ugandans. Journal of Acquired Immune Deficiency Syndrome. 2014;65:456–462. doi: 10.1097/QAI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Molecular psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. Journal of affective disorders. 2007;98:143–151. doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Neupane SP, Bramness JG. Who seeks treatment for alcohol problems? Demography and alcohol-use characteristics of patients in taboo and non-taboo drinking groups attending professional alcohol services in Nepal. Asian Journal of Psychiatry. 2014 doi: 10.1016/j.ajp.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Neupane SP, Lien L, Hilberg T, Bramness JG. Vitamin D deficiency in alcohol-use disorders and its relationship to comorbid major depression: a cross-sectional study of inpatients in Nepal. Drug Alcohol Depend. 2013;133:480–485. doi: 10.1016/j.drugalcdep.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Neupane SP, Lien L, Martinez P, Aukrust P, Ueland T, Mollnes TE, Hestad K, Bramness JG. High Frequency and Intensity of Drinking may Attenuate Increased Inflammatory Cytokine Levels of Major Depression in Alcohol-use Disorders. CNS Neuroscience & Therapeutics. 2014;20:898–904. doi: 10.1111/cns.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, Lestage J, Castanon N, Kelley KW, Dantzer R. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenkrug GF. Tryptophan kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: the serotonin hypothesis revisited 40 years later. The Israel journal of psychiatry and related sciences. 2010;47:56–63. [PMC free article] [PubMed] [Google Scholar]
- Quak J, Doornbos B, Roest AM, Duivis HE, Vogelzangs N, Nolen WA, Penninx BW, Kema IP, de Jonge P. Does tryptophan degradation along the kynurenine pathway mediate the association between pro-inflammatory immune activity and depressive symptoms? Psychoneuroendocrinology. 2014;45:202–210. doi: 10.1016/j.psyneuen.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Molecular psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schrocksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clinica chimica acta; international journal of clinical chemistry. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Shrestha NM, Sharma B, van Ommeren M, Regmi S, Makaju R, Komproe I, Shrestha GB, De Jong JT. Impact of torture on refugees displaced within the developing world: symptomatology among Bhutanese refugees in Nepal. JAMA. 1998;280:443–448. doi: 10.1001/jama.280.5.443. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW, Forrest CM, Darlington LG. Kynurenine pathway inhibition as a therapeutic strategy for neuroprotection. The FEBS journal. 2012;279:1386–1397. doi: 10.1111/j.1742-4658.2012.08487.x. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Fiellin DA, O'Connor PG. The prevalence and impact of alcohol problems in major depression: a systematic review. Am J Med. 2005;118:330–341. doi: 10.1016/j.amjmed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Van Ommeren M, Sharma B, Thapa S, Makaju R, Prasain D, Bhattarai R, de Jong J. Preparing Instruments for Transcultural Research: Use of the Translation Monitoring Form with Nepali-Speaking Bhutanese Refugees. Transcultural Psychiatry. 1999;36:285–301. [Google Scholar]
- Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clinical chemistry. 1997;43:2424–2426. [PubMed] [Google Scholar]
- Wittchen HU. Reliability and validity studies of the WHO Composite International Diagnostic Interview (CIDI): a critical review. J Psychiatr Res. 1994;28:57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Composite International Diagnostic Interview (core version 2.1), in Series Composite International Diagnostic Interview (core version 2.1) Geneva, Switzerland: 1997. [Google Scholar]


