Abstract
The main objective of this study was to assess the influence of slaughter methods on the quality of Chilean jack mackerel (Trachurus murphyi) during refrigerated storage on board. Fishes were slaughtered by asphyxia in air (AA), asphyxia in ice water (AI) or stunning fish heads (SH), and the rigor mortis, pH, total volatile basic nitrogen (TVB-N), trimethylamine (TMA), 2-thiobarbituric acid reactive substances (TBARS) and sensory properties for the fishes were analyzed. On day 0, Chilean jack mackerel samples of AI group displayed higher pH values than those of AA and SH groups. TVB-N, TMA and TBARS values of all samples increased with the storage time, and these values of AI had a lower increase than AA and SH. Moreover, samples of AI had a better sensory score than AA and SH during storage. It can be concluded that slaughter method of asphyxia in ice water for Chilean jack mackerel exhibit the better efficiency on maintaining the fish quality during refrigerated storage on board.
Keywords: Trachurus murphyi, Slaughter method, Quality change, Asphyxia, Stunning
Introduction
Chilean jack mackerel (Trachurus murphyi) is a species of jack mackerel in the genus Trachurus of the family Carangidae (Angel and Ojeda 2001). It is one of the most important commercial pelagic resources with high volumes harvest. Chilean jack mackerels are found in the south Pacific off the coasts of Chile and Peru, around New Zealand and South Australia, and in a band across the open ocean in between (FAO 2012). They are usually canned or marketed fresh for human consumption (FAO 2012), and they are a staple food in Africa.
Slaughter methods for fish are usually recognized as a critical processing in terms of the fish quality. Greater fish muscle activity during slaughter leads to a rapid decrease in energy reserves in the fish, i.e. in adenosine triphosphate (ATP), as well as to a rise in the lactic acid level and with a consequent drop in post mortem pH. Fishes struggling during slaughter are easy to go into rigor rapidly (Chafer et al. 2001a). So that fishes have the possibility of being higher in quality and welfare if the method of slaughter is chosen carefully.
Fishes are usually slaughtered either by immersion in ice-water or asphyxia in ice. One of the reasons cited by producers for using these methods is that rapid chilling maintains flesh quality by reducing both autolytic degradation and muscular activity immediately before death (Robb and Kestin 2002). Slaughter method of asphyxia in ice-water is usually chosen in fisheries or aquaculture, because it is easy to implement for commercialization of fishery’s factories. However, it is not considered humane due to it do not induce immediate loss of brain function of fish (Robb and Kestin 2002; van de Vis et al. 2003). Therefore, sudden slaughter methods such as human or electrical stunning are also used for slaughtering of fish to reduce the stress and pain of fish during fish slaughter.
The aim of this study was to investigate the effects of the slaughter methods, asphyxia in air (AA), asphyxia in ice-water (AI) and stunning fish heads (SH) on the quality of Chilean jack mackerel during refrigerated storage on board, and the physical, chemical and sensory characteristics were studied to assess the quality of fish during storage, including rigor mortis, pH, total volatile basic nitrogen (TVB-N), trimethylamine (TMA), 2-thiobarbituric acid reactive substances (TBARS) and sensory evaluation.
Materials and methods
Fish and handling
Chilean jack mackerels were captured in March, 2012 by “Kaifuhao” (Shanghai Kaichuang Pelagic Fishery Limited Company, China) in the southwest Pacific ocean near Chile. One hundred fifty alive fishes with average weight and length of 864 ± 92.8 g and 40.7 ± 2.0 cm, respectively, were divided into three groups (50 individuals in each group), were slaughtered by stunning fish heads by hand (SH), immersing into 1.0 ± 1.0 °C ice-water (AI) about 15 min and asphyxia in air (AA) about 30 min, respectively. After slaughtering, fishes from these groups were immediately transferred to polystyrene bags and stored at 4.0 ± 1 °C. Chemical traits, rigor development analyses and sensory evaluation were performed on day 0 (2 h after death) and 1, 2, 4, and 6 days after slaughtering on board. Three samples taken randomly were used to analyze the freshness changes at each sampling time. The experiment was performed in triplicate.
Rigor mortis development
The degree of stiffening (rigor mortis) was assessed by measuring the angle of sag from the perpendicular to the vertical where the tail was clamped. In this rigor meter, the pre-rigor stage is shown by no stiffening, giving an angle lower than 30°, the onset or the development of rigor is shown by an angle between 30° and 70°, full rigor is shown by full stiffening which means an angle higher than 70°. Subsequently rigor angles were transformed to percent values in order to analyze changes in rigor of jack mackerel during storage (Chafer et al. 2003).
Chemical analysis
pH
Ten grams samples of the fish flesh were homogenized in 90 mL of distilled water and the mixture was filtered. The pH of filtrate was measured using a pH meter PHS-3C (Shanghai precision and scientific instrument Co., LTD, China) at the ambient temperature.
Determination of TVB-N
TVB-N value was estimated by the semi micro steam distillation with some modifications. Ten grams samples of the jack mackerel were dispersed in 100 ml of distilled water and stirred for 30 min, and the mixture was filtered. Adding 5 ml MgO (10 g L−1) to 5 ml filtrates through Kjeldahl Apparatus, and 5 ml distilled water was as control. The distillate was collected in a flask containing 10 ml aqueous solution of boric acid (20 g L−1) and a mixed indicator produced from dissolution of 0.1 g of methyl red and 0.1 g of methylene blue to 100 ml of ethanol. Afterward, the boric acid solution was titrated with a 0.01 mol L−1 hydrochloric acid solution. The TVB-N value was determined according to the consumption of hydrochloric acid (Zhang et al. 2011).
Determination of TMA
TMA was determined by the method of Mol et al. (2007). Samples were mixed with trichloracetic acid solution (10 %). After filtration, filtrate was shaken with toluene, formaldehyde (20 %) and potassium hydroxide (50 %). The upper layer was mixed with picric acid (0.2 %) and immediately measured by UV visible spectrophotometer uv-1200 (Meipuda instrument Co., LTD, Shanghai, China) at 450 nm. Results were compared with the standard curve and TMA content of the sample was expressed as mg/100 g fish.
Determination of TBARS
Determination of TBARS was described by Zhang et al. (2011) with some modifications. Jack mackerel fleshes (5 g) were dispersed in 20 ml of thiobarbituric acid solution (0.375 % thiobarbituric acid (w/v), 15 % trichloroacetic acid. The mixture was filtered and the filtrate at the high temperature (95–97 °C) for 40 min, cooled at room temperature. The absorbance of the supernatant was measured at 532 nm. Results were expressed as mg malondialdehyde/100 g muscle.
Sensory assessment
Sensory assessment was estimated as described by Fan et al. (2008) with some modifications. The sensory quality of fish sample was evaluated by five trained panelists from the kitchen and laboratory staff. Panelists scored for appearance color, odor, flavor, general acceptability and texture, using a nine-point hedonic scale (1, dislike extremely to 9, like extremely). A sensory score of four was taken as the borderline of acceptability.
Statistical analysis
The SPSS 17 statistical software was used for the analysis of the experimental results. Analysis of variance (ANOVA) was used to calculate significant difference (p < 0.05) between samples. Data were expressed as means ± SD and mean separations were determined by Duncan’s multiple range test.
Results and discussion
Rigor mortis analysis
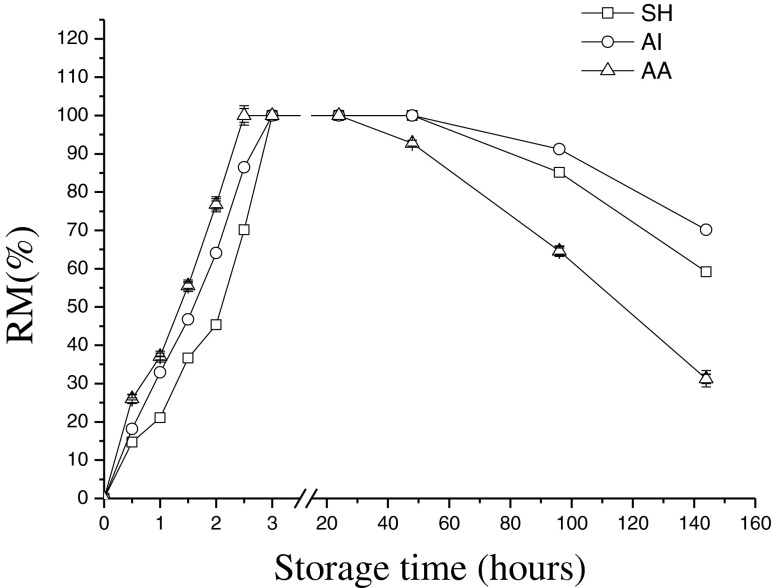
Rigor mortis (RM) was examined, as it is widely used as an indicator of premortem stress both in duration and intensity (Chafer et al. 2001b). The dynamics of rigor mortis onset and resolution, considering that partially determines the tenderness of meat. Changes in RM of fishes treated with different killing procedures on tail bending are illustrated in Fig. 1. Slaughtering procedures, like the measurements described above, were found to affect the timing of rigor. The full rigor time of SH group was longer than AI group, and AI group was longer than AA group (147 ± 5, 126 ± 5, and 109 ± 5 min, respectively). Fish in SH group had less struggling time that led to less muscle activity, so the rigor process was extended. AI group had longer rigor time than AA group. It was consistent with the result of Skjervold et al. (1999). In this study, jack mackerel fishes were wild-caught with fishing nets, in which the condition of pre-slaughter as stress, starvation and body temperature are difficult to control and measure, rigor was reported to take place within 6 h of catching. Fishes increased physical activity during catching time resulted in earlier onset of rigor (Gregory 2008). So it showed SH group only prolongs 38 min in full stiffening time and the onset and resolution of rigor mortis were similar for all groups.
Fig. 1.
Changes in rigor mortis of Chilean jack mackerel during storage at 4 °C. SH stunning fish heads; AI asphyxia in ice water; AA asphyxia in air
Chemical analysis
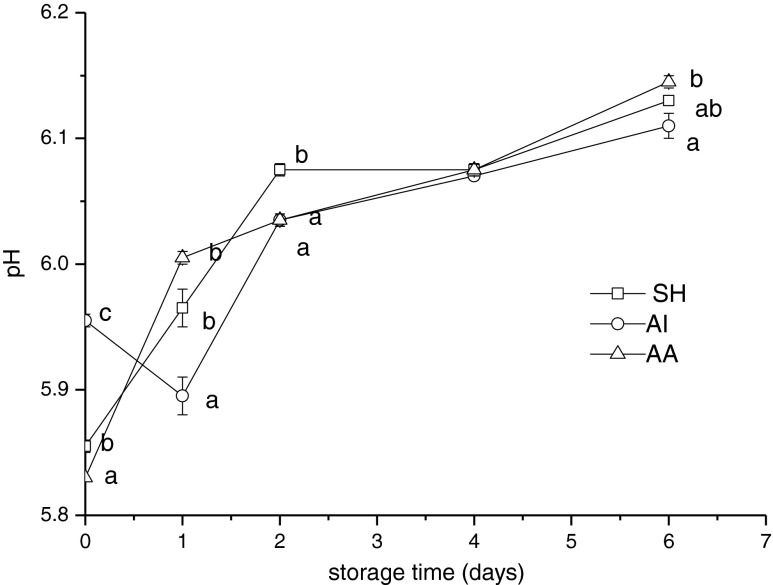
pH value decreases due to the formation of lactic acid in the flesh just after fish death, but then the decomposition of nitrogenous compounds leads to an increase in pH value. Increases in pH value indicated the accumulation of alkaline compounds, such as ammonia compounds and TMA, mainly derived from microbial and endogenous enzyme action. In Fig. 2, pH levels of all samples were lower than 6.00 at the beginning of storage and pH values measured were similar with the result of Quitral et al. (2009). The initial pH values of AI, SH and AA were 5.96 ± 0.01, 5.86 ± 0.01 and 5.83 ± 0.01, respectively. It showed significant differences in these values (p < 0.05). It may be due to the difference of the pre-slaughter muscle activity of fishes treated by these slaughter methods. The formation of lactic acid in muscle increased with the increase of intensity of muscle activity and the struggling time during slaughter. On day 6, pH values of AI, SH and AA group increased to 6.13 ± 0.03, 6.11 ± 0.01 and 6.15 ± 0.02, respectively. It shows significant differences (P < 0.05) between AI group and AA group. It indicated that the method of slaughter must be chosen carefully after fish catching, and the fish struggling time in fishing nets must be decreased.
Fig. 2.
Changes in pH of Chilean jack mackerel during storage at 4 °C. SH stunning fish heads; AI asphyxia in ice water; AA asphyxia in air. Data were expressed as means ± SD, and different letters denote significant differences (p < 0.05) of the mean values at the same day
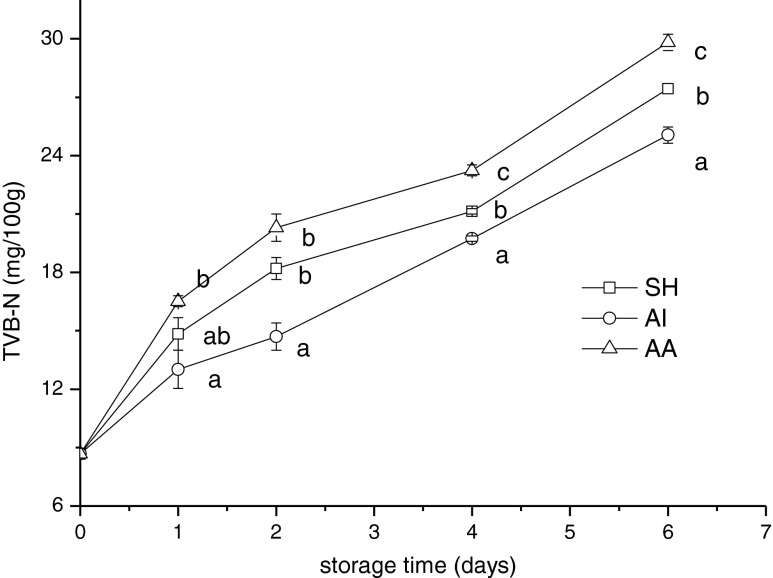
TVB-N content of SH, AI and AA group jack mackerel samples stored at 4 °C is shown in Fig. 3. TVB-N, which is mainly composed of ammonia and primary, secondary and tertiary amines, resulted from degradation of proteins and non-protein nitrogenous compounds, which are chiefly caused by microbial activity (El-Hanafy et al. 2011). So TVB-N was commonly considered as an indicator to evaluate the spoilage of marine fish (Wang et al. 2011). However, various authors have reported different acceptability levels for different fish species, specific treatments, and processing conditions for TVB-N value. However, a level of 30 mg TVB-N/100 g of fish muscle is usually regarded as spoiled. At the beginning of storage, the TVB-N value was 8.68 mg/100 g flesh for jack mackerel stored in refrigerator room. The release of total volatile bases increased up to 25.06 ± 0.42, 27.44 ± 0.34, 29.82 ±0.28 mg/100 g muscle for AI, SH and AA group samples stored in a refrigerator room on day 6. Significant differences (p < 0.05) were found between jack mackerel stored in all group after the 4-day day period of storage. From the results obtained, it can be concluded that AI group produced a significant decrease in TVB-N as compared with SH and AA, because of its decreasing intensity of muscle activity and chilling process. This finding was agreement with Sobhi et al. (2010) who reported intense exercise prior to death causes a higher proteolysis during storage.
Fig. 3.
Changes in total volatile base nitrogen of Chilean jack mackerel during storage at 4 °C. SH stunning fish heads; AI asphyxia in ice water; AA asphyxia in air. Data were expressed as means ± SD, and different letters denote significant differences (p < 0.05) of the mean values at the same day
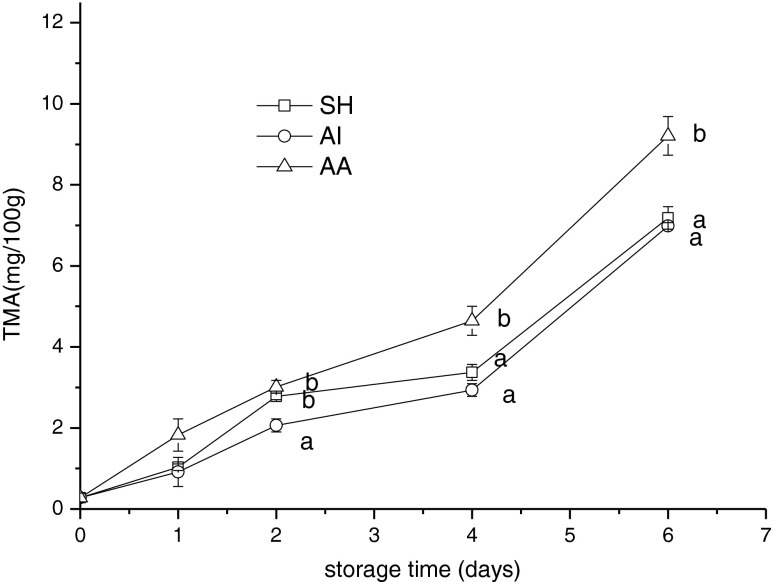
Most species of marine fish and shellfish produce in their digestive process trimethylamine oxide (TMAO) which plays a role in osmoregulation. In fresh or iced fish TMAO is reduced by bacterial enzymes to TMA (Krzymien and Elias 1990). TMA is widely used as a valuable tool to evaluate the quality of fish stored in ice because of its related to the deterioration of the fish under refrigerated conditions. The TMA content of mackerel increased slightly on the first 2 days, but increased rapidly after the subsequent storage period (Fig. 4). The initial mean TMA content of jack mackerel muscle was 0.28 ± 0.12 mg/100 g. A sharp increase in TMA content was observed on day 6. The TMA content of samples on day 6 in SH, AI and AA group was 7.18 ± 0.28, 6.98 ± 0.08 and 9.21 ± 0.48 mg/100 g, respectively. During storage, samples in AA showed the significantly higher TMA content than those in AI and SH group. But no significant difference was found between SH group and AI group. In general, the upper limit for TMA before consumer rejection of fish is usually 5.0 to 10.0 mg/100 g (Abreu et al. 2009). On day 6, the TMA contents of all samples were below the maximal proposed level (10 mg/100 g).
Fig. 4.
Changes in trimethyl amine nitrogen of Chilean jack mackerel during storage at 4 °C. SH stunning fish heads; AI asphyxia in ice water; AA asphyxia in air. Data were expressed as means ± SD, and different letters denote significant differences (p < 0.05) of the mean values at the same day
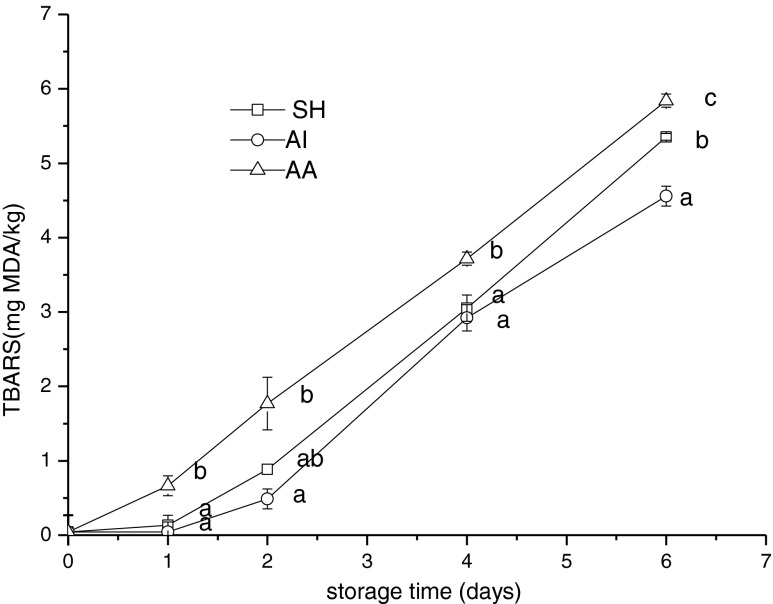
Lipid oxidation is an important quality problem in jack mackerel with 3–5 % fat (crude) with 20–27 % polyunsaturated fatty acids (PUFA) (Aranda et al. 2006). At the beginning of the storage period, TBARS values of all groups were 0.44 ± 0.03 mg MDA/kg (Fig. 5). On day 6, TBARS values of SH, AI and AA group was 5.35 ± 0.04, 4.56 ± 0.13 and 5.84 ± 0.09 mg MDA/kg, respectively. Significant difference (p < 0.05) was found between AA group and others during storage. It was explained that when fishes were forced to take strenuous exercise and under higher temperature, fish muscles were easier to form lipid peroxidation metabolites (Gregory 2008). According to TBARS results, placing fishes into ice-water can be attributed to the better quality of the fish. In addition, the concentration of TBARS in good quality frozen and chilled fish or fish stored on ice is typically between 5 and 8 mg MDA/kg, whereas levels of 8 mg MDA/kg flesh are generally regarded as the limit of acceptability for most species (Kilinc et al. 2007). The statistical result obtained from the present study suggested that TBARS concentration of all groups was with in the quality limit.
Fig. 5.
Changes in TBARS of Chilean jack mackerel during storage at 4 °C. SH stunning fish heads; AI asphyxia in ice water; AA asphyxia in air. Data were expressed as means ± SD, and different letters denote significant differences (p < 0.05) of the mean values at the same day
Sensory analysis
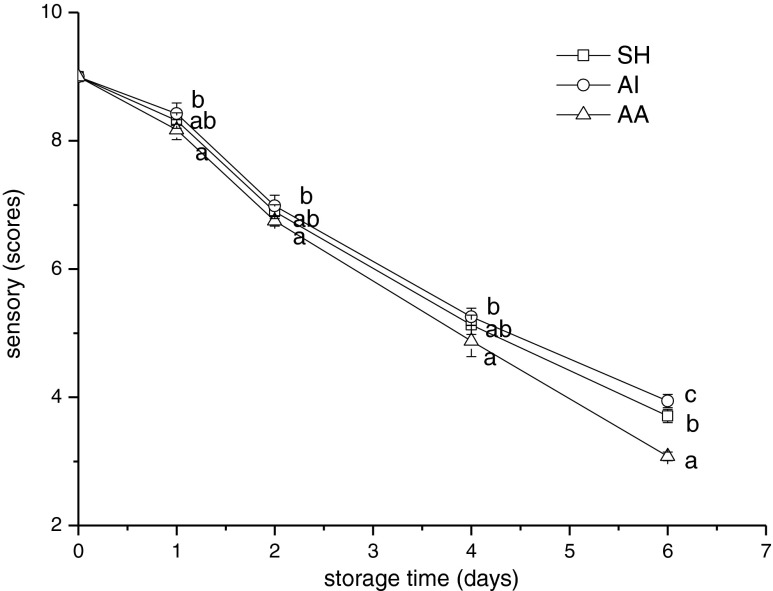
The results of the sensory assessment of samples are given in Fig. 6. Sensory scores showed a significant decline in fish samples of all groups during storage. Samples of AI group got a higher score than SH group and AA group. Changes of sensory scores illustrated that slaughter method of asphyxia in ice-water could maintain fish better quality characteristics. The result was in accordance with the results of chemical analysis above. In addition, it is well known that fish spoilage gives rise to the subsequent development of strongly fishy, rancid and putrid odors, and fish are then clearly rejected for consumption (Mol et al. 2007). On day 6, jack mackerel of AA (scores, 3.42 ± 0.07) was considered not to be acceptable for human consumption.
Fig. 6.
Changes in sensory scores of Chilean jack mackerel during storage at 4 °C. SH stunning fish heads; AI asphyxia in ice water; AA asphyxia in air. Data were expressed as means ± SD, and different letters denote significant differences (p < 0.05) of the mean values at the same day
Conclusions
In this study, influences of three slaughter methods on the quality of jack mackerel muscle were studied. Asphyxia in air is the almost universal industrial procedure to slaughter fish and it is easy to obtain. Slaughter method of stunning fish heads was difficult to manipulate, and it was easy to damage fish appearance and make fish bled. Asphyxia in ice-water appears to be a best choice to slaughter fish due to its convenience. In addition, the analysis of TVB-N, TBARS, pH, TMA and sensory evaluation showed that slaughter method of asphyxia in ice-water could maintain better quality of fish during refrigerated storage. Based on the present results, slaughter method of asphyxia in ice-water is highly recommended as the commercial and industrial slaughter method for Chilean jack mackerel to maintain the fish quality.
Acknowledgment
This study was funded by the National High Technology Research and Development Program of China (863 project, 2012AA092301).
References
- Abreu VKG, Zapata JFF, Figueiredo EAT, Garruti DS, Freitas ER, Pereira ALF, Braga ARC. Gamma irradiation on frozen and packaged headed shrimp. J Food Qual. 2009;32:425–435. doi: 10.1111/j.1745-4557.2009.00268.x. [DOI] [Google Scholar]
- Angel A, Ojeda FP. Structure and trophic organization of subtidal fish assemblages on the northern Chilean coast: the effect of habitat complexity. Mar Ecol Prog Ser. 2001;217:81–91. doi: 10.3354/meps217081. [DOI] [Google Scholar]
- Aranda M, Mendoza N, Villegas R. Lipid damage during frozen storage of whole jack mackerel (Trachurus symmetricus murphyi) J Food Lipids. 2006;13:155–166. doi: 10.1111/j.1745-4522.2006.00041.x. [DOI] [Google Scholar]
- Chafer M, Gonzalez-Martinez C, Ortola MD, Chiralt A, Fito P. Kinetics of osmotic dehydration in orange and mandarin peels. J Food Process Eng. 2001;24:273–289. doi: 10.1111/j.1745-4530.2001.tb00544.x. [DOI] [Google Scholar]
- Chafer M, Gonzalez-Martinez C, Ortola MD, Chiralt A. Long term osmotic dehydration processes of orange peel at atmospheric pressure and by applying a vacuum pulse. Food Sci Technol Int. 2001;7:511–520. [Google Scholar]
- Chafer M, Perez S, Chiralt A. Kinetics of solute gain and water loss during osmotic dehydration of orange slices. Food Sci Technol Int. 2003;9:389–396. doi: 10.1177/1082013203040545. [DOI] [Google Scholar]
- El-Hanafy AEA, Shawky HA, Ramadan MF. Preservation of Oreochromis niloticus fish using frozen green tea extract: impact on biochemical, microbiological and sensory characteristics. J Food Process Preserv. 2011;35:639–646. doi: 10.1111/j.1745-4549.2011.00513.x. [DOI] [Google Scholar]
- Fan WJ, Chi YL, Zhang S. The use of a tea polyphenol dip to extend the shelf life of silver carp (Hypophthalmicthys molitrix) during storage in ice. Food Chem. 2008;108:148–153. doi: 10.1016/j.foodchem.2007.10.057. [DOI] [Google Scholar]
- FAO (2012) Trachurus murphyi (Nichols, 1920) species fact sheets
- Gregory NG. Animal welfare at markets and during transport and slaughter. Meat Sci. 2008;80:2–11. doi: 10.1016/j.meatsci.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Kilinc B, Cakli S, Cadun A, Dincer T, Tolasa S. Comparison of effects of slurry ice and flake ice pretreatments on the quality of aquacultured sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) stored at 4 °C. Food Chem. 2007;104:1611–1617. doi: 10.1016/j.foodchem.2007.03.002. [DOI] [Google Scholar]
- Krzymien ME, Elias L. Feasibility study on the determination of fish freshness by trimethylamine headspace analysis. J Food Sci. 1990;55:1228–1232. doi: 10.1111/j.1365-2621.1990.tb03903.x. [DOI] [Google Scholar]
- Mol S, Erkan N, Ucok D, Tosun SY. Effect of psychrophilic bacteria to estimate fish quality. J Muscle Foods. 2007;18:120–128. doi: 10.1111/j.1745-4573.2007.00071.x. [DOI] [Google Scholar]
- Quitral V, Donoso ML, Ortiz J, Herrera MV, Araya H, Aubourg SP. Chemical changes during the chilled storage of Chilean jack mackerel (Trachurus murphyi): effect of a plant-extract icing system. LWT Food Sci Technol. 2009;42:1450–1454. doi: 10.1016/j.lwt.2009.03.005. [DOI] [Google Scholar]
- Robb DHF, Kestin SC. Methods used to kill fish: field observations and literature reviewed. Anim Welf. 2002;11:269–282. [Google Scholar]
- Skjervold PO, Fjaera SO, Ostby PB. Rigor in Atlantic salmon as affected by crowding stress prior to chilling before slaughter. Aquaculture. 1999;175:93–101. doi: 10.1016/S0044-8486(99)00037-X. [DOI] [Google Scholar]
- Sobhi B, Adzahan NM, Karim MSA, Karim R. Physicochemical and sensory properties of a traditional chilli shrimp paste. J Food Agric Environ. 2010;8:38–40. [Google Scholar]
- van de Vis H, Kestin S, Robb D, Oehlenschlager J, Lambooij B, Munkner W, Kuhlmann H, Kloosterboer K, Tejada M, Huidobro A, Ottera H, Roth B, Sorensen NK, Akse L, Byrne H, Nesvadba P. Is humane slaughter of fish possible for industry? Aquac Res. 2003;34:211–220. doi: 10.1046/j.1365-2109.2003.00804.x. [DOI] [Google Scholar]
- Wang W, Han JX, Wu YJ, Yuan F, Chen Y, Ge YQ. Simultaneous detection of eight food allergens using optical thin-film biosensor chips. J Agric Food Chem. 2011;59:6889–6894. doi: 10.1021/jf200933b. [DOI] [PubMed] [Google Scholar]
- Zhang LN, Li X, Lu W, Shen HX, Luo YK. Quality predictive models of grass carp (Ctenopharyngodon idellus) at different temperatures during storage. Food Control. 2011;22:1197–1202. doi: 10.1016/j.foodcont.2011.01.017. [DOI] [Google Scholar]