Abstract
Mung bean was subjected to different processing conditions (soaking, germination, cooking and autoclaving) and their textural, pasting and in vitro starch digestibility characteristics were studied. A significant reduction in textural properties (hardness, cohesiveness, gumminess and chewiness) after cooking and autoclaving treatment of mung bean was observed. Flours made from differently processed mung bean showed significant differences (P < 0.05) in their pastin g characteristics. Peak and final viscosity were the highest for flour from germinated mung bean whereas those made from autoclaved mung bean showed the lowest value. in vitro starch digestibility of mung bean flours was assessed enzymatically using modified Englyst method and the parameters studied were readily digestible starch (RDS), slowly digestible starch (SDS), resistant starch (RS) and total starch (TS) content. Various processing treatments increased the RDS contents of mung bean, while the SDS content was found to be the highest for soaked and the lowest for the autoclaved sample. Germinated sample showed higher amount of digestible starch (RDS + SDS) as compared to raw and soaked samples. Flours from raw and soaked samples showed significantly low starch hydrolysis rate at all the temperatures with total hydrolysis of 29.9 and 31.2 %, respectively at 180 min whereas cooked and autoclaved samples showed high hydrolysis rates with 50.2 and 53.8 % of these hydrolyzing within 30 min of hydrolysis.
Keywords: Mung bean, In vitro starch digestibility, Pasting, Texture
Introduction
Grain legumes are an important and inexpensive source of protein, dietary fiber and starch for a large part of the world’s population, mainly in developing countries (Perla et al. 2003). Mung bean (Vigna radiata) is one of the most important grain legumes in India. About 85 % of mung bean produced is consumed in several countries of Asia (Singh and Singh 1992) with India producing about 70 % of world production (Fery 2002). Seed color of mung beans are usually a dark olive green or yellow, but some cultivars produce brown or black seed (Rubatzky and Yamaguchi 1997). Mung beans provide a major source of protein in cereal-based diets. The dried mung beans may be eaten whole or split, cooked or fermented, milled and ground into flour. In India, it is mainly consumed as dhal and whole seed after boiling and frying. Traditionally, mung beans are cooked either as whole seed or sprouts as a vegetable dish or sometimes used in soup or with sugar as a snack or a dessert. Among the grain legumes, mung bean is known for its easy digestibility, low flatulence potential and high protein content (Doughty and Walker 1982). Mung beans have been reported to contain 23.8–27 % protein, 1.2 % fat, 3.3 % ash, 62.6 % carbohydrates, and 16.3 % fiber (El-Adawy 2000)
The role of legumes as a major source of dietary nutrients appears to be limited because of several factors including their low protein and starch digestibility (Kataria et al. 1989; Negi et al. 2001). The most serious drawback in the utilization of legumes is their long cooking time (Singh and Rao 1995). Pre-soaking has been recommended to facilitate the cooking process in several legumes. Soaking is known to reduce antinutrients and improve cooking quality (Wah et al. 1997). Soaking in water overnight followed by germination or sprouting of the grain is a very common household practice, especially for the processing of pulses. During germination several enzyme systems become active and bring about profound changes in the nutritive value and digestibility of legumes (Subramanian et al. 1976; Mohd et al. 1980). During germination, starch is broken down to dextrin and maltose and proteins are broken down to poly peptides, peptides and amino acids.
Thermal treatment of legumes (as cooking) also makes the consumption of these foods possible. The process considerably decreased naturally existing antinutritional factors, increasing the availability of other nutrients, such as proteins and starch (Domene and Oliveira 1993). Carbohydrates constitute the main fraction of grain legumes, accounting for up to 55–65 % of the dry mass. Of these, starch and non starch polysaccharide (dietary fiber) are the major constituents, with smaller but significant amounts of oligosaccharides. In recent years, glycemic index (GI) has become a useful tool for planning diets for the patients of diabetic, dyslipidemia, cardiovascular disease, and even certain cancers in the general population (Jenkins et al. 1981). Due to poor digestibility compared to that of other cereals, legume starches promote slow and moderate postprandial glucose and insulin responses, and have low GI values (Jenkins et al. 1980).
There have been reports regarding the effect of processing on the in vitro starch digestibility of legumes such as peas (Skrabanja et al. 1999), black gram (Rehman 2007), moth bean and horse gram (Bravo et al. 1998). The effect of soaking and germination individually and in combination with dehulling on proximate composition; antinutrients and in vitro starch and protein digestibility of chickpea (Ghavidel and Prakash 2007; El-Adawy et al. 2003), mung bean (Mubarak 2005; El-Adawy et al. 2003), cowpea and lentil (Ghavidel and Prakash 2007) has been studied. The objective of the present work was to study the effect of different processing methods such as soaking, germination, cooking and autoclaving on the in vitro digestibility, pasting and textural properties of mung bean so as to enhance the utilization of mung bean which is a pulse of economic importance in India.
Materials and methods
Materials
Commercial mung bean was procured from the local market of Amritsar, India.
Sample preparation
Mung bean was subjected to following different processing methods:
Soaking
Mung beans were soaked in distilled water for 18 h at 30 °C, the seed-to-water ratio used was 1:5 (w/v). The soaked mung beans were rinsed with distilled water and drained.
Germination
Mung beans were soaked in distilled water for 18 h and then germinated in sterile petri dish lined with wet filter paper for 72 h at 30 °C in the dark.
Cooking
Mung beans (without prior soaking) were directly cooked in boiling distilled water in a beaker using a seed-to-water ratio of 1:5. Samples were cooked until soft as felt between the fingers (cooking time was ~ 41 min) and drained.
Autoclaving
Mung beans (without prior soaking) were autoclaved at 1.05 kg/cm2 pressure at 121 °C for 15 min. The excessive water after autoclaving was drained off.
All of the above processed samples were freeze dried and ground into flour in a mill and sieved using 0.5 mm sieve. The flours so obtained were used for further analysis.
Proximate composition
Mung beans were estimated for their moisture, ash, fat, fiber and protein (N×6.25) content by employing the standard methods of analysis (AOAC 1984). Carbohydrate content was calculated by difference. The analyses were conducted in triplicates.
Pasting properties
The pasting properties of flours from raw, soaked, germinated, cooked and autoclaved mung bean were studied in triplicate by using Rapid Visco Analyzer (Newport Scientific Pty Ltd, Warriewood NSW 2102, Australia) as described earlier (Kaur and Singh 2005). Viscosity profiles of flours from differently processed mung bean were recorded using flours suspensions (8 %, w/w; 28 g total weight). The temperature-time conditions included a heating step from 50 to 95 °C at 6 °C/min (after an equilibration time of 1 min at 50 °C), a holding phase at 95 °C for 5 min, a cooling step from 95 to 50 °C at 6 °C/min and a holding phase at 50 °C for 2 min. Each sample was analyzed in triplicate.
Textural properties
Texture profile analysis (TPA) of raw and processed mung bean was performed using a single grain by TA/XT2 texture analyzer (Stable Micro Systems, Surrey, England). Mung bean was subjected to 80 % compression with a cylindrical probe (38 mm diameter) at a crosshead speed of 1 mm/s twice in two cycles using a 50 kg load cell. The textural parameters of hardness, springiness, cohesiveness, gumminess and chewiness were computed using TPA (Bourne 1978). Eight measurements were performed for each sample.
In vitro starch digestibility
In vitro starch digestibility was analyzed following the method described by Englyst et al. (1992) with slight modifications (Sandhu and Lim 2008). Amyloglucosidase (No. 9913, Sigma–Aldrich) (1 ml) was added to deionized water (2 ml). Porcine pancreatic alpha-amylase (No. 7545, Sigma–Aldrich, St. Louis, MO) (3.89 g) was dispersed in water (25.7 ml) and centrifuged for 10 min at 2,500 g, and 18.7 ml of supernatant was collected. This supernatant was mixed with 1 ml of diluted amyloglucosidase for making the enzyme solution. The solution was freshly prepared for the digestion analysis.
Aliquots of guar gum (10 ml, 5 g/l) and sodium acetate (5 ml, 0.5 M) were added to the flour samples (0.5 g, db) in a test tube. Seven glass balls (10 mm diameter) and 5 ml of enzyme solution were then added to each tube; following the incubation in shaking water bath at 37 °C with agitation (170 rpm). Aliquots (0.5 ml) were taken at intervals and mixed with 4 ml of 80 % ethanol, and the glucose contents in the mixture were measured using glucose oxidase and peroxidise assay kits (No. GAGO-20, Sigma–Aldrich). The total starch content was measured according to Englyst et al. (1992).
The starch classification based on its digestibility was: RDS as the starch that was hydrolyzed within 20 min of incubation, RS as the starch not hydrolyzed with 120 min, and SDS as the starch digested during the period between 20 and 120 min.
Estimated glycemic index (GI)
Using the hydrolysis curve (0–180 min), the hydrolysis index (HI) was calculated as the percentage of total glucose released from the sample, to that released from white bread (Goñi et al. 1997; Granfeldt et al. 1992). The glycemic indices of the samples were estimated according to the equation proposed by Goñi et al. (1997): GI = 39.71 + 0.549HI.
Statistical analysis
The data reported in all the tables were subjected to one-way analysis of variance (ANOVA) using Minitab Statistical Software version 14 (Minitab, Inc., State College, USA).
Results and discussion
Proximate composition and textural properties
The ash, fat, protein and fiber content of mung bean were 3.82, 2.86, 22.8, and 12.3 %, respectively (Table not shown). The carbohydrate content (58.3 %) was calculated by difference.
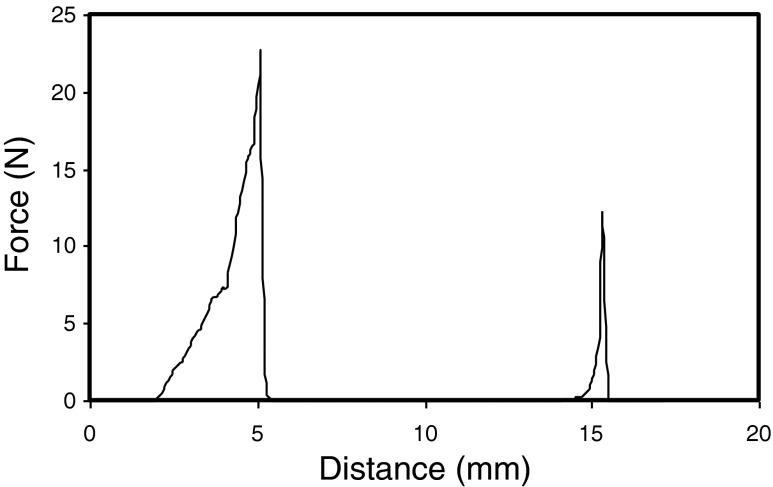
As mung beans are consumed in variety of forms including soaked and cooked forms, so the study of textural properties after these treatments is important. The textural properties of raw and processed single mung bean grain differed significantly (Table 1) and characteristic curve is shown in Fig. 1. The hardness (maximum height of the force peak on the first compression cycle) of raw mung bean was the highest (15,809 g) whereas the lowest was observed for autoclaved (1,207 g) sample. Textural measurements of cooked legume seeds indicated softening with heating in water at 100 °C. The cooking process of legumes involves water absorption to an equilibrium condition, followed by softening of the texture by heat. Softening of beans during cooking has been reported to be accompanied by structural changes in the seed (Rockland and Jones 1974; Sefa-Dedeh et al. 1978). During cooking of legumes, the seeds undergo important physicochemical changes involving gelatinization and swelling of starch, denaturation of protein, solubilization of some of the polysaccharides, softening of the structure, and other physical and chemical changes, which result in a palatable texture (Stanley and Aguilera 1985). Cohesiveness (ratio of the positive force areas under the first and second compressions), gumminess and chewiness of the autoclaved mung bean were also found to be the lowest as compared to its soaked, germinated and cooked counterparts. Springiness (height to which the sample recovers during the time elapse between the end of the first bite and the start of second bite) on the other hand was found to be the highest (1.114) for autoclaved sample and the lowest (0.341) being observed for soaked sample. Textural characteristics of legumes may be dependent upon seed microstructure, chemical and physical changes occurring during processing (Kaur et al. 2005). It was observed that the textural parameters of cooked and autoclaved mung bean had lower value as compared to their counterpart soaked sample. This may be attributed to the softening of certain constituents upon cooking which resulted in lower value for textural parameters of cooked seeds in comparison to those of soaked seeds.
Table 1.
Textural properties of raw and differently processed mung bean
| Mung bean | Hardness (g) | Cohesiveness | Gumminess | Chewiness | Springiness |
|---|---|---|---|---|---|
| Raw | 15,809 ± 62e | – | – | – | – |
| Soaked | 7,038 ± 36d | 0.208 ± 0.011b | 1,467 ± 19c | 500 ± 11c | 0.341 ± 0.004a |
| Germinated | 6,487 ± 52c | 0.285 ± 0.017c | 1,847 ± 22d | 756 ± 17d | 0.409 ± 0.017c |
| Cooked | 4,431 ± 26b | 0.211 ± 0.009b | 933 ± 29b | 333 ± 13b | 0.357 ± 0.005b |
| Autoclaved | 1,207 ± 19a | 0.144 ± 0.007a | 173 ± 12a | 25 ± 3a | 1.144 ± 0.016d |
Values are means of eight readings. Values (±standard deviation) within the same column followed by the same superscript are not significantly different (p < 0.05)
Fig. 1.
Typical texture profile analysis curve for a single grain
Pasting properties
Pasting properties of flours from raw, soaked, germinated, cooked and autoclaved mung bean are summarized in Table 2. Significant differences (P < 0.05) in pasting properties among different flours were observed. All the flours showed gradual increase in viscosity with increase in temperature. The increase in viscosity with temperature may be attributed to the removal of water from the exuded amylose by the granules as they swell (Ghiasi et al. 1982). Granule swelling is accompanied by leaching of granular constituents, predominantly amylose, into the external matrix resulting in a dispersion of swollen granules in a continuous matrix (Noel et al. 1993). Sharp peaks for thermograms were observed for flours from raw and soaked mung bean whereas, reverse was observed for flours from cooked and autoclaved samples (figure not shown). Peak and final viscosity (indicates the ability of starch to form a viscous paste) were the highest (4,601 & 5,167 cP, respectively) for flour from germinated mung bean whereas that from autoclaved mung bean had the lowest value (353 & 739 cP, respectively). Low peak and final viscosity of flours from cooked and autoclaved samples may be due to thermal degradation of the starch granules during the heat treatment. Breakdown (measure of the susceptibility of cooked starch to disintegration) was not observed for flours of cooked and autoclaved samples which may be due to already disintegrated starch granules. During the final cooling (from 95 to 50 °C), the viscosity increased owing to the alignment of the chains of amylose (Flores-Farias et al. 2000). Setback viscosity (measure of syneresis of starch upon cooling of cooked starch pastes) ranged between 387 to 2,277 cP, the lowest being for autoclaved flour and the highest for germinated flour.
Table 2.
Pasting properties of flours from raw and differently processed mung bean
| Mung bean flour | Peak viscosity (cP) | Trough viscosity (cP) | Breakdown (cP) | Final viscosity (cP) | Setback (cP) |
|---|---|---|---|---|---|
| Raw | 2,075 ± 20c | 1,397 ± 15c | 678 ± 8b | 2,264 ± 14b | 867 ± 8b |
| Soaked | 4,539 ± 24d | 2,863 ± 19d | 1,676 ± 11c | 5,132 ± 17d | 2,269 ± 11d |
| Germinated | 4,601 ± 17e | 2,890 ± 13d | 1,711 ± 10d | 5,167 ± 22d | 2,277 ± 13d |
| Cooked | 1,088 ± 16b | 1,089 ± 9b | 1 ± 0a | 2,357 ± 9c | 1,268 ± 17c |
| Autoclaved | 353 ± 9a | 352 ± 11a | 1 ± 0a | 739 ± 11a | 387 ± 6a |
Values are means of triplicate determinations. Values (±standard deviation) within the same column followed by the same superscript are not significantly different (p < 0.05).
Digestibility studies
The enzymatically assessed readily digestible starch (RDS), slowly digestible starch (SDS), resistant starch (RS) and total starch (TS) contents of raw and processed mung bean are shown in Table 3. RDS is readily and completely digested in small intestine and is associated with more rapid elevation of postprandial plasma glucose. The raw mung bean contained the lowest RDS and TS of 2.1 and 29.9 %, respectively. Various processing treatments increased the RDS contents of mung bean with autoclaving method exhibiting the highest content (49.3 %). Legumes, in general are markedly resistant to pancreatic amylase attack but cooking led to dramatic increase in susceptibility to this enzyme (Rehman 2007). In fact, cooking improves the digestibility of starch through gelatinization and destruction of anti nutrients (Yu-Hui 1991). SDS is completely but more slowly, digested in small intestine and attenuates postprandial plasma glucose and insulin levels. It is generally the most desirable form of dietary starch (Jenkins et al. 1981). SDS content was found to be the highest for soaked and the lowest for autoclaved sample. Germination significantly (P < 0.05) increased the in vitro starch digestibility of mung bean as compared to raw and soaked samples which is supported by the findings of Kataria et al. (1989), Negi et al. (2001) and Archana Sehga and Kawatra (2001). It has been reported that protein and starch digestibility (Kataria et al. 1992; Preet and Punia 2000) increased during germination of legumes due to the activation of the amylolytic enzymes during germination. Germinated mung beans are widely consumed in India and it serves as a major energy source. RS has been defined as the sum of starch and the products of starch degradation not absorbed in the small intestine but are fermented in large intestine of healthy individuals (Asp 1992). Raw mung bean contained high RS content (8.9 %), mainly RS2, which contributed to its low starch digestibility whereas cooking and autoclaving treatment decreased the RS significantly (P < 0.05). The decrease might be due to the partial loss of the soluble components, such as oligosaccharides and phenoloic substances (Siddhuaraju and Becker 2005). The starch in raw samples is contained with the granules that are poorly affected by hydrolytic enzymes and it is therefore most indigestible (Colonna et al. 1992) and this accounts for higher RS content of raw legumes (RS2). Multiple factors are involved in the reduced bioavailability of legume starches. The presence of intact cell/tissue structure, enclosing starch granules, hinders the swelling and solubilization of starch and the formation of retrograded starch (RS3), resulting in a reduced digestion rate in vitro (Tovar et al. 1990). Other factors affecting the legume starch digestibility are the high amylose/amylopectin ratios and the presence of various antinutrients, such as polyphenols, phytic acid and other antinutrients (Deshpande and Cheryan 1984; Thomson and Yoon 1984).
Table 3.
Digestibility and glycemic index of starch fractions from raw and differently processed mung bean
| Mung bean flour |
RDS (%) | SDS (%) | RS (%) | HI | GI |
|---|---|---|---|---|---|
| Raw | 2.1 ± 0.5a | 18.9 ± 0.4d | 8.9 ± 0.5d | 17.0 ± 0.8a | 49.1 ± 0.4a |
| Soaked | 3.0 ± 0.4b | 19.7 ± 0.6e | 8.4 ± 0.4d | 19.1 ± 0.5b | 50.2 ± 0.6b |
| Germinated | 14.4 ± 0.3c | 15.5 ± 0.5c | 6.0 ± 0.4c | 26.8 ± 0.7c | 54.4 ± 0.6c |
| Cooked | 46.6 ± 0.8d | 9.2 ± 0.5b | 2.3 ± 0.3a | 54.4 ± 0.9d | 71.8 ± 0.3d |
| Autoclaved | 49.3 ± 0.7e | 7.6 ± 0.3a | 3.2 ± 0.4b | 60.4 ± 0.11e | 72.8 ± 0.5e |
Values are means of triplicate determinations. Values (±standard deviation) within the same column followed by the same superscript are not significantly different (p < 0.05)
Sample size: 0.5 g (dwb); RDS: readily digestible starch; SDS: slowly digestible starch; RS: resistant starch; HI: hydrolysis index; GI: glycemic index. GI was calculated using the equation proposed by Goñi et al. (1997): GI = 39.71 + 0.549 HI
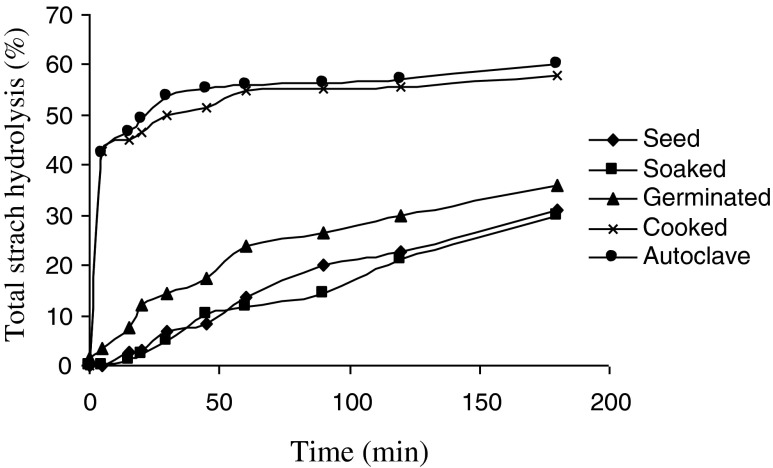
The in vitro starch hydrolysis rate of flours from raw and processed mung beans is shown in Fig. 2. Flour from raw and soaked samples showed significantly low starch hydrolysis rate at all the temperatures with total hydrolysis of 29.9 and 31.2 %, respectively at 180 min. Cooked and autoclaved samples on the other hand showed high hydrolysis rates with 50.2 and 53.8 % of these hydrolyzing within 30 min of hydrolysis. Digestion of both cooked and autoclaved samples reached their plateaus after 60 min of incubation with hydrolysis curve not changing thereafter but for other samples (raw, soaked and germinated) the curve was still increasing after 180 min of incubation. In general, cooked and autoclaved mung bean was digested more rapidly than soaked and germinated counterpart samples.
Fig. 2.
Rate of starch hydrolysis of seeds and differently processed mung bean. Area under curves was used for calculation of HI
Hydrolysis index (HI) and estimated glycemic index
The hydrolysis index (HI) is a useful tool, from the nutritional point of view, for comparison of starch digestibility. The index expresses the digestibility of the starch in foods in relation to the digestibility of starch in a reference material, namely white bread. Glycemic index (GI) is calculated from HI and is defined as a percentage of the corresponding area after injection of an equicarbohydrate portion of the reference product (Jenkins et al. 1983). HI and GI of flour from raw and processed mung beans are shown in Table 3. Both HI and GI followed the order: autoclaved > cooked > germinated > soaked > raw sample. Cooked and autoclaved samples showed significantly high GI as compared to other samples. Uncooked cereals consumed in ground forms as a common meal are recently gaining importance as they are considered promoting health (Han et al. 2008). Thus, flour from soaked and germinated mung bean can be quite beneficial for planning the diets of diabetic, obese and patients suffering from cardiovascular diseases.
Conclusions
The present study indicated that different processing methods influenced the textural and pasting characteristics and in vitro starch digestibility of mung bean. Thermal processing caused a significant reduction in textural properties due to softening of the seeds. Flours made from differently processed mung bean differed significantly in their pasting characteristics. Various processing treatments increased the RDS contents of mung bean. Germination significantly increased the in vitro starch digestibility as compared to raw and soaked mung bean samples. In general, cooked and autoclaved mung bean was digested more rapidly than soaked and germinated counterpart samples. Also, consumption of germinated mung bean which is quite common in India can be beneficial in terms of health promoting benefits.
References
- AOAC . Official methods of Analysis. 14. Arlington, VA: Association of Official Analytical chemists; 1984. [PubMed] [Google Scholar]
- Archana Sehga S, Kawatra A. In vitro protein and starch digestibility of pearl millet (Pennisetum gluacum L.) as affected by processing techniques. Nahrung. 2001;45(1):25–27. doi: 10.1002/1521-3803(20010101)45:1<25::AID-FOOD25>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Asp N-G (1992) Resistant starch. Proceedings from the second plenary meetings of EURESTA. Eur J Clin Nut 46 (Suppl 2), S1 [PubMed]
- Bourne MC. Texture profile analysis. Food Technol. 1978;32(7):62–66,72. [Google Scholar]
- Bravo L, Siddhurajo P, Saura-Calixto F. Effect of various processing methods on the in vitro starch digestibility and resistant starch content of Indian pulses. J Agric Food Chem. 1998;46:4667–4674. doi: 10.1021/jf980251f. [DOI] [Google Scholar]
- Colonna P, Leloup V, Buleon A. A limiting factor of starch hydrolysis. Eur J Clin Nut. 1992;46:S17–S32. [PubMed] [Google Scholar]
- Deshpande SS, Cheryan M. Effects of phytic acid, divalent cations and their interactions on α-amylase activity. J Food Sci. 1984;49:516–519. doi: 10.1111/j.1365-2621.1984.tb12456.x. [DOI] [Google Scholar]
- Domene SMA, Oliveira AC. The use of nitrogen-15 labeling for the assessment of leguminous protein digestibility. J Nut Sci Vitaminol. 1993;39(1):47–53. doi: 10.3177/jnsv.39.47. [DOI] [PubMed] [Google Scholar]
- Doughty J, Walker A. Legumes in human nutrition. FAO, Food and Nutrition paper 20. Rome: FAO; 1982. p. 46. [PubMed] [Google Scholar]
- El-Adawy TA. Functional properties and nutritional quality of acetylated and succinylated mung bean protein isolate. Food Chem. 2000;70:83–91. doi: 10.1016/S0308-8146(00)00079-0. [DOI] [Google Scholar]
- El-Adawy TA, Rahma EH, El-Bedawey AE. Nutritional potential and functional properties of germinated mung bean, pea and lentil seeds. Plant Food Hum Nut. 2003;58:1–13. doi: 10.1023/B:QUAL.0000040339.48521.75. [DOI] [Google Scholar]
- Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nut. 1992;46:S33–S50. [PubMed] [Google Scholar]
- Fery FL. New Opportunities in Vigna. In: Janick J, Whipkey A, editors. Trends in new crops and new uses. Alexandria, VA: ASHS press; 2002. pp. 424–428. [Google Scholar]
- Flores-Farias R, Martinez-Bustos F, Salinas-Moreno Y, Chang YK, Hernandez JS, Rios E. Physicochemical and rheological characteristics of commercial nixtamalised Mexican corn flours for tortillas. J Sci Food Agric. 2000;80:657–664. doi: 10.1002/(SICI)1097-0010(20000501)80:6<657::AID-JSFA576>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Ghavidel RA, Prakash J. The impact of germination and dehulling on nutrients, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. Lebensm-Wiss Technol. 2007;40:1292–1299. doi: 10.1016/j.lwt.2006.08.002. [DOI] [Google Scholar]
- Ghiasi K, Varriano-Marston K, Hoseney RC. Gelatinization of wheat starch. II. Starch-surfactant interaction. Cereal Chem. 1982;59:86. [Google Scholar]
- Goñi I, Garcia-Alonso A, Saura-Calixto F. A starch hydrolysis procedure to estimate glycemic index. Nut Res. 1997;17:427–437. doi: 10.1016/S0271-5317(97)00010-9. [DOI] [Google Scholar]
- Granfeldt Y, Björck I, Drews A, Towar J. An in vitro procedure based on chewing to predict metabolic responses to starch in cereal and legume products. Eur J Clin Nut. 1992;46:649–660. [PubMed] [Google Scholar]
- Han J-A, Jang S-H, Lim S-T. In vitro digestibility of rice and barley in forms of raw flour and cooked kernels. Food Sci Biotech. 2008;17:180–183. [Google Scholar]
- Jenkins DJA, Wolever TMS, Taylor RH, Barker H, Fielden H. Exceptionally low blood glucose responses to dried beans, comparison with other carbohydrate foods. Brit Med J. 1980;281:578–580. doi: 10.1136/bmj.281.6240.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins DJA, Wolever TMS, Taylor RH, Barker H, Fielder H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nut. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- Jenkins DJA, Wolever TMS, Jenkins AL, Thorne MJ, Kalmusky J, Reichert R, Wong G. The glycemic index of foods tested in diabetic patients: A new basis for carbohydrate exchange favouring the use of legumes. Diabetolog. 1983;34:257–264. doi: 10.1007/BF00282710. [DOI] [PubMed] [Google Scholar]
- Kataria A, Chauhan BM, Punia D. Antinutrients and protein digestibility (in vitro) of mungbean as affected by domestic processing and cooking. Food Chem. 1989;32:9–17. doi: 10.1016/0308-8146(89)90003-4. [DOI] [Google Scholar]
- Kataria A, Chauhan BM, Punia D. Digestibility of proteins and starch (in vitro) of amphidiploids (black gram × mung bean) as affected by domestic processing and cooking. Plant Food Hum Nut. 1992;42(2):117–125. doi: 10.1007/BF02196464. [DOI] [PubMed] [Google Scholar]
- Kaur M, Singh N. Studies on functional, thermal and pasting properties of flours from different Chickpea (Cicer arietinum L.) cultivars. Food Chem. 2005;91:403–411. doi: 10.1016/j.foodchem.2004.06.015. [DOI] [Google Scholar]
- Kaur M, Singh N, Sodhi NS. Physicochemical, cooking, textural and roasting characteristics of chickpea (Cicer arietinum L.) cultivars. J Food Eng. 2005;69(4):511–517. doi: 10.1016/j.jfoodeng.2004.09.002. [DOI] [Google Scholar]
- Mohd IN, Bressani R, Elias LG. Changes in chemical and selected biochemical components. Protein quality and digestibility of mung bean (Vigna radiata) during germination and cooking. Plant Food Hum Nut. 1980;30:135–144. doi: 10.1007/BF01099051. [DOI] [Google Scholar]
- Mubarak AE (2005) Nutritional composition and antinutritional factors of mung bean seeds (Phaseolus aureus) as affected by some home traditional processes. Food Chem 89:489–495
- Negi A, Boora P, Khetarpaul N. Starch and protein digestibility of newly released moth bean cultivars: Effect of soaking, germination and pressure-cooking. Nahrung. 2001;45(4):251–254. doi: 10.1002/1521-3803(20010801)45:4<251::AID-FOOD251>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Noel TR, Ring SG, Whittam MA. Physical properties of starch products: Structure and function. In: Dickinson E, Walstra P, editors. Food Colloids and Polymers: Stability and mechanical properties. Royal Society of Chemistry: Cambridge; 1993. pp. 126–137. [Google Scholar]
- Perla O, Luis AB, Sonia GS, Maria PB, Juscelino T, Octavio PL. Effect of processing and storage time on in vitro digestibility and resistant starch content of two bean (Phaseolus vulgaris L.) varieties. J Sci Food Agric. 2003;83:1283–1288. doi: 10.1002/jsfa.1413. [DOI] [Google Scholar]
- Preet K, Punia D. Antinutrients and digestibility (in vitro) of soaked, dehulled and germinated cowpea. Nut Health. 2000;14(2):109–117. doi: 10.1177/026010600001400203. [DOI] [PubMed] [Google Scholar]
- Rehman Z-U. Domestic processing effects on available carbohydrate content and starch digestibility of black grams (Vigna mungo) and chick peas (Cicer arietium) Food Chem. 2007;100:764–767. doi: 10.1016/j.foodchem.2005.10.041. [DOI] [Google Scholar]
- Rockland LB, Jones FT. Scanning electron microscope study on dry beans- Effect of cooking on cellular structure of cotyledons in rehydrated lima beans. Journal of Food Sci Technol. 1974;30:342–346. [Google Scholar]
- Rubatzky VE, Yamaguchi M. World vegetable principles, production and nutritive values. 2. New York: Chapman and Hall; 1997. [Google Scholar]
- Sandhu KS, Lim S-T. Digestibility of legume starches as influenced by its physical and structural properties. Carbohydr Polym. 2008;71:245–252. doi: 10.1016/j.carbpol.2007.05.036. [DOI] [Google Scholar]
- Sefa-Dedeh S, Stanley DW, Voisey PW. Effect of soaking time and cooking condition on texture and microstructure of Cowpea (Vigna unguiculata) J Food Sci. 1978;43:1832. doi: 10.1111/j.1365-2621.1978.tb07426.x. [DOI] [Google Scholar]
- Siddhuaraju P, Becker K. Nutritional and antinutritional composition, in vitro amino acid availability, starch digestibility and predicted glycemic index of differentially processed mucuna beans (Mucuna pruriens var. utilis): as under-utilised legume. Food Chem. 2005;91:275–286. doi: 10.1016/j.foodchem.2004.02.044. [DOI] [Google Scholar]
- Singh U, Rao PV. Quick –cooking quality of pigeonpea dhal as influenced by soaking solutions and enzyme treatment. J Food Sci Technol. 1995;32:122–125. [Google Scholar]
- Singh U, Singh B. Tropical grain legumes as important human foods. Economic Bot. 1992;46:310–321. doi: 10.1007/BF02866630. [DOI] [Google Scholar]
- Skrabanja V, Liljeberg HGM, Hedlay CL, Kreft I, Björk ME. Influence of genotype and processing on the in vitro rate of starch hydrolysis and resistant starch formation in peas (Pisum sativum L.) J Agric Food Chem. 1999;47:2033–2039. doi: 10.1021/jf981060f. [DOI] [PubMed] [Google Scholar]
- Stanley DW, Aguilera JM. A review of textural defect in cooked reconstituted legumes-the influences of structure and composition. J Food Biochem. 1985;9:277–323. doi: 10.1111/j.1745-4514.1985.tb00355.x. [DOI] [Google Scholar]
- Subramanian V, Manickam A, Pathmanabhan G. Biochemical changes during early germination of red gram (Cajanus cajan L.) seeds. Ind J Exper Bio. 1976;14:736–737. [Google Scholar]
- Thomson LU, Yoon JH. Starch digestibility as affected by polyphenols and phytic acid. J Food Sci. 1984;49:1228–1229. doi: 10.1111/j.1365-2621.1984.tb10443.x. [DOI] [Google Scholar]
- Tovar J, Björk IM, Asp N-G. Starch content and α-amylolysis rate in precooked legume flours. J Agric Food Chem. 1990;38:1818–1823. doi: 10.1021/jf00099a007. [DOI] [Google Scholar]
- Wah CS, Sharma K, Jackson MG. Studies on various chemical treatments of seed meal to improve or inactivate tannins. Ind J Animal Sci. 1997;47:8–11. [Google Scholar]
- Yu-Hui T. Effect of the hard-to-cook defect and processing on protein and starch digestibility of cow-peas. Cereal Chem. 1991;68:413–418. [Google Scholar]