Abstract
The study was carried out, to explore the potentiality of extrusion technology for elimination of antinutritional components of cereal brans. Extrusion variables were moisture content (14, 17 and 20 %) and temperatures (115 °C, 140 °C, 165 °C). Phytic acid, polyphenols, oxalates, trypsin inhibitor, bulk density and color of brans after extrusion were analyzed. All four raw bran samples had high concentration of phytic acid, polyphenols, oxalates and trypsin inhibitors. Extrusion cooking was found effective in reduction of these antinutritients. Extrusion processing reduced the phytic acid by 54.51 %, polyphenol by 73.38 %, oxalates by 36.84 %, and trypsin inhibitor by 72.39 %. The heat treatment caused the highest reduction in polyphenols followed by trypsin inhibitors, phytic acid and oxalates. The highest reduction in antinutrients was observed at 140 °C and 20 % moisture content. Bulk density increased significantly compared to raw brans and increase in redness and decrease in yellowness of brans was observed after extrusion treatment.
Keywords: Extrusion cooking, Cereal brans, Phytic acid, Polyphenols, Oxalates, Trypsin inhibitors
Introduction
Although cereal brans are rich in dietary fiber, omega-3-fatty acids and contain modest amounts of starch, proteins, certain minerals and vitamins, but their use in food and feed is still limited because of the presence of several antinutritional factors (ANFs). These include phytic acids, trypsin inhibitors, polyphenols, oxalates, saponins, lipases and heamagglutinin. These toxic factors present in cereal brans must be eliminated or minimized in order to utilize its entire nutritional potential. Many efforts have been made in the past but all were focused on removing one or more of the toxic factors (Rehman and Mahmood 1996; Sayre et al. 1988; Shivaji and Jagrahan 1983). Processing treatments viz. wet, dry, microwave heating, chemical treatment and extrusion cooking are useful for elimination of undesirable components in cereal brans.
Extrusion cooking, a multi-step, multi-functional and thermal/mechanical process, has permitted a large number of food applications. Beneficial effects include destruction of antinutritional factors, gelatinization of starch, increased soluble dietary fiber and reduction of lipid oxidation. Recently, extrusion cooking has become one of the most popular technologies in food processing. It is a low cost, high temperature short time (HTST) process, used world-wide for processing of a number of food products (Frame 1994; Harper 1981; Smith and Singh 1996). Extrusion is being considered as the best method to abolish α-amylase inhibitors, trypsin, and chymotrypsin and heamagglutinin activity without modifying protein content in food products (Soetan and Oyewole 2009). Therefore, this study was carried out to investigate the effect of extrusion cooking on the ANFs in wheat, rice barley and oat brans.
Materials and methods
Raw materials
Wheat bran was collected from Ludhiana Flour Mill, Ludhiana, India. Rice bran was purchased from A.P. Solvex Pvt. Ltd., Dhuri, Punjab, India. Barley bran was prepared by milling of barley in grain pearler (Central Institute of Agricultural Engineering (CIAE), Bhopal, India). Oat bran (Baggry’s India Ltd., New Delhi, India) was purchased from local market. Defatting of brans was achieved by treating with the cold hexane (food grade). The dried cereal brans were ground to a uniform particle size using Cemotec mill (Foss, Hoganas, Sweden) having setting at No. 1.
Extrusion cooking
Samples were extruded using Clextral BC 21 (Clextral, Firminy, France) co-rotating intermeshing twin screw extruder having 400 mm useful length and 2.5 mm screw diameter and was equipped with a single screw volumetric feeder. Moisture content of samples was adjusted to 14, 17 and 20 % by adding calculated amount of water. Feeder speed was set to feed at the rate of 20 kg/h. Barrel temperature was set at 25 °C, 75 °C and 100 °C for Ist, 2nd and 3rd zones respectively. Temperature of fourth zone was varied as 115 °C, 140 °C and 165 °C. Screw speed was maintained at 400 rpm. The die was fitted with one circular insert having 6 mm diameter. The product was cooled and packed in polythene bags for futher analysis.
Analysis of antinutritional compounds
The antinutritional compounds such as phytic acid (Davies and Reid 1979), total polyphenol (Swain and Hills 1959), and oxalates (Abaza et al. 1968), were quantified. Trypsin inhibitor activity was determined by the enzyme assay (Hajela et al. 1999) by using benzoyl-DL-arginine-p-nitroanilide (BAPNA) as a substrate. One trypsin inhibitor unit (TIU) is expressed as an increase of 0.01 absorbance units per 10 ml of reaction mixture at 410 nm. Trypsin inhibitor activity was defined in terms of trypsin units inhibited per g protein.
Functional characteristics
Bulk density (weight per unit volume) of cereal brans were determined by method of Egan et al. 1981. Color analysis was performed by using Hunter Lab Colorimeter, MiniScan XE Plus (Hunter Lab, Reston, Virginia). Color readings were expressed by Hunter values for L, a, and b. L values measure black to white (0–100); + a = red, − a = green; + b = yellow; − b = blue.
Statistical analysis
The data was statistically analyzed as described by Snedecor and Cochran (1980) using three replications.
Results and discussion
Phytic acid
Table 1 presents the data with respect to the effect of extrusion treatment at different temperature and moisture levels on the phytic acid content (mg/g) of cereal brans. Phytic acid content (on 14 % moisture basis) of different cereal bran was 41.74 mg/g (wheat bran), 42.82 mg/g (rice bran), 40.30 mg/g (barley bran) and 27.69 mg/g (oat bran). Statistically significant reduction was noticed among cereal brans with regard to phytic acid. At 20 % moisture content mean phytic acid in untreated bran was 38.14 mg/g which was reduced to 17.35, 18.89 and 19.65 mg/g at 115 °C, 140 °C and 165 °C respectively. Lower moisture content during extrusion resulted into lower degradation in phytic acid of cereal brans. At 115 °C, the mean phytic acid values were 19.92, 18.63 and 17.35 mg/g at 14, 17 and 20 % moisture respectively. Similar pattern in reduction was observed at higher temperature with regard to moisture content of the bran during extrusion. These values showed that lower temperature resulted into highest reduction in phytic acid. At temperature of 115 °C and moisture content of 20 %, extrusion treatment was most effective for reducing phytic acid upto 64.40 % in wheat bran, 63.55 % in barley bran and 26.47 % in oat bran. However highest reduction (55.83 %) in rice bran was observed at 140 °C with 20 % moisture content during extrusion. Earlier work by Chauhan et al. 1988; Asp et al. (1989) showed a significant reduction in phytic acid during extrusion processing of foods. Sharma et al. (2004) found that extrusion treatment significantly reduced the phytic acid content of rice bran from 29.33 mg/g in raw rice bran to 24.66 mg/g in extruded rice bran. Phytic acid reductions presumably vary in cereal brans due to variation in phytate content which may be attributed to differences in milling extraction rates, genotypic and environmental effects. Khattab and Arntfield (2009) found that autoclaving was the most effective in reducing phytic acid content by 65.04–70.49 % in legume seeds. The observed reduction in phytic acid content of legume seeds during heat treatments may be partly due to the heat labile nature of phytic acid and the formation of insoluble complexes between phytate and other components. Fairweather-Tait et al. (1989) reported that phytic acid was reduced during extrusion but total phytate was not affected. According to Gualberto et al. (1997) screw speed had no effect on the phytate content in wheat, rice and oat brans.
Table 1.
Effect of extrusion treatment on phytic acids* (mg/g) content of different cereal brans
| Temp. (°C) | Moisture content (%) | Type of bran | Bran means | |||
|---|---|---|---|---|---|---|
| Wheat | Rice | Barley | Oat | |||
| Control | 41.74 ± 0.74g | 42.82 ± 0.82d | 40.30 ± 0.30f | 27.69 ± 0.69e | 38.14 ± 0.14g | |
| 115 | 14 | 19.28 ± 0.28cd | 20.76 ± 0.20c | 18.13 ± 0.13c | 21.50 ± 0.50bc | 19.92 ±0.92cd (47.77) |
| 17 | 16.41 ± 0.41b | 19.52 ± 0.50ab | 17.82 ± 0.82c | 20.76 ± 0.76ab | 18.63 ± 0.63b (51.15) | |
| 20 | 14.86 ± 0.86a | 19.48 ± 0.48ab | 14.69 ± 0.69a | 20.36 ± 0.36a | 17.35 ± 0.35a (54.51) | |
| 140 | 14 | 22.43 ± 0.43f | 19.89 ± 0.89bc | 22.72 ± 0.72e | 23.21 ± 0.21d | 22.06 ± 0.28f (42.16) |
| 17 | 19.73 ± 0.73cd | 19.57 ± 0.57ab | 17.73 ± 0.73bc | 22.88 ± 0.88d | 19.98 ± 0.98cd (47.61) | |
| 20 | 18.82 ± 0.82c | 18.92 ± 0.10a | 15.46 ± 0.46a | 22.36 ± 0.36cd | 18.89 ± 0.89bc (50.47) | |
| 165 | 14 | 21.43 ± 0.43ef | 20.26 ± 0.60bc | 21.40 ± 0.40d | 21.46 ± 0.46bc | 21.14 ± 0.24ef (44.57) |
| 17 | 21.17 ± 0.17de | 20.03 ± 0.40bc | 19.41 ± 0.41d | 21.40 ± 0.61b | 20.5 ± 0.50de (46.25) | |
| 20 | 19.96 ± 0.96d | 19.81 ± 0.80abc | 17.73 ± 0.51bc | 21.10 ± 0.44ab | 19.65 ± 0.65bcd (48.47) | |
*on 14 % moisture basis
Values are means ± Standard deviation of triplicate determinations
Figures in parentheses indicate present reduction over control values
Means within columns with different letters are significantly different (P ≤ 0.05)
Polyphenols
Data with respect to total polyphenols in cereal bran is depicted in Table 2. Treatment at higher moisture content found to be more effective in reduction of polyphenols. With feed moisture content of 20 %, reduction in polyphenol content was 64.68 %, 73.38 % and 62.44 % at 115 °C, 140 °C and 165 °C, respectively. Moderate temperature was more effective in degradation of polyphenols. At temperature of 140 °C with 20 % moisture content reduction varied between 64.84 and 80.74 % for individual cereal brans. About 62.44 % mean reduction in polyphenol was detected at 165 °C with 20 % moisture content, however, the degradation was more at low temperature i.e. 115 °C (64.68 %) and 140 °C (73.38 %) with 20 % moisture content. This might be attributed to greater loss of endogenous polyphenols in cereal brans at low temperature and release of polyphenols at higher temperature. Viscidi et al. (2004) reported that extrusion process decreased the level of polyphenols in plant materials, that was also confirmed by the results of Gumal et al. (2007) where other extrusion parameters were applied for rye grains (14 %/120 °C, 20 %/120 °C, 20 %/180 °C).
Table 2.
Effect of extrusion treatment on polyphenols*(mg/g) content of different cereal brans
| Temp. (°C) | Moisture content (%) | Type of bran | Bran means | |||
|---|---|---|---|---|---|---|
| Wheat | Rice | Barley | Oat | |||
| Control | 3.20 ± 0.09f | 5.80 ± 0.11e | 6.53 ± 0.21d | 0.54 ± 0.09de | 4.02 ± 0.12d | |
| 115 | 14 | 2.17 ± 0.11e | 3.53 ± 0.23d | 3.65 ± 0.06c | 0.54 ± 0.04de | 2.47 ± 0.10c (38.56) |
| 17 | 1.46 ± 0.06bc | 3.50 ± 0.10d | 2.65 ± 0.05b | 0.38 ± 0.02bcd | 2.00 ± 0.15bc (50.25) | |
| 20 | 1.20 ± 0.01ab | 1.74 ± 0.20a | 2.43 ± 0.03b | 0.30 ± 0.05abc | 1.42 ± 0.13ab (64.68) | |
| 140 | 14 | 1.30 ± 0.03ab | 2.59 ± 0.07c | 2.34 ± 0.09b | 0.53 ± 0.01de | 1.69 ± 0.07ab (57.69) |
| 17 | 1.26 ± 0.02ab | 2.32 ± 0.01abc | 1.50 ± 0.25a | 0.21 ± 0.04ab | 1.32 ± 0.09ab (67.16) | |
| 20 | 1.12 ± 0.06a | 1.75 ± 0.04ab | 1.26 ± 0.08a | 0.15 ± 0.02a | 1.07 ± 0.11a (73.38) | |
| 165 | 14 | 1.80 ± 0.08d | 4.00 ± 0.02d | 3.89 ± 0.10c | 0.57 ± 0.06e | 2.56 ± 0.20c (36.32) |
| 17 | 1.44 ± 0.01bc | 3.96 ± 0.05d | 2.27 ± 0.01b | 0.45 ± 0.02cde | 2.03 ± 0.19bc (49.50) | |
| 20 | 1.12 ± 0.05a | 2.41 ± 0.10c | 2.19 ± 0.19b | 0.31 ± 0.03abc | 1.51 ± 0.05ab (62.44) | |
*on 14 % moisture basis
Values are means ± Standard deviation of triplicate determinations
Figures in parentheses indicate present reduction over control values
Means within columns with different letters are significantly different (P ≤ 0.05)
Oxalate content
Mean values for percent oxalate content showed that extrusion temperature and moisture results into non-significant reduction impact in reducing the oxalate concentration in all brans (Table 3). The oxalate content in untreated bran ranged from 0.309 to 0.445 %, being minimum for oat bran (0.309 %) and maximum for rice bran (0.445 %). At 115 °C temperature of extrusion, the maximum reduction in oxalate content was achieved at 20 % moisture content (10.53 %). Mean values showed that maximum reduction (36.84 %) was observed at 140 °C and 20 % moisture content. Similar pattern of non-significant decrease in oxalate content was observed at 165 °C with different moisture content, but reduction was comparatively lower than extrusion temperature of 140 °C. The percent reduction in oxalates at 140 °C temperature, 20 % moisture content was 36.88 %, 42.02 %, 18.77 % and 54.69 % for wheat, rice, barley, and oat bran respectively. Siener et al. 2006 compared the soluble and total oxalate contents among cereals and cereal products such as wheat, rye, barley, oat, maize and rice etc. and concluded that a high amount of oxalate was contained in outer layers of cereal grains. Judprasong et al. 2006 found a very small amount (about 3 mg/100DM) of oxalate in whole grain rice both before and after boiling. Our data suggests that treated bran, would supply a low level of oxalate content when compared to raw brans.
Table 3.
Effect of extrusion treatment on oxalates* (%) content of different cereal brans
| Temp. (°C) | Moisture content (%) | Type of bran | Bran means | |||
|---|---|---|---|---|---|---|
| Wheat | Rice | Barley | Oat | |||
| Control | 0.442 ± 0.12a | 0.445 ± 0.12a | 0.325 ± 0.11a | 0.309 ± 0.12a | 0.380 ± 0.10a | |
| 115 | 14 | 0.438 ± 0.11a | 0.430 ± 0.07a | 0.322 ± 0.02a | 0.277 ± 0.11a | 0.367 ± 0.05a (3.42) |
| 17 | 0.437 ± 0.12a | 0.392 ± 0.12a | 0.316 ± 0.09a | 0.267 ± 0.07a | 0.353 ± 0.01a (7.11) | |
| 20 | 0.437 ± 0.11a | 0.343 ± 0.09a | 0.312 ± 0.01a | 0.262 ± 0.04a | 0.338 ± 0.02a (10.53) | |
| 140 | 14 | 0.368 ± 0.11a | 0.271 ± 0.10a | 0.311 ± 0.08a | 0.159 ± 0.11a | 0.277 ± 0.06a (27.11) |
| 17 | 0.324 ± 0.12a | 0.265 ± 0.04a | 0.305 ± 0.11a | 0.156 ± 0.10a | 0.263 ± 0.03a (30.79.) | |
| 20 | 0.279 ± 0.08a | 0.258 ± 0.12a | 0.264 ± 0.03a | 0.140 ± 0.09a | 0.236 ± 0.11a (36.84) | |
| 165 | 14 | 0.329 ± 0.10a | 0.359 ± 0.05a | 0.312 ± 0.06a | 0.238 ± 0.11a | 0.310 ± 0.09a (18.42) |
| 17 | 0.320 ± 0.06a | 0.356 ± 0.12a | 0.311 ± 0.09a | 0.221 ± 0.11a | 0.302 ± 0.03a (20.53) | |
| 20 | 0.312 ± 0.11a | 0.261 ± 0.08a | 0.308 ± 0.05a | 0.214 ± 0.12a | 0.273 ± 0.01a (28.95) | |
*on 14 % moisture basis
Values are means ± Standard deviation of triplicate determinations
Figures in parentheses indicate present reduction over control values
Means within columns with different letters are significantly different (P ≤ 0.05)
Trypsin inhibitors
Trypsin inhibitor (TI) being heat labile, degraded significantly with the extrusion processing as illustrated in Table 4. Raw wheat, rice, barley and oat bran was having 54.25, 53.82, 50.39 and 49.74 TIU/g of trypsin inhibitors, respectively before processing. Mean values showed that the extrusion temperature of 140 °C resulted into maximum inhibition in TI. At 140 °C with 14, 17 and 20 % moisture content mean values of trypsin inhibitor were 15.58, 14.92 and 14.34 TIU/g respectively. The percent reduction in trypsin inhibitor activity at 20 % moisture content and 115 °C, 140 °C and 165 °C was 56.37, 72.38 and 63.48 %, respectively. Stabilization of these brans using extrusion technology was effectively achieved at 140 °C and 20 % moisture content. A remarkable reduction in trypsin inhibitor to 71.17, 73.11, 73.07 and 72.21 % in wheat, rice, barley, and oat bran respectively was achieved at 140 °C for 20 % moisture content. Purushotham et al. (2007) reported that trypsin inhibitor activity decreased with increase in extrusion temperature in soybeans. Trypsin Inhibitor activity in raw soybeans were reduced from 51 to <2 TUI between 40 and 140 °C extrusion temperatures. Though initial values at 40–80 °C indicated slow inactivation of Trypsin Inhibitor activity units from 51 to 34 TUI, the inactivation was increased with further rise in extrusion temperature from 80 to 140 °C. This phenomenon of inactivation of trypsin inhibitors points out the information that Trypsin inhibitor was sensitive to extrusion temperature. They summarized that trypsin Inhibitors was inactivated to desired levels during extrusion at temperatures between 120 and 140 °C making extruded soybean a stable and suitable ingredient for pet food formulations. Cooking generally inactivates heat sensitive factors such as trypsin inhibitors as a result of denaturation of these heat liable proteins (Vidal-Valvarde et al. 1994).
Table 4.
Effect of extrusion treatment on trypsin inhibitor *(TIU/g) content of different cereal brans
| Temp. (°C) | Moisture content (%) | Type of bran | Bran means | |||
|---|---|---|---|---|---|---|
| Wheat | Rice | Barley | Oat | |||
| Control | 54.25 ± 0.29f | 53.82 ± 0.82e | 50.39 ± 0.39e | 49.74 ± 0.74d | 52.05 ± 2.21f | |
| 115 | 14 | 26.25 ± 0.25e | 25.82 ± 0.20d | 22.26 ± 0.26d | 21.30 ± 0.30c | 23.91 ± 0.91e (54.06) |
| 17 | 26.18 ± 0.18e | 25.04 ± 1.10d | 21.71 ± 0.71cd | 21.30 ± 1.30c | 23.56 ± 0.56de (54.74) | |
| 20 | 26.08 ± 0.08e | 23.03 ± 1.00c | 20.88 ± 0.84c | 20.86 ± 0.86c | 22.71 ± 0.71d (56.37) | |
| 140 | 14 | 17.75 ± 0.75b | 15.82 ± 0.90a | 14.62 ± 0.62a | 14.13 ± 0.13a | 15.58 ± 0.58b (70.07) |
| 17 | 16.84 ± 0.84b | 14.74 ± 0.70a | 13.97 ± 0.97a | 14.13 ± 0.10a | 14.92 ± 0.92ab (71.34) | |
| 20 | 15.64 ± 0.64a | 14.47 ± 1.40a | 13.57 ± 0.57a | 13.82 ± 0.82a | 14.37 ± 0.37a (72.39) | |
| 165 | 14 | 20.76 ± 0.66d | 19.29 ± 0.09b | 19.61 ± 0.61b | 18.98 ± 0.98b | 19.66 ± 0.66c (62.33) |
| 17 | 19.77 ± 0.67c | 18.94 ± 0.94b | 19.58 ± 0.58b | 18.56 ± 0.56b | 19.21 ± 0.21c (63.09) | |
| 20 | 19.52 ± 0.52c | 18.73 ± 0.05b | 19.30 ± 0.30b | 18.48 ± 0.48b | 19.01 ± 0.13c (63.48) | |
*on 14 % moisture basis
Values are means ± Standard deviation of triplicate determinations
Figures in parentheses indicate present reduction over control values
Means within columns with different letters are significantly different (P ≤ 0.05)
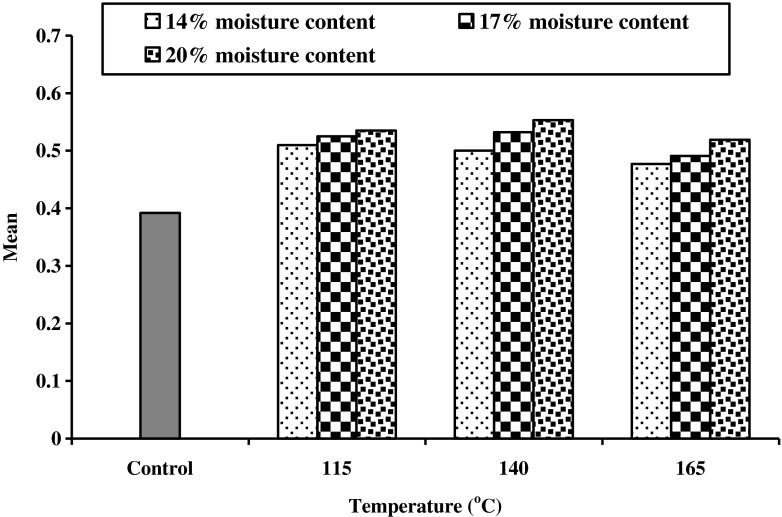
Bulk density
Effects of extrusion processing on bulk density of cereal brans are shown in Fig. 1. The value for untreated brans was 0.373, 0.355, 0.253 and 0.589 g/ml for wheat, rice, barley and oat brans respectively with the mean value of 0.392 g/ml. Non significant variation was observed in mean bulk density of cereal brans during extrusion processing. Mean value of bulk density increased up to 0.553 g/ml at 140 °C with 20 % moisture content from 0.392 g/ml of untreated bran. Treatment at high temperature and moisture content showed lower effect on bulk density. Kim et al. (1987a, b) reported similar findings and concluded that bulk density of extruded rice bran increased compared to raw rice bran. According to Gutkoski and El-Dash (1999) the apparent density ranged from 449 to 457 Kg/m3, with an average value of 454 Kg/m3, which was higher than 438 Kg/m3 found in raw material of oat products with similar granularity (<500 μm).
Fig. 1.
Effect of extrusion treatment on bulk density of different cereal brans
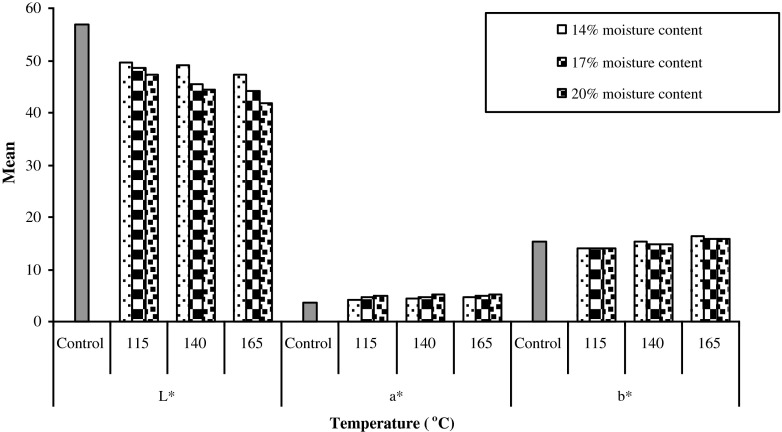
Color
Changes in the color value with extrusion treatment are shown in Fig. 2. Color is important to attract consumers before they consume a product (Francis 1991). Extruded brans becomes darker (lower L* values) in color with increase in temperature and moisture content of extrusion. The L* value at 165 °C and 20 % moisture content of cereal brans was lowest which explain the fact that lightness of brans decreased as the temperature and moisture content for extrusion cooking increased. At higher temperature and moisture content (165 °C for 20 % moisture content) higher redness (a*) was observed for all brans. However, b* values (yellowness) decreased as the temperature and moisture content increased. Our results are in accordance with Gutkoski and EL-Dash (1999). They reported that the products with lower values of L* (luminosity) and greater values of a* (red) were obtained after extrusion cooking. The color acquired by extruded products might be due to caramelization or maillard reaction. Lysine and other amino acids present in the raw material probably reacted with reducing sugars, favored by the processing conditions, which led to darkening of the extruded products.
Fig. 2.
Effect of extrusion treatment on color values of different cereal brans
Conclusion
Extrusion processing is a suitable technique to improving the quality of cereal brans for use into the food preparation. A temperature of 115 °C and 140 °C with 20 % moisture content could reduce the phytic acid content to more than half of its original concentration. Extrusion cooking of bran at 140 °C with 20 % moisture content resulted into the reduction of polyphenols and trypsin inhibitor to about three fourth. Affectivity of extrusion cooking for reduction of oxalates is comparatively low. Extrusion processing resulted into increase in bulk density value and darkens the color of the brans. Further studies are required to be carried out in this area.
Acknowledgments
The financial support from the Council of Scientific and Industrial Research (CSIR) New Delhi is gratefully acknowledged.
Contributor Information
Satinder Kaur, Email: Satinderpau@yahoo.co.in.
Savita Sharma, Email: savitasharmans@yahoo.co.in.
Baljit Singh, Email: baljitsj@yahoo.co.in.
B. N. Dar, Email: darnabi@gmail.com
References
- Abaza RH, Blake JT, Fisher EJ. Oxalate determination: analytical problems encountered with certain plant species. J Agric Food Chem. 1968;51:963–967. [Google Scholar]
- Asp NG, Bjorck-Mercier IB, Linko P, Harper JM. Nutritional properties of extruded foods. In: Harper JM, editor. Extrusion cooking. St. Paul: American Association of Cereal Chemists; 1989. pp. 399–434. [Google Scholar]
- Chauhan GS, Verma NS, Bains GS. Effect of extrusion process on the nutritional quality of rice legume blend. Nahrung. 1988;32:43. doi: 10.1002/food.19880320113. [DOI] [PubMed] [Google Scholar]
- Davies NT, Reid H. An evaluation of phytate, zinc, copper, iron and manganese content of and availability from soya based textured vegetable protein meat substitutes of meat extruders. Br J Nutr. 1979;41:579–588. doi: 10.1079/BJN19790073. [DOI] [PubMed] [Google Scholar]
- Egan H, Kirk RS, Sawyer R. Pearson’s chemical analysis of foods. 8. New York: Churchill Livingstone, Logman Inc.; 1981. [Google Scholar]
- Fairweather-Tait SJ, Portwood DE, Symss LL, Eagles J, Minski MJ. Iron and zinc absorption in human subjects from a mixed meal of extruded and non-extruded wheat bran flour. Am J Clin Nutr. 1989;49(1):151–155. doi: 10.1093/ajcn/49.1.151. [DOI] [PubMed] [Google Scholar]
- Frame ND. The technology of extrusion cooking. London: Blackie Academic and Professional; 1994. [Google Scholar]
- Francis FJ. Color measurements and interpretation. In: Fung D, Matthews RF, editors. Instrumental methods of quality assurance in foods. New York: Marcel Dekker; 1991. pp. 189–209. [Google Scholar]
- Gualberto DG, Bergman CJ, Kazemzadeh M, Weber CW. Effect of extrusion processing on the soluble and insoluble fiber and phytic acid contents of cereal bran. Plant Foods Hum Nutr. 1997;51:187–198. doi: 10.1023/A:1007941032726. [DOI] [PubMed] [Google Scholar]
- Gumal D, Korus J, Achremowicz B. The influence of extrusion on the content of polyphenols and antioxidant/antiradical activity of rye grains (Secale cereal L.) Acta Sci Pol Technol Aliment. 2007;6(4):103–111. [Google Scholar]
- Gutkoski LC, El-Dash AA. Effect of extrusion process variables on physical and chemical properties of extruded oat products. Plant Foods Hum Nutr. 1999;54:315–325. doi: 10.1023/A:1008101209353. [DOI] [PubMed] [Google Scholar]
- Hajela N, Pande AH, Sharma S, Rao DN, Hajela K. Studies on a doubleheaded protease inhibitors from Phaseolus mungo. J Plant Biochem Biotechnol. 1999;8:57–60. doi: 10.1007/BF03263059. [DOI] [Google Scholar]
- Harper JM. Extrusion of starches and starches materials. In: Harper JM, editor. Extrusion of food. Boca Raton: CRC Press; 1981. pp. 41–60. [Google Scholar]
- Judprasong K, Charoenkiatkul S, Sungpuag P, Vasanachitt K, Nakjamanong Y. Total and soluble oxalate contents in Thai vegetables, cereal grains and legume seeds and their changes after cooking. J Food Compos Anal. 2006;19:340–347. doi: 10.1016/j.jfca.2005.04.002. [DOI] [Google Scholar]
- Khattab RY, Arntfield SD. Nutritional quality of legume seeds as affected by some physical treatments 2. Antinutritional factors. LWT Food Sci Technol. 2009;42:1113–1118. doi: 10.1016/j.lwt.2009.02.004. [DOI] [Google Scholar]
- Kim CJ, Byun SM, Chiegh HS, Know TW. Optimization of extrusion bran stabilization process. J Food Sci. 1987;52:1355. doi: 10.1111/j.1365-2621.1987.tb14081.x. [DOI] [Google Scholar]
- Kim CJ, Byun SM, Chiegh HS, Know TW. Compression of solvent extraction characteristics of rice bran pre treated by hot air drying, steam cooking and extration. J Am Oil Chem Soc. 1987;64:514. doi: 10.1007/BF02636385. [DOI] [Google Scholar]
- Purushotham B, Radhakrishna PM, Sherigara BS. Effects of steam conditioning and extrusion temperature on some anti-nutritional factors of soyabean (Glycine max) for pet food applications. Am J Anim Vet Sci. 2007;2(1):1–5. doi: 10.3844/ajavsp.2007.1.5. [DOI] [Google Scholar]
- Rehman MYA, Mahmood S. Anti-nutritional factors, metabolizable energy and chemical composition of rice bran. MARDI Res J. 1996;24:135–145. [Google Scholar]
- Sayre RN, Earl L, Kratzer FH, Saunders RM. Effects of diets containing raw and extrusion-cooked rice bran on growth and efficiency of food utilization of broilers. Br J Poult Sci. 1988;29:8–15. [Google Scholar]
- Sharma HR, Chauhan GS, Agarwal K. Physico-chemical characteristics of rice bran processed by dry heating and extrusion cooking. Int J Food Prop. 2004;7:603–614. doi: 10.1081/JFP-200033047. [DOI] [Google Scholar]
- Shivaji SZ, Jagrahan SI. Nutritive value of raw, parboiled, stabilized and deoiled rice bran for growing chicks. J Sci Food Agric. 1983;34:783–788. doi: 10.1002/jsfa.2740340804. [DOI] [Google Scholar]
- Siener R, Honow R, Voss S, Seidler A, Hesse A. Oxalate content of cereals and cereal products. J Agric Food Chem. 2006;54:3008–3011. doi: 10.1021/jf052776v. [DOI] [PubMed] [Google Scholar]
- Smith AC, Singh N. New applications of extrusion technology. Indian Food Ind. 1996;15:14–23. [Google Scholar]
- Snedecor GW, Cochran WG (1980) Statistical methods. 7th Ed. Oxford and IBIT Public. Co, New York
- Soetan KO, Oyewole OE. The need for adequate processing to reduce the antinutritional factors in plants used as human foods and animal feeds: a review. Afr J Food Sci. 2009;3(9):223–232. [Google Scholar]
- Swain TS, Hills WE. The phenolic constituents of Prunus domestica I. – The quantitative analysis of phenolic constituents. J Sci Food Agric. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- Vidal-Valvarde C, Frias J, Estrella I, Gorospe MJ, Ruiz R, Bacon J. Effect of processing on some antinutritional factors of lentils. J Agric Food Chem. 1994;42:2291–2295. doi: 10.1021/jf00046a039. [DOI] [Google Scholar]
- Viscidi KA, Dougherty MP, Briggs J, Camire ME. Complex phenolics compounds reduce lipid oxidation in extruded oat cereals. LWT Food Sci Technol. 2004;37:789–796. doi: 10.1016/j.lwt.2004.03.005. [DOI] [Google Scholar]