Abstract
Chemical composition, antioxidant potential and corresponding lipid preoxidation of Indian commercial beers were evaluated. The presence of polyphenolic compounds such as tannic acid, gallic acid, catechol, vanillin, caffeic acid, quercetin, p-coumaric acid and rutin was quantified using LC-MS while the organic acids including tartaric, malic, acetic, citric and succinic acids were analysed using HPLC. Beer sample B8 had the greatest concentration of phenolic and flavonoid components (0.620 ± 0.084 mg/mL and 0.379 ± 0.020 mg/mL respectively) among the beer samples studied. The DPPH radical scavenging activity was observed in the range of 68.34 ± 0.85 % to 89.90 ± 0.71 % and ABTS radical cation scavenging activity was in the range of 59.75 ± 0.20 % to 76.22 ± 0.50 %. Percent protection in lipid peroxidation was quantified to be maximum (54.45 ± 3.39 %) in sample B5. Total phenolic content positively correlates with antioxidant assays, DPPH and ABTS (r = 0.35 and r = 0.58 respectively) with p < 0.001 and also with lipid peroxidation (r = 0.04) with p < 0.001. Negative correlation was observed between total flavonoid content with ABTS and lipid peroxidation (r = −0.1 and r = −0.05) respectively. The process of brewing warrants additional research to determine how the concentration of selected phenolic compounds can be increased.
Keywords: Indian beers, Phenolic content, Flavonoids, Antioxidant potential, Rutin, HPLC, LC-MS
Introduction
Beer is an alcoholic beverage widely consumed throughout the world. It is the third most popular drink overall after water and tea (Nelson 2005; Piazzon et al. 2010). Beer is produced by the saccharification of starch and fermentation of the resulting sugar solution. The starch and saccharification enzymes are often derived from malted cereal grains; most commonly malted barley and malted wheat. The preparation of beer is called brewing. Beer is flavoured with hops, which add bitterness and acts as a natural preservative, though other flavourings such as herbs or fruit may occasionally be included (Barth 2011).
Beer contains a variety of polyphenols which can be important for its chemical stability which include prenylated flavonoids, phenolic acids, simple phenols, flavanols, hydroxycoumarins, flavones (Gerloff et al. 2010), proanthocyanidins, tannins, and amino phenolic compounds (Zhao et al. 2010). Apart from their antioxidant potential, these compounds play an important role in flavour (bitterness, astringency, harshness) and colour (Gerloff et al. 2010). The majority of polyphenol compounds are derived from the malt and remainder come from the hops (Montanari et al. 1999). These compounds are involved in the chemical stability and shelf life of the beer. Phenolic compounds can also function as antioxidants in the human body as a protective agent against the oxidation of ascorbic acid and unsaturated fatty acids (Zhao et al. 2010).
Rutin, another phenolic compound has anti-inflammatory activity (Guardia et al. 2001), anti-arthritic and antifungal effects (Han 2009). It inhibits aldose reductase activity and can acts as an inhibitor of angiogenesis (Luo et al. 2008). Thus, it helps in controlling some cancers. Rutin may have the possibility of a protective effect against some neurological disorders such as Parkinsson’s diseases (Khan et al. 2012). Studies have shown that moderate consumption of beer may be associated with reduced risk of coronary heart disease (Bamforth 2002) as well as an inhibitory effect on pancreatic cancer growth (Gerloff et al. 2010). Ferulic acid, a phenolic acid found in barley, malted barley and beer, is an effective antioxidant and it possesses anticancer properties in vitro (Szwajgier et al. 2005). It has been observed that beer improves human plasma antioxidant capacity due to the presence of phenolic compounds (Nardini et al. 2006).
India is one of the fastest growing markets for beer and per capita consumption of beer in India is increasing at approximately 7 % to 9 % per year (http://www.foodregulatorysummit.org/pdf/aiba.pdf). Various factors that include lifestyle, tourism and favourable government policies have facilitated the steady growth of beer consumption in India. The annual consumption of beer in India is 16.2 million hectolitres (http://www.indianexpress.com/news/maharashtra-ranks-second-in-terms-of-beer-consumption/947997). These figures could increase substantially due to a change in the beer drinking culture in India. The consumption of beer is increasing day by day, but a literature search has revealed that no research has been published on the chemical composition and antioxidant activity of the beers manufactured in India.
In the present study selected Indian beers were characterized for their chemical composition, polyphenolic content and radical scavenging activity. Chemical characterization such as pH, total acidity, alcohol content, reducing sugars, carbohydrate and protein concentration were also determined.
Materials and methods
Samples
Fifteen different beer brands including 13 domestic and two imported were purchased from local market (Maharashtra, India) (Table 1) and a sample of one batch for each brand was examined in the study. All samples were pale lager types and stored in a refrigerator at 4 °C till further analysis. The beer samples were dealcoholized according to Ghatak et al. (2012) by concentrating them using rotary evaporation at 25 °C. An equal volume of distilled water was added to beer and the volume was reduced to its original volume in order to remove alcohol without altering the concentration of phenolic compounds.
Table 1.
Place of brewing and storage style of beers
| Beers | Place of brewing | Storage style |
|---|---|---|
| B1 | Perumbavoor (Kerala), Cochin | Pale lager |
| B2 | Perumbavoor (Kerala), Cochin | Pale lager |
| B3 | Perumbavoor (Kerala), Cochin | Pale lager |
| B4 | Yeshwantpur (Banglore) | Pale lager |
| B5 | Aurangabad (Maharashtra) | Pale lager |
| B6 | Banglore | Pale lager |
| B7 | Hyderabad (Andhra Pradesh) | Pale lager |
| B8 | Mumbai and Delhi | Pale lager |
| B9 | Mumbai | Pale lager |
| B10 | Taloja (Navi-Mumbai), | Pale lager |
| B11 | Aurangabad (Maharashtra) | Pale lager |
| B12 | Andhra Pradesh | Pale lager |
| B13 | Goa | Pale lager |
| B14 | Mexico | Pale lager |
| B15 | Belgium | Pale lager |
Chemical characterization
pH and total acidity
The pH was determined according to Joslyn (1950). The total acidity of the beer samples was determined by potentiometric titration. Ten mL of beer was added to 50 mL of distilled water and the sample was titrated against 0.05 N sodium hydroxide. The volume of NaOH required to attain a pH of 8.2 was determined using a pH meter. Total acidity was expressed in terms of tartaric acid equivalents (AOAC Method 2000).
Alcohol content
The ethanol content was estimated using a potassium dichromate reagent (Seo et al. 2009) with slight modifications. Beer samples (3.5 mL) were added to 10 mL of acidified potassium dichromate and incubated at 30 °C for 30 min. After incubation 100 mL of distilled water and 4 mL of potassium iodide (25 %) were added to each sample. The samples were titrated against 0.1 N sodium thiosulphate using starch (1 %) as an indicator. Titration determines excess dichromate. Subtracting this amount of excess dichromate from the initial stock gives the amount of ethanol present. Results were interpreted using ethanol as the standard.
Reducing sugar content
Reducing sugars were estimated based on the method of Sengupta et al. (2000) using 3, 5—dinitrosalicylic acid (DNSA) reagent. A 1 mL sample (1:10 diluted) was added to 2 mL of DNSA reagent, the tubes were incubated at 85 °C in water bath for 5 min. Ten mL of distilled water was added and the absorbance was recorded at 540 nm. The reducing sugar content was expressed as maltose equivalents (mg/mL) using maltose as a standard.
Carbohydrates content
The carbohydrate content was estimated using anthrone reagent according to the method of Yemm and Willis (1954). Anthrone reagent (4 mL) was added to 1 mL of beer (1:1000 diluted) and incubated in a boiling water bath for 10 min. After cooling the absorbance was recorded spectrophotometrically at 620 nm. Results were expressed as glucose equivalents (mg/mL) using glucose as the standard.
Protein content
The protein content of beer samples was estimated according to Lowry et al. (1951). Five mL of Folin’s solution was added to 1 mL of beer sample (1:100 diluted). The mixture was incubated at 30 °C in dark for 10 min. Folin’s reagents (0.5 mL) was added to all the tubes and incubated at 30 °C for 30 min. The absorbance was measured at 660 nm. Bovine serum albumin was used as a standard to interpret the results.
Parameters related to antioxidant capacity
Total phenolic content
Folin-Ciocalteu reagent was used for the determination of the total phenolic content (TPC) as per Chaturvedi et al. (2012). The beer samples were mixed with Folin-Ciocalteu reagent and aqueous sodium carbonate. The mixtures were incubated for 30 min at 30 °C. The absorbance was read at 650 nm. Total phenolic values were expressed as mg/mL of tannic acid, using tannic acid as the standard equivalent.
Profile of phenolic compounds by LC-MS
Liquid Chromatography-Mass Spectrometry for the determination of phenolic compounds in beer was carried out at Reliable Analytical Laboratories, Maharashtra using Agilent Technologies 6460 Triple Quadrupole LC/MS. Samples were centrifuged at 12,000 rpm for 10 min before analysis. The HPLC system consisted of two pumps and an automated injector. Separation was achieved on a C-18 column (Agilent Eclipse, 5 μ, 15 cm, 4.6 mm id). Two mobile phases used were: 0.1 % formic acid in water and acetonitrile.
Total flavonoid content
The total flavonoid content was estimated using 2 % aluminium chloride (AlCl3) solution in methanol according to Luximon-Ramma et al. (2002). To 1.5 mL dealcoholized beer (1:5 diluted), 1.5 mL AlCl3 in methanol was added. The samples were incubated at 30 °C for 10 min. The absorbance was recorded at 368 nm using quercetin as the standard.
DPPH radical scavenging activity
Free radical scavenging activity was estimated using DPPH as described by Ghatak et al. (2012). A solution of 0.3 mM DPPH in methanol was prepared and 0.5 mL of this solution was mixed with 100 μL of beer. The reaction mixture was left in the dark at 30 °C for 30 min. The absorbance of the mixture was taken at 518 nm. The ability to scavenge DPPH radical was calculated using following formula:
ABTS radical cation scavenging activity
The radical scavenging activity of beer against the ABTS radical cation was measured using the method of Lobo et al. (2010). The stock solutions included 7 mM ABTS solution and 2.4 mM potassium persulfate solution. The working solution was prepared by mixing two stock solutions in equal quantities and allowing them to react for 12 h at 37 °C in the dark. The resulting ABTS+ solution (1 mL) was diluted with 60 mL methanol to obtain an absorbance of 0.706 ± 0.001 units at 734 nm using the spectrophotometer. To 1 mL of 1:100 diluted beer samples, 1 mL of ABTS was added and absorbance was recorded after 7 min at 734 nm. Percentage ABTS radical cation scavenging activity was calculated as percent inhibition using ABTS+ solution as control.
Lipid peroxidation assay
Isolation of mitochondrial fraction from rat liver
Wistar rats (weighing about 240 ± 20 g and 3 months old) were used for this assay due to their close resemblance to humans for the preparation of mitochondria. Rat liver was excised and homogenized in 0.25 M sucrose containing 1 mM EDTA. The homogenate was centrifuged at 3000 × g for 10 min, to remove cell debris and the nuclear fractions. The supernatant was centrifuged at 10,000 × g for 10 min to sediment the mitochondria (Chaturvedi et al. 2012).
Protein estimation of mitochondrial extract by Bradford reagent
Bradford reagent was prepared according to Chaturvedi et al. (2012) by dissolving 100 mg Coomassie Brilliant Blue G-250 in 50 mL 95 % ethanol. To this 100 mL 85 % (w/v) phosphoric acid was added and volume was made to 1 L with distilled water. Protein was estimated at 595 nm and mitochondrial pellets were suspended in potassium phosphate buffer at the concentration of 10 mg protein/mL.
Exposure of rat liver mitochondria to oxidative stress
Oxidative damage was induced by ascorbate-Fe2+-system as described by Ghatak et al. (2012). The pink color of thiobarbituric acid reactive substances formed was estimated spectrophotometrically at 532 nm as malondialdehyde equivalents after accounting for the appropriate blanks. The malondialdehyde standard was prepared by the acid hydrolysis of tetraetoxypropane.
Profile of organic acids by HPLC
High Performance Liquid Chromatography for the detection of organic acids in the beer samples was carried out according to Kordis-Krapez et al. (2001). Samples were centrifuged at 12,000 rpm for 10 min before injection. Chromatographic separation was achieved on a C-18 reversed phase column (Princeton SPHER, 5 μm, 250 mm). An injection volume of 20 μL and wavelength 210 nm was used. Aqueous solution of H3PO4 (6 × 10−3 mol/L, pH = 2.1) was used as the mobile phase and flow rate of 1.0 mL/min was employed.
Statistical analysis
All the observations were taken in triplicate and the data was presented as ± standard deviation (SD). Analysis of Variance (one way ANOVA) was further performed using SPSS software (version 14.0). Correlation coefficient was calculated using Pearson Product Moment Correlation. A probability of p < 0.001 was considered to be statistically significant and correlation coefficient (r) values were determined.
Results and discussion
pH and total acidity
The pH of the beer samples ranged from 3.83 to 4.49 (Table 2). B8 was least acidic of all the samples with a pH of 4.49, while B9 was the most acidic with a pH of 3.83. The total acidity of the beer samples was in the range 0.0957 ± 0.0027 % to 0.2252 ± 0.0 % tartaric acid equivalent (Table 2). B7 had the least total acidity of 0.0957 ± 0.0027 %, while B12 had the greatest total acidity of 0.2252 ± 0.0 %. The pH and total acidity are considered the two most important criteria by the brewing industries which strongly influence sanitation and other physiological parameters like color, odour, taste, biological and chemical stability. Brewing industry usually prefer pH in a range of 3.90–4.20 for light lager beers which also plays important role during brewing process that include enzyme effectiveness, hop utilization, protein coagulation and to monitor yeast activity in clean beer fermentation. Alcoholic beverages from Tanzania reported to have pH and total acidity in the range of 3.9–5.5 and 0.41–0.062, 0.28–0.38 and 0.06–0.09 g/100 mL respectively (Tüsekwa et al. 2000).
Table 2.
pH, total acidity, alcohol content, reducing sugars, protein content and carbohydrate content in beer samples
| Sample | pH | Total acidity (%) | Alcohol content (% v/v) | Reducing sugars (mg/mL) | Protein content (mg/mL) | Carbohydrate content (mg/mL) |
|---|---|---|---|---|---|---|
| B1 | 3.84 | 0.1163 ± 0.0 | 6.68 | 1.466 ± 0.027 | 3.93 ± 0.21 | 47.33 ± 1.007 |
| B2 | 4.27 | 0.1332 ± 0.0027 | 8.91 | 2.682 ± 0.008 | 5.32 ± 0.0 | 54.4 ± 0.283 |
| B3 | 4.00 | 0.1238 ± 0.0 | 7.13 | 2.430 ± 0.015 | 2.85 ± 0.28 | 55.6 ± 0.283 |
| B4 | 4.21 | 0.1426 ± 0.005 | 7.13 | 2.293 ± 0.0 | 4.74 ± 0.02 | 63.3 ± .0141 |
| B5 | 3.94 | 0.1201 ± 0.0 | 7.58 | 1.982 ± 0.012 | 2.41 ± 0.05 | 42.87 ± 0.702 |
| B6 | 3.94 | 0.1595 ± 0.0027 | 7.13 | 2.070 ± 0.012 | 3.43 ± 0.02 | 56.5 ± 0.141 |
| B7 | 4.20 | 0.0957 ± 0.0027 | 6.24 | 1.344 ± 0.003 | 2.86 ± 0.02 | 45.9 ± 0.424 |
| B8 | 4.49 | 0.1164 ± 0.005 | 5.79 | 1.906 ± 0.018 | 4.45 ± 0.47 | 43.1 ± 0.141 |
| B9 | 3.83 | 0.152 ± 0.0027 | 4.90 | 1.006 ± 0.0 | 3.78 ± 0.09 | 52.8 ± 0.283 |
| B10 | 3.84 | 0.1088 ± 0.0038 | 6.24 | 1.153 ± 0.009 | 2.96 ± 0.31 | 37.3 ± 0.424 |
| B11 | 4.39 | 0.1351 ± 0.0053 | 5.79 | 1.225 ± 0.082 | 5.41 ± 0.12 | 30.2 ± 0.283 |
| B12 | 3.98 | 0.137 ± 0.0027 | 7.58 | 2.564 ± 0.009 | 4.23 ± 0.02 | 92.1 ± 0.424 |
| B13 | 4.16 | 0.2252 ± 0.0 | 6.24 | 1.025 ± 0.057 | 3.33 ± 0.02 | 41.2 ± 0.8 |
| B14 | 3.84 | 0.1126 ± 0.0 | 4.45 | 0.469 ± 0.021 | 2.04 ± 0.04 | 16.3 ± 0.424 |
| B15 | 4.45 | 0.1407 ± 0.0027 | 6.68 | 1.469 ± 0.042 | 4.52 ± 0.09 | 54.8 ± 0.283 |
Values represent mean ± standard deviation (SD)
Alcohol content
Most of the alcohols formed during beer fermentation are linked to the yeast-protein synthesis, they are formed from keto-acids which may be determined by transamination or deamination of the amino acids in wort or synthesized from wort carbohydrates. The ethanol content was in the range 4.45 %–8.91 % v/v (Table 2). B2 had the greatest alcohol content of 8.91 % v/v, while B14 had the least alcohol content of 4.45 %v/v (Table 2). According to studies performed by Bamforth (2002), many beers have alcohol content approximately in the range of 3 %–6 % (v/v). These keto-acids are then converted to higher alcohols by decarboxlyation and reduction (Pollock 1981). However beer is one the most abundant alcoholic beverages consumed worldwide and presence of alcohol provides protection against several heart diseases and also along with the presence polyphenols can reduce oxidative stress produced by various metabolic process and stress conditions (Arranz et al. 2012).
Reducing sugars
The reducing sugar content in the beer samples ranged from 2.682 ± 0.008 mg/mL to 0.469 ± 0.021 mg/mL (Table 2). The maximum reducing sugar content was found in beer sample B2 (2.682 ± 0.008 mg/mL) while the minimum was found in beer sample B14 (0.469 ± 0.021 mg/mL). Yeast can only utilise selected lower molecular weight sugars, such as fructose, glucose, maltose, sucrose and maltotriose. Oligosaccharides containing more than three glucose units cannot be fermented. Therefore, when the fermentation is allowed to go to completion, only small amounts of smaller molecular weight sugars are found in beer (Lehtonen and Hurme 1994). For some styles of beer, the fermentation is halted before completion.
Carbohydrate content
Carbohydrates can be present in large amounts in beer, since it is made from barley, which is rich in carbohydrates. The carbohydrate content in the beer samples was found in the range of 92.1 ± 0.424–16.3 ± 0.424 mg/mL (Table 2). The greatest carbohydrate content was observed in sample B12 (92.1 ± 0.424 mg/mL) which is a strong beer whereas least content was observed in beer sample B14 (16.3 ± 0.424 mg/mL) which is a mild beer. Major carbohydrates present in beer include glucose, maltose, maltotriose, dextrins and arabinoxylans (Cortacero-Ramirez et al. 2003). Fermentable sugars directly contribute to the sweetness of beer, whereas larger carbohydrates may contribute to body or mouthfeel (Iola et al. 2003).
Protein content
Beers vary in the protein and polypeptide content which affects important parameters, such as the shelf-life of the product. The maximum protein content was found in beer sample B11 (5.41 ± 0.12 mg/mL) whereas the minimum protein content was found in beer sample B14 (2.04 ± 0.04 mg/mL) (Table 2). The proteins come from malted barley, which contain approximately 10 %–12 % protein (Cortacero-Ramirez et al. 2003). The proteins and amino acids present in the beer can influence foam and haze stability (Mainente et al. 2011).
Total phenolic content and profiling by LC-MS
Beer contains a complex mixture of phenolic compounds ranging from 150 mg/L to 350 mg/L (Ng and Mocek 1973; Narziss 1972). The total phenolic content of beer samples was determined using Folin-Ciocalteu reagent and it ranged from 0.16 ± 0.016 to 0.620 ± 0.084 mg/mL (Table 3) of which B8 showed the greatest (0.620 ± 0.084 mg/mL) phenolic content and B3 showed the least (0.16 ± 0.016 mg/mL) (Table 3). Traces of vanillin, catechol, quercetin, tanic acid were identified using LC-MS (Table 4). Interestingly rutin, caffeic acid and p-coumaric acid were identified in all the beer samples. The highest concentration of rutin is observed in B12 (111.94 ±0.36 ppb) and least concentration was observed in B14 (0.20 ± 0.08 ppb) (Table 4). Rutin (Fig. 2) is a flavonoid glycoside found in buckwheat, which is a derivative of quercetin with positive physiological effects (Roussis et al. 2005). It is an effective inhibitor of iron ion-dependent lipid peroxidation and since it can chelate iron ions and form inert iron complexes that are unable to initiate lipid peroxidation (Afanasev et al. 1989). In recent years considering the importance of rutin, buckwheat has been used as substitute for other grains in gluten free beer. Although it is not a cereal, buckwheat can be used similarly to barley and produce malt that can form the basis of a mash that will brew beer without gliadin or hordein (proteins that together constitute gluten) and therefore can be suitable for coeliacs and sensitive to certain glycoprotein’s (Smagalski 2006). Trace amount of other phenolics like gallic acid is present in beer samples B1, B2 and B3, while catechin is absent in all the beer samples (Table 4). These obtained results of phenolic content are lower than those from Zhao et al. (2010). So far more than 50 polyphenolic compounds have been identified in beer and 75 %–85 % of these are derived from malt (Gerloff et al. 2010). Phenols contribute to beer flavor and product stability (Iola et al. 2003). However, Nardini and Ghiselli (2004) reported that addition of ascorbic acid as antioxidant and ethylenediaminetetraacetic acid as a chelator which helps in stability of phenolic compounds in beer.
Table 3.
Phenolic content, flavonoids content, DPPH, ABTS radical scavenging activity and percent protection in lipid peroxidation
| Sample | Phenolic content (mg/mL) | Flavonoids content (mg/mL) | DPPH radical scavenging activity (%) | ABTS radical scavenging activity (%) | Percentage protection |
|---|---|---|---|---|---|
| B1 | 0.247 ± 0.018 | 0.093 ± 0.003 | 80 ± 0.0 | 69.99 ± 0.70 | 40.41 ± 3.87 |
| B2 | 0.303 ± 0.008 | 0.123 ± 0.001 | 84.44 ± 0.0 | 71.68 ± 0.30 | 43.15 ± 0.0 |
| B3 | 0.160 ± 0.016 | 0.076 ± 0.0004 | 75.56 ± 1.12 | 65.61 ± 0.40 | 31.17 ± 0.49 |
| B4 | 0.377 ± 0.005 | 0.151 ± 0.006 | 80.56 ± 0.79 | 68.01 ± 0.10 | 33.22 ± 2.42 |
| B5 | 0.244 ± 0.010 | 0.140 ± 0.001 | 74.44 ± 0.0 | 65.82 ± 0.40 | 54.45 ± 3.39 |
| B6 | 0.457 ± 0.005 | 0.225 ± 0.001 | 76.86 ± 0.26 | 67.44 ± 0.10 | 40.07 ± 4.36 |
| B7 | 0.226 ± 0.017 | 0.098 ± 0.003 | 71.11 ± 1.11 | 61.80 ± 0.50 | 43.15 ± 0.0 |
| B8 | 0.620 ± 0.084 | 0.379 ± 0.020 | 76.56 ± 0.47 | 72.18 ± 0.20 | 38.70 ± 2.42 |
| B9 | 0.219 ± 0.004 | 0.115 ± 0.002 | 72.41 ± 0.26 | 64.41 ± 1.70 | 29.80 ± 3.39 |
| B10 | 0.168 ± 0.007 | 0.103 ± 0.002 | 68.34 ± 0.85 | 62.36 ± 0.90 | 24.32 ± 1.45 |
| B11 | 0.290 ± 0.014 | 0.147 ± 0.003 | 81.48 ± 1.05 | 76.22 ± 0.50 | 19.18 ± 1.94 |
| B12 | 0.319 ± 0.028 | 0.178 ± 0.001 | 80.00 ± 0.0 | 75.78 ± 0.30 | 38.02 ± 2.43 |
| B13 | 0.375 ± 0.019 | 0.147 ± 0.001 | 89.90 ± 0.71 | 72.67 ± 0.10 | 35.62 ± 2.91 |
| B14 | 0.177 ± 0.001 | 0.014 ± 0.002 | 72.73 ± 0.0 | 59.75 ± 0.20 | 33.57 ± 0.97 |
| B15 | 0.333 ± 0.002 | 0.087 ± 0.001 | 89.14 ± 0.35 | 68.79 ± 0.80 | 55.47 ± 0.96 |
Values represent mean ± standard deviation (SD)
Table 4.
Phenolic characterization in the beer samples by LC-MS
| Sample | Gallic Acid (ppb) | Tannic acid (ppb) | Caffeic acid (ppb) | Catechol (ppb) | Rutin (ppb) | p-coumaric acid (ppb) | Vanillin (ppb) | Quercetin (ppb) |
|---|---|---|---|---|---|---|---|---|
| B1 | 3.01 ± 0.45 | 15.93 ± 0.34 | 1.45 ± 0.22 | 2.69 ± 0.19 | 27.11 ± 0.93 | 2.37 ± 0.76 | 8.47 ± 1.22 | 12.79 ± 0.54 |
| B2 | 5.06 ± 0.12 | 3.19 ± 0.54 | 1.99 ± 0.45 | 2.39 ± 0.77 | 18.52 ± 1.04 | 0.34 ± 0.67 | 9.44 ± 0.48 | – |
| B3 | 3.15 ± 0.23 | 15.3 ± 0.68 | 2.50 ± 0.39 | 0.30 ± 0.71 | 13.61 ± 0.89 | 2.79 ± 0.88 | 4.28 ± 0.34 | 4.71 ± 0.93 |
| B4 | – | 7.65 ± 0.35 | 3.25 ± 0.17 | 0.30 ± 0.52 | 33.05 ± 0.2 | 2.46 ± 0.57 | 8.37 ± 0.68 | 6.73 ± 0.78 |
| B5 | – | 3.19 ± 0.84 | 4.70 ± 0.31 | 14.08 ± 0.84 | 41.85 ± 1.28 | 3.34 ± 1.08 | 2.43 ± 0.24 | 4.71 ± 0.11 |
| B6 | – | 10.2 ± 0.76 | 2.06 ± 0.62 | 3.59 ± 0.14 | 32.84 ± 0.78 | 1.35 ± 0.8 | 18.99 ± 0.17 | 4.04 ± 0.42 |
| B7 | – | 12.11 ± 0.23 | 2.84 ± 0.57 | 2.99 ± 0.28 | 5.12 ± 0.44 | 1.55 ± 0.34 | 3.12 ± 0.11 | 6.06 ± 0.23 |
| B8 | – | 15.3 ± 0.55 | 2.30 ± 0.42 | 0.30 ± 0.08 | 19.03 ± 0.39 | 0.61 ± 0.22 | 3.89 ± 0.67 | 18.17 ± 0.84 |
| B9 | – | 0.64 ± 0.29 | 2.71 ± 0.1 | 0.30 ± 0.02 | 4.91 ± 0.78 | 1.43 ± 0.77 | 5.55 ± 0.08 | 4.04 ± 0.77 |
| B10 | – | 10.2 ± 0.75 | 1.26 ± 0.17 | 0.60 ± 0.45 | 12.38 ± 0.18 | 0.51 ± 0.81 | 4.58 ± 0.45 | 9.42 ± 0.57 |
| B11 | – | 4.46 ± 0.93 | 5.56 ± 0.44 | 1.5 ± 0.29 | 12.99 ± 0.68 | 1.68 ± 0.7 | 11.20 ± 0.14 | 15.48 ± 0.6 |
| B12 | – | 24.22 ± 1.22 | 0.35 ± 0.08 | 0.29 ± 0.57 | 111.94 ± 0.36 | 0.24 ± 0.32 | 11.59 ± 0.42 | 2.08 ± 0.34 |
| B13 | – | 10.2 ± 0.64 | 0.21 ± 0.13 | 1.2 ± 0.24 | 55.66 ± 0.79 | 0.34 ± 0.19 | 20.54 ± 0.55 | – |
| B14 | – | 16.57 ± 1.37 | 1.04 ± 0.88 | 0.29 ± 0.09 | 0.20 ± 0.08 | 2.83 ± 0.17 | 4.09 ± 1.37 | 24.23 ± 1.47 |
| B15 | – | 5.74 ± 0.69 | 2.92 ± 0.53 | 0.6 ± 0.04 | 54.13 ± 0.98 | 0.169 ± 0.68 | 10.81 ± 0.57 | 9.42 ± 0.75 |
Values represent mean ± standard deviation (SD)
ppb parts per billion
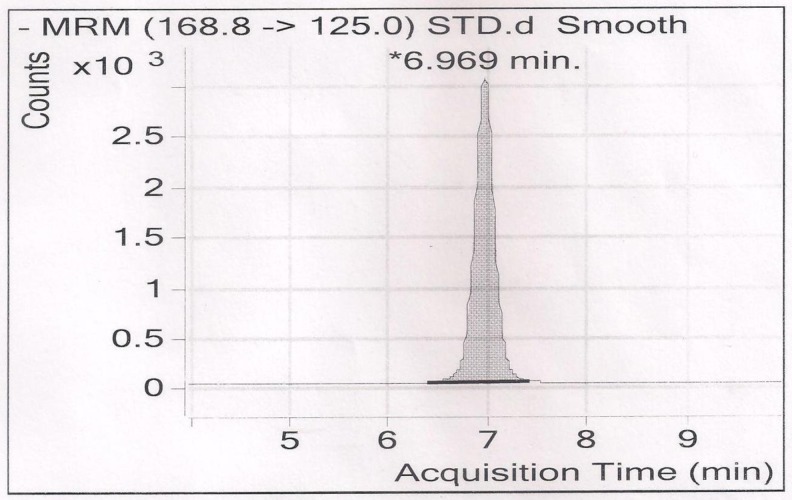
Fig. 2.
LC/MS chromatogram for the determination of standard phenolic compound (Rutin) was carried out by Agilent Technologies 6460 Triple Quadrupole LC/MS on a C-18 column (Agilent Eclipse, 5 μ, 15 cm, 4.6 mm ID)
Phenolic acids like p-coumaric acid, caffeic acid, gallic acid have been quantified in beers through LC-MS. Absorption of the phenolic acids and there bioavaibility can be identified by measuring plasma levels for free and conjugated forms which has been reported by Nardini et al. (2006). According to this report phenolic acids in beer which shows content in free (nonhydrolyzed beer) and total (free + bound, hydrolyzed beer), includes p-coumaric acid present in the free form (76.2 % and 69.8 % of total) and other identified phenolics such as caffeic acid is present in conjugated form (18.6 %) respectively. As beer has high phenolic content which overall contributes to the antioxidant activity and hence make beer as highly dietary consumable beverage. According to data analysis of human plasma after beer administration, Nardini et al. (2006) reported that phenolic acids absorbs beer based on glucuronidation and sulfatation, the best two ways known for detoxification and solubility of compound. In facts, plasma phenolic acids of beer reach 0.05–0.07 μM for caffeic acid and p-coumaric acid at 30 min after ingestion (Nardini et al. 2006). As previous research and current research completely reveals the antioxidant potentiality of beer which has beneficial health effects over dreadful disease like cancer and cardiovascular along with the corresponding reveal of their metabolism in humans makes possibility to enhance the quality of beer which can be proven more beneficial.
Total Flavonoid Content
Maximum flavonoid content was observed in beer sample B8 (0.379 ± 0.02 mg/mL), whereas the least amount was found in beer sample B14 (0.014 ± 0.002 mg/mL) (Table 3). Flavonoids exist in a wide range of concentration in beer, from 0.001 mg/L to 20 mg/L, likely due to variations in the barley and hop variety, growing conditions, brewing methodology, and the style of beer.
DPPH radical scavenging and ABTS radical cation scavenging activity
The DPPH radical scavenging activity of the beer samples was 68.34 ± 0.85 % to 89.90 ± 0.71 % (Table 3), while the percentage ABTS radical cation scavenging activity of the beer samples was 59.75 ± 0.20 % to 76.22 ± 0.50 % (Table 3). Antioxidants with DPPH radical scavenging activities can donate hydrogen to free radicals, particularly to the lipid peroxide or hydroperoxide generated free radicals, which are the major propagators of the chain autoxidation reaction of lipids, to form non radical species, resulting in the inhibition of further lipid peroxidation (Zhao et al. 2010). Beer with greater DPPH radical scavenging activity could have enhanced flavour stability because beer staling can occur from the formation of trans-2-nonenal and other saturated and unsaturated aldehydes as a result of oxidation (Zhao et al. 2010). Beer with greater ABTS radical cation scavenging activity stabilises active oxygen radicals and provide better flavour stability (Zhao et al. 2010). Different reaction kinetics between phenol, ABTS radical cation and DPPH radical over a similar range of concentrations might lead to the different results (Campos and Lissi 1996). Samples with more DPPH radical scavenging activity also had more ABTS radical cation scavenging activity and vice versa. Some studies reported a strong correlation between phenolic content and antioxidant activity in fruits, vegetables and grains, while others do show no correlation (Osman et al. 2009). The amount of certain phenolic compounds rather than the quantity of total phenols determines the biological activity of alcoholic beverages with respect to antioxidant activity (Gorinstein et al. 2001).
Lipid peroxidation
The percentage protection provided by the various beer samples against lipid peroxidation as determined by TBARS analysis was 19.18 ± 1.94 % to 55.47 ± 0.96 % (Table 3). Sample B15 provided the most protection, while B11 provided the least protection. Gorinstein et al. (1999) showed that after 4 weeks of feeding beer to rats, there was a significant decrease in the amount of lipid peroxidation. Flavonoids, an important class of antioxidants, effectively suppress lipid peroxidation in biological tissues and subcellular fractions such as mitochondria, microsomes, liposomes, low-density lipoproteins and erythrocyte membrane (Yang et al. 2001).
HPLC characterization for detection of organic acids
Tartaric and succinic acids were found in most of the beer samples (Table 5). Tartaric acid was present in the range 0 to 43.84 ± 1.33 mg/L, while succinic acid was present in the range 0 to 677.49 ± 0.67 mg/L. Malic and citric acids were also found in some beer samples (Fig. 1). Acetic acid was present in samples B1, B13 and B15. Organic acids (in addition to CO2) affect acidity and pH as well as the taste of beer (sourness, tartness, acidity) and they can have positive physiological effects (diuretic, reduction in uric acid) (Montanari et al. 1999). The kind and amount of organic acid generated during fermentation is highly dependent on the type of yeast and overall process control (Taing and Taing 2007). Various studies have shown a wide variety of organic acids content in beers. The total content of organic acids was between 451 mg/L and 712 mg/L as found by Montanari et al. (1999). The most common organic acid found was lactic (average 128 mg/L), followed by citric (116 mg/L), acetic (108 mg/L), succinic (68 mg/L), malic (63 mg/L), pyruvic and fumaric (44 mg/L). Also, ketoglutaric (25 mg/L) and citramalic acids (9 mg/L) were observed. Perez-Ruiz et al. (2004) detected lactic acid (550–630 mg/L), malic acid (40–70 mg/L), tartaric acid (0–22 mg/L), oxalic acid (12–25 mg/L) and citric acid (70–200 mg/L) (Fig. 2).
Table 5.
Organic acids content in the beer samples by HPLC analysis
| Sample | Tartaric acid (ppm) | Malic acid (ppm) | Acetic acid (ppm) | Citric acid (ppm) | Succinic acid (ppm) |
|---|---|---|---|---|---|
| B1 | 15.224 ± 1.244 | – | 98.48 ± 1.67 | 56.62 ± 1.94 | 88.23 ± 0.92 |
| B2 | 9.576 ± 1.3 | – | – | – | 105.86 ± 1.345 |
| B3 | 14.934 ± 0.62 | – | – | 277.79 ± 1.74 | 41.51 ± 1.49 |
| B4 | 22.54 ± 1.253 | 10.26 ± 1.780 | – | – | 513.09 ± 1.38 |
| B5 | 9.07 ± 1.092 | 35.55 ± 0.689 | – | 15.31 ± 1.28 | 214.19 ± 1.75 |
| B6 | 16.15 ± 1.854 | – | – | – | 266.97 ± 0.94 |
| B7 | 20.71 ± 1.602 | 39.53 ± 0.723 | – | – | 119.92 ± 1.345 |
| B8 | 21.52 ± 0.9 | 27.33 ± 1.898 | – | 227.16 ± 1.37 | 71.00 ± 1.87 |
| B9 | 14.52 ± 1.02 | – | – | – | – |
| B10 | 43.84 ± 1.333 | 53.92 ± 1.768 | – | – | 117.31 ± 1.12 |
| B11 | 26.56 ± 1.45 | 88.32 ± 1.32 | – | – | 555.63 ± 1.79 |
| B12 | 18.21 ± 1.678 | 11.67 ± 1.29 | – | – | 543.08 ± 1.93 |
| B13 | – | – | 1024.25 ± 1.797 | – | 217.49 ± 0.88 |
| B14 | 5.28 ± 1.432 | 33.42 ± 0.887 | – | – | 68.41 ± 1.57 |
| B15 | 23.01 ± 1.223 | 95.32 ± 0.873 | 58.43 ± 0.564 | – | 677.49 ± 0.67 |
Values represent mean ± standard deviation (SD)
ppm parts per million
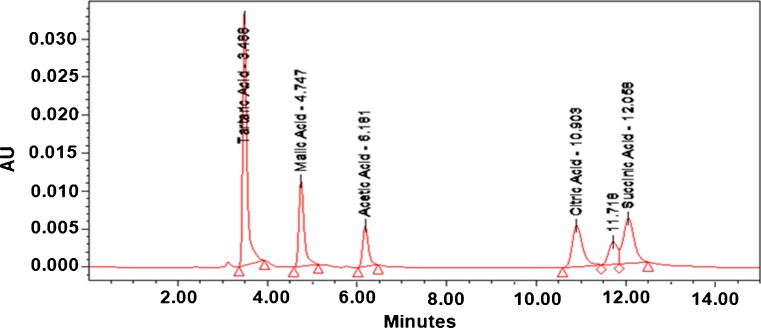
Fig. 1.
HPLC fingerprinting: HPLC chromatogram of standard, separated on C18 column, hypersil, USA (Revere phase column 15 cm; particle size 5 μm) using gradient elution—Aqueous solution of H3PO4 (6 × 10−3 mol/L, pH = 2.1) at a flow rate of 1.0 mL/min. The chromatograms at 210 nm were analyzed
Correlation analysis between phenolic content, flavonoid content, antioxidant activity and lipid peroxidation
A correlation analysis was performed using Pearson correlation coefficient to check linear correlation among the assays. It was observed that there is a strong positive correlation between total phenol content and total flavonoid content (r = 0.92) as well as positive correlation with DPPH antioxidant capacity (r = 0.35, p < 0.001) and ABTS radical cation scavenging activity (r = 0.58, p < 0.001). A week positive correlation was also setup between total phenols and TBARS lipid peroxidation (r = 0.04, p < 0.001). Nevertheless positive correlation was also set between total flavonoid and antioxidant assays ranging from (r = 0.1–0.92, p < 0.001), however there was negative correlation between total flavonoids and TBARS lipid peroxidation assay (r = −0.1) (Table 6). There was also a negative correlation between ABTS radical cation scavenging activity and TBARS lipid peroxidation (r = −0.05). Hence this correlation analysis clearly determines that each assay such as total phenols and total flavonoids correlates with one another and might partly correspond with each other, which can also include antioxidant assays. But this hypothesis is not true in the case of correlation between total flavonoid content, ABTS radical cation scavenging activity and TBARS assay. Results reported by Zhao et al. (2012) showed similar kind of correlation.
Table 6.
Correlation between total phenolic content, antioxidant activity and lipid peroxidation
| Comparison n = 15 | TPC | TFC | DPPH | ABTS | LP |
|---|---|---|---|---|---|
| TPC | 1 | 0.92 | 0.35* | 0.58* | 0.04* |
| TFC | – | 1 | 0.1* | 0.53* | −0.1* |
| DPPH | – | – | 1 | 0.73 | 0.29 |
| ABTS | – | – | – | 1 | −0.05 |
| LP | – | – | – | – | 1 |
Correlation coefficient (r) values are represented in the table
*Indicates significance P < 0.001
TPC total phenol content, TFC total flavonoid content, LP lipid peroxidation, n number of samples
Conclusion
The present study describes the variations in phenolic content and antioxidant activities of commercial beers among several commercial brands of beer sold in India. DPPH radical scavenging activity and ABTS radical cation scavenging activity were positively correlated to each other and the phenolic content of the beer. The phenolic compounds are responsible for beer antioxidant activity. Phenolic compounds provide an option for brewers to increase the phenolic content during brewing for improve flavour stability in beer. The concentration of various phenolic compounds was determined in the beer samples examined. The process of brewing warrants additional research to determine how the concentration of selected phenolic compounds can be increased.
Acknowledgments
Authors are thankful to Dr. Prof. Vijai KS Shukla, IFSC, Lystrup, Denmark for critically evaluating the manuscript, Mr. Subhash Kudale for his help in RP-HPLC analysis, and higher authorities of University for providing necessary facilities.
References
- Afanasev IB, Dorozhko AI, Brodskii AV, Kostyuk VA, Potapovitch AI. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol. 1989;38(11):1763–1769. doi: 10.1016/0006-2952(89)90410-3. [DOI] [PubMed] [Google Scholar]
- AOAC Method (2000) Official methods of analysis of AOAC International, 17th edn, Vol II Chapter 27, pp 6–7
- Arranz S, Chiva-Blanch G, Valderas-Martínez P, Medina-Remón A, Lamuela-Raventós RM, Estruch R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients. 2012;4:759–781. doi: 10.3390/nu4070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamforth CW. Nutritional aspects of beer-a review. Nutr Res. 2002;22:227–237. doi: 10.1016/S0271-5317(01)00360-8. [DOI] [Google Scholar]
- Barth R (2011) The chemistry of beer, 3rd edn. Owl’s Nest Publishing, pp 250–258, ISBN: 978-0984795031
- Campos A, Lissi E. Total antioxidant potential of Chilean wines. Nutr Res. 1996;16:385–389. doi: 10.1016/0271-5317(96)00020-6. [DOI] [Google Scholar]
- Chaturvedi PA, Ghatak AA, Desai NS. Evaluation of antioxidant activity and polyphenol content in Woodfordia fruticosa from different altitude. J Plant Biochem Biotechol. 2012;21:17–22. doi: 10.1007/s13562-011-0066-1. [DOI] [Google Scholar]
- Cortacero-Ramirez S, Castro MHB, Carretero AS, Blanco CC, Gutierre AF. Analysis of beer components by capillary electrophoresis method. Trends Anal Chem. 2003;22:440–456. doi: 10.1016/S0165-9936(03)00704-0. [DOI] [Google Scholar]
- Gerloff A, Singer MV, Feick P. Beer and its non alcoholic compounds: role in pancreatic exocrine secretion, alcoholic pancreatitis and pancreatic carcinoma. Int J Environ Res Public Health. 2010;7:1093–1104. doi: 10.3390/ijerph7031093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatak AA, Chaturvedi PA, Desai NS (2012) Indian grape wines: a potent source of phenols, polyphenols and antioxidants. Int J Food Prop (in press)
- Gorinstein S, Zemser M, Albores FV, et al. Proteins and amino acids in beers, their contents and relationships with other analytical data. Food Chem. 1999;67:71–78. doi: 10.1016/S0308-8146(99)00071-0. [DOI] [Google Scholar]
- Gorinstein S, Caspi A, Pawelzik E, Deldago-Licon E, Libman I, Trakhtenberg S, Weisz M, Martin-Belloso O. Proteins of beer affect lipid levels in rats. Nutr Res. 2001;21:1159–1169. doi: 10.1016/S0271-5317(01)00311-6. [DOI] [Google Scholar]
- Guardia T, Rotelli AE, Juares AO, Pelzer LE. Anti-inflammatory properties of plant flavonoids and effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56(9):683–687. doi: 10.1016/S0014-827X(01)01111-9. [DOI] [PubMed] [Google Scholar]
- Han Y. Rutin has therapeutic effect on septic arthritis caused by Candida albicans. Int Immunopharmacol. 2009;9(2):207–211. doi: 10.1016/j.intimp.2008.11.002. [DOI] [PubMed] [Google Scholar]
- http://www.foodregulatorysummit.org/pdf/aiba.pdf
- http://www.indianexpress.com/news/maharashtra-ranks-second-in-terms-of-beer-consumption/947997
- Iola F, Duarte IF, Godejohann M, Braumann U, Spraul M, Gil AM. Application of NMR spectroscopy and LC-NMR/MS to the identification of carbohydrates in beer. J Agric Food Chem. 2003;51:4847–4852. doi: 10.1021/jf030097j. [DOI] [PubMed] [Google Scholar]
- Joslyn MA. Methods in food anal. New York: Academic; 1950. p. 528. [Google Scholar]
- Khan M, Raza SS, Javed H, Ahmad A, Khan A, Islam F, Safhi MM, Islam F. Rutin protects dopaminergic neurons from oxidative stress in an animal model of Parkinson’s disease. Neurotox Res. 2012;22(1):1–15. doi: 10.1007/s12640-011-9295-2. [DOI] [PubMed] [Google Scholar]
- Kordis-Krapez M, Abram V, Kac M, Ferjancic S. Determination of organic acids in white wines by RP-HPLC. Food Technol Biotechnol. 2001;39(2):93–99. [Google Scholar]
- Lehtonen P, Hurme R. Liquid chromatographic determination of sugars in beer by evaporative light scattering detection. J Inst Brew. 1994;100:343–346. doi: 10.1002/j.2050-0416.1994.tb00834.x. [DOI] [Google Scholar]
- Lobo VC, Phatak A, Chandra N. Antioxidant and free radical scavenging activity of Hygrophila schulli (buch.-ham.) Almeida and Almeida seeds. Adv Biores. 2010;1(2):72–78. [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–270. [PubMed] [Google Scholar]
- Luo H, Jiang BH, King SM, Chen YC. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr Cancer. 2008;60(6):800–809. doi: 10.1080/01635580802100851. [DOI] [PubMed] [Google Scholar]
- Luximon-Ramma A, Bahorum T, Soobratee MA, Aruoma OI. Antioxidant activities of flavonoid components in extracts of Cassia fistula. J Agric Food Chem. 2002;50(18):5042–5047. doi: 10.1021/jf0201172. [DOI] [PubMed] [Google Scholar]
- Mainente F, Simonato B, Zoccatelli G, Rizzi C. A method for the preparative separation of beer proteins and glycocompounds. J Inst Brew. 2011;117(3):435–439. doi: 10.1002/j.2050-0416.2011.tb00490.x. [DOI] [Google Scholar]
- Montanari L, Perretti G, Natella F, Guidi A, Fantozzi P. Organic and phenolic acids in beer. Lebensm Wiss Technol. 1999;32:535–539. doi: 10.1006/fstl.1999.0593. [DOI] [Google Scholar]
- Nardini M, Ghiselli A. Determination of free and bound phenolic acids in beer. Food Chem. 2004;84:137–143. doi: 10.1016/S0308-8146(03)00257-7. [DOI] [Google Scholar]
- Nardini M, Natella F, Scaccini C, Ghiselli A. Phenolic acids from beer are absorbed and extensively metabolized in humans. J Nut Biochem. 2006;17:14–22. doi: 10.1016/j.jnutbio.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Narziss L. Abriss der Bierbrauerei. 3. Stuttgart: Ferdinand Enke Verlage; 1972. pp. 264–300. [Google Scholar]
- Nelson M (2005) The barbarian’s beverage: a history of beer in ancient Europe, 1st edn. Routledge Publisher, pp 110, ISBN: 0-415-31121-7
- Ng E, Mocek M. A method for estimating total polyphenols in beer. J Inst Brew. 1973;79:165–169. doi: 10.1002/j.2050-0416.1973.tb03519.x. [DOI] [Google Scholar]
- Osman H, Rahim AA, Isa NM, Bakhir NM. Antioxidant activity and phenolic content of Paederia foetida and Syzygium aqueum. Molecules. 2009;14:970–978. doi: 10.3390/molecules14030970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Ruiz T, Martınez-Lazano C, Tomas V, Martin J. High-performance liquid chromatographic separation and quantification of citric, lactic, malic, oxalic and tartaric acids using a post column photochemical reaction and chemiluminescence detection. J Chromatogr A. 2004;1026:57–64. doi: 10.1016/j.chroma.2003.10.130. [DOI] [PubMed] [Google Scholar]
- Piazzon A, Forte M, Nardini M. Characterization of phenolics content and antioxidant activity of different beer types. J Agric Food Chem. 2010;58(19):10677–10683. doi: 10.1021/jf101975q. [DOI] [PubMed] [Google Scholar]
- Pollock JRA. Brewing science. London: Academic; 1981. [Google Scholar]
- Roussis IG, Lambropoulos I, Soulti K. Scavenging capacities of some wines and wine phenolic extracts. Food Technol Biotechnol. 2005;43(4):351–358. [Google Scholar]
- Sengupta K, Singh BD, Mustai CC. A study on the effect of time of harvest of mulberry leaf on silkworm (Bombyx mori L.) cocoon crop and cocoon quality. Ind J Seric. 2000;10:1–5. [Google Scholar]
- Seo HB, Kim HJ, Lee OK, Ha JH, Lee HY, Jung KH. Measurement of ethanol concentration using solvent extraction and dichromate oxidation and its application to bioethanol production process. J Industrial Microbiol Biotechnol. 2009;36(2):285–292. doi: 10.1007/s10295-008-0497-4. [DOI] [PubMed] [Google Scholar]
- Smagalski C (2006) CAMRA & the first international gluten free beer festival. Carolyn Smagalski, Bella Online, http://www.bellaonline.com/articles/art39558.asp
- Szwajgier D, Pielecki J, Targonski Z. Changes of free ferulic acids and coumaric acid contents during malting of barley grain. Pol J Food Nutr Sci. 2005;14(4):423–429. [Google Scholar]
- Taing O, Taing K. Production of malic and succinic acids bysugar-tolerant yeast Zygosaccharomyces rouxii. Eur Food Res Technol. 2007;224:343–347. doi: 10.1007/s00217-006-0323-z. [DOI] [Google Scholar]
- Tüsekwa AB, Mosha TC, Laswai HS, Towo EE. Traditional alcoholic beverages of Tanzania: production, quality and changes in quality attributes during storage. Int J Food Sci Nutr. 2000;51(2):135–143. doi: 10.1080/096374800100831. [DOI] [PubMed] [Google Scholar]
- Yang B, Kotani A, Arai K, Kusu F (2001) Estimation of the antioxidant activities of flavonoids from their oxidation potentials. Anal Sci 599–604 [DOI] [PubMed]
- Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biogeosciences. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Chen W, Lu J, Zhao M. Phenolic profiles and antioxidant activities of commercial beers. Food Chem. 2010;119:1150–1158. doi: 10.1016/j.foodchem.2009.08.028. [DOI] [Google Scholar]
- Zhao H, Li H, Sun G, Yang B, Zhao M. Assessment of endogenous antioxidative compounds and antioxidant activities of lager beers. J Sci Food and Agri. 2012;93:910–917. doi: 10.1002/jsfa.5824. [DOI] [PubMed] [Google Scholar]