Abstract
The effect of vacuum packaging technique on the shelflife of eviscerated pabda catfish (Ompok pabda) during chill storage at 4 ± 2 °C was studied. The shelflife of pabda fish was predicted based on the cumulative observations of biochemical, microbiological and sensory parameters. There was no significant difference in the lipid quality parameters studied between the samples during the chill storage period, whereas, vacuum packaging significantly improved the sensory and microbiological quality. Based on microbiological parameters such as Total Viable Count, Enterobacteriaceae, Escherichia coli, Salmonella Spp, total Vibrios, Listeria monocytogenes, Staphylococcus aureus, faecal Streptococcus and anaerobic sulphite reducers, the shelflife of chilled gutted pabda catfish was estimated to be 14–16 and 18–20 days for air packed and vacuum packed samples, respectively. The sensory parameters showed extended shelflife by four more days for both the samples.
Keywords: Vacuum packaging, Pabda catfish, Evisceration, Chill storage
Introduction
Fresh seafood undergoes spoilage faster than other food commodities. The high content of moisture, free amino acids and other low molecular weight extractives account for the faster spoilage rate in fish flesh. Shelflife of such products is limited under normal atmospheric condition by the chemical effects of atmospheric oxygen and the growth of aerobic spoilage microorganisms (Ozogul et al. 2004). Apart from microbial action, loss of quality to a greater extent is related to oxidative processes affecting not only lipids, but also proteins. Fish lipid contains high levels of polyunsaturated fatty acids, and is highly susceptible to oxidative deterioration. Eventhough, chilling is the most prevalent method of preservation which retains the original characteristics of the fish meat, chilling alone does not ensure the required quality and safety to fresh fish. The continuous demand for processed foods without added chemical preservatives is putting pressure on manufacturers to expel synthetic additives from labels. Hence, recent interventions in fish processing sector such as modified atmospheric packaging, vacuum packaging, active and intelligent packaging are gaining importance. In these techniques, spoilage reactions are primarily controlled by limiting the availability of reactive oxygen molecule inside the package, and thereby allowing minimal bacterial growth than that normally encounters. Studies have shown that evisceration and vacuum packaging along with icing considerably increased the shelflife of fresh fish like salmon (Hansan et al. 2009), carp (Krizek et al. 2004) and sardine (Ozogul et al. 2004). Evisceration or gutting of fish reduces the load of intestinal microorganisms and endogenous enzyme associated with protein and lipid degradation. Vacuum packaging represents a static form of hypobaric storage which decreases the supply of oxygen to the aerobic bacteria in the flesh, leading to reduced microbial growth and oxidative reactions. It is widely used in the food industry and is one of the packaging methods that enhance the shelflife and overall quality of muscle foods at relatively low cost. In addition to enhanced quality aspects, evisceration and vacuum packaging also adds value to the final products which enables better price realisation in the retail markets. Hence, in the present study, the synergistic effects of gutting, vacuum packaging and chilling on the quality characteristics of pabda fish have been evaluated.
Ompok pabda, commonly known as pabda catfish, is an indigenous freshwater fish species widely cultured along India, Bangladesh, Pakistan, Afghanistan and Myanmar. It is a highly valuable fresh water fish for its superior flesh with a soft texture, flexible bones and good taste. It is commonly sold locally as fresh or ice preserved. Compared to marine fish species, very few studies have been reported in literature on the quality and shelflife characteristics of freshwater fishes except for a few on major carps. To best of our knowledge, so far no organised studies have been reported on preservation and shelflife characteristics of pabda cat fish. In this context, the present study was undertaken to assess the biochemical, microbiological and sensory characteristics of Ompok pabda and to extend the shelflife by gutting and vacuum packing technique during chill storage.
Materials and methods
Raw material
The freshly caught pabda catfish (Ompok pabda), having an average weight of 300 g and length of 25 cm were procured from a fish market near Navi Mumbai, India. Immediately after procuring, the fish were properly washed with potable water, iced and brought to the laboratory in insulated boxes. Further, the whole batch was assorted to two lots of uniform weight, after beheading, gutting and washing. Lot I was immediately packed in polyethylene pouches (conventional polyethylene pack- hereafter designated as CPE pack) and lot II was vacuum-packed in laminated pouches (size: 15 × 22 cm; 12 μ-polyester laminated with 300 gauge low-density polyethylene) (hereafter designated as VP pack). It was then sealed in a vacuum packaging machine (Model TTM 363708, Winner electronics, Mumbai, India) at 1 bar pressure. Both the samples were kept in chilled atmosphere maintained at 4 ± 2 °C. The samples from each batch were drawn regularly in triplicate for biochemical, microbiological and sensory analyses.
Chemical analysis
All the chemicals used for the study were of analytical grade. For chemical analysis, the fish muscle was homogenised using a mixer grinder. Proximate composition of the raw fish was determined by AOAC (2002) method. pH of the homogenised sample in distilled water (1: 5 w/v) was determined by using a glass electrode digital pH meter (Cyberscan 510, Eutech instruments, Singapore). Drip loss of the fish during chill storage was measured gravimetrically by taking the weight difference of the sample with and without exudate and expressed as ml of water/100 g fish meat. Total volatile base nitrogen (TVB-N) was estimated by the microdiffusion method (Conway 1950). Histamine content of the sample was determined by colourimetric assay according to the method of Hardy and Smith (1976) and expressed as mg%. Oxidative stability of the sample was assessed by measuring Thiobarbituricacid (TBARS) value (Tarladgis et al. 1960) and Peroxide value (Yildiz et al. 2003) of the fish sample. Free Fatty Acid (FFA) value was determined as per AOAC (2002) to assess the hydrolytic rancidity and the result was expressed as percentage oleic acid.
Microbiological analysis
Microbiological analysis was carried out for the enumeration of total viable count (TVC), total Enterobacteriaceae, Escherichia coli (E. coli), Salmonella spp, total Vibrios, Listeria monocytogenes, Staphylococcus aureus, faecal Streptococcus and anaerobic sulphite reducers. Briefly, 25 g of fish were aseptically weighed and homogenized with 225 ml sterile 0.85 % normal saline for 1 min, in a Stomacher 400 lab blender (Seward Medical, London, UK). The homogenized sample was serially diluted using 9 ml sterile saline for bacteriological analysis. Total viable counts (TVC) were determined in Plate Count Agar by the spread plate method (AOAC 2002). Counts of Staphylococcus aureus (AOAC 2002), Faecal Streptococci (AOAC 2002) and total Enterobacteriaceae (Koutsoumanis and Nychas 1999) were determined at regular intervals. The total anaerobic sulphite reducers were enumerated on Differential Reinforced Clostridial Medium (DRCM) by following three tubes MPN technique (APHA 1976).
Sensory analysis
The sensory analysis was carried out for both raw and cooked samples of uniform sizes cut from VP and CPE fish samples. For cooked sample scoring, fish pieces were cooked in boiling brine containing 1.5 % NaCl for 10 min and were assessed by a panel of 5 experienced members. Scoring was based on a 9 point hedonic scale as described by Amerine et al. (1965) with slight modifications. Various sensory characteristics like colour and appearance, texture, flavour and taste were evaluated by the trained panellists. The panelists were requested to rinse their mouth with water after tasting each sample to avoid bias in judgement/evaluation. The overall impression of the samples by each panel member was expressed as the ‘overall acceptability’. The overall acceptability score was determined taking into account of the total score obtained for raw and cooked samples (by adding the scores for all the attributes and dividing by the total number of attributes). A score of 9–7 was given to samples with no off-flavours, bright silvery pink colour and firm and elastic texture (Like extremely to like moderately), 7–5 for trace off-flavour, pale grey to colourless and less elastic texture (Like slightly), and 4–5 for medium off-flavour, slight brownish colour and slight soft texture (Neither like or dislike). An overall score below 4 was considered as ‘rejected’.
Statistical analysis
All experiments were done in triplicate (n = 3) and results were expressed as means ± standard deviation (SD). Analysis of variance (ANOVA) was carried out to find out the average effect of treatments and storage days on various spoilage indices using the statistical software SPSS 20 (IBM, SPSS Inc. Chicago). Treatment means were compared by using Tukey’s studentised range test. Kruskal-Wallis test was performed to compare the ranking of sensory evaluation scores. The statistical significance for all the parameters was identified at 95 % confidence level (P < 0.05).
Results and discussion
Proximate composition of fresh pabda catfish meat
The mean (±SD) values of compositional contents of moisture, protein, fat and ash in fresh pabda cat fish are presented in Table 1. The average protein content of pabda catfish meat was found to be 18.69 % which indicates that it can be considered as a good table fish rich in protein. Freshwater fish in general contain 17–22 % protein and fat content in a broad range of less than 2 % to as high as 20 % depending on type of feed given, age, size etc. (Natarajan and Sreenivasan 1961). In the present study, pabda catfish indicated a fat content of about 3 % and can be categorised as medium fatty fish suitable for evaluating the efficacy of vacuum packaging during chill storage.
Table 1.
Proximate composition and biochemical quality parameters of fresh gutted pabda catfish
| Parameters | Mean value |
|---|---|
| Moisture (g%) | 79.54 ± 1.52 |
| Protein (g%) | 18.69 ± 0.98 |
| Fat (g%) | 2.67 ± 0.28 |
| Ash (g%) | 0.83 ± 0.04 |
| pH | 6.75 ± 0.06 |
| TVB-N (mg%) | 7. 18 ± 0.57 |
| FFA | 0.320 ± 0.01 |
| PV | 0.94 ± 0.08 |
| TBA | 0.309 ± 0.012 |
| Histamine | 1.85 + 0.04 |
Values in parenthesis represents standard deviation for n = 3
Changes during chill storage
Moisture
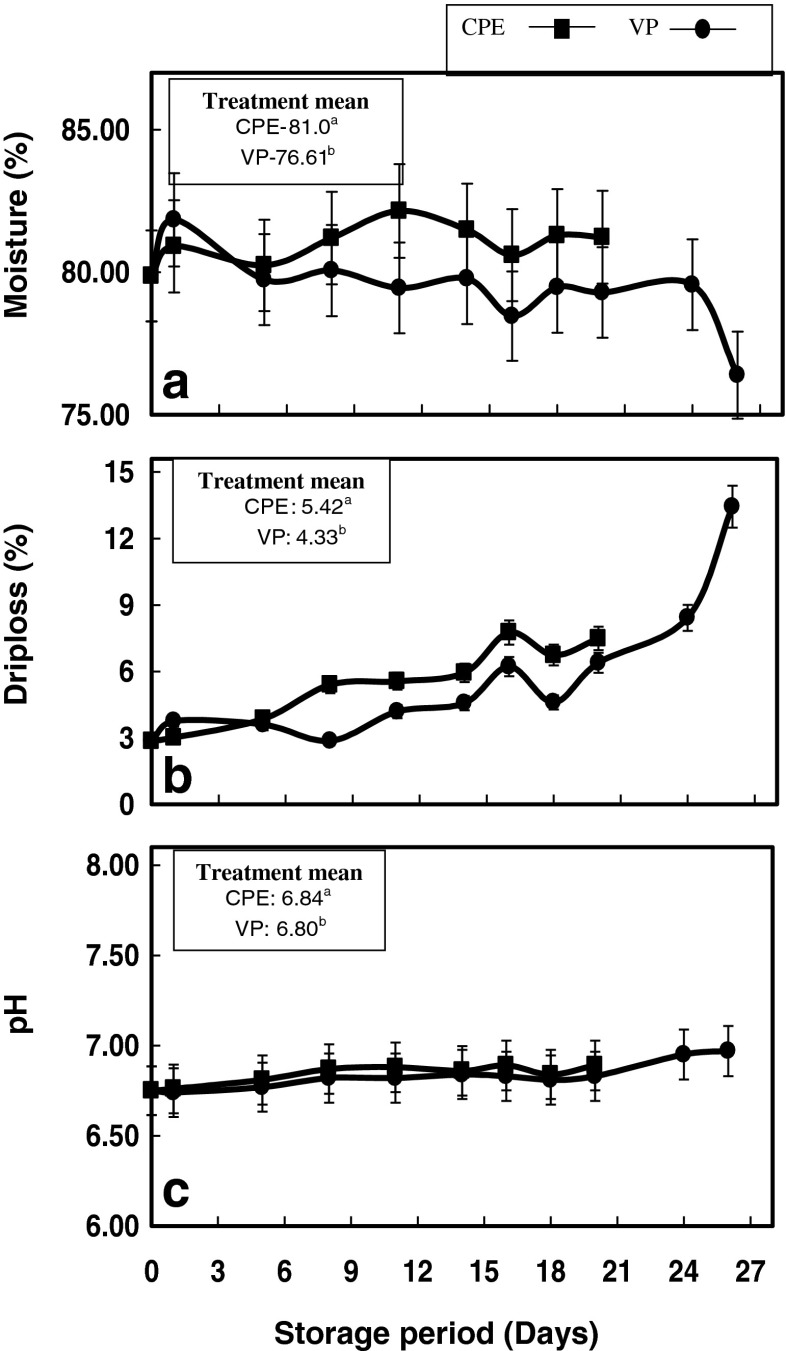
Variations in the moisture content of the samples during chill storage are depicted in the Fig. 1a. Eventhough not significant, the moisture content of CPE samples showed an increasing trend during chill storage (p < 0.05). Similarly, the moisture content of VP samples also did not change appreciably during initial span of chill storage, though a slight decline was observed towards the end of storage period. This may be associated with loss of waterholding capacity of myofibrillar proteins during extended chill storage. The increase in the moisture content observed in CPE samples may be associated with the poor barrier property of packaging material and pack-seal which might have promoted the entry of moisture from storage environment.
Fig. 1.
Changes in (a) Moisture (b) Drip loss and (c) pH of pabda catfish fillets during chill storage. Treatment mean values with same letters are not significantly different from each other (p < 0.05)
Drip loss
Drip loss refers to the most loosely bound water in muscle, and is generally associated with the loss of water holding capacity of myofibrillar proteins (Sikorski and Ruiter 1994). Extended chill storage quite often results in denaturation and aggregation of myofibrillar proteins, which in turn reduces the water holding capacity of proteins. In the present study, a significant increase in drip loss was observed during chill storage period in both the samples (p < 0.05). Vacuum packed samples showed higher driploss on the first day of storage, and thereafter CPE samples showed significantly higher values till the end of storage period (Fig. 1b) (p < 0.05). However, this higher driploss observed in CPE pack cannot be accounted as loss from fish samples, as higher moisture content was observed in the meat during chill storage. The probable reason for this may be the penetration of moisture from the storage environment in to the package, owing to the poor barrier properties of the packaging material.
pH
In general, pH values of both CPE and VP samples showed negligible variation during the initial period of chill storage and thereafter showed an increasing trend during extended period of chill storage (Fig. 1c). CPE samples showed significantly higher values (p < 0.05) of pH from 5th day of chill storage onwards, whereas VP samples presented stable pH values during the initial 8 days of storage and thereafter showed significantly higher values (p < 0.05) up to the end of storage period. Moreover, the mean pH values of CPE samples over the storage period was significantly higher than that of VP samples at any day of sampling (p < 0.05). The increase in pH observed during the later period of chill storage may be due to the release of basic components associated with the degradation of muscle components. The shelflife of vacuum packaged fish depends to a great extent on the pH of muscle tissue, especially at the time of packaging. This is because, the anoxic condition coupled with the lower pH (less than 6) of the fish muscle has got a synergistic inhibitory effect on the growth of bacteria associated with fish spoilage. In the present study, the pH of fish meat at the time packing was 6.75, which is a higher value compared to other freshwater fishes (Puchala et al. 2005). The pH value is regarded as an important parameter to evaluate fish spoilage, and changes of less than or equal to 0.1 units indicate a first grade quality, while changes in the range of 0.1 to 0.2 units are referred to as an acceptable quality, with those higher than 0.2 units suggesting deterioration (Mazorra-Manzano et al. 2000). In the present study, eventhough CPE samples showed significantly higher mean pH value compared to VP samples over the chill storage period, the change in pH at the time of sensory rejection was less than 0.2 units. This may be because, in fresh water fishes like pabda catfish, the development of basic components of muscle deterioration is limited compared to marine fishes due to the negligibly lower content of Tri Methyl Amine Oxide (TMAO) in muscle tissue.
Total volatile base nitrogen (TVB-N)
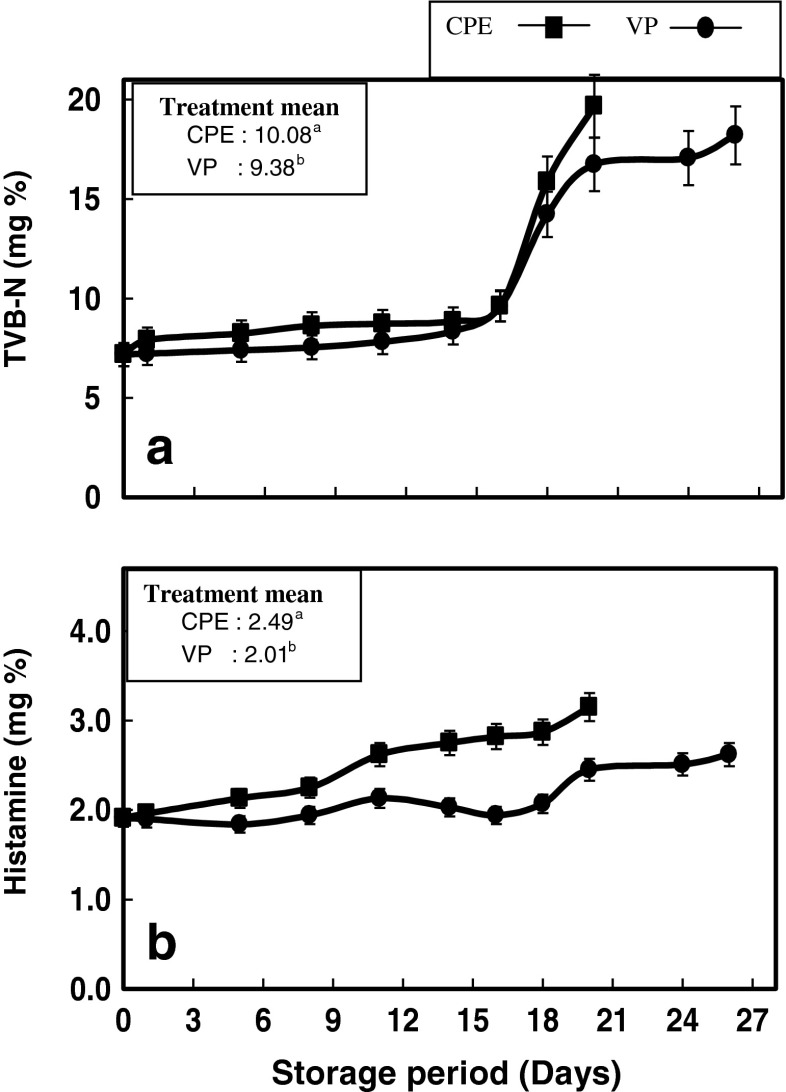
TVB-N content of gutted pabda catfish stored in two different packaging conditions are depicted in Fig. 2a. The TVB-N content of fresh fish was 7.5 mg% which gradually increased till 16th day of storage in both the samples, and thereafter showed a significant increase up to their respective day of sensory rejection (p < 0.05). However, the mean TVB-N value of VP samples were significantly lower than that of CPE samples throughout the chill storage period (p < 0.05) (Fig. 2a inset). According to Yeh et al. (1978), the release of ammonium compounds in fish during postmortem stage may be initially offset by lixiviation, especially if the exposed area is large. However, after some days, the increase becomes evident and generally coincides with the increase in pH. The more alkaline the medium becomes, the more deaminase activity is favored. Cobb and Vanderzant (1975) related the higher values of TVB-N to bacterial spoilage and that some bacterial species produces volatile bases more readily than others. It has been reported that TVB-N, which is the standard chemical indicator of seafood spoilage, is appropriate for advanced spoilage but is an insufficient sign of quality during the initial stages of seafood spoilage (Clancy et al. 1995; Tejada and Huidobro 2002). This is in agreement with the observation of Ismail et al. (2013) during the chill storage of meagre fillet under vacuum. The concentration of TVB-N in freshly caught fish is typically between 5 and 20 mg N/100 g, whereas levels of 30–35 mg N/100 g fish are generally regarded as the limit of acceptability in marine fishes (Connel 1995). However, no statutory TVB-N limit values have been laid down for TVB-N values of freshwater fish. Scherer et al. (2006) noted that TVB-N values are more suitable for spoilage assessment in marine fish than in freshwater fish. TVB-N values of 25 mg/100 g has been suggested as the highest acceptable level for rainbow trout (Arashisar et al. 2004; Gimenez et al. 2002; Stansby 1963), nile perch (Amegovu et al. 2012) and 15 mg/100 g in chilled common carp packed under modified atmosphere (Jezek and Buchtova 2007). In the present study, the maximum TVB-N value registered was 19.67 and 18.2 mg/100 g meat for CPE and VP samples, respectively on the day of sensory rejection. Hence, the results of present study suggests a TVB-N value of 18–20 mg/100 g as the highest acceptable limit for gutted freshwater pabda fish.
Fig. 2.
Changes in (a) TVB-N and (b) histamine content of pabda catfish fillets during chill storage. Treatment mean values with same letters are not significantly different from each other (p < 0.05)
Histamine content
Histamine is a major component of biogenic amines which is formed after the bacterial decomposition of the aminoacid, histidine. Histamine is regarded as a serious hazard in marine fish as far as food certification is concerned, but imposes less health risk in freshwater fish (Kordiovská et al. 2006). However, in the present study, since the presence of histamine was detected in the fresh fish itself, changes in histamine content over the storage period was monitored. A number of the histamine-forming bacteria are facultative anaerobes that can grow in reduced oxygen environments (FDA 2011). Evisceration and removal of the gills may reduce, but not eliminate, the number of histamine-forming bacteria (Visciano et al. 2012). Variations in the histamine content of air and vacuum packed pabda samples are depicted in the Fig. 2b. In the present study, the histamine content was increased from an initial value of 1.91 mg/100 g meat to 3.15 and 2.62 mg/100 g on the day of rejection in CPE and VP samples, respectively. In VP samples, histamine content was not significantly differed till 18 days of storage; however, thereafter showed a significant increase (p < 0.05). On the other hand, CPE samples showed significantly higher histamine content after 8 days of chill storage which remained more or less constant upto 18th day of storage. This is in contrary to the previous reports where no significant difference was found between the vacuum packed and non vacuum packed flesh of carp during chill storage (Krizek et al. 2004). In the present study, the histamine content in both the samples during the entire storage period was very well below the limit (20 mg/100 g) set by EEC (1991), possibly owing to the reduced decarboxylation of histidine to histamine in freshwater fishes. However, there is difference in opinion about the maximum limit of histamine in the case of freshwater fishes. Mietz and Karmas (1978), has suggested a critical limit value for Biogenic Amine Index (BAI), which indicates the total biogenic amine content, at 10 mg/kg, for freshwater fish. In the present study, when the samples were rejected based on the sensory changes during the chill storage period, the histamine content was around 3 mg/100 g.
Free fatty acid value (FFA)
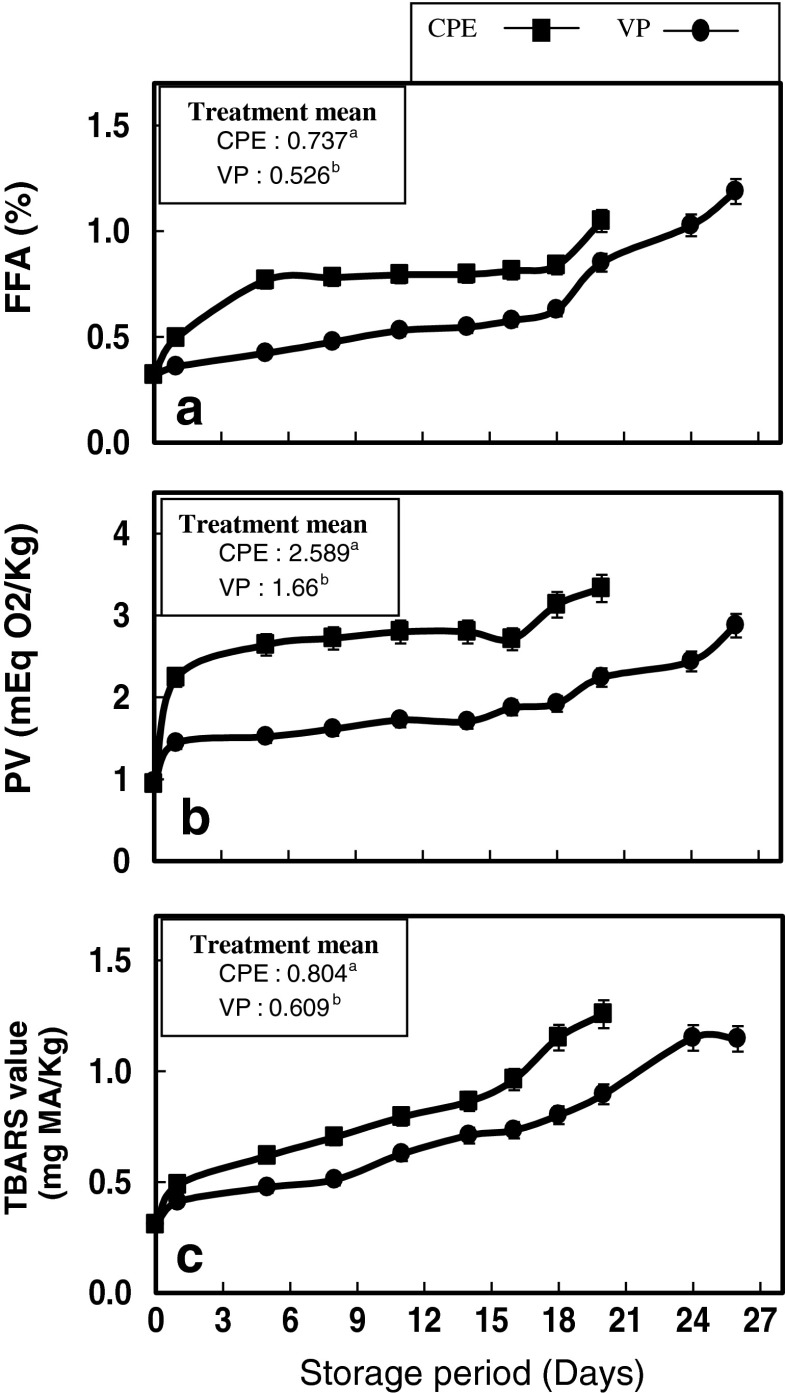
FFA content of gutted pabda catfish was determined and the results are depicted in Fig. 3a. In brief, FFA values of both the samples showed a significant increase during chill storage (p < 0.05), and a good correlation between FFA and storage days was observed (R = 0.92). The release of FFA was increased from an initial value of 0.32 % to the maximum values of 1.18 and 1.04 % for CPE and VP samples respectively, on the day of sensory rejection. The higher FFA values observed during chill storage period may be attributed to the hydrolysis of triglycerides and phospholipids mediated by lypolytic enzymes. This maximum values observed in the present study can be considered low when compared to FFA values of fatty fish species stored under the same conditions. However, VP samples showed significantly lower mean FFA content compared to CPE samples over the chill storage period (p < 0.05) (Fig. 3a inset). Presence of FFA in freshwater fish is not regulated from the safety point of view in legislation (Jezek and Buchtova 2007). It has been reported that during the early stages of chill storage period, FFA is mainly produced by the endogenous enzyme (lipases and phospholipases) and later on by microbial lipases (Aubourg et al. 2010). Lipase, phospholipase A2, and phospholipase B are believed to be the major endogenous enzymes associated with lipid hydrolysis of fish, while phospholipase C is derived mostly from microorganisms (Brockerhoff and Jensen 1974). Accordingly, in the present study, the endogenous enzyme activity was neglible in both samples, while microbial activity became prominant after 15 days of chill storage.
Fig. 3.
Changes in (a) FFA (b) PV (c) TBARS values of pabda catfish fillets during chill storage. Treatment mean values with same letters are not significantly different from each other (p < 0.05)
Peroxide Value (PV)
PV gives a measure of hydroperoxides formed as a result of primary lipid oxidation. Variations in the mean PV of the samples are given in Fig. 3b. Both CPE and VP samples showed a significant increase in PV throughout the chill storage period (p < 0.05). However, vacuum packaging delayed the development of hydroperoxides significantly over the storage days compared to polyethylene packed samples as indicated by lower mean PV values (p < 0.05) (Fig. 3b inset). This could be due to the absence of oxygen which is the major initiator of lipid peroxidation. It has been stated that, lipids in fresh fish tissue held in ice or under refrigeration exhibit a small tendency towards oxidative rancidity (Stansby 1963). The highest mean values of PV observed for both the samples during the chill storage period were well below the established limit. Generally, peroxide value of 20 meq O2 kg−1 of fat is considered necessary for oils to become rancid (Hras et al. 2000). However, undesirable flavours and odours may develop at very low peroxide values. In the present study, eventhough PV remained well below 20 meq O2 kg−1 throughout the storage period, all the samples developed an off odour during the later period of chill storage principally due to the deterioration of muscle components owing to microbial and enzymatic activity and hence, rejected based on sensory evaluation.
Thio Barbituric Acid Reactive Substances (TBARS)
TBARS is a major indicator of secondary lipid oxidation which measures the amount of malonaldehyde formed as a result of oxidation of lipid hydroperoxides. The level of TBARS shows the amount of peroxidation that has already been occurred. Changes in the TBARS values of CPE and VP samples over the storage period are presented in Fig. 3c. Both the samples showed a significant increase in TBARS values over the storage days (p < 0.05), eventhough VP samples presented significantly lower TBARS values compared to that of CPE samples (p < 0.05) (Fig. 3c inset). There is a direct relationship with the presence of oxygen and progress of lipid oxidation in food. Gimenez et al. (2002) reported that lipid oxidation was significantly higher in packages with 20 % and 30 % oxygen than in those with 10 % oxygen in rainbow trout fillets. TBARS value in the range of 1–2 mg malonaldehyde/kg of fish sample is usually taken as the limit of acceptability (Lakshmanan 2000), beyond which fish will normally develop an objectionable odour and taste. In the present study, the values for TBARS has crossed 1 mg malonaldehyde/kg on 19th and 24th day of storage in CPE and VP samples, respectively. This observation is in good agreement with the results of sensory evaluation. The observed stability of the vacuum packed samples to lipid oxidation could also be attributed to the removal of oxygen from the pack as well as the superior barrier properties of the packaging material. While deriving conclusion, it has to be noted that, TBARS values may not always reflect the actual rate of lipid oxidation since malonaldehyde can interact with other components of fish muscle and thus making unavailable for TBARS. However,once the value crosses the maximum limit of acceptability, it should be taken as a definite indication of fat deterioration.
Microbiological analysis
Total Viable Count (TVC)
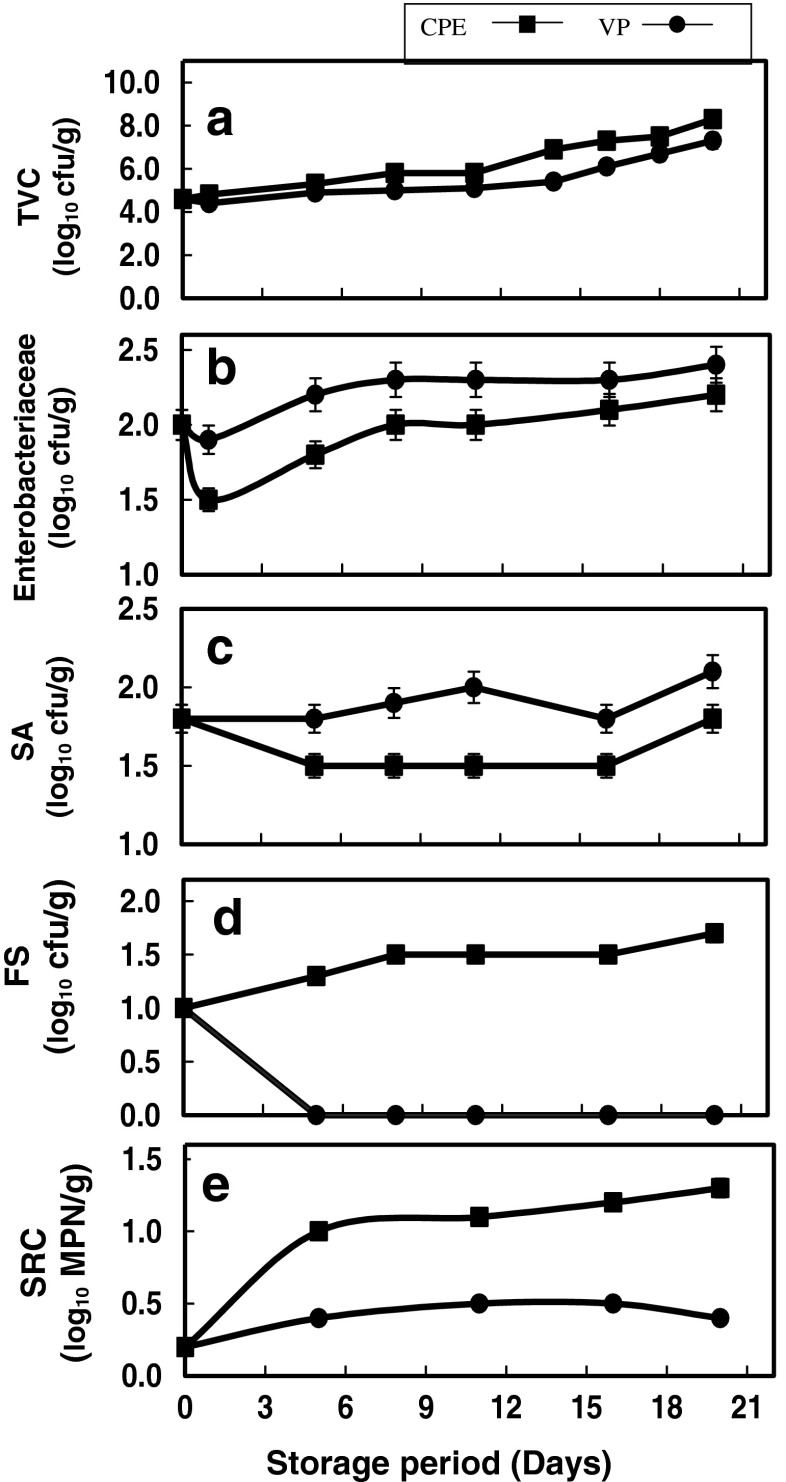
Fresh pabda catfish meat had a total viable count of 4.6 log10 cfu/g. During chill storage, bacteria grew quickly in CPE samples than in VP samples. TVC of CPE samples showed a progressive increase up to 11th day of chill storage and thereafter, increased sharply till they crossed the maximum limit of 107 cfu/g (ICMSF 1986) on 16th day (Fig. 4a). On the contrary, the VP samples showed an initial reduction in the count after one day of chill storage, and therafter increased gradually and reached 107 cfu/g on the 20th day of storage. The initial decrease in bacterial count observed in VP samples may be attributed to the immediate effect of removal of O2 from the package environment. In general, the level of vacuum in the pack determines the amount of residual oxygen in the product and thereby the microbial development. After evacuation, the residual oxygen will be consumed for both the metabolic activities of muscle tissue and the bacteria present on the surface, as a result of which oxygen concentration within the pack falls below 1 % while the carbon dioxide concentration rises to about 20 % or more (Eustace 1981). The CO2 released by the respiration of microorganisms inhibits the growth of gram-negative bacteria, yeasts and moulds (Eklund 1982). In the present study, the general trend in TVC of both the samples indicated a positive correlation with TVB-N and histamine content. The correlation analysis indicated higher correlation for TVB-N in VP samples (r = 0.97), whereas, for histamine production higher correlation was observed in CPE samples (r = 0.97). TVB-N is a product of both autolytic and bacterial degradation, where as histamine is produced by bacterial action alone. The major histamine forming bacteria are Enterobacter aerogenes, Proteus morganii, E. coli, Vibrio sp, Klebsiella pneumoniae etc. which grow quickly in aerobic atmosphere.
Fig. 4.
Changes in counts of (a) Total Viable Count (TVC) (b) Enterobacteriaceae (c) Staphylococcus aureus (SA) (d) Faecal Streptococci (FS) and (e) Sulphite reducing Clostridia (SRC) of pabda cat fish fillets during chill storage
Enterobacteriaceae and E. coli
Enterobacteriaceae counts are often used to assess the overall quality of a food and the temperature history of the product during processing. They can survive in microaerophilic conditions and develop unpleasant odours or discolouration in vacuum packed products (Tsiligianni et al. 2012). Hence, the enumeration of these microorganisms is important in vacuum packed products. The low initial count of Enterobacteriaceae observed in fresh gutted pabda (2 log10 CFU/g) indicates good hygienic environment of the aquaculture farm as well as the adequate hygiene handling practices followed during gutting and packaging of fish samples. After chilling, a 0.5 log reduction in Enterobacteriaceae count was observed in VP samples on the first day, whereas, only 0.1 log reduction was observed in CPE samples (Fig. 4b). The greater reduction observed in vacuum packed samples may be due to the synergistic effect of cold shock and removal of O2 from the pack environment. Further, from the third day onwards, a progressive increase in the count was observed both in CPE and VP samples. Enterobacteriaceae, being psychrotolerant, are capable of growing at refrigeration temperatures; however, they cannot compete well with other Gram-negative spoilers (ICMSF 1998). At the time of sensory rejection, a 0.2 log increase was noticed in VP samples whereas, 0.4 log increase was observed in CPE samples. As majority of histamine forming bacteria belong to Enterobacteriaceae family, a correlation anlysis was carried out between Enterobacteriaceae count and histamine content of pabda catfish during chill storage. The results indicated high positive correlation between Enterobacteriaceae count and histamine content in control air pack (r = 0.88), whereas poor correlation was observed in vacuum pack (r = 0.5). This also justifies the reduced histamine production observed in the VP samples compared to that in CPE samples.
Escherichia coli was not detected in fresh pabda catfish as well as during any stage of chill storage period. This indicates that the flesh was not contaminated with gut content of fish during gutting and cleaning steps. Besides, this also indicates the best management practices followed in the aquaculture farm.
Vibrio cholerae, Salmonella spp and Listeria monocytogenes
The count of three major pathogenic organisms common in fish viz. Salmonella spp, Vibrio cholera and Listeria monocytogenes was determined in fresh as well as chill stored samples, as they can survive in microaerophilic conditions prevailed in vacuum packs. The results indicated complete absence of these organisms in fresh fish and throughout the chill storage period. According to Leitão (1977), fish from non-polluted waters are free from Salmonella spp. as this bacteria is not naturally found in the fish flora and its presence in fish is mainly due to handling by potential human carriers or contact with poorly disinfected surfaces. Besides, this bacteria hardly proliferates in food containing other microorganisms.
Staphylococcus aureus
In fresh pabda catfish, the count of S. aureus was 1.8 log10 cfu/g. In CPE samples, the count remained static till 5th day and then progressively increased and crossed the limit on 20th day (Fig. 4c). On the contrary, 0.3 log cfu reduction was observed in S. aureus count in vacuum packed samples on 5th day of chill storage. Thereafter, the count remained stagnant upto 16th day and then increased upto 1.9 log10 cfu/g on 20th day. In vacuum pack, the count of S. aureus was within the limit prescribed for fresh fish by ICMSF (1986) even when the fish was rejected based on sensory analysis.
Faecal Streptococci (FS) and Sulphite Reducing Clostridia (SRC)
Faecal Streptococci and sulphite reducing clostridia are widely distributed in the environment because they can withstand harsh environmental conditions and their presence indicates possibility of faecal contamination of the sample (Hill et al. 1993). Faecal Streptococci count in fresh pabda catfish was 1 log10 cfu/g. In CPE sample, the count increased up to 1.7 log10 cfu/g on the day of sensory rejection, whereas, in VP sample the complete absence of faecal Streptococci count was observed from 5th day of chill storage onwards (Fig. 4d).
SRC was monitored at 5 days interval. The most important representatives of SRC species are Cl. perfringens, Cl. bifermentans, Cl. sporogenes and Cl. botulinum (Dijk and Grootenhuis 2003). The hardiness of SRC spores makes it useful to indicate recent faecal contamination compounded by chemical pollutants, where other faecal pollution indicators have been destroyed (Horan 2003). The total anaerobic sulphite reducing bacterial population in fresh pabda was 0.2 log10 cfu/g meat. In CPE samples a progressive increase in count was observed, where as VP sample showed only a marginal increase in the count over the storage period (Fig. 4e). It has been previously reported that eventhough temperature abuse for short period will not permit significant growth of these organisms, cyclic and static temperature abuse of chilled products for relatively long periods may lead to high and dangerous numbers of SRC.
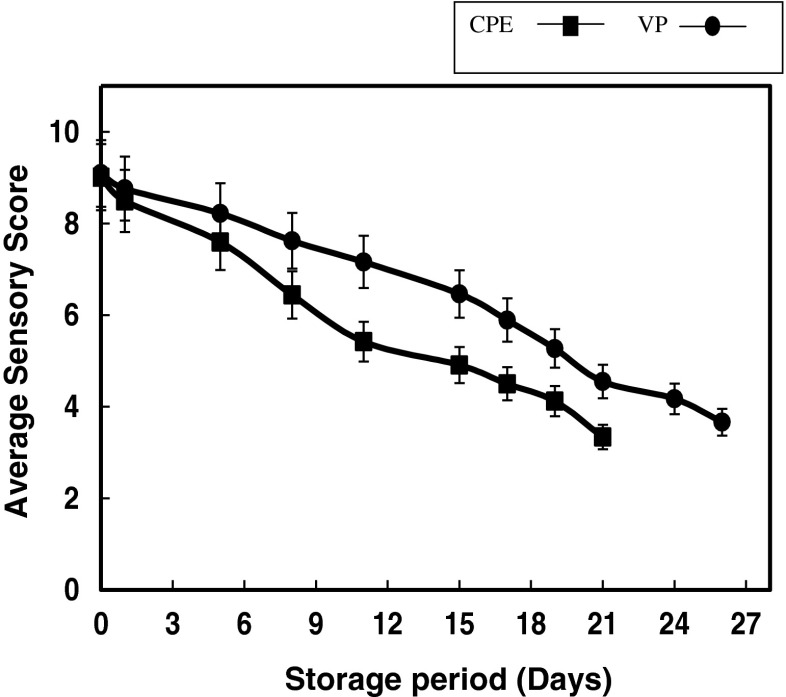
Sensory evaluation
Eventhough both the samples crossed the limit of microbiological count within 20th day of chill storage, study was continued till the samples were rejected by the sensory panel. Changes in the overall acceptability score for appearance, colour, odour and flavour are presented in the Fig. 5. The sensory score has shown a declining trend, however, the sensory deterioration was delayed in VP sample compared to CPE sample. Quality deterioration was characterized by off-odour, discolouration from bright pink to pale brownish green, and softening of the muscle. Accordingly, the panelists rejected CPE and VP samples on 20th and 26th days of chill storage, respectively. The correlation analysis of TVC and sensory scores during chill storage indicated a high negative correlation in both CPE and VP samples (r = −0.96), the normal trend expected in spoilage process of muscle foods. The overall sensory scores obtained for each sample were further subjected to Kruskal-Wallis ranking. As per statistical ranking, VP samples ranked higher to CPE samples at any point of time during chill storage (Table 2).
Fig. 5.
Average overall sensory acceptability scores of pabda cat fish fillets during chill storage
Table 2.
Statistical ranking of overall sensory scores of Pabda catfish during chill storage based on Kruskal-Wallis test
| Storage period (days) | Mean rank | |
|---|---|---|
| CPE | VP | |
| 0 | 43.00 | 53.00 |
| 1 | 38.00 | 47.80 |
| 5 | 33.00 | 43.20 |
| 8 | 28.00 | 37.90 |
| 11 | 23.00 | 33.10 |
| 14 | 18.00 | 28.00 |
| 16 | 13.00 | 23.00 |
| 18 | 8.00 | 18.00 |
| 20 | 3.00 | 13.00 |
| 24 | … | 7.90 |
| 26 | … | 3.10 |
In conclusion, the shelflife of chilled gutted pabda fish was predicted based on the cumulative obervations of biochemical, microbiological and sensory parameters. Accordingly, the shelflife of chilled gutted pabda catfish was estimated to be 14–16 and 18–20 days for CPE and VP samples, respectively, based on the maximum microbial load limit of 7 log10 cfu/g recommended for freshwater fish (ICMSF 1986). Eventhough, the sensory parameters showed extended shellife by 4 more days for both the samples, emphasis was given to microbiological safety rather than the progress of spoilage indicated by sensory analysis.
Conclusion
All the biochemical indices of protein and fat deterioration indicated values well within the prescribed limit of acceptability irrespective of the packaging technique throughout the chill storage period. However, the sensory and microbiological parameters indicated a 4 days extension in storage life for both control and vacuum packed samples. The results of present study indicated that vacuum packaging technique is not effective for fresh water medium fatty fish like pabda catfish unlike that reported for marine fatty fishes. However, as far as retail sale is considered adding 4 days to the shelflife is advantageous as it allows the fish vendor to get extended sale period and good profitability.
Acknowledgments
The authors would like to thank Director, CIFT, Cochin for providing the facilities to undertake this work. The support offered by Mr. Joshy C.G, Scientist, CIFT, Cochin for statistical analysis of the data is highly acknowledged.
References
- Amegovu AK, Sserunjogi ML, Ogwok P, Makokha V. Nucleotide degradation products, total volatile basic nitrogen, sensory and microbiological quality of nile perch (Lates niloticus) fillets under chill storage. J Microbiol Biotechnol Food Sci. 2012;2:653–666. [Google Scholar]
- Amerine MA, Pongborn RH, Roescler EB. Principles of sensory evaluation of food. New York: Academic; 1965. p. 602. [Google Scholar]
- AOAC (2002) Official methods of analysis. Association of Official Analytical Chemists International, Ch. 17, Vol. 1., p. 4–5, 52., Gaithersburg
- APHA . In: Compendium of methods for the microbiological examination of foods. Speck ML, editor. New York: American Public Health Association; 1976. [Google Scholar]
- Arashisar S, Hisar O, Kaya M, Yanik T. Effects of modified atmosphere and vacuum packaging on microbiological and chemical properties of rainbow trout (Oncorynchus mykiss) fillets. Int J Food Microbiol. 2004;97:209–214. doi: 10.1016/j.ijfoodmicro.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Aubourg S, Alvarez V, Pena J. Lipid hydrolysis and oxidation in farmed gilthead seabream (Sparus aurata) slaughtered and chilled under different icing condition. Grasas Y Aceites. 2010;61:183–190. doi: 10.3989/gya.108909. [DOI] [Google Scholar]
- Brockerhoff H, Jensen RG. Lipolytic enzymes. New York: Academic; 1974. [Google Scholar]
- Clancy GS, Bearnes DA, Higgs DA, Dosanjh BS. Effect of methodology on the determination of total volatile nitrogen and trimethylamine levels in previously frozen Pacific herring (Clupea harengus pallasi) stored at 2–5°C for up to 15 days. Can Tech Rep Fish Aquat Sci. 1995;2047:10. [Google Scholar]
- Cobb BF, Vanderzant C. Development of a chemical test for shrimp quality. J Food Sci. 1975;40:121. doi: 10.1111/j.1365-2621.1975.tb03751.x. [DOI] [Google Scholar]
- Connel JJ. Control of fish quality, fishing new books. Cambridge, London: Blackwell Science Ltd.; 1995. p. 241. [Google Scholar]
- Conway EJ. Micro-diffusion analysis and volumetric error. London: Crosby Lockwood and Son Ltd; 1950. [Google Scholar]
- Dijk R, Grootenhuis A. Microbiologie van voedingsmiddelen; methoden, principes en criteria. Houten: Keesing Noordervliet BV; 2003. pp. 433–561. [Google Scholar]
- EEC (1991) Laying down the health conditions for the production and the placing on the market of fishery products. Council Directive (EEC) 91/493/EEC
- Eklund MW (1982) Effect of CO2-modified atmospheres and vacuum packaging on Clostridium botulinum and spoilage organisms of fishery products. In Proceedings of the First National Conference on Seafood Packaging and Shipping, Washington, DC. pp. 298–33
- Eustace IJ. Some factors affecting oxygen transmission rates of plastic films for vacuum packaging of meat. J Food Technol. 1981;16:73–80. doi: 10.1111/j.1365-2621.1981.tb00998.x. [DOI] [Google Scholar]
- Food and Drug Administration (FDA) Fishand fishery products hazards and controls guidance. 4. Washington, DC: Department of Health and Human Services, Food and Drug Administration, Center for Food Safety and Applied Nutrition; 2011. [Google Scholar]
- Gimenez B, Roncales P, Beltran JA. Modified atmosphere packaging of filleted rainbow trout. J Sci Food Agric. 2002;82:1154–1159. doi: 10.1002/jsfa.1136. [DOI] [Google Scholar]
- Hansan AA, Morkore T, Rudi K, Rodbotten M, Bjerke F, Eie T ( 2009) Quality changes of prerigor filleted Atlantic salmon (Salmo salar L.) packaged in modified atmosphere using CO2 emitter, traditional MAP and vacuum. J Food Sci 74:242–249. [DOI] [PubMed]
- Hardy R, Smith JGM. The storage of mackerel (Scomber scombrus). Development of histamine and rancidity. J Sci Food Agric. 1976;27:595–599. doi: 10.1002/jsfa.2740270702. [DOI] [PubMed] [Google Scholar]
- Hill RT, Knight IT, Anikis MS, Colwell RR. Benthic distribution of Sewage Sludge indicated by Clostridium perfringens at a deep-ocean dump site. Appl Environ Microbiol. 1993;59:47–51. doi: 10.1128/aem.59.1.47-51.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan NJ. Fecal indicator organisms. In: Mara D, JHoran N, editors. The handbook of water and wastewater microbiology. London: Academic; 2003. pp. 105–112. [Google Scholar]
- Hras AR, Hadolin M, Knez Z, Bauman D. Comparison of antioxidative and synergistic effects of rosemary extract with α -tocopherol, ascorbyl palmitate and citric acid in sunflower oil. Food Chem. 2000;71:229–233. doi: 10.1016/S0308-8146(00)00161-8. [DOI] [Google Scholar]
- ICMSF (1986) International Commission on Microbiological Specification for Foods: Microorganisms in foods. Sampling for microbiological analysis: principles and specific applications, 2nd Ed. International Commission on Microbiological Specifications for Foods
- International Commission on Microbiological Specifications for Foods (ICMSF) Microorganisms in foods. New York: Blackie Academic & Professional; 1998. [Google Scholar]
- Ismail YK, Eduardo E, Jaime A, Abdullah D. Effect of chill storage on quality of vacuum packed mearge fillets. J Food Eng. 2013;115:486–496. doi: 10.1016/j.jfoodeng.2012.09.007. [DOI] [Google Scholar]
- Jezek F, Buchtova H. Physical and chemical changes in fresh chilled muscle tissue of common carp (Cyprinus carpio L.) packed in a modified atmosphere. Acta Vet Brn. 2007;76:S83–S100. doi: 10.2754/avb200776S8S083. [DOI] [Google Scholar]
- Kordiovská P, Vorlová L, Borkovcová I, Karpíšková R, Buchtová H, Svobodová Z, Křížek M, Vácha F. The dynamics of biogenic amine formation in muscle tissue of carp (Cyprinus carpio) Czech J Anim Sci. 2006;51(6):262–270. [Google Scholar]
- Koutsoumanis K, Nychas GJE. Chemical and sensory changes associated with microbial flora of Mediterranean boque (Boops boops) stored aerobically at 0, 3, 7 and 10°C. Appl Environ Microbiol. 1999;65:698–706. doi: 10.1128/aem.65.2.698-706.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek M, Vacha F, Varlova L, Lukasova J, Cupakova S. Biogenic amines in vacuum-packed flesh of carp (Cyprinus carpio) stored at different temperatures. Food Chem. 2004;88:185–191. doi: 10.1016/j.foodchem.2003.12.040. [DOI] [Google Scholar]
- Lakshmanan PT. Fish spoilage and quality assessment. In: Iyer TSG, Kandoran MK, Thomas M, Mathew PT, editors. Quality assurance in seafood processing. Cochin: Society of Fisheries Technologists (India); 2000. pp. 26–40. [Google Scholar]
- Leitão MF d F. Microbiologia do pescado e controle sanitário no processamento. Bol Inst Tecnol Aliment. 1977;14:1–35. [Google Scholar]
- Mazorra-Manzano MA, Pacheco-Aguilar R, Díaz-Rojas EI, Lugo-Sánchez ME. Postmortem changes in black skipjack muscle during storage in Ice. J Food Sci. 2000;65(5):774–779. doi: 10.1111/j.1365-2621.2000.tb13585.x. [DOI] [Google Scholar]
- Mietz JL, Karmas E. Polyamine and histamine content of rockfish, salmon, lobster and shrimp as an indicator of decomposition. J AOAC Int. 1978;61:139–145. [Google Scholar]
- Natarajan MV, Sreenivasan A. Proximate and mineral composition of freshwater fishes. Ind J Fish. 1961;8:422–429. [Google Scholar]
- Ozogul F, Polat A, Ozogul Y. The effect of modified atmosphere packaging and vacuum packaging on chemical, sensory and microbiological change of sardines (Sardina pilchardus) Food Chem. 2004;85:49–57. doi: 10.1016/j.foodchem.2003.05.006. [DOI] [Google Scholar]
- Puchala R, Białowąs H, Pilarczyk M. Influence of cold and frozen storage on carp (cyprinus carpio) flesh quality. Pol J Food Nutr Sci. 2005;1:103–106. [Google Scholar]
- Scherer R, Augusti PR, Bochi VC, Steffens C, Fries LLM, Daniel AP, Kubota EH, Neto JR, Emanuelli T. Chemical and microbiological quality of grass carp (Ctenopharyngodon idella) slaughtered by different methods. Food Chem. 2006;99:136–142. doi: 10.1016/j.foodchem.2005.06.048. [DOI] [Google Scholar]
- Sikorski ZE, Ruiter A. Changes in proteins and nonprotein nitrogen compounds in cured, fermented, and dried seafoods. In: Sikorski ZE, Pan BS, Shahidi F, editors. Seafood proteins. New York: Chapman and Hall; 1994. pp. 113–126. [Google Scholar]
- Stansby ME. Industrial fishery technology. Huntington: R.E. Krieger Publishing Co.; 1963. [Google Scholar]
- Tarladgis BG, Watts BM, Younthan MT. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J Am Oil Chem Soc. 1960;37:44. doi: 10.1007/BF02630824. [DOI] [Google Scholar]
- Tejada M, Huidobro A. Quality of farmed gilthead seabream (Sparus aurata) during ice storage related to the slaughter method and gutting. Eur Food Res Technol. 2002;215:1–7. doi: 10.1007/s00217-002-0494-1. [DOI] [Google Scholar]
- Tsiligianni M, Papavergou E, Soultos N, Magra T, Savvaidis IN. Effect of chitosan treatments on quality parameters of fresh refrigerated swordfish (Xiphias gladius) steaks stored in air and under vacuum conditions. Int J Food Microbiol. 2012;159:101–106. doi: 10.1016/j.ijfoodmicro.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Visciano P, Schirone M, Tofalo R, Suzzi G. Biogenic amines in raw and processed seafood. Front Microbiol. 2012;3:1–10. doi: 10.3389/fmicb.2012.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CS, Ranzell Nickelson II, Finne G. Ammonia producing enzymes in white shrimp tails. J Food Sci. 1978;43(5):1400–1404. doi: 10.1111/j.1365-2621.1978.tb02501.x. [DOI] [Google Scholar]
- Yildiz G, Wehling RL, Cuppett SL. Comparison of four analytical methods for the determination of peroxide value in oxidized soybean oils. J Am Oil Chem Soc. 2003;80:103–107. doi: 10.1007/s11746-003-0659-3. [DOI] [Google Scholar]