Abstract
Artocarpus heterophyllus shell powder was investigated in terms of its nutritional and biological potential. A thorough examination of shell powder demonstrated its potential as a source of minerals, β carotene and dietary fiber, which were assessed gravimetrically & spectrophotometrically. This showed 3.05 ± 0.19 g 100 g−1 DW of alkaloids followed by saponins and tannins. Three different extracts; acetone, methanol, & mix solvent were used to evaluate phenolic & flavonoid content, antioxidant & antimicrobial activity, GC/MS screening and quantitative analysis of polyphenols. Among all, the methanol extract showed highest antioxidant activity evaluated by DPPH, FRAP & ABTS assays and was significantly correlated with phenolic and flavonoid contents. Phenolic & flavonoid content was found to be 158 ± 0.34 mg (GAE) and 10.0 ± 0.64 mg (CE) respectively. The results of antimicrobial activity showed that L. monocytogenes was more susceptible to all extracts followed by other microorganisms. Catechin, ascorbic & chlorogenic acids were identified as major polyphenols analyzed by LC-MS/MS. GC/MS analysis showed that it contains a variety of compounds with different therapeutic activities. The study revealed that A. heterophyllus shell is a good source of natural antioxidants & other bioactive compounds and can be used in cosmetics, medicines and functional food application.
Keywords: Artocarpus heterophyllus, Antioxidant, Polyphenols, LC-MS/MS, Antibacterial, GC/MS, Dietary fiber
Introduction
The current life style and rising health concerns have led to an increase in the demand for food and food products, which provide health benefits beyond the basic nutrition. Natural antioxidants play an important role in human health care and used in various industrial applications such as cosmetic, medicine and functional food. Besides natural antioxidants, artificial antioxidants butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) and propyl gallate etc. are also used as well as natural ones. Research has shown that artificial antioxidants may produce toxic substances, which shall harm human health (Nurul et al. 2011). Fruits & vegetables are important sources of natural antioxidants. Higher intake of fruits and vegetables has been proven to reduce the risk of chronic diseases (Ames et al. 1993). This positive characteristic can be attributed to phytochemicals such as carotenoids, alkaloids, vitamins, minerals and polyphenols (Krishna et al. 2011). These compounds directly contribute to the antioxidant capacity and are usually used in the prevention of oxidative rancidity (Guanghou and Lai 2006; Lipi et al. 2012). Generally, these compounds are highly present in the outer layers of fruits & vegetables (Kanti and Syed 2009). During the processing stage, these parts are usually removed and retained in the press residues or by-products. In recent times, the utilization of food processing by-products has been gaining a more focused attention. Dumping or burning of this industrial waste is a potential cause of air and water pollution. Utilization of these industrial by-products may be economically worthwhile. Researchers have shown a strong correlation between the antioxidant properties of these residues and their health promoting and/or disease preventing property (Lavelli et al. 2000). In view of the growing interest in these compounds, there is a need to identify and quantify these important compounds to supply the present needs from the natural sources. Jackfruit (A. heterophyllus Lam. Fam. Moraceae), a wild plant found throughout the tropics, is bears the largest known edible fruit (up to 35 kg) (Purseglove 1986; Rowe-Dutton 1985). It is cultivated in many parts of the tropics particularly in Southeast Asia i.e., Africa, Australia, Brazil, China, Indonesia, India, Malaysia, Philippines, Srilanka and Thailand. Jackfruit is used in several ways. Young fruits and seeds are used as vegetables. The pulp of ripe is eaten fresh and used in fruit salads. A significant amount of peel (approx. 2,714–11,800 kg per tree per year) is discarded as agricultural wastes (Inbaraj and Sulochana 2004).
The root, leaves, bark, pulp and seeds of jackfruit have been the subject of study for research carried out till date. Literature proves the antibacterial and antioxidant activity of leaf extract (Loizzo et al. 2010), polyphenols and antitumor activity of seeds (Rajendran and Ramkrishnan 2009) and carotenoid composition in the kernel (Chandrika et al. 2005). However, its peel is almost completely neglected. Therefore, the peel emerges as a potential subject of study. The aim of the present study was to investigate the nutritional and biological potential of the powder prepared by shell of A. heterophyllus.
Material and methods
Plant material
Sample of A. heterophyllus were collected in 2009 from areas in and around Delhi. It was manually peeled and cut into small pieces, which were dried in oven at 60–70 °C before being grinded and powdered (DW).
Extraction
Powdered air-dried sample was extracted by a slight modification in the method of Rehman (2006) and Demiray et al. (2009). Different solvent systems were used to evaluate the effectiveness of solvent type. The sample was extracted with aqueous acetone (Acetone: water, 80:20 v/v; AAE), aqueous methanol (methanol: water, 80:20 v/v; AME) & mix solvent (ethanol: hexane: water, 80:10:10 MSE). It was then homogenized by using high efficiency homogenizer (IKA® Ultra Turrax T25) and kept for three days in shaker at room temperature. All extracts were centrifuged (DuPont, model Sorvall RC-5C) to obtain the supernatant and the residues were re-extracted under same conditions. Supernatants were pooled and combined and evaporated with a rotary evaporator (Nutronix, Jain Brothers India). After the evaporation of organic solvents, extract yield (EY) was calculated (g 100 g−1 of DW). All extracts were stored at 4 °C until analyses. The whole procedure was performed in triplicates at three different times.
Chemical properties & elemental constituents
The moisture and total ash content was determined by gravimetric method at temperature 103 ± 0.5 °C (Ref. 935.29, AOAC 2006) and at 525 ± 0.5 °C (Ref. 900.02A, AOAC 2006) respectively. The total nitrogen content was analyzed using the Kjeldahl method Ref. 976.05 (AOAC 2006) and the obtained nitrogen was transformed into protein content by multiplying the total nitrogen by a conversion factor of 6.25. Crude fat content was assessed using the AOAC method, Ref. 2003.06. The amount of total carbohydrates was determined with the following formula: total carbohydrates (% fresh weight) = 100—moisture (%)—protein content (% fresh weight)—crude fat (% fresh weight)—ash (% fresh weight) and reported as total carbohydrates in g 100−1 g DW. Crude fiber content was calculated using Ref. 978.10 of AOAC 2006. (All AOAC methods were according to AOAC 2006).
Mineral analysis was determined using Optima 2100 DV ICP-OES (Perkin-Elmer, USA), after prior mineralization in an Anton Paar Multiwave microwave digester (Anton Paar Ltd., Hertford, UK) as per Ref 956.52 (AOAC 2005). The correlation coefficients for the calibration curves were obtained more than 0.99. The results were calculated as mg 100 g−1 of DW.
Phytochemical constituents
Alkaloid, saponins and tannins
Alkaloid & saponins were calculated gravimetrically (Herborne 1973 and Obadoni and Ochuko 2001). Tannin was calculated spectrophotometrically by mixing the sample with 0.1 M FeCl3 in 0.1 N HCl and 0.008 M potassium ferrocyanide. The absorbance was measured at 605 nm. Tannic acid was used as reference. Results were reported as mg 100 g−1 of DW (Van-Burden and Robinson 1981).
β carotene analysis
β carotene was analyzed according to the method described by Kongsuwan et al. (2009). Sample was extracted with petroleum ether, evaporated to dry and reconstituted with iso-propanol. Analysis was performed using Agilent HPLC series 1200 with a mobile phase constituting Acetonitrile: methanol (70:30). C18 column was used for separation. β carotene content was expressed as μg g−1 of DW.
Total dietary fiber (TDF), insoluble dietary fiber (IDF) and soluble dietary fiber (SDF)
Fat free sample was analyzed for the determination of TDF, IDF & SDF content by enzymatic and gravimetric method of the Association of Official (AOAC-985.29; 2005) and results were expressed in g 100 g−1 on DW basis.
Total phenolic, flavonoid & flavonol
Total phenolic content was determined by Folin-Ciocalteu method and the results were expressed as gallic acid equivalents (GAE, mg 100 mg−1 extract) (Slinkard and Singleton 1997). Flavonoid content was calculated colorimetrically. The absorbance was measured at 510 nm and results were reported in mg as catechin equivalents (CE, mg 100 mg−1 extract) (Jia et al. 1995). Flavonol content was analyzed as rutin equivalents and measured at 440 nm (Miliauskas et al. 2004).
Antioxidant activities
ABTS, FRAP and DPPH radical scavenging activity
The ability of the test sample to scavenge ABTS.+ radical cation was compared to trolox standard. Extracts were mixed with 1 ml ABTS reagent and measured at 734 nm after 6 min incubation at room temperature. To calculate the TEAC, the gradient of the plot for the sample was divided by the gradient of the plot for trolox (Re et al. 1991; Bibhabasu et al. 2008). Stable DPPH was used for determination of radical scavenging activity of extracts. Catechin was used as a positive control and the inhibition ratio (IC50) was calculated (Chang et al. 2001). FRAP assay was carried out using freshly prepared FRAP reagent and calculated as trolox equivalent (TE, mg 100 mg−1 extract) (Stratil et al. 2006).
Quantification of polyphenols by LC-MS/MS
Agilent 1100 series HPLC instrument and 6460 triple quad MS detector was used for the estimation of polyphenols. C18 column was used at a flow rate of 0.8 ml min−1, with a two solvent mobile phase (eluent A = 10 mM ammonium acetate and 1 % Acetic acid in water; eluent B = 1 % Acetic acid in methanol). The eluent gradient used for all extracts was 0–3 min, 15–40 % A; 3–5.5 min, 40–90 % A; 5.5–9 min, 90 % A; 9–9.5 min, 90–15 % A; 9.5–10 min, 15 % A (Demiray et al. 2009). Quantitation was based on external standardization by employing calibration curves in the range of 1–50 ng ml−1 based on the peak area calculated from selected ion chromatograms of the corresponding [M–H]− ion. Results were expressed as μg 100 mg−1 of extract.
Characterization of GC/MS analysis
Extraction of shell powder was carried out according to the soxhlet method. Agilent 6890 gas chromatograph and 5975B mass spectrometer was used with a capillary column of fused silica HP-5 ms. Extract was injected in the split mode (1:50) at 280 °C. The oven programming was set according (Medini et al. 2009). Eluents were detected in EI mode and scan range was (m/z 40–1050). All the mass spectra of the identified peaks were compared with the spectra from the NIST’05, WILAY spectral library and F.A.M.E mix (C8:C24). The results (quality match >90 %) for individual compounds were only reported as their percentage of the total area of peaks in the total ion chromatogram.
Antibacterial activity
Agar well diffusion method was used against four Gram-positive and five Gram-negative bacteria (Table 4). MIC was determined through a standard two-fold micro dilution technique in sterile flat-bottom 96-well microplates (Difco Laboratories, Detroit, MI, US) and expressed in mg ml−1 as the lowest extract concentration for which the wells were optically cleared (Mathabe et al. 2006; Basri and Fan 2005). Streptomycin was used as a positive control.
Table 4.
GC/MSD analysis of A. heterophyllus shell powder extracts
| RT ± 0.5 (min) | Components | MR/Base peak/Characterization mass spectral ion (EI) m/z relative abundance (%) | %Area(SD) AAE | (%)Area(SD) AME | %Area(SD) MSE | Identification |
|---|---|---|---|---|---|---|
| Fatty acids | ||||||
| 22.480 | C14:0 (Myrisitic acid) | 228 (M+), 73, 60(95.2), 43(86.3) | nd | 20.10 ± 0.90 | nd | a & b |
| 23.044 | C16:0 (Palmitic acid) | 270(M+), 74,87(69.7),43(41.5) | nd | 6.53 ± 0.94 | 3.65 ± 0.94 | a & b |
| 27.585 | C16:1(Palmitoleic acid) | 294 (M+),67,81(77.5),55(74.2) | nd | nd | 1.98 ± 0.98 | a & b |
| 29.410 | C18:0 (Stearic acid) | 284(M+), 43,73(84),60(80.6) | nd | nd | 2.30 ± 0.47 | a & b |
| 28.374 | C18:1 (Oleic acid) | 270 (M+), 55,69(75.7),41(75.2) | nd | 4.10 ± 0.94 | nd | a & b |
| 28.419 | C18:2 (Linoleic acid) | 294 (M+),67,81(87.1),55(60.2) | 1.70 ± 0.83 | 2.53 ± 0.98 | 8.03 ± 0.90 | a & b |
| 27.146 | C18:3 (Linolenic acid) | 292 (M+),79,67(93.7),95(83.7) | nd | 1.41 ± 0.91 | 0.93 | a & b |
| Hydrocarbans | ||||||
| 42.606 | C17(Heptadecane) | 240 (M+), 57, 43(64.6), 71(64) | nd | nd | 1.60 ± 0.90 | a |
| 38.414 | C20 (Eicosane) | 282 (M+), 57, 43(78.7), 71(58.7) | nd | nd | 1.28 ± 0.90 | a |
| 48.714 | C28(Octacosane) | 39 (M+), 57,43(68.3), 71(67.6) | nd | nd | 1.60 | a |
| 50.934 | C29 (Nonacosane) | 408 (M+), 57, 43(71.8), 71(66) | nd | nd | 1.49 | a |
| 46.054 | C30(Henicosane) | 296 (M+), 57, 43(58.7), 71(69.5) | nd | nd | 1.89 | a |
| 28.205 | C16 (7-hexadecyne) | 222 (M+), 67,81(84.8),55(70.8) | nd | 4.46 ± 0.90 | nd | a |
| 35.101 | C24 (Tetracosane) | 338 (M+), 57,71(67.9),43(56.1) | nd | nd | 0.77 | a |
| 41.693 | Squalene | 410 (M+), 69,81(61.2),41(25.7) | nd | 0.88 ± 0.94 | nd | a |
| 28.577 | Cyclododecene | 166 (M+), 67,55(97.5),82(90.2) | 2.22 ± 0.80 | nd | nd | a |
| Sterols | ||||||
| 58.560 | Campesterol | 400 (M+), 43,55(61.7),41(47) | nd | nd | 1.37 ± 0.54 | a |
| 59.610 | Stigmasterol | 412 (M+), 55,43(65.1),81(47.9) | nd | nd | 1.12 ± 0.48 | a |
| 51.441 | β.-Sitosterol | 414 (M+), 44,55(35.5),41(33.1) | nd | 1.47 ± 0.83 | nd | a |
| 59.768 | Lanosterol | 426 (M+), 69,43(91.3),55(64.3) | 3.50 ± 0.60 | nd | nd | a |
| Others | ||||||
| Carboxylic acids & esters | ||||||
| 49.097 | 12-oleanen-3-yl acetate, (3. alpha.)- | 468(M+),218,203(25.6),219(18.5) | 12.30 ± 0.64 | nd | nd | a |
| 60.129 | 9, 19-cyclolanost-24-en-3 ol, acetate, (3. beta.)- | 468 (M+), 43,69(75.2),41(57.2) | 5.98 ± 0.64 | 1.68 ± 0.94 | 24.87 ± 0.94 | a |
| 36.465 | Hexadecanoic acid, 2,3-dihydroxypropyl ester, (+/−.)- | 330 (M+), 43,57(69), 41(67) | nd | 1.22 ± 0.97 | nd | a |
| 28.870 | 2-Chloroethyl linoleate | 342 (M+),67,81(82.9),55(60.9) | nd | nd | 23.01 ± 0.96 | a |
| 33.523 | Ethyl oleate | 310 (M+), 43,55(68.3),69(66.9) | nd | nd | 1.09 ± 0.96 | a |
| 61.278 | Lanosta-8, 24- dien-3-one | 310 (M+),426,69,43(91.3),55(64.3) | nd | nd | 4.58 ± 0.97 | a |
| 28.374 | Oleic acid | 270 (M+), 55,69(75.7),41(75.2) | nd | 4.10 ± 0.94 | nd | a |
| 9.093 | 4 H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 144 (M+), 43,44(75.5),144(375) | nd | 3.47 ± 0.50 | nd | a |
| Amines, alcohol, aldehyde & ketones | ||||||
| 55.216 | Pyridine-3-carboxamide, oxime, N-(2-trifluoromethylphenyl)- | 281 (M+),69,244(86.3),78(74.7) | nd | 1.02 ± 0.80 | nd | a |
| 10.288 | 2-Furancarboxaldehyde, 5-(hydroxymethyl) | 126 (M+),97,126(77.9),41(73.2) | nd | 4.99 ± 0.42 | nd | a |
RT retention time of the compound, in minutes, %Area the percentages of the area of the total ion chromatogram represented by the peaks of each of the compounds identified, SD standard deviation(n = 3), (a) Identified by NIST and WILAY spectral library, mass fragmentation, (b) Identified by NIST, WILAY spectral library, mass fragmentation and co-injection with authentic material. One-way analysis of variance (ANOVA) was performed to determine the significant differences among the means. Differences at p < 0.05 were considered as significant
Statistical analysis
All the data was reported as mean ± SD. The mean values were calculated based on the data taken from at least three independent experiments conducted on separate days using freshly prepared reagents. To determine the significant differences among the means one-way analysis of variance (ANOVA) was performed for all data. Differences at p < 0.05 were considered as significant. Correlation analysis was done with SPSS (version 10) statistical package.
Results and discussion
Chemical properties & elemental constituents
The obtained values (Table 1) were compared to those reported in other studies conducted on the different parts of jackfruit. A 100 g edible portion of ripe jackfruit contains carbohydrate (18.9 g), protein (1.9 g), fat (0.1 g), moisture (77 %), fiber (1.1 g), calcium (20 mg), phosphorous (30 mg) and iron (500 mg). (Bose 1985). Potassium and iron content in seeds of jackfruit was recorded 2,470.00 ppm & 148.50 ppm respectively by Ajayi (2008). In our study, the values of chemical & mineral contents were found relatively low when compared to edible part of jackfruit but level of fat and calcium was high. Fairly good amount of calcium (Ca), phosphorus (P), magnesium (Mg), potassium (K) and sodium (Na) were found in the shell part of jackfruit. These minerals play a crucial role in providing rigidity to skeleton, involvement in neuromuscular function, blood clotting and many other metabolic processes and are recognized as dietary essentials. Low amount of Pb, Cd, As and Hg showed that it is free from toxic metals. A preliminary investigation of food materials intended for future uses as supplemental ingredients and gives an idea about their possible functional behaviour during processing as well as their effect on the quality of the end product.
Table 1.
Chemical properties and elemental constituents of A. heterophyllus shell powder
| Composition | Content ± SD |
|---|---|
| Chemical constituents (g 100 g−1) | |
| Moisture (FW) | 65.05 ± 0.68 |
| Ash (DW) | 3.50 ± 0.22 |
| Protein (DW) | 1.50 ± 0.17 |
| Fat (DW) | 1.93 ± 0.59 |
| Crude fiber (DW) | 3.55 ± 0.41 |
| Carbohydrates (DW) | 13.45 ± 0.29 |
| Elemental constituents (mg 100 g−1 DW) | |
| Calcium | 456.1 ± 0.94 |
| Potassium | 273.1 ± 0.50 |
| Magnesium | 182.6 ± 0.91 |
| Sodium | 38.91 ± 0.55 |
| Phosphorous | 72.40 ± 0.12 |
| Iron | 16.25 ± 0.24 |
| Cupper | 1.45 ± 0.97 |
| Manganese | 7.56 ± 0.37 |
| Zinc | 2.65 ± 0.54 |
| Lead | 0.015 |
| Chromium | 0.060 |
| Tin | < 0.01 |
| Arsenic | < 0.01 |
| Cadmium | <0.01 |
| Mercury | <0.01 |
Values are means ± SD; n = 3
FW fresh weight, DW dry weight
Phytochemical constituents
Alkaloids, saponins & tannins
Crude phytochemicals are therapeutically, the most significant plant substances. The presence of these constituents have been reported to account for the exertion of antibacterial property and exhibit various pharmacological and biochemical actions when ingested by humans. Quantitative phytochemicals estimate of powder in g 100 g−1 DW were obtained as tannins, saponins and alkaloids; 1.45 ± 0.29, 2.67 ± 0.17 and 3.05 ± 0.19 respectively. The presence of tannins can support jackfruit’s peel use in wound healing, varicose ulcers, hemorrhoids, frostbite and burn (Okwu and Okwu 2006). Saponins are known as anti-nutritional elements and reduce the uptake of certain nutrients including cholesterol and glucose (Supradip et al. 2010).
β carotene
β carotene content of A. heterophyllus shell powder was assessed (5.1 ± 0.34, μg g−1 of DW) by HPLC analysis. β carotene content (5.6 ± 0.3 μg g−1) was also reported by Chandrika et al. (2005) in the kernel of A. heterophyllus that was in close agreement with our results. β carotene functions both as an antioxidant and as an essential nutrient. Results show that shell powder of jackfruit could be a significant source of vitamin A in both conventional foods and dietary supplements. People with high dietary intake of beta-carotene or high blood levels of this nutrient have a reduced risk of various diseases, including cancer and heart diseases (Chandrika et al. 2005).
TDF, IDF and SDF content
It is believed that dietary fiber acts as a protective agent against cardiovascular diseases, constipation, colon cancer and diabetes. It also facilitates and improves the absorption of minerals (Pietinen et al. 2001; Arun and Rituparna 2010). The insoluble fraction of the fiber is related to the intestinal regulation and soluble fiber decreases the cholesterol level. (Larion 1996). Our study revealed the amount of TDF, IDF and SDF to be 2.2 ± 0.13, 1.27 ± 0.13 and 0.93 ± 0.05 respectively g 100 g−1 on DW basis. Dietary fiber is naturally present in cereals, fruits and vegetables and nuts. Amount and composition of fiber differ from one food to another. The content of DF in vegetables could be in the range of 28–30 % of dry weight, even in some vegetables such as white and red beans, much higher values may reach. However, due to high water content, lower level of DF (only 1–3.5 % dry weight) is found in fruits. (Johnson and Southgate 1994).
Total phenolic, flavonoid and flavonol
All the results are reported in Table 2. In this study, observation was made on the results of phenolic content, where the value of phenolic content in the shell powder is higher than that reported earlier in the results of pulp (0.21 ± 0.012 mg GAE/g) and seed (27.7 mg GAE/g) (Umesh et al. 2010; Soong and Barlow 2004). The reason could be the production of more alkaloids and phenolic constituents in outer part during fruiting, in order to protect and preserve the seeds from the microbial attack (Soong and Barlow 2004). Flavonoid (10.5 ± 0.21 mg 100 mg−1) was also present in a high amount in our study as reported earlier in the result of the edible part (1.20 mg of RE/g) (Umesh et al. 2010). A fairly good amount of flavonoid suggested that shell powder may be used for management of cardiovascular diseases and oxidative stress. Clinical studies have provided evidence of a potential role of flavonoids in multiple health benefits. Flavonol content in methanol extract was found to be 6.9 ± 0.66 mg 100 mg−1 in our study. Flavonol content of food is high if it is >50 mg/kg. Onions, apples, tea and red wine are the other major dietary sources of flavonols used in many countries. Flavonol intake is inversely proportional to the risk of heart diseases and lung cancer (Hakkinen et al. 1994).
Table 2.
Total phenolic, flavonoids and antioxidant activity of A. heterophyllus shell powder extracts
| Solvent | EY (g 100 g−1 DW) | TPC (mg GAE) 100 mg−1 extract | TFC (mg CE) 100 mg−1 extract | Flavanol (mg RE) 100 mg−1 extract | FRAP (mg TE) 100 mg−1 extract | Total antioxidant activity (TEAC) of extract | IC50 of DPPH radical (mg ml−1 extract) |
|---|---|---|---|---|---|---|---|
| AAE | 19.95 ± 0.82 | 148 ± 0.72 | 8.5 ± 0.12 | 6.2 ± 0.72 | 2.62 ± 0.25 | 0.65 ± 0.12 | 0.61 ± 0.10 |
| AME | 20.54 ± 0.51 | 158 ± 0.34 | 10.0 ± 0.64 | 6.9 ± 0.66 | 2.86 ± 0.12 | 0.78 ±0.11 | 0.56 ± 0.10 |
| MSE | 21.91 ± 0.73 | 145.0 ± 0.13 | 10.5 ± 0.21 | 6.5 ± 0.55 | 2.54 ± 0.15 | 0.70 ± 0.10 | 0.59 ± 0.12 |
TPC total phenolic content, TFC total flavonoid content, TEAC trolox equivalent antioxidant concentration, TE trolox equivalent, EY extraction yield, GAE Gallic acid equivalent, CE catachein equivalent
Values are means ± SD; n = 3. One-way analysis of variance (ANOVA) was performed to determine the significant differences among the means. Differences at p < 0.05 were considered as significant
Antioxidant activities
Total antioxidant activity of the extracts was calculated from the decolorization of ABTS.+, which was measured spectrophotometrically at 734 nm. Interaction with the extract or standard trolox suppressed the absorbance of the ABTS.+ radical cation and the results, expressed as percentage inhibition of absorbance. The TEAC value of AME was obtained as 0.78 followed by other extracts. The radical-scavenging activity of DPPH was expressed as IC50. IC50 of AME was assessed to be 0.56 ± 0.10 mg ml1. The result of FRAP assay as trolox equivalent are reported in Table 2. Previously IC50 (DPPH value) 0.7 mg ml−1 and FRAP (to reduce Fe+3 to Fe+2 1.7 Mm TEAC g−1) was reported as 5 mg ml−1 for pulp methanolic extract (Umesh et al. 2010). The obtained results suggest that not only the edible part but the shell of Artocarpus heterophyllus also exhibits a high antioxidant and free radical scavenging activity.
Phenolic and flavonoid compounds present in the plants are mainly responsible for antioxidant activity, which has been proved already (Zhou and Yu 2004). Therefore, antioxidant ability of the extracts was strongly correlated with the phenolic and flavonoid content. Statistically, a significant (P < 0.05) correlation was found between total phenolic content and antioxidant activities assessed by FRAP and ABTS assays with r-values, 0.88 and 0.82 respectively. A moderate correlation was found between total flavonoid and ABTS assays (r = 0.62).
Quantification of polyphenols by LC-MS/MS
In the quantification of polyphenolic compounds by LC-MS/MS, the concentrations of six major polyphenols were quantified: flavanols, hydroxybenzoic acids, ellagitannins, hydroxycinnamic acids, and flavonols (Fig. 1). Quantification was achieved by integration of the peak using an external standard method. In this study, the highest extraction efficiency was found in AME for phenolic compounds while, total flavonoid content was highest in mix solvent (Table 3). Out of the nine compounds only six compounds were detected and quantitated. Chlorgenic and ascorbic acid were the major compounds in all the extracts and were ranged as 0.64–3.8 μg and 0.29–2.18 μg respectively in 100 mg extract. Ascorbic acid is one of the most effective antioxidant in fruits & vegetables and is associated with a reduced risk of chronic diseases due to its ability to scavenge free radicals in biological system. A significant correlation was found to exist for total phenolic and flavonoid content; between LC-MS/MS data and the results obtained by colorimetric assays were r2 = 63.85 %, r2 = 98.01 respectively. A moderate correlation was found between total polyphenolic and FRAP assay (r2 = 49.33 %). There was no significant correlation found between either the DPPH or FRAP assay and the total polyphenols detected by LC-MS/MS. This can be explained by fact that individual polyphenols have different relative antioxidant potencies. There is a wide degree of variation between different phenolic compounds in their effectiveness as antioxidants. Determination of an absolute value for the antioxidant capacity of an extract is difficult because it depends on the actual concentration of the radical, its degradation during analysis or the matrix interference (Almela et al. 2006).
Fig. 1.
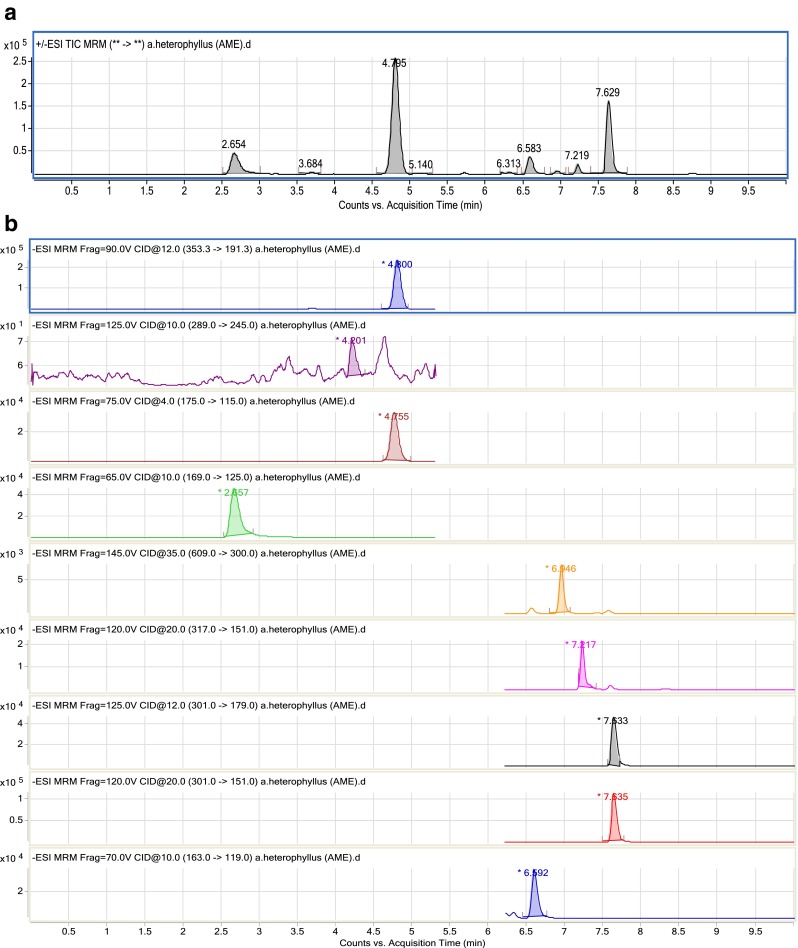
LC-MS/MS chromatograms of A. heterophyllus shell powder extract (a) TIC (total ion chromatogram) and (b) MRM (multiple reaction monitoring system)
Table 3.
Quantification of polyphenols by LC-MS/MS
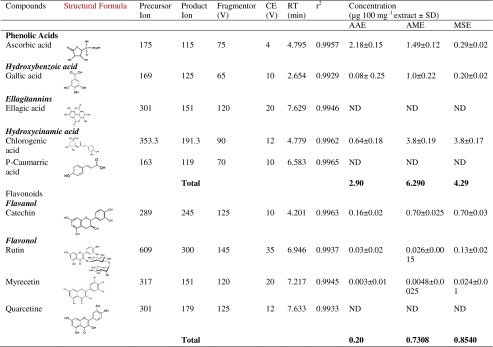
CE collision energy, RT retention time, r 2 coefficient of regression, ND not detected
All data are expressed as mean ± SD. (n = 3)
Characterization of GC/MS analysis
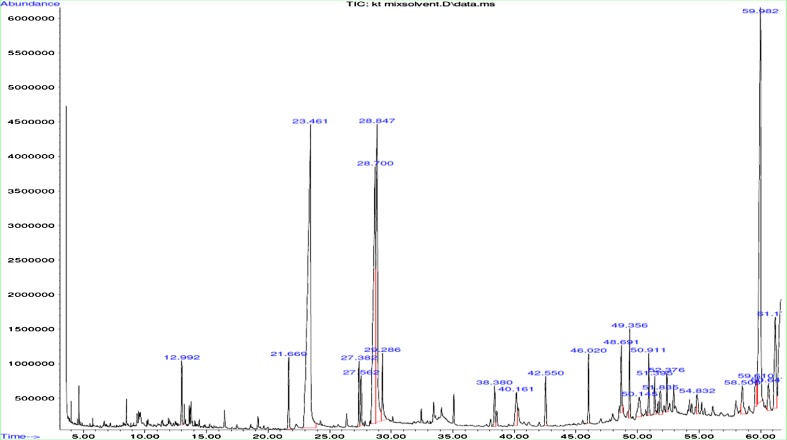
Major identified compounds by GC/MS are grouped according to their chemical nature in Table 4. Some of the eluted compounds are best described for their health benefits (Fig. 2). Phytosterols have been reported as a positive influence for diabetic state by directly lowering fasting blood glucose levels by cortisol inhibition (Devaraj and Jialal 2006). Palmitic acid, linoleic acid, linolenic acid, oleic acid, stearic acid and myrisitic acid are most common fatty acids found in animals and plants and are primarily used to produce hormone like substances that regulate a wide range of functions, including blood pressure, blood clotting, blood lipid levels, the immune response, and the inflammation response to injury infection. (Altieri et al. 2009). Squalene is commonly used in cosmetic industry. β-sitosterol has been shown to positively influence a diabetic state by directly lowering fasting blood glucose levels by cortisol inhibition (McAnuff et al. 2005). Phytochemical constituents were also studied by Chowdhury et al. 1997 by Gas liquid chromatography and listed capric, myristic, lauric, palmitic, oleic, stearic, linoleic and arachidic acids as major compounds with varying proportions in different parts of the jackfruit. The GC/MSD and LC-MS/MS profiling shows the presence of hydrocarbons, sterols, saturated and unsaturated fatty acids, phenolic and flavonoid compounds and may be contributed for its good antibacterial and antioxidant properties (Cushnie and Lamb 2005; Maisuthisakul et al. 2008).
Fig. 2.
GC/MS Chromatogram for mix solvent extract of Artocarpus heterophyllus shell powder
Antibacterial activity
Results of antibacterial activity of the obtained extracts are evaluated in Table 5. In this study, aqueous methanol and mix solvent showed appreciable zone of inhibition. Among bacteria, L. monocytogenes and S. aureus were found to be more susceptible to extract followed by other microorganisms. MIC of the obtained extracts against all tested bacteria was ranged between 4.2 and 5.9 mg ml−1. It appears that components extracted from the sample are active against the food borne pathogens. Earlier, the study was carried out on A. heterophyllus leaves extract with a MIC (488.1 μg ml−1) against food borne pathogens (Loizzo et al. 2010). Infectious diseases caused by food borne pathogens are remains a major threat to public health despite a tremendous progress in medicine, particularly in developing countries. Antimicrobial activities of alkaloids, tannins, saponins and flavonoids have already been documented (Supradip et al. 2010; Okoli and Iroegbu 2005; Altieri et al. 2009). Preliminary phytochemical analysis has shown that it is a good source of various phytoconstitutents and the antimicrobial activity in this study could be mainly due to the presence of these phytoconstitutents. The results in this study suggest that the extract could be used to control food borne infections.
Table 5.
Antibacterial activity of A. heterophyllus shell powder extracts
| Microorganisms | AAE | AME | MSE | Streptomycin | ||||
|---|---|---|---|---|---|---|---|---|
| ZI ± SD | MIC | ZI ± SD | MIC | ZI ± SD | MIC | ZI ± SD | MIC | |
| Gram positive bacteria | ||||||||
| Staphylococcus aureus | 18 ± 0.6 | 4.2 ± 0.1 | 22 ± 0.5 | 4.2 ± 0.5 | 18 ± 0.3 | 4.2 ± 0.4 | 19 ± 0.5 | 0.014 ± 0.5 |
| Bacillus subtilis | 13 ± 0.9 | 5.5 ± 0.2 | 14 ± 0.6 | 5.4 ± 0.1 | 13 ± 0.4 | 5.2 ± 0.2 | 12 ± 0.2 | 0.018 ± 0.3 |
| Bacillus cereus | 11 ± 0.5 | 5.5 ± 0.2 | 11 ± 0.3 | 5.5 ± 0.5 | 12 ± 0.9 | 5.1 ± 0.1 | 12 ± 0.5 | 0.022 ± 0.1 |
| Listeria monocytogenes | 19 ± 0.5 | 4.2 ± 0.5 | 23 ±0.3 | 4.2 ± 0.5 | 22 ± 0.5 | 4.2 ± 0.3 | 20 ± 0.9 | 0.017 ± 0.4 |
| Streptococcus faecalis | 11 ± 0.6 | 5.6 ± 0.5 | 10 ± 0.6 | 5.6 ± 0.4 | 11 ± 0.6 | 5.6 ± 0.5 | 13 ± 0.4 | 0.020 ± 0.5 |
| Gram negative bacteria | ||||||||
| Salmonella typhimurium | 16 ± 0.3 | 4.7 ± 0.3 | 18 ± 0.3 | 4.5 ± 0.2 | 16 ± 0.3 | 4.7 ± 0.5 | 19 ± 0.5 | 0.012 ± 0.1 |
| Shigella flexneri | 18 ± 0.4 | 4.7 ± 0.1 | 18 ±0.7 | 4.5 ± 0.2 | 16 ± 0.4 | 4.5 ± 0.4 | 17 ± 0.6 | 0.014 ± 0.4 |
| Pseudomonas aeroginosa | 09 ± 0.4 | 5.9 ± 0.5 | 11 ± 0.5 | 5.8 ± 0.1 | 09 ± 0.7 | 5.8 ± 0.2 | 10 ± 0.2 | 0.019 ± 0.5 |
| Escherichia coli | 11 ± 0.4 | 5.6 ± 0.2 | 12 ± 0.2 | 5.5 ± 0.5 | 12 ± 0.4 | 5.6 ± 0.5 | 14 ± 0.2 | 0.015 ± 0.2 |
ZI mean zone of inhibition (mm), MIC minimum inhibitory concentration (mg ml−1)
Values are mean SD; n = 3
Conclusion
By far, this is a pioneer study on shell of A. heterophyllus. No published data on shell portion of A. heterophyllus is available till date. Epidemiological evidence supports the hypothesis that food rich in natural antioxidants plays an important role in the prevention of several chronic diseases. On the basis of results presented here, a majority of the compounds identified in A. heterophyllus shell powder extract are beneficial for health, as they show strong antioxidant, antibacterial and putative therapeutic activity. Plant based antibacterial and antioxidant compounds have various biological potential as they serve the purpose without any side effect that is often associated with the synthetic compounds. The presented data might be helpful to ascertain A. heterophyllus shell as a potential source of natural antioxidants and nutraceutical/functional food applications. However, there is scope for further clinical studies, which can be carried out to explore the utility and efficiency in the treatment of chronic diseases.
Acknowledgments
The authors would like to acknowledge the state Grading Laboratory, Directorate of Agricultural Marketing, Delhi, India for their financial support.
References
- Ajayi IA. Comparative study of the chemical composition and mineral element content of Artocarpus heterophyllus and Treculia africana seeds and seed oils. Biores Technol. 2008;99:5125–5129. doi: 10.1016/j.biortech.2007.09.027. [DOI] [PubMed] [Google Scholar]
- Almela L, Sanchez-Munoz B, Fernandez-Lopez JA, Roca M, Rabe V. Liquid chromatographic mass spectrometric analysis of phenolics and free radical scavenging activity of rosemary extract from different raw material. J Chromatogr A. 2006;1120:221–229. doi: 10.1016/j.chroma.2006.02.056. [DOI] [PubMed] [Google Scholar]
- Altieri C, Bevilacqua A, Cardillo D, Sinigagha M. Effectiveness of fatty acids and their monoglycerides against gram-negative pathogens. Int J Food Sci Tech. 2009;44:359–366. doi: 10.1111/j.1365-2621.2008.01744.x. [DOI] [Google Scholar]
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis. 18. USA: Association of official Analytical Chemist; 2006. [Google Scholar]
- Arun KV, Rituparna B. Dietary fiber as functional ingredients in meat products: a novel approach for healthy living—a review. J Food Sci Technol. 2010;47:247–257. doi: 10.1007/s13197-010-0039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basri DF, Fan SH. The potential of aqueous and acetone extracts of galls of Quercus infectoria as antibacterial agents. Ind J Pharmacol. 2005;37:26–29. doi: 10.4103/0253-7613.13851. [DOI] [Google Scholar]
- Bibhabasu H, Santanu B, Nripendranath M. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement Altern Med. 2008;8:63. doi: 10.1186/1472-6882-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose TK. Jackfruit. In: Mitra BK, editor. Fruits of India: tropical and subtropical. Naya Prokas: Culcutta; 1985. pp. 488–497. [Google Scholar]
- Chandrika UG, Jansz ER, Warnasuriya ND. Analysis of carotenoids in ripe jackfruit (Artocarpus heterophyllus) kernel and studied the bioconversion in rats. J Sci Food Agr. 2005;85:186–190. doi: 10.1002/jsfa.1918. [DOI] [Google Scholar]
- Chang ST, Wu JH, Wang SY, Kang PL, Yang NS, Shyur LF. Antioxidant activity of extracts from Acacia confusa bark and heartwood. J Agric Food Chem. 2001;49:3420–3424. doi: 10.1021/jf0100907. [DOI] [PubMed] [Google Scholar]
- Chowdhury FA, Raman Md A, Milan AJ. Distribution of free sugars and fatty acids in jackfruit (Artocarpus heterophyllus) Food Chem. 1997;60:25–28. doi: 10.1016/S0308-8146(96)00294-4. [DOI] [Google Scholar]
- Cushnie TPT, Lamb JA. Review: antimicrobial activity of flavonoids. Int J Antim Agent. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demiray S, Pintado ME, Castro LMP. Evaluation of Phenolic profiles and antioxidant activities of Turkish medicinal plants Tilia argentea, Crataegi folium leaves and Polygonum bistorta roots. World Acad Sci Eng Technol. 2009;54:312–317. [Google Scholar]
- Devaraj S, Jialal I. The role of dietary supplementation with plant sterols and stanols in the prevention of cardiovascular disease. Nutr Rev. 2006;64:348–354. doi: 10.1111/j.1753-4887.2006.tb00219.x. [DOI] [PubMed] [Google Scholar]
- Guanghou S, Lai PL. Residue from star fruit as valuable source for functional food ingredients and antioxidant nutraceuticals. Food Chem. 2006;97:277–284. doi: 10.1016/j.foodchem.2005.03.048. [DOI] [Google Scholar]
- Hakkinen SH, Karenlampi SO, Heinonen IM, Mykkanen HM, Torronen AR. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J Agric Food Chem. 1994;47:2274–2279. doi: 10.1021/jf9811065. [DOI] [PubMed] [Google Scholar]
- Herborne JB. Phytochemical methods. London: Chapman and Hall; 1973. pp. 110–113. [Google Scholar]
- Inbaraj BS, Sulochana N. Carbonised: jackfruit peel as an adsorbent for the removal of Cd (II) from aqueous solution. Biores Technol. 2004;94:49–52. doi: 10.1016/j.biortech.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Jia Z, Tang M, Wu J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1995;64:555–559. [Google Scholar]
- Johnson IT, Southgate DAT. Dietary fiber and related substances. In: Edelman J, Miller S, editors. Food safety science. London: Champman and Hill; 1994. pp. 39–65. [Google Scholar]
- Kanti BP, Syed IR. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Long. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsuwan A, Suthiluk P, Theppakorn T, Srilaong V, Setha S (2009) Bioactive compounds and antioxidant capacities of phulae and nanglae pineapple. Asian J Food Agro Ind Special Issue:S44–S50
- Krishna DS, Kathrin S, Bronwen S, Laurie M. Antioxidant capacity, polyphenolics and pigments of broccoli–cheese powder blends. J Food Sci Technol. 2011;48(4):510–514. doi: 10.1007/s13197-010-0211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larion D. Dietary fiber: effects on lipid metabolism and mechanisms of action. Eur J Clin Nut. 1996;50:125–133. [PubMed] [Google Scholar]
- Lavelli V, Peri C, Rizzolo A. Antioxidant activity of tomato products as studied by model reactions using xanthine oxidase, myeloperoxidase, and copper-induced lipid peroxidation. J Agric Food Chem. 2000;48:1442–1448. doi: 10.1021/jf990782j. [DOI] [PubMed] [Google Scholar]
- Lipi DEB, Utpal R, Runu C. Role of nutraceuticals in human health. J Food Sci Technol. 2012;49(2):173–183. doi: 10.1007/s13197-011-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizzo MR, Tundis R, Chandrika UG, Abeyesekera AM, Menichini F, Frega NG. Antioxidant and antibacterial activities of Foodborne pathogens of Artocrapus heterophyllus Lam. (Moraceae) leaves extracts. J Food Sci. 2010;75:M291–M295. doi: 10.1111/j.1750-3841.2010.01558.x. [DOI] [PubMed] [Google Scholar]
- Maisuthisakul P, Pasuk S, Ritthiruangdej P. Relationship between antioxidant properties and chemical composition of some Thai plants. J Food Com Anal. 2008;21:229–240. doi: 10.1016/j.jfca.2007.11.005. [DOI] [Google Scholar]
- Mathabe MC, Nikolova RV, Lall N, Nyazema NZ. Antibacterial activities of medicinal plants used for the treatment of diarrhoea in Limpopo Province. South Africa. J Ethano. 2006;105:286–293. doi: 10.1016/j.jep.2006.01.029. [DOI] [PubMed] [Google Scholar]
- McAnuff MA, Harding WW, Omoruyi FO, Jacobs H, Morrison EY, Asemota HN. Hypoglycemic effects of steroidal sapogenins isolated from Jamaican bitter yam, Dioscorea polygonoides. Food Chem Toxicol. 2005;43:1667–1672. doi: 10.1016/j.fct.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Medini H, Marzouki H, Chemli R, Khouja LM, Marongiu M. Comparison of antimicrobial activity and the essential oil composition of juniperus oxycedrus subsp.macrocarpa and j, oxycedrus subsp. rufescens obtained by hydrodistillation and supercritical carbon dioxide extraction methods. Chem Nat Comp. 2009;45:739–741. doi: 10.1007/s10600-009-9416-9. [DOI] [Google Scholar]
- Miliauskas G, Venskutonis PR, Beek TAV. Screening of radical scavenging activity of some medicinal and aromatic plant extract. Food Chem. 2004;85:231–237. doi: 10.1016/j.foodchem.2003.05.007. [DOI] [Google Scholar]
- Nurul AZ, Darah I, Shaida FS, Nor AS. Assessment of antioxidant activity, total phenolic content and invitro toxicity of Malaysian red seaweed, Acanthophora spicifera. J Chem Pharm Res. 2011;3:182–191. [Google Scholar]
- Obadoni BO, Ochuko PO. Phytochemical studies and comparative efficacy of some homeostatic plants in Edo and Delta States of Nigeria. Global J Pure Appl Sci. 2001;8:203–208. [Google Scholar]
- Okoli S, Iroegbu CU. In vitro antibacterial activity of Synclisa scabrida whole root extracts. Afr J Biotechnol. 2005;4:946–952. [Google Scholar]
- Okwu DE, Okwu ME. Chemical composition of Spondias mombin Linn plant parts. J Sustain Agric Environ. 2006;6:140–147. [Google Scholar]
- Pietinen P, Lahti-Koski M, Vartiainen E, Puska P. Nutrition and cardiovascular disease in Finland since the early 1970s: a success story. J Nutr Health Aging. 2001;5:150–154. [PubMed] [Google Scholar]
- Purseglove P. Tropical crops, dicotyledons artocarpus heterophyllus jackfruit. London: Longman; 1986. pp. 384–386. [Google Scholar]
- Rajendran N, Ramkrishnan J (2009) Polyphenol analysis and antitumer activity of crude extracts from Tegmen of Artocarpus heterophyllus. Internet J Alternat Med 7(No-2)
- Rehman ZU. Citrus peel extract—a natural source of antioxidant. Food Chem. 2006;99:450–454. doi: 10.1016/j.foodchem.2005.07.054. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Bio Med. 1991;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rowe-Dutton P. Artocarpus heterophyllus-jackfruit. In: Garner JR, Chaudhury SA, editors. The propagation of tropical fruit trees. London: FAO/CAB; 1985. pp. 269–29. [Google Scholar]
- Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic. 1997;28:49–55. [Google Scholar]
- Soong YY, Barlow PJ. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004;88:411–417. doi: 10.1016/j.foodchem.2004.02.003. [DOI] [Google Scholar]
- Stratil P, Klejdus B, Kuban V. Determination of total content of Phenolic compounds and their antioxidant activity in vegetables–evaluation of spectrophotometric methods. J Agric Food Chem. 2006;54:607–616. doi: 10.1021/jf052334j. [DOI] [PubMed] [Google Scholar]
- Supradip S, Suresh W, Jitendra K, Swaran D, Balraj SP. Screening for feeding deterrent and insect growth regulatory activity of Triterpenic Saponins from Diploknema butyracea. J Agric Food Chem. 2010;58:434–440. doi: 10.1021/jf902439m. [DOI] [PubMed] [Google Scholar]
- Umesh BJ, Shrimant NP, Bapat VA. Evaluation of antioxidant capacity and phenol content in jackfruit (Artocarpus heterophyllus Lam.) fruit pulp. Plant Foods Hum Nutr. 2010;65:99–104. doi: 10.1007/s11130-010-0155-7. [DOI] [PubMed] [Google Scholar]
- Van-Burden TP, Robinson WC. Formation of complexes between protein tannic acid. J Agric Food Chem. 1981;1:77–82. [Google Scholar]
- Zhou K, Yu I. Effects of extractions solvent on the wheat bran antioxidant activity estimation. LWT-Food Sci Technol. 2004;37:717–721. doi: 10.1016/j.lwt.2004.02.008. [DOI] [Google Scholar]