Abstract
Antioxidants are components which prevent auto-oxidation of oils and fats by giving their hydrogen to free radicals formed in the initiation and propagation stages of autoxidation. During the past two decades, a lot of researches using natural plants extract in edible oils have been carried out due to the trend to minimize or avoid the use of synthetic food additives. According to the most studies, there are various natural antioxidants which can be extracted from low cost resources, such as most parts of olive plant, green tea, sesame, medicinal plants, etc. One of the most important requirements for a suitable antioxidant in oils and fats is the thermal stability during heat processing. It has been shown that most of natural additives have more antioxidants activity and thermal stability than synthetic ones in different edible oils. In this review, recent advances in the application of natural antioxidants in the food industry will be covered.
Keywords: Natural antioxidants, Synthetic antioxidants, Stability, Plant extracts, Edible oils
Introduction
The oxidative reaction is responsible for rancid odors and flavors within fats and oils which reduces nutritional quality of foods. Oxidation reactions consist of auto-oxidation, photo-oxidation, enzymatic oxidation and ketonic oxidation, whereas auto-oxidation is the most common deterioration during storage of edible oils. Autoxidation is the reaction between oxygen and unsaturated fatty acids via an auto-catalytic process consisting of a free radical chain mechanism. This chain includes initiation, propagation, and termination reactions that could be cyclical once started:
The initiation process generates free radicals from the substrate. Antioxidants are components which prevent auto-oxidation of oils and fats by giving their hydrogen to free radicals formed in the initiation and propagation stages of autoxidation by following reactions (AH is antioxidant molecule):
There are many factors that affect the auto-oxidation reaction such as unsaturation (the most important factor), temperature, presence of oxygen, light, moisture, heavy metals and antioxidants. Antioxidants prevent free radical induced cell and biological targets damage by preventing the formation of radicals, scavenging them, or by promoting their decomposition (Young and Woodside 2001). Generally, they can be considered as two main groups, natural and synthetic antioxidants. Among the synthetic types, the most frequently used are butylated hydroxyl anisole (BHA), butylated hydroxyl toluene (BHT), propyl gallate (PG) and tert-butyl hydroquinone (TBHQ). In most countries, the usage level of synthetic antioxidants is regulated and the safety of the compounds involved has been tested based on long-term toxicity studies. Much of the interest on naturally occurring antioxidants is developed because of the trend to minimize or avoid the use of synthetic food additives (Shahidi 2005). Yanishlieva and Marinova (2001) have reviewed the studies applying natural antioxidants for stabilization of edible oils until the year 2000. In the present study, it has been tried to cover relevant works in the last 12 years with a focus on researches which have assessed the antioxidant activity in oil matrix through Schaal oven test instead of model systems.
Safety and regulatory considerations of synthetic antioxidants
Synthetic antioxidants have been tested for safety and approval for use in food at low concentrations on the basis of complex toxicity studies. Although synthetic antioxidants have been widely used in most countries, there is still doubt about their safety (Shahidi 2005). An antioxidant should have two conditions to be considered as safe: its LD50 must not be less than 1,000 mg/kg body weight, and the antioxidant should not have any significant effect on the growth of the experimental animal in long-term studies at a level 100 times greater than that proposed for human consumption (Lehman et al. 1951). Approval of an antioxidant for food application also requires extensive toxicological studies of its possible mutagenic, teratogenic and carcinogenic effects (Pokorny et al. 2000), It has been proved that antioxidants may share a number of toxic properties at high concentrations (Shahidi 2005). According to the studies of Lanigan and Yamarik (2002), BHT had bad effects on rat’s liver, kidney and lung because of their potential action as carcinogenesis. Some studies have proved BHA and BHT to be cytotoxic because of the carcinogenicity of BHA in the forestomach of rodents (Saito et al. 2003; Verhagen et al. 1991; Sarafian et al. 2002; Farag et al. 2003), thus, some decisions have been made by governments to reduce the use of synthetic antioxidants in foods. It is notable that adverse effects of synthetic antioxidants are observed only at high concentrations. On the other hand, some studies reported that the dosage of synthetic antioxidants which currently are using in foods, not only have no adverse effect on human but also have anticarcinogenic and antimutagenic properties and some other beneficial effects (Whysner et al. 1994; Hirose, et al. 1999; Slamenova et al. 2003; Valenzuela et al. 2003; Williams et al. 1999). According to Williams et al. (1999), BHA and BHT pose no cancer hazard and to the contrary, may be anticarcinogenic at current levels of use. The safety of synthetic antioxidants is always a controversial discussion because of their possible toxic effects during long-term intake. It seems logical that if there is a little possibility of being harmful of synthetic antioxidants, we try to replace them with natural antioxidants that are more compatible with human nature.
The usage level, approval, type and application of an antioxidant may be different among countries. Many countries have adopted regulations similar to the United States regarding the usage of these antioxidants. Table 1 shows regulations governing the use of synthetic antioxidants in Canada and the United States and Acceptable Daily Intake (ADI) Levels of them.
Table 1.
Regulations governing the use of synthetic antioxidants and their ADI levels
| Antioxidant | United States | Canada | ADI | Reference |
|---|---|---|---|---|
| BHA | 50 ppm alone or in combination with BHT | Not to exceed 0.02%, alone or in combination with BHT, PG or TBHQ | 0–0.5 mg/kg body weight (bw) | Shahidi 2005; JECFA 2003a |
| BHT | 50 ppm alone or in combination with BHA | Not to exceed 0.02%, alone or in combination with BHA, PG or TBHQ | 0–0.3 | Shahidi 2005; JECFA 2003b |
| Propyl gallate | Not to exceed 0.02% of fat content including essential (volatile) oil content | Not to exceed 0.02%, alone or in combination with BHA, BHT or TBHQ | 0–1.4 | Shahidi 2005; JECFA 2003c |
| TBHQ | Not to exceed 0.02% of fat content including essential (volatile) oil, Alone or in combination with BHA and/or BHT | Not to exceed 0.02% alone or in combination with BHA or BHT or PG | 0–0.7 | Shahidi 2005; JECFA 2003d |
Use of natural antioxidants in edible oils
In recent years, phenolic compounds of plant origin have attracted considerable attention due to their beneficial functional and nutritional effects including antioxidant and antimicrobial activity (Bubonja-Sonje et al. 2011). During the past two decades, a lot of researches about the use of natural plant extracts with antioxidant activities in various edible oils have been carried out. Some of relevant cases have been shown in Table 2. The main goal of these researches was to reduce the application of synthetic compounds as antioxidants because of their potential negative health effects and as a result of consumer demand.
Table 2.
Use of natural extracts with antioxidant properties in edible oils
| Reference | Major antioxidant compounds | Applied edible oil | Solvent | Antioxidant source |
|---|---|---|---|---|
| Leonardis et al. (2007) | Hydroxytyrosol tyrosol, Caffeic acid and Ferulic acid | Lard | Ethyl acetate | Olive oil mill wastewater |
| Abd-ElGhany et al. (2010) | N.D.a | Sunflower oil | Ethanol | Olive waste cake extract |
| Bera et al. (2006) | N.D. | Flaxseed oil | N-hexan | Ajowan (Carum copticum) |
| Assimopoulou et al. (2004) | Alkannin and Shikonin | Lard, corn oil, olive oil and sunflower oil | Dichloromethane | Tinctoria roots |
| Jennings and Akoh (2009) | Carnosic acid α-tocopherol, EDTA | Rice bran oil | – | Rosemary extract, Carnosic acid, Ethylenediamine tetraacetic acid (EDTA), and α-tocopherol |
| Di Mattia et al. (2009) | Gallic acid, Catechin and Quercetin | Olive oil-in-water emulsions | – | Gallic acid, Catechin and Quercetin |
| Mariod et al. (2006) | N.D. | sunflower oil | Methanol | Guiera senegalensis leaves, Guiera senegalensis roots and Combretum hartmannianum leaves |
| Michotte et al. (2011) | Myricetin (flavonol), Catechin (flavanol), Genistein (isoflavone), and Caffeic acid (Hydroxycinnamic acid) | Linseed oil | – | Myricetin (Flavonol), Catechin (Flavanol), Genistein (Isoflavone), and Caffeic acid (Hydroxycinnamic acid) |
| Bandak et al. (2011) | N.D. | Corn oil | Ethyl acetate | Majorana syriaca |
| Mohamed and Awatif (1997) | Sesamol | sunflower oil | N-hexane | Sesame oil unsaponifiable matter |
| Suja et al. (2004) | Sesamol | Soybean, sunflower, and safflower oils | Methanol | Sesame cake |
| Singh et al. (2009) | N.D. | – | Water | Residues of Artemisia scoparia (Sagebrush or Wormweed) |
| Du and Li (2008) | N.D. | Rapeseed oil, soybean oil, Palm oil, peanut oil, and sunflower seed oil | Water | Steam distillated extract of Cinnamomum cassia haulm and peel |
| Ahn et al. (2008) | N.D. | Microencapsulated high oleic sunflower oil | Essential oil, water, and ethyl alcohol (4:5:4,w/w) | Rosemary, Broccoli sprout and Citrus |
| Ozcan and Derya (2011) | N.D. | Hazelnut and Poppy oils | Water | Rosemary (Rosmarinus officinalis), Clove (Syzygium aromaticum) and Cinnamon (Cinnamomum zeylanicum) |
| Bouaziz et al. (2008) | Oleuropein and Hydroxytyrosol | Refined and husk olive oils | Methanol and water (80:20 v/v) | Chemlali olive leaves |
| Erkan et al. (2009) | Carnosic acid and Sesamol | Sunflower oil | – | Carnosic acid and Sesamol |
| Devi et al. (2007) | Oryzanols, Tocols and Ferulic acid | Soybean oil | Methanol | Defatted Rice bran |
| Farhoosh et al. (2011) | N.D. | Sunflower oil | Ethanolic KOH(1 N) | Unsaponifiable matter of Bene hull oil |
| Iqbal and Bhanger (2007) | N.D. | Sunflower oil | Methanol, Ethanol, iethyl ether, acetone, hexane and ethyl acetate | Garlic |
| Kiokias et al. (2009) | β-Carotene, Lycopene, Marigold, Paprika and Bixin | Sunflower oil in water emulsions | – | Carotenoids |
| Kulisica et al. (2004) | N.D. | Water and n-pentane | Oregano | |
| Wanasundara and Shahidi (1998) | N.D. | Seal blubber oil and Menhaden oil | Ethyl acetate | Green tea |
| Pan et al. (2007a) | N.D. | Peanut oil | Ethanol | Cortex fraxini |
| Pan et al. (2007b) | N.D. | Peanut oil | Ethanol | Polygonum cuspidatum |
a ND Not determined
Olive antioxidants
Natural antioxidants could be present in any parts of plants including leaf, stem, root, seed, fruit, bark, etc. Most parts of olive plant are a suitable source for natural antioxidants like olive oil, kernel and leaf which have been tested for antioxidant activity in edible oils by many researchers such as Bouaziz et al. (2008). They used Chemlali olive leaves and its hydrolysate extract in refined and husk olive oils. In this case, peroxide values, absorption coefficients K270 and K232, Rancimat induction time, sterols and fatty acid contents were determined during 6 months storage at 50 °C in order to evaluate oxidative stability. It was concluded that hydrolysate extracts at 400 ppm had the highest protective effect against oil oxidation (even more than BHT). During storage, there was no significant variation in fatty acid composition. However, the total sterol concentration of the oils treated with hydrolysate extract increased. The application of the leaf extract of two varieties (Koroneiki and Roghani) of olive plant in sunflower oil has been studied by Rafiee et al. (2011a, b, c). The results of peroxide value and thiobarbitoric acid index revealed that methanol extract of Cronaiky at 1,000 ppm could control the oxidation of sunflower oil comparable to BHA and BHT at 100 and 200 ppm levels.
Extraction of antioxidants from olive waste materials is more economical. Leonardis et al. (2007) used phenolic compounds extracted from olive oil mill wastewater for oxidative stabilization of lard. Every 100 g of waste water yielded 50 mg of total phenol. Their results showed that natural extracted phenolic compounds had a good antioxidant activity and could be used for oxidative stabilization of lard. Cytotoxicity assay showed that a phenolic extract at the dose of 100–200 ppm, did not inhibit cell growth. In another research by Abd-Elghany et al. (2010), olive waste cake extract (which is considered as an agricultural waste), was used to improve oxidative stability of sunflower oil. Antioxidants were extracted by ethanol and were added at levels of 100, 200, 400 and 600 ppm to samples of sunflower oil heated at 180 ± 5°C for 4 h/day, for 5 consecutive days. As it has been shown in Table 3, changes in peroxide value, TBA value, free fatty acids, oxidized fatty acids, polar compounds, iodine value and Rancimat oxidative stability of sunflower oil compared to crude sunflower oil and sunflower oil treated with 200 ppm BHT were analyzed. In all cases, the crude sunflower oil showed the lowest oxidative stability whereas the quality of sunflower oil increased at higher concentrations of olive waste extract. They concluded that concentration of 200 ppm and higher of olive cake extract is more effective in increasing the protection factor and calculated oxidative stability at ambient temperature and shelf-life than BHT. Other works on olive natural antioxidants have been mentioned in Table 2.
Table 3.
Changes in qualitative parameters (Data from Abd-Elghany et al. (2010)) of sunflower oil treated with different levels of olive waste cake extract (100, 200, 400 and 600 ppm named as, SFO + OWC1, SFO + OWC2, SFO + OWC3 and SFO + OWC4, respectively) compared to crude sunflower oil (SFO) and sunflower oil treated with 200 ppm BHT (SFO + BHT) during heating at 180 ± 5°C
| Heating period (h) | SFO | SFO + BHT | SFO + OWC1 | SFO + OWC2 | SFO + OWC3 | SFO + OWC4 |
|---|---|---|---|---|---|---|
| Peroxide value (meq O2/kg) | ||||||
| 0 | 0.87 | 0.87 | 0.87 | 0.87 | 0.87 | 0.87 |
| 4 | 1.98 | 1.21 | 1.35 | 1.20 | 1.10 | 1.01 |
| 8 | 3.25 | 2.98 | 3.16 | 2.97 | 2.50 | 2.25 |
| 12 | 5.22 | 4.82 | 4.98 | 4.78 | 4.35 | 4.12 |
| 16 | 6.38 | 5.43 | 5.68 | 5.40 | 5.12 | 4.93 |
| 20 | 7.12 | 6.29 | 6.85 | 6.30 | 6.05 | 5.88 |
| TBA value (mg malonaldehyde/kg) | ||||||
| 0 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 |
| 4 | 2.68 | 2.25 | 2.34 | 2.20 | 2.01 | 1.82 |
| 8 | 5.76 | 4.36 | 4.52 | 4.21 | 4.03 | 2.24 |
| 12 | 7.89 | 6.62 | 6.78 | 6.35 | 6.10 | 5.62 |
| 16 | 8.25 | 7.73 | 7.89 | 7.46 | 7.22 | 6.75 |
| 20 | 8.93 | 7.89 | 7.98 | 7.58 | 7.30 | 6.89 |
| Free fatty acids(as % oleic acid) | ||||||
| 0 | 0.078 | 0.078 | 0.078 | 0.078 | 0.078 | 0.078 |
| 4 | 0.091 | 0.086 | 0.088 | 0.087 | 0.084 | 0.080 |
| 8 | 0.108 | 0.094 | 0.096 | 0.095 | 0.090 | 0.086 |
| 12 | 0.139 | 0.109 | 0.112 | 0.109 | 0.105 | 0.100 |
| 16 | 0.192 | 0.130 | 0.134 | 0.132 | 0.126 | 0.118 |
| 20 | 0.235 | 0.189 | 0.200 | 0.191 | 0.172 | 0.165 |
| Oxidized fatty acids (%) | ||||||
| 0 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 |
| 4 | 0.13 | 0.22 | 0.26 | 0.20 | 0.18 | 0.18 |
| 8 | 0.59 | 0.45 | 0.50 | 0.41 | 0.38 | 0.35 |
| 12 | 0.98 | 0.76 | 0.81 | 0.65 | 0.50 | 0.50 |
| 16 | 1.29 | 0.99 | 1.12 | 0.92 | 0.86 | 0.80 |
| 20 | 2.35 | 1.96 | 2.13 | 1.83 | 1.70 | 1.65 |
| Polar compounds (%) | ||||||
| 0 | 2.12 | 2.12 | 2.12 | 2.12 | 2.12 | 2.12 |
| 4 | 2.85 | 2.68 | 2.72 | 2.66 | 2.46 | 2.32 |
| 8 | 3.75 | 2.95 | 2.98 | 2.92 | 2.70 | 2.48 |
| 12 | 4.86 | 3.85 | 3.98 | 3.80 | 3.58 | 3.29 |
| 16 | 6.79 | 4.98 | 5.21 | 4.98 | 4.39 | 3.98 |
| 20 | 8.38 | 7.65 | 7.88 | 7.56 | 7.20 | 6.75 |
| Iodine value | ||||||
| 0 | 130.21 | 130.21 | 130.21 | 130.21 | 130.21 | 130.21 |
| 4 | 125.20 | 127.35 | 126.45 | 127.45 | 128.12 | 129.11 |
| 8 | 122.62 | 125.15 | 123.82 | 125.50 | 126.35 | 128.34 |
| 12 | 118.35 | 120.65 | 119.23 | 120.75 | 123.25 | 127.15 |
| 16 | 110.22 | 115.34 | 114.46 | 117.38 | 120.15 | 124.28 |
| 20 | 104.50 | 110.23 | 109.45 | 112.25 | 116.50 | 121.26 |
| Induction period in Rancimat oxidative stability test (h) | ||||||
| 8 | 10 | 10.6 | 12.5 | 17.8 | 25.5 | |
*Data from Abd-Elghany et al. (2010)
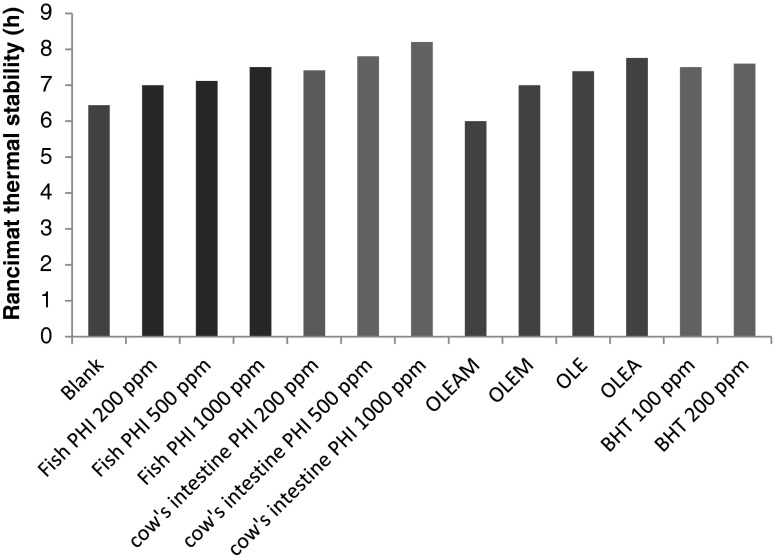
Application of olive plant antioxidants have been shown to be very effective in stabilization of various edible oils whereas the usage of antioxidants from olive waste materials is more economical. On the other hand, applying olive plant extract changes not only the color of oil to green (similar to olive oil), but also it gives an unpleasant bitter taste to the oil. These are the most important problems which should be considered in the future studies. The encapsulation of olive leaf extract could be a possible solution for these problems which was examined by Taghvaei et al. (2013). The microwave-assisted olive leaf extract (OLE) encapsulated by Arabic gum and maltodextrin were used in order to stabilization of soybean oil. As it has been illustrated in Fig. 1, OLE encapsulated with Arabic gum showed the most oxidative protection activity (more than BHT at 100 and 200 ppm).
Fig. 1.
Rancimat analysis of soybean oil samples containing fish protein hydrolysis isolate (PHI), cow’s intestine PHI, olive leaf extract (OLE), OLE encapsulated by Arabic gum (OLEA), OLE encapsulated by maltodextrin (OLEM), OLE encapsulated by a mixture of Arabic gum and maltodextrin (OLEAM) and BHT at 110 °C (Taghvaei et al. 2013)
Sesame antioxidants
Sesame oil has a special and strong antioxidant called sesamol which is made of two parts: sesamin and sesamolin. In a study by Mohamed and Awatif (1997), sesame oil unsaponifiable matter was used as a natural antioxidant in sunflower oil. The sesame seeds were roasted at 180 °C for 30 min before saponification and unsaponifiable matter were added individually in sunflower oil at levels of 0.02, 0.05 and 0.1% and their effectiveness were compared with a control (no additives) sample at 63°C. Their result showed an effective antioxidative activity which increased at higher concentrations of natural antioxidants. Also, roasting treatment increased the level of effective antioxidant compounds and these researchers recommended sesame oil unsaponifiable matter as an alternative natural antioxidant for food applications. In another study, Suja et al. (2004) used methanol extract of sesame cake in soybean, sunflower, and safflower oils, and antioxidant activity of the extract was evaluated by Schaal oven method and differential scanning calorimetry analysis. Their results revealed that sesame cake extract, at concentrations of 5, 10, 50 and 100 ppm, could significantly (P < 0.05) decrease the diene value, peroxide value and p-anisidine value of vegetable oils during storage at 60 °C. They found that sesame cake extract had better antioxidant effect than BHT at 200 ppm.
Although sesame has a strong oxidation prevention capacity, but it’s not economical to apply sesame just for antioxidant extraction. The consumption of synthetic antioxidants is very economical for edible oil producers, hence in order to substitute a natural antioxidant with its synthetic rival; it needs to be more effective in oxidation prevention, and at the same time, more economical. Mohamed and Awatif (1997) and Suja et al. (2004) proved that it is possible to extract sesame antioxidants more economically with sesame oil production byproducts.
Green tea antioxidants
Green tea is well known for its phenolic compounds with various medicinal effects such as antioxidant effects. The activity of green tea extracts on the oxidation of seal blubber oil and menhaden oil was examined under Schaal oven test at 65°C by Wanasundara and Shahidi (1998). The dechlorophyllized green tea extract was applied to both seal blubber oil and menhaden oil at 100, 200, 500 and 1,000 ppm levels. The antioxidant activity of extract was compared with BHA, BHT and TBHQ at 200 ppm and α-tocopherol at 500 ppm. Green tea extracts at higher concentrations than 200 ppm showed excellent antioxidant activity in both oils and its efficacy was higher than that of BHA, BHT and α-tocopherol, but less than that of TBHQ. Green tea extract also used to protect Turkish dry-fermented sausage (sucuk) against oxidation during the ripening periods and it showed more effectiveness than BHT (Bozkurt 2006). The most important antioxidant compound of green tea is catechin which along with apicatechins, epicatechin gallate, epigallocatechin, and epigallocatechin gallate and other flavonoids makes the green tea extract as a suitable mixture of natural antioxidants with scavenging oxygen radicals and chelating metal ions activity.
Natural antioxidants from medicinal and other plant
Bera et al. (2006) used a new natural antioxidant (soluble ajowan1extract) for preventing oxidation of flaxseed compared with synthetic antioxidants (TBHQ, BHT, EQ). They observed that TBHQ had a higher thermal stability than other antioxidants but considering the cost with ajowan and its use as a spice in food preparations, the mentioned natural antioxidant was more preferred. The antioxidant activity of Alkannin and Shikonin (food colorants) and their derivatives, bearing both the quinone and the hydroquinone moiety, have been studied by Assimopoulou et al. (2004). Lard, corn oil, olive oil and sunflower oil were used as oil substrates for the antioxidant assay. Dichloromethane extract of A. tinctoria roots, containing mainly alkannin esters, presented a good antioxidant activity in lard. The antioxidant activity of alkannin in corn oil was not satisfactory, while monomeric shikonin, combined with citric acid, showed a very moderate antioxidant activity in sunflower oil. The combination of the dichloromethane extract of A. tinctoria roots with caffeic acid resulted in a synergistic effect. Mariod et al. (2006) used two local plant phenolic extracts to enhance the stability of sunflower oil. The antioxidative activities of extracts from Guiera senegalensis leaves, (GSL), Guiera senegalensis roots (GSR) and Combretum hartmannianum leaves (CHL), were evaluated by beta-carotene linoleic acid and DPPH assays in sunflower oil at 70°C. Total phenolic compounds were 240.1, 275.6 and 253.4 mg/g respectively. These researchers showed that plant extracts can effectively inhibit the formation of peroxides. Also, antioxidant activity of 500 mg of these natural extracts was higher than 20 mg BHA in sunflower oil. The GSL extract was the most effective antioxidant followed by the GSR and CHL extract.
Bandak et al. (2011) used Majorana syriaca extract to protect bulk corn oil against oxidation. The addition of Majorana syriaca ethyl acetate extract (200 ppm) led to 28.9–43.2% protection against lipid oxidation. In another study, the chemical composition of hydro-distilled oil (yield 0.17%, w/v), from the residues of Artemisia scoparia (sagebrush or wormweed), was analyzed by Singh et al. (2009). These authors indicated that the residue essential oils (25–200 lg/ml) exhibited a strong antioxidant and radical scavenging activity and it had a good potential for the food and pharmaceutical industry. In a research performed by Du and Li (2008), the steam distillated extract of Cinnamomum cassia haulm and peel was used as an antioxidant in rapeseed oil, soybean oil, palm oil, peanut oil, and sunflower seed oil. The antioxidant activity was determined by evaluating TBA and peroxide values during the frying process of beef. Their results revealed that the antioxidant effect of cassia oil during the frying process was optimum under the conditions of 30 μL cassia oil/250 mL palm oil, 1.5 min at 150 °C.
Ahn et al. (2008) demonstrated antioxidant effect of four natural plants extracts (rosemary, broccoli sprout and citrus) on the microencapsulated high oleic sunflower oil. According to their results, these natural plant extracts can effectively inhibit the lipid oxidation of microencapsulated high oleic sunflower oil. They used a mixture of mentioned natural extracts rather than single one and found that lipid oxidation of microencapsulated high oleic sunflower oil remarkably reduced under the accelerated storage conditions. In another research, antioxidant effects of essential oils from rosemary (Rosmarinus officinalis), clove (Syzygium aromaticum) and cinnamon (Cinnamomum zeylanicum) compared with butylated hydroxyanisole (BHA), were studied on hazelnut and poppy oils by Ozcan and Derya (2011). The essential oils were added to the oils at concentrations of 0.25% and 0.5% and BHA at 0.02% level was added for comparison. Peroxide values were examined at regular intervals under storage at 50 °C in darkness for 14 days. Essential oils showed stronger antioxidant effect when compared with control groups, but BHA was more effective than essential oils. Among the investigated essential oils, the cinnamon oil was the most effective antioxidant on preventing lipid oxidation, which was followed by clove and rosemary oils. Devi et al. (2007) used methanol extracts of defatted rice bran in soybean oil. They used crude methanol extract (CME) and it’s partially purified acetone extract (AE). For further purification of the acetone extract, sequential solvent extraction was employed yielding a lipophilic phase (AE-LP) with hexane and a polar phase (AE-PP) with acetone. The antioxidant activity measured by peroxide value, diene value and p-anisidine value and it followed the order of AE-PP > AE-LP = AE > CME with the activity of AE-PP being equivalent to that of BHT at a 200 ppm level. It was concluded that enhancing in antioxidant activity of extracts might be due to increasing concentration of antioxidants in purified extracts compared with CME.
In another study, Farhoosh et al. (2011) used unsaponifiable matter of bene hull oil as an antioxidant which was composed of hydrocarbons, carotenes, tocols, linear and triterpenic alcohols, methyl sterols, sterols, and triterpenic dialcohols. The sunflower oil had oxidative stability index of 3.54 h which increased by using these fractions. It was concluded that all fractions had significant antioxidant activity in sunflower oil at 50 °C. Efficacy of garlic extract in stabilizing sunflower oil during accelerated storage has been studied by Iqbal and Bhanger (2007). Methanol extract of garlic at three different concentrations, i.e. 250, 500 and 1,000 ppm were added to sunflower oil and BHA and BHT at 200 ppm used for comparison. Evaluation parameters for antioxidant efficacy were weight gain, antioxidant activity index, free fatty acid content, peroxide value, conjugated dienes, conjugated trienes and thiobarbituric acid-reactive substance. The authors suggested garlic as a potential antioxidant for stabilization of sunflower oil. Antioxidant efficacy in decreasing order followed by garlic extract at concentration of 1,000 ppm, BHT, BHA, garlic extract at concentration of 500 ppm, garlic extract at concentration of 250 ppm and control. The antioxidant effect of essential oil from oregano was examined by Kulisica et al. (2004). The β-carotene bleaching test, the 2, 20-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method and TBA value assay were carried out to investigate antioxidant capacity. It was found that antioxidant activity of the oregano essential oil is less effective than ascorbic acid, but comparable with α-tocopherol and BHT. Pan et al. (2007a) used Cortex fraxini ethanol extract in peanut oil to investigate antioxidant activity by the linoleic acid peroxidation method and the free radical scavenging assay. Use of Cortex fraxini extract significantly reduced lipid oxidation and no significant differences were detected between use of Cortex fraxini extract and BHT. In another research by the same authors, Polygonum cuspidatum ethanol extract was used in peanut oil and results almost was the same (Pan et al. 2007b).
The antioxidant activity of toyserkani variety of walnut husk has been examined by Rezai et al. (2011). The TBA and Peroxide value evaluated during 16 days at 60 °C. Result of this study showed that walnut husk is a potential source of natural antioxidants since methanol extract with the concentration of 1,000 ppm could control the oxidation of soybean oil fairly equal to BHT at concentration of 200 ppm. The effect of nettle (Urtica dioica) leaf extract on the inhibition of soybean oil oxidation has been carried out by Gharekhani et al. (2009). The effects of nettle extracts in three concentration levels (200, 500 and 800 ppm) and synthetic antioxidants (BHA and BHT) in two levels (100 and 200 ppm) on the stability of soybean oil were compared through determination of peroxide value and TBA value during 25 days at 60 °C. The results showed the extracts with the concentration of 800 ppm had the highest antioxidant activity comparable with BHT, and more than BHA.
These studies prove that there are so many medicinal plants which can be used for extraction of natural antioxidants but, there are two important factors that should be considered: a) A natural antioxidant resource is better to be ubiquities, not just cultivated in a certain part of world. b) The other uses of these medicinal plants should be considered; maybe it’s not an economical idea to cultivate a plant only for antioxidants extraction. It’s more logical to use well known, ubiquities and proved natural resource for extraction of natural antioxidants.
Use of individual and pure natural antioxidants
Some studies have been performed using each antioxidant compounds individually. Use of four phenolic compounds as antioxidants namely myricetin and catechin as a flavanol), genistein (as isoflavone), and caffeic acid (as a hydroxycinnamic acid) at a concentration of 555 μmol/kg in refined linseed oil, have been examined by Michotte et al. (2011). Their antioxidative effect was assessed according to the Schaal oven test procedure compared with BHA. Caffeic acid, catechin and myricetin were found to be more efficient than BHA. Jennings and Akoh (2009) examined the effectiveness of natural versus synthetic antioxidants in rice bran oil. Oxidative stability index of rice bran oil containing rosemary extract, carnosic acid, TBHQ, ethylenediamine tetraacetic acid (EDTA), and α-tocopherol, and 50:50 (w/w) combinations in concentrations of 200, 300, 400 and 500 ppm were determined in this work. Mean peroxide and p-anisidine values for carnosic acid and carnosic acid/rosemary extract were comparable to TBHQ.
The effect of gallic acid, catechin and quercetin on physical and chemical stability of olive oil-in-water emulsions have been investigated by Di Mattia et al. (2009). Gallic acid showed a low activity towards secondary oxidation. Catechin showed an interfacial localisation which was reflected in the enhancement of primary oxidation and in the inhibition of secondary oxidation. Quercetin was proven not to slow down the bimolecular phase of auto-oxidation. Erkan et al. (2009) investigated antioxidative effects of carnosic acid and sesamol on sunflower oil under temperature-controlled microwave heating. In order to investigate oxidative stability of oil, the concentration of conjugated diene hydroperoxide and p-anisidine value were determined and the temperature of microwave treatments at a frequency of 2450 MHz, was 40 °C, 60 °C and 80 °C. Carnosic acid and sesamol were both effective in reducing the oxidation of sunflower oil upon microwave heating and carnosic acid was found to be a more effective antioxidant than sesamol. The amounts of added antioxidants remaining in sunflower oil after different microwave heating periods at different temperatures were determined by HPLC-DAD in order to specify the effect of microwave on antioxidant composition. Carnosic acid decomposed more than sesamol during microwave heating due to not only the more consumption of carnosic acid as an antioxidant but also the more rapid decomposition of carnosic acid than sesamol under microwave heating. The more antioxidant activity of carnosic acid in spite of its decomposition may be attributed to the possibility that decomposition products of carnosic acid could also show antioxidant activity. Kiokias et al. (2009) examined antioxidant activity of natural carotenoid extracts in the emulsions of sunflower oil in water. A concentration of 2 grl−1 of carotenoids (β-Carotene, tomato (lycopene-rich), marigold (lutein-rich), paprika and bixin) were added to the emulsion and lipid hydro-peroxides and secondary oxidation products were examined. The tested carotenoids did not inhibit production of hydro-peroxides but they significantly retarded the formation of volatile aldehydes during the storage at 30 °C.
In these researches, instead of a mixture of antioxidants in a natural extract, the behavior of an individual antioxidant has been investigated in various edible oils. It has to be mentioned that application of individual natural antioxidants is not applicable in edible oil industry since the separation and purification of an antioxidant compound from a natural extract is not economical but these studies give researchers a better understanding about what they deal with. For instance, which compound in a natural extract is responsible for antioxidant properties?
Use of natural antioxidants from animal resources
Protein hydrolysates isolate (PHI) from animal resources also have been reported to own suitable oxidative prevention activities, such as chicken intestinal mucosa (Damle et al. 2010), smooth hound (Bougatef et al. 2009), sheep visceral mass (Bhaskar et al. 2007) and sardinelle by-products (Bougatef et al. 2010). Sarmadi and Ismail (2010) and Bernardini et al. (2011) also reviewed the antioxidant properties of bioactive peptides from animal sources. In a comparative study, Taghvaei et al. (2013) applied natural antioxidants from both plant resource (olive leaf extract) and animal resources (fish PHI and cow’s intestine PHI) in soybean oil. The antioxidant activity of PHIs and OLE samples during 20 days storage at 55°C was evaluated by peroxide value, TBA value, p-anisidine value and Rancimat stability test. It has been concluded that PHI samples had higher antioxidant activity than OLE and cow’s intestine PHI at 1,000 ppm showed best oxidative protection activity (Fig. 1).
The exact mechanism of oxidation prevention for these peptides has not fully been understood yet. Some studies have revealed that free radicals scavenging, metal ions chelating and oxygen quenching can be the main reasons of oxidation prevention of bio-active peptides (Sarmadi and Ismail 2010). Antioxidant activity of these peptides is more related to their composition, structure, and hydrophobicity.
Thermal stability of natural antioxidants
Many factors such as antioxidant concentration, temperature, pH and processing treatment influence the antioxidant activity (Gazzani et al. 1998). A natural Antioxidant should satisfy several requirements before being accepted for application in food products. The antioxidant should be effective for a least 1 year at a temperature of between 25 and 30 °C; it should be stable to heat processing and protect the finished product (Coppen 1989). The presence of food moisture, atmospheric oxygen and high temperatures could cause various chemical changes and loss of antioxidants such as steam distillation of antioxidants, oxidation of phenolic compounds, decrease in their pro-oxidative activity due to reaction with fried materials and polymerisation (Pokorny et al. 2000). Synthetic antioxidants such as BHA and BHT are more susceptible to steam distillation (Warner et al. 1986). Losses of natural antioxidants are only small since their volatility is much lower than that of common synthetic antioxidants (Pokorny et al. 2000).
Chamorro et al. (2012) investigated the effect of thermal treatments on polyphenolic content and antioxidant activity in grape pomace and grape seed extract. The heat treatments were 100 °C in a furnace or autoclave for 15, 30 and 60 min. The antioxidant activity was determined by ABTS, DPPH and photo-chemiluminescence methods. Their results showed the antioxidant activity and total polyphenols content of grape seed extract or grape pomace did not change during autoclave treatment but it caused an extensive hydrolysis of gallocatechin (70%), catechin (61%), epicatechin (65%), procyanidin B1 (75%) and procyanidin B2 (73%) in grape seed extract, and an increase in gallic acid (71%), gallocatechin (100%) and epicatechin gallate (129%) in grape pomace was determined by HPLC–MS. These modifications were not related to the changes in antioxidant activity. Thermal stability of Aegle marmelos leaf extracts were evaluated by Reddy and Urooj (2011). Aegle marmelos leaves were extracted with methanol (ME), ethanol (EE), and water (WE) at a temperature of 100 °C for 15 min. WE showed maximum stability to high temperature as measured by RSA. The thermal stability of antioxidants in four vegetable oils by heat flux differential scanning calorimetry (DSC) and electron spin resonance spectroscopy (ESR) were studied by Giffrida et al. (2007). The sample oils were sunflower oil and high oleic sunflower oil, low trans fatty acid oil and partially hydrogenated palm oil and the studied antioxidants were ascorbyl palmitate, α-tocopherol, γ-tocopherol and propyl gallate at a concentration of 300 mg/kg. Their results confirmed that strongest antioxidant was γ-tocopherol and propyl gallate for sunflower oil and partially hydrogenated palm oil, respectively.
Santos et al. (2012) evaluated thermal stability of commercial synthetic antioxidants and some natural antioxidants by TG/DTA technique with both dynamic and isothermal (110 °C) analysis methods. They found that synthetic antioxidants exhibited thermal resistances in the following order: PG > TBHQ > BHA > BHT and natural antioxidants displayed stabilities in the following order: α-tocopherol > caffeic acid > ferulic acid > gallic acid. Cruz et al. (2007) analyzed the thermal stability of three biomass-derived fractions with antioxidant activity (ethyl acetate soluble-fraction from Eucalyptus globulus acid hydrolysates, ethyl acetate soluble-fraction of auto-hydrolysis liquors from red grape pomace after fermentation and distillation and washing water of the same feedstock) and two synthetic food antioxidants, BHA and BHT. The non-volatile fraction, the antioxidant activity and the percentage of recovered phenolic in solid phase were measured at 100, 150 or 200 °C in assays lasting up to 120 min. The result showed the ethyl acetate solubles from acid hydrolysates of Eucalyptus globulus wood, from auto-hydrolysis and from washing of distilled red grape pomace showed higher thermal stability than BHA or BHT. In addition, the natural derived antioxidants showed more antioxidant activity after the heating treatment. It has been proved that encapsulation could improve the thermal stability of natural antioxidants (Taghvaei et al. 2013). As it has been shown in Fig. 1, the OLE encapsulated by Arabic gum enhanced the thermal stability of soybean oil more than OLE. The reason could be attributed to protection of natural antioxidants against destructive factors by encapsulation.
It can be concluded that some natural antioxidants not only have a stronger oxidation prevention capacity than synthetic antioxidants, but also they have more thermal stability and can remain more active after heat treatments in comparison with synthetic antioxidants.
Conclusions and further remarks
Considering adverse effects of synthetic antioxidants at high concentrations and low thermal stability of them in heat processing and frying of food products, it seems logical to substitute synthetic antioxidants with natural ones. According to many studies performed in the past two decades, there are lots of natural antioxidants which can be extracted from low cost resources with more antioxidant activity and thermal stability than synthetic ones in various edible oils. For example, green tea extract which is prepared from tea wastes can be introduced as suitable natural antioxidants in the food industry. Also, rosemary extract and β-carotene and tocopherols rich extracts may be used more frequent in the future. Many of these studies show that it is possible to extract and apply natural antioxidants instead of the synthetic ones in the edible oil industry. In the study of Yanishlieva and Marinova (2001) Also tea extract and rosemary extract has been suggested as a suitable substitute for synthetic antioxidants. The authors have concluded that the safety concerns must be addressed in order to use natural antioxidants in future studies. In the past decade, the main focus of studies in this area was to introduce more influential natural antioxidants which most of them were from plant resources and generally were considered as safe. For instance, if we use olive extract at very low concentrations, there is no need to investigate the toxic effects of such extract in human. But some unrecognized natural antioxidants have been introduced and it is necessary to examine their possible toxic effects on human body.
It has been shown that animal PHIs also have suitable antioxidant activity and could be more effective than some natural antioxidants from plant resources and synthetic antioxidants. There is need for more studies to apply animal PHIs in edible oils and assess the long term stability of oil since in the most studies in this area the antioxidant activity have been examined through model systems.
It sounds that more works also need to be done to scale up and commercialize the application of these natural compounds in the food formulations. The synergistic effects of natural antioxidants with each other also need to be examined.
Footnotes
Carum copticum
Contributor Information
Mostafa Taghvaei, Email: m.taghvaii@gmail.com.
Seid Mahdi Jafari, Phone: +98-9113551373, Email: smjafari@gau.ac.ir.
References
- Abd-ElGhany ME, Ammar MS, Hegazy AE. Use of olive waste cake extract as a natural antioxidant for improving the stability of heated sunflower oil. World Appl Sci J. 2010;11(1):106–113. [Google Scholar]
- Ahn JH, Kim YP, Seo EM, Choi YK, Kim HS. Antioxidant effect of natural plant extracts on the microencapsulated high oleic sunflower oil. J Food Eng. 2008;84:327–334. doi: 10.1016/j.jfoodeng.2007.05.029. [DOI] [Google Scholar]
- Assimopoulou AN, Boskou D, Papageorgiou VP. Antioxidant activities of alkannin, shikonin and Alkanna tinctoria root extracts in oil substrates. Food Chem. 2004;87:433–438. doi: 10.1016/j.foodchem.2003.12.017. [DOI] [Google Scholar]
- Bandak G, Dermesonlouglou EK, Taoukis PS, Oreopoulou V. Antioxidant effect of Majorana syriaca extract in bulk corn oil and o/w emulsion after applying high hydrostatic pressure. Food Chem. 2011;125:1166–1170. doi: 10.1016/j.foodchem.2010.10.022. [DOI] [Google Scholar]
- Bera D, Lahiri D, Nag A. Studies on a natural antioxidant for stabilization of edible oil and comparison with synthetic antioxidants. J Food Eng. 2006;74:542–545. doi: 10.1016/j.jfoodeng.2005.03.042. [DOI] [Google Scholar]
- Bernardini RD, Harnedy P, Bolton D, Kerry J, O’Neill E, Mullen AM, Hayes M. Antioxidant and antimicrobial peptidic hydrolysates from muscle protein sources and by-products. Food Chem. 2011;124:1296–1307. doi: 10.1016/j.foodchem.2010.07.004. [DOI] [Google Scholar]
- Bhaskar N, Modi KV, Govindavaju K, Radh C. Utilization of meat industry by products: protein hydrolysate from sheep visceral mass. J Bioresour Technol. 2007;98:388–394. doi: 10.1016/j.biortech.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Bouaziz M, Fki I, Jemai H, Ayadi M, Sayadi S. Effect of storage on refined and husk olive oils composition: stabilization by addition of natural antioxidants from Chemlali olive leaves. Food Chem. 2008;108:253–262. doi: 10.1016/j.foodchem.2007.10.074. [DOI] [Google Scholar]
- Bougatef A, Hajji M, Balti R, Lassoued I, Triki-Ellouz Y, Nasri M. Antioxidant and free radical-scavenging activities of smooth hound (Mustelus mustelus) muscle protein hydrolysates obtained by gastrointestinal proteases. Food Chem. 2009;114:1198–1205. doi: 10.1016/j.foodchem.2008.10.075. [DOI] [Google Scholar]
- Bougatef A, Nedjar-Arroume N, Manni L, Ravallec R, Barkia A, Guillochon D, Nasri M. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 2010;118:559–565. doi: 10.1016/j.foodchem.2009.05.021. [DOI] [Google Scholar]
- Bozkurt H. Utilization of natural antioxidants: green tea extract and Thymbravspicata oil in Turkish dry-fermented sausage. Meat Science. 2006;73:442–450. doi: 10.1016/j.meatsci.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Bubonja-Sonje M, Giacometti J, Abram M. Antioxidant and antilisterial activity of olive oil, cocoa and rosemary extract polyphenols. Food Chem. 2011;127:1821–1827. doi: 10.1016/j.foodchem.2011.02.071. [DOI] [Google Scholar]
- Chamorro S, Goni I, Viveros A, Hernandez DH, Brenes A. Changes in polyphenolic content and antioxidant activity after thermal treatments of grape seed extract and grape pomace. Eur Food Res Technol. 2012;234:147–155. doi: 10.1007/s00217-011-1621-7. [DOI] [Google Scholar]
- Coppen PP. Rancidity in foods. London: Elsevier Applied Science; 1989. [Google Scholar]
- Cruz JM, Conde E, Dominguez H, Parajo JC. Thermal stability of antioxidants obtained from wood and industrial wastes. Food Chem. 2007;100:1059–1064. doi: 10.1016/j.foodchem.2005.11.012. [DOI] [Google Scholar]
- Damle MV, Harikumar P, Jamdar SN. Debittering of protein hydrolysates using immobilized chicken intestinal mucosa. Process Biochem. 2010;45:1030–1035. doi: 10.1016/j.procbio.2010.03.016. [DOI] [Google Scholar]
- Devi RR, Jayalekshmy A, Arumughan C. Antioxidant efficacy of phytochemical extracts from defatted rice bran in the bulk oil system. Food Chem. 2007;104:658–664. doi: 10.1016/j.foodchem.2006.12.014. [DOI] [Google Scholar]
- Di Mattia CD, Sacchetti G, Mastrocola D, Pittia P. Effect of phenolic antioxidants on the dispersion state and chemical stability of olive oil O/W emulsions. Food Res Int. 2009;42:1163–1170. doi: 10.1016/j.foodres.2009.05.017. [DOI] [Google Scholar]
- Du H, Li H. Antioxidant effect of Cassia essential oil on deep-fried beef during the frying process. Meat Science. 2008;78:461–468. doi: 10.1016/j.meatsci.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Erkan N, Ayranci G, Ayranci E. A kinetic study of oxidation development in sunflower oil under microwave heating: effect of natural antioxidants. Food Res Int. 2009;42:1171–1177. doi: 10.1016/j.foodres.2009.06.003. [DOI] [Google Scholar]
- Farag RS, El-Baroty GS, Basuny A. Safety evaluation of olive phenolic compounds as natural antioxidants. Int J Food Sci Nutr. 2003;54:159–174. doi: 10.1080/0963748031000136306. [DOI] [PubMed] [Google Scholar]
- Farhoosh R, Tavassoli-Kafrani M, Sharif A. Antioxidant activity of the fractions separated from the unsaponifiable matter of bene hull oil. Food Chem. 2011;126:583–589. doi: 10.1016/j.foodchem.2010.11.047. [DOI] [Google Scholar]
- Gazzani G, Papetti A, Massolini G, Daglia M. Antioxidative and pro-oxidant activity of water soluble components of some common diet vegetables and the effect of thermal treatment. J Agric Food Chem. 1998;6:4118–4122. doi: 10.1021/jf980300o. [DOI] [Google Scholar]
- Gharekhani M, Ghorbani M, Ebrahimzadeh MA, Jafari SM, Sadeghi MA. Effect of nettle (Urtica dioica) leaves extract on the inhibition of soybean oil oxidation. Food Process Preserv. 2009;1:85–102. [Google Scholar]
- Giuffrida F, Destaillats F, Egart MH, Hug B, Golay PA, Skibsted LH, Dionisi F. Activity and thermal stability of antioxidants by differential scanning calorimetry and electron spin resonance spectroscopy. Food Chem. 2007;101:1108–1114. doi: 10.1016/j.foodchem.2006.03.010. [DOI] [Google Scholar]
- Hirose M, Takhashi S, Ogawa K, Futakuchi M, Shirai T, Shibutani M, Uneyama C, Toyoda K, Iwata H. Chemoprevention of heterocyclic amine-induced carcinogenesis by phenolic compounds in rats. Cancer Lett. 1999;143:173–178. doi: 10.1016/S0304-3835(99)00120-2. [DOI] [PubMed] [Google Scholar]
- Iqbal S, Bhanger MI. Stabilization of sunflower oil by garlic extract during accelerated storage. Food Chem. 2007;100:246–254. doi: 10.1016/j.foodchem.2005.09.049. [DOI] [Google Scholar]
- JECFA (2003a) Additive-068 Butylated hydroxyanisole, URL http://www.fao.org/ag/agn/jecfa-additives/search.html. Accessed 19.08.11
- JECFA (2003b) Additive-069 Butylated hydroxytoluene, URL http://www.fao.org/ag/agn/jecfa-additives/search.html. Accessed 19.08.11
- JECFA (2003c) Additive-357 Propyl gallate, URL http://www.fao.org/ag/agn/jecfa-additives/search.html. Accessed 19.08.11
- JECFA (2003d). Additive-459 Tertiary butylhydroquinone, URL http://www.fao.org/ag/ agn/jecfa- additives/search.html. Accessed 19.08.11
- Jennings BH, Akoh CC. Effectiveness of natural versus synthetic antioxidants in a rice bran oil-based structured lipid. Food Chem. 2009;114:1456–1461. doi: 10.1016/j.foodchem.2008.11.031. [DOI] [Google Scholar]
- Kiokias S, Dimakou C, Oreopoulou V. Activity of natural carotenoid preparations against the autoxidative deterioration of sunflower oil-in-water emulsions. Food Chem. 2009;114:1278–1284. doi: 10.1016/j.foodchem.2008.10.087. [DOI] [Google Scholar]
- Kulisica T, Radonicb A, Katalinicc V, Milosa M. Use of different methods for testing antioxidative activity of Oregano essential oil. Food Chem. 2004;85:633–640. doi: 10.1016/j.foodchem.2003.07.024. [DOI] [Google Scholar]
- Lanigan RS, Yamarik TA. Final report on the safety assessment of BHT. Int J Toxicol. 2002;21:19–94. doi: 10.1080/10915810290096513. [DOI] [PubMed] [Google Scholar]
- Lehman AJ, Fitzhugh OG, Nelson AA, Woodard G. The pharmacological evaluation of antioxidants. Adv Food Res. 1951;3:197–208. doi: 10.1016/S0065-2628(08)60261-X. [DOI] [PubMed] [Google Scholar]
- Leonardis A, Macciola V, Lembo G, Aretini A, Nag A. Studies on oxidative stabilisation of lard by natural antioxidants recovered from olive-oil mill wastewater. Food Chem. 2007;100:998–1004. doi: 10.1016/j.foodchem.2005.10.057. [DOI] [Google Scholar]
- Mariod A, Matthäus B, Hussein IH. Antioxidant activities of extracts from Combretum hartmannianum and Guiera senegalensis on the oxidative stability of sunflower oil. J Agricaltural Sci. 2006;18:20–28. [Google Scholar]
- Michotte D, Rogez H, Chirinos R, Mignolet E, Campos D, Larondelle Y. linseed oil stabilisation with pure natural phenolic compounds. Food Chem. 2011;129:1228–1231. doi: 10.1016/j.foodchem.2011.05.108. [DOI] [PubMed] [Google Scholar]
- Mohamed HMA, Awatif II. The use of sesame oil unsaponifiable matter as a natural antioxidant. Food Chem. 1997;62:269–276. doi: 10.1016/S0308-8146(97)00193-3. [DOI] [Google Scholar]
- Ozcan MM, Derya A. Antioxidant effect of essential oils of rosemary, clove and cinnamon on Hazelnut and poppy oils. Food Chem. 2011;129:171–174. doi: 10.1016/j.foodchem.2011.01.055. [DOI] [Google Scholar]
- Pan Y, Zhu J, Wang H, Zhang X, Zhang Y, He C, Ji X, Li H. Antioxidant activity of ethanol extract of Cortex fraxini and use in peanut oil. Food Chem. 2007;103:913–918. doi: 10.1016/j.foodchem.2006.09.044. [DOI] [Google Scholar]
- Pan Y, Zhang X, Wang H, Liang Y, Zhu J, Li H, Zhang Z, Wua Q. Antioxidant potential of ethanol extract of Polygonum cuspidatum and application in peanut oil. Food Chem. 2007;105:1518–1524. doi: 10.1016/j.foodchem.2007.05.039. [DOI] [Google Scholar]
- Pokorny J, Yanishlieva N, Gordon M. Antioxidants in food practical applications. New York: CRC press; 2000. [Google Scholar]
- Rafiee Z, Jafari SM, Alami M, Khomeiri M (2011a) Antioxidant effect of microwave-assisted extracts of olive leaves on sunflower oil. Journal of Agricultural Science and Technology, In press
- Rafiee Z, Jafari SM, Alami M, Khomeiri M. Antioxidant properties of olive leaf extract and its application in sunflower oil. J Food Sci Res. 2011;21:12–24. [Google Scholar]
- Rafiee Z, Jafari SM, Alami M, Khomeiri M. Microwave-assisted extraction of phenolic compounds from olive leaves; A comparison with maceration. J Animal Plant Sci. 2011;21:738–745. [Google Scholar]
- Reddy VP, Urooj A (2011) Antioxidant properties and stability of aegle marmel os leaves extracts. Journal of Food Scientists & Technology. In press [DOI] [PMC free article] [PubMed]
- Rezai ES, Jafari SM, Khomeiri M, Bayat H. Antioxidant activity of toyserkani variety of walnut husk and comparison of its antiradical activity with synthetic antioxidants. J Food Sci Res. 2011;21:40–50. [Google Scholar]
- Saito M, Sakagami H, Fujisawa S. Cytotoxicity and apoptosis induction by butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) Anticancer Res. 2003;23:4693–4701. [PubMed] [Google Scholar]
- Santos NA, Cordeiro AMTM, Damasceno SS, Aguiar RT, Rosenhaim R, Filho JRC, Carvalho JR, Santos IMG, Maia AS, Souza AG (2012) Commercial antioxidants and thermal stability evaluations. Fuel, In press
- Sarafian TA, Kouyoumjian S, Tashkin D, Roth MD. Synergistic cytotoxicity of 9-tetrahydrocannabinol and butylated hydroxyanisole. Toxicol Lett. 2002;133:171–179. doi: 10.1016/S0378-4274(02)00134-0. [DOI] [PubMed] [Google Scholar]
- Sarmadi BH, Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010;31:1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Shahidi F. Bailey’s industrial oil and fat products. New Jersey: John Wiley & Sons; 2005. [Google Scholar]
- Singh HP, Mittal S, Kaur S, Batish DR, Kohli RK. Chemical composition and antioxidant activity of essential oil from residues of Artemisia scoparia. Food Chem. 2009;114:642–645. doi: 10.1016/j.foodchem.2008.09.101. [DOI] [Google Scholar]
- Slamenova D, Horvathova E, Robichova S, Hrusovska L, Gabelova A, Keibl K, Jakubikova J, Sedlak J. Molecular and cellular influences of butylated hydroxyanisole on Chinese hamster V79 cells treated with N-methyl-N′-nitro-N nitrosoguanidine: Antimutagenicity of butylated hydroxyanisole. Environ Mol Mutagen. 2003;41:28–36. doi: 10.1002/em.10127. [DOI] [PubMed] [Google Scholar]
- Suja KP, Abraham JT, Thamizh SN, Jayalekshmy A, Arumughan C. Antioxidant efficacy of sesame cake extract in vegetable oil protection. Food Chem. 2004;84:393–400. doi: 10.1016/S0308-8146(03)00248-6. [DOI] [Google Scholar]
- Taghvaei M, Jafari SM, Sadeghi-Mahoonak A, Mehregan-Nikoo A, Rahmanian N, Hajitabar J, Meshginfar N (2013) The effect of natural antioxidants extracted from plant and animal resources on the oxidative stability of soybean oil. LWT - Food Science and Technology, under review
- Valenzuela A, Sanhueza J, Nieto S. Cholesterol oxidation: health hazard and the role of antioxidants in prevention. Biol Res. 2003;36:291–302. doi: 10.4067/s0716-97602003000300002. [DOI] [PubMed] [Google Scholar]
- Verhagen H, Schilderman PAEL, Kleinjans JCS. Butylated hydroxyanisole in perspective. Chem Biol Interact. 1991;80:109–134. doi: 10.1016/0009-2797(91)90019-4. [DOI] [PubMed] [Google Scholar]
- Wanasundara UN, Shahidi F. Antioxidant and pro-oxidant activity of green tea extracts in marine oils. Food Chem. 1998;63(3):335–342. doi: 10.1016/S0308-8146(98)00025-9. [DOI] [Google Scholar]
- Warner CR, Brumley WC, Daniels DH, Joe JFL, Fazio T. Reactions of antioxidants in foods. Food Chem Toxicol. 1986;24:10–15. doi: 10.1016/0278-6915(86)90282-6. [DOI] [PubMed] [Google Scholar]
- Whysner J, Wang CX, Zang E, Iatropoulos MJ, Williams GM. Dose response of promotion by butylated hydroxyanisole in chemically initiated tumours of the rat forestomach. Food Chem Toxicol. 1994;32:215–222. doi: 10.1016/0278-6915(94)90193-7. [DOI] [PubMed] [Google Scholar]
- Williams GM, Iatropoulos MJ, Whysner J. Safety assessment of butylated hydroxyanisole and butylated hydroxytoluene as antioxidant food additives. Food Chem Toxicol. 1999;37:1027–1038. doi: 10.1016/S0278-6915(99)00085-X. [DOI] [PubMed] [Google Scholar]
- Yanishlieva NV, Marinova EM. Stabilisation of edible oils with natural antioxidants. European J Lipid Sci Technol. 2001;103:752–767. doi: 10.1002/1438-9312(200111)103:11<752::AID-EJLT752>3.0.CO;2-0. [DOI] [Google Scholar]
- Young IS, Woodside JV. Antioxidants in health and disease. J Clin Patholology. 2001;54:176–186. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]