Abstract
Optimum water extraction conditions for phenolics of pomegranate peels were investigated by fractional factorial and face-centered central composite designs. Five potential factors were selected for the fractional factorial design: extraction technique, extraction temperature, extraction time, particle size and solvent to solid ratio. After eliminating statistically unimportant factors, a face-centered central composite design was set up with two controllable factors and with two responses: total phenolics and α-glucosidase inhibition activity. Optimum conditions were found as 100 °C for extraction temperature and 1 min for extraction time. There were no statistically significant differences (p > 0.05) between water extracts at optimized conditions and classical methanol extracts. Total phenolic content by HPLC was192.0 mg/g of pomegranate peels on dry matter basis. Phenolics of pomegranate peels showed α-glucosidase inhibition activity with an IC50 (concentration of phenolics required to inhibit 50 % of the enzyme activity) value of 5.56 ± 2.23 μg/ml. Pomegranate peel phenolics with its antioxidant and α-glucosidase inhibition properties might be a suitable ingredient for functional food applications.
Keywords: Pomegranate peel, Central composite design, Fractional factorial design, Alpha glucosidase inhibition
Introduction
Pomegranate (Punica granatum L.), mainly produced in Middle Eastern countries, has been reported to have several nutritional and health benefits (Lansky and Newman 2007; Mirsaeedghazi et al. 2012). Beneficial health effects of pomegranate fruit are due to the components in flowers, seeds, arils and peels. The peels constitute approximately 40 % of the whole fruit and are rich in ellagic acid derivatives such as the ellagitannins, punicalagin, and punicalin. In addition, some ellagic acid derivatives (ellagic acid hexoside, pentoside, etc.) are also present, although in lesser amounts (Seeram et al. 2005). Aqueous extracts of pomegranate peel has been shown to display antioxidant (Malviya et al. 2013) and antibacterial activity (Devatkal et al. 2013). Punicalagin, the main phenolic compound of the peel, has shown remarkable biological activities including anti-inflammatory, hepatoprotective and antigenotoxic activities (Aqil et al. 2012).
Researchers mostly express the beneficial effects of any plant material by antioxidant capacity/activity, however, certain components have more specific abilities beyond antioxidant action. There is increasing evidence that individual phenolics or classes of phenolics may cause other beneficial effects, independent of their antioxidant capacities, by directly influencing the activities of key enzymes (McDougall et al. 2005). There have been reports that phenolic fractions from plants can cause insulin-like effects in glucose utilization. Phenolic extracts from a number of materials were found to be effective inhibitors of intestinal α-glucosidase activity (McDougall et al. 2005; Nwosu et al. 2011). Phenolic compounds of certain fruits have been shown to exert α-glucosidase inhibition properties such as muscadine (You et al. 2012), blueberry, and blackcurrant (McDougall et al. 2005), elderberry, and boysenberry (Matsui et al. 2001).
Phenolics of pomegranate peels have been extracted mostly by methanol and/or combinations of methanol and other organic solvents by classical extraction techniques (Cam and Hisil 2010; Wang et al. 2011). The main advantage of water as extraction solvent is its low price, good availability and environmental safety. However, water has some drawbacks in phenolic extractions such as limited solvation power on phenolics at mild temperatures. The properties of water change markedly when it is heated as the hydrogen-bonded lattice is disrupted as thermal motion increases. As the temperature rises there is a marked and systematic decrease in permittivity, an increase in diffusion rate and a decrease in the viscosity and surface tension (Smith 2002). Phenolic extraction might be improved by increasing the extraction temperature, however, certain phenolics are prone to the oxidation at elevated temperatures with elongated extraction time. Optimization of process factors in phenolic extraction would be beneficial in order to limit phenolic oxidation as well as to use process factors at optimum levels which prevent unnecessary costs.
Experimental design and optimization are tools that are used to systematically examine different types of problems that arise within research, development and production (Lundstedt et al. 1998; Myers and Montgomery 1995). The first step of any optimization study should be started with assigning of candidate factors which might be obtained from a literature search or careful examination of the system to study. A suitable design such as factorial or fractional factorial is constructed to screen the candidates and eliminate the unimportant factors. After assigning significant factors and levels a convenient experimental design, Box-Behnken, Central composite etc., is set up to gather data and optimize the system responses based on predetermined inputs and outputs.
The aim of present study, for the first time, was to optimize the extraction of phenolics from pomegranate peels with water by applying principles of experimental design, and to determine α-glucosidase inhibition potentials of phenolics of pomegranate peels.
Materials and methods
Materials
Pomegranate peels were obtained from a pomegranate juice production plant (Göknur A.Ş, Niğde, Turkey). The peels were open air dried at room temperature avoiding direct sunlight, and then the dried peels were ground. The grinded peels were passed through two different standard sieves (Prüfsieb Jel 200, Germany). Three different particle size fractions were collected (<212 μm, 212–500 μm, and >500 μm). The fractions were kept in a polyethylene pouches in a freezer at −18 °C. For comparison, 500 g of the peels were freeze dried by a bench top freeze dryer (Labconco, Missouri, USA).
Chemicals
HPLC grade water (18.2 MΩ cm) was prepared using a Millipore Simplicity 185 water purification system (Millipore Corp, Darmstad, Germany). Folin-Ciocalteu’s phenol reagent, methanol, gallic acid, ellagic acid, KH2PO4, Na2CO3, NaOH, α-amylase, Baker’s yeast α-glucosidase, p-nitrophenyl α-D-Glucoside, L-Glutathione, were obtained from Sigma-Aldrich Chemical Company (St. Louis, USA). Punicalagin standard was purified from pomegranate peels (Cerda et al. 2003). Syringe filters made of nitrocellulose (Millipore, Bedford, USA) or polytetrafluoroethylene (Sartorious, Goettingen, Germany) with pore size of 0.45 μm were used to filter aqueous or organic extracts, respectively.
Extraction
Phenolics of pomegranate peels were extracted with water using different combinations of the factors. At basic conditions, 1 g of grinded pomegranate peels (particle sizes <212 μm) was weighed into a screw-capped vial, and water at 25 °C was added to adjust the exact solvent to solid ratio of 50 ml/g. A magnetic stirrer bar (25 mm × 8 mm) was placed into the vial. Extraction was performed at 25 °C for 30 min using a magnetic stirrer (Daihan Sci. Co.,Seoul, Korea). After centrifuge (Nüve, Ankara, Turkey) at 3,000 g for 5 min, resulting supernatant was collected, filtered through a 0.45 μm membrane filter and stored at −20 °C until analyzed. The extracts were stored no more than 1 day. Extractions were performed in two stages by changing the factor levels using fractional factorial design principles in the first and central composite design principles in the second stage as indicated in the related section.
Phenolic extracts were further purified into anthocyanins, and non-anthocyanin phenolics using C18 Sep-pak cartridges (Skrede et al. 2000).
Pomegranate peels dried either at atmospheric conditions or freeze dried were also extracted with methanol and water at optimum conditions using a magnetic stirrer in order to determine the effects of drying method and solvent type on phenolic yield. One gram of the peels was extracted with 50 ml of methanol at 40 °C for 30 min. Methanolic extracts were filtered by a 0.45 μm filter prior to analysis.
Experimental design
In order to select appropriate factors on extraction process six potential factors were listed as extraction solvent, extraction technique, temperature, time, particle size, and solvent to solid ratio after literature search and evaluating the whole extraction system. A fractional factorial design was selected to conduct appropriate and limited number of experiments. At this stage of the study, it was aimed to determine the main effects of the factors as interaction effects were out of the scope. Factorial designs are convenient for selection of the variables, however with some fractional factorial experiments the number of experiments may be more reduced without losing the necessary information. Five of the 6 factors were included into the fractional factorial design. Solvent type was assigned as fixed factor, thus, water was selected as extraction solvent because of one of the aim of the study that was extraction of the phenolics with water.
After determining the important factors, a new design was set up with two variables to explore the effects of factors on extraction results after assigning the particle size factor as fixed. Experiments were randomized and obtained extracts analyzed with minimum 2 times and results were averaged.
Analysis of the extracts
Total phenolic content
Total phenolic content (TPC) of the extracts was determined according to a modified colorimetric method (Singleton et al. 1999). Briefly, 0.5 ml of a 10–100-fold diluted extract was mixed with 2.5 ml of 10 fold diluted Folin & Ciocalteu’s phenol reagent and incubated for 1 min, before 2 ml of 7.5 % Na2CO3 was added. The mixture was allowed to stand for 30 min. The absorbance versus prepared blank was read at 765 nm (Shimadzu UV-1700 spectrophotometer, Shimadzu Corp, Kyoto, Japan). Five different concentrations of gallic acid solutions (20–100 mg/l) were used for calibrations. The final results were expressed as mg gallic acid equivalent (GAE) per g of dry matter.
Alpha-amylase inhibition assay
Sigma-Aldrich’s quality control test procedure (Bernfeld 1955) for enzymatic assay of α-amylase was used with slight modification to determine the α-amylase inhibitory activity of the extracts. Porcine pancreatic α-amylase solution (1 unit/ml) was prepared in 20 mmol/l NaH2PO4 buffer (pH 6.9) containing 6.7 mmol/l NaCl. As substrate starch solution (1 %, w/v) was prepared using the same buffer solution. Colour reagent to detect the reducing sugar resulted from hydrolysis of starch by α-amylase enzyme was prepared by mixing a 5.31 mol/l sodium potassium tartrate solution in 2 mol/l NaOH and 96 mmol/l 3,5-dinitrosalicylic acid solution. One milliliter of starch solution, extract solution and buffer were transferred into a screw capped vial. The mixtures were vortexed, placed in a rack and let to equilibrate at 37 °C in a water bath (Heat Tech., Thermo Sci., Darmstad, Germany). The reaction was started with the addition of 1 ml α-amylase enzyme solution. One milliliter of colour reagent was added into the vials which then incubated in a boiling water bath for 15 min. The vials cooled on ice to room temperature. The absorbance of the resulting orange-yellow to red mixture was determined at 540 nm.
Alpha-glucosidase inhibition assay
Alpha glucosidase inhibition activity of the extracts was determined according to the method of Ryu et al. (2010) with slight modifications. Briefly, 0.2 unit/ml of α-glucosidase (50 μl) in cold 67 mmol/l KH2PO4 (pH 6.8), 3 mmol/l glutathione solution (50 μl) in order to activate supply proper enzyme action, and properly diluted extracts (50 μl) were pipetted into a screw capped vial followed by addition of 1,250 μl of phosphate buffer for proper dilution. The mixtures were vortexed, placed in a rack and let to equilibrate at 37 °C in a water bath. Ten millimoles per litre of p-Nitropheyl α-D-Glucoside (125 μl) was pipetted into each mixture to start the enzyme action. The reaction was stopped by addition of 100 mmol/l Na2CO3 (2 ml) at 20 min. Control samples were prepared by adding water instead of extracts, and blank sample was prepared adding equal volume of water instead of extracts and enzyme solution. Absorbance of the resulting pale yellow colour, resulted from releasing p-Nitropheyl from the substrate, was recorded at 400 nm.
Calculation of %inhibition and IC50 values
Alpha amylase and α-glucosidase inhibitory activities of the extracts were expressed as percentage inhibition by following equation:
IC50 values were also calculated for tested materials. By definition, IC50 is the concentration of phenolics required to inhibit 50 % of the enzyme activity (Shobana et al. 2009). In order to determine the IC50 values, the enzyme activity of α-glucosidase was determined in the presence of the pomegranate peel extract at various concentrations (1–10 mg/l). IC50 values were determined based on graphs constructed by % inhibition vs. phenolic concentration. Phenolics of pomegranate peels were ineffective on α-amylase, therefore IC50 value for α-amylase inhibition was not calculated.
HPLC-DAD analysis of phenolics
Phenolics of the extracts were determined according to an HPLC method (Cam and Hisil 2010) by a Shimadzu (Kyoto, Japan) Prominence UFLC system equipped with a CBM-20A communications bus module, two LC-20ADXR pumps, a SIL-20ACXR auto-sampler, a DGU-20A5 degasser, a CTO-20AS VP column oven and a SPD-M20A photodiode array detector (DAD). Data were collected and analyzed using LC Solution 1.25 software. Chromatographic separations of the extracts were carried out on a Zorbax C18 column (250 × 4.6 mm, particle size 5 μm, Agilent) using water/acetic acid (98:2, v/v) (Solvent A) and methanol (Solvent B) as the mobile phases at a flow rate of 1 ml/min. The elution program used was as follows: 5 % B for 5 min, 5–70 % B for 25 min and 70–5 % B for 10 min. The column temperature was maintained at 35 °C and the detection was monitored at 280, 365 and 378 nm. The amounts of punicalagin and its derivatives, ellagic acid and its derivatives were calculated from chromatograms that recorded at 378 nm and 365 nm, respectively. UV spectra of the components were taken continuously between 200 and 800 nm throughout the elution in order to determine component identity and peak purity. Stock solutions of punicalagin (1,000 mg/l) and ellagic acid (80 mg/l) were prepared in water/methanol (1:1). The stock solutions were then further diluted to target concentrations. The extracts were filtered by 0.45 μm filter prior to 10 μl of injection to HPLC.
Statistical analyses
Designing of experimental points, randomization, analysis of variance, and fitting of quadratic models were carried out by Design Expert 7.0.0 (Stat-Ease Inc., Minneapolis, MN). One-way analysis of variance (ANOVA) and Tukey test were performed using the SPSS 17.0.1 statistical package for Windows (SPSS Inc., Chicago, IL).
Results and discussion
Screening experiments by fractional factorial designs
In this part of the study, it was aimed to gather maximum information about phenolic extraction process by conducting minimum number of experiments. This was done by following the principles of response surface methodology. TPC by Folin & Ciocalteu’s Phenol reagent was selected as system response to observe the effects of factors on extraction yield. TPC assay, technically simple and quick, gives precise results in the experiments, measures the sample’s reducing capacity, and gives excellent correlations with other antioxidant capacity methods (Huang et al. 2005). TPC was considered as a suitable method because of the aforementioned properties, and the method was in harmony with the idea of getting robust and fast information about the studied system. Response surface methodology comprises a group of statistical techniques for empirical model building and model exploitation. By careful design and analysis of experiments, it seeks to relate a response or output variable to the levels of a number of predictors or input variables that affect it. A variable initially believed unimportant turns out to have a major effect. A good compromise is to employ a preliminary screening design such as a two-level fractional factorial design to pick out variables worthy of further study (Box and Draper 2007). In order to select the most important factors a fractional factorial design was selected both to observe main effects of the factors and keeping the number of experiments in manageable number. To investigate the k variables in a full factorial design, 2k experiments are needed. An experimenter needs to perform 32 experiments in full factorial design with 5 variables. However, if interaction is not required at this stage, the number of the experiments can be reduced by applying the principles of fractional factorial design. In fractional factorial designs, the number of experiments is reduced by a number p according to a 2k−p design where p is the size of the fraction. The size of the fraction will influence the possible number of effects to estimate and, of course, the number of experiments needed. A half fraction (25−1) design was used to determine the effective factors on extraction system. This type of designs are also known as resolution V designs in which the main effects are confounded with four-variable interaction effects, and the two-variable interaction effects are confounded with the three-variable interaction effects (Lundstedt et al. 1998).
Table 1 shows the factors and their levels in 25−1 design. Sixteen experiments were performed and responses as TPC were evaluated with analysis of variances (ANOVA). In ANOVA, all main effects had one degree of freedom, the rest of the variance sources were assigned as residuals. Particle size, temperature and time affected the system response significantly in descending importance order.
Table 1.
Experimental factors, their levels and statistical analysis of fractional factorial design
| Factor | Type | Notation | Levels | F value | P value* | |
|---|---|---|---|---|---|---|
| −1 | +1 | |||||
| Temperature | Numerical | A | 25 | 75 | 13.6 | 0.0042 |
| Time | Numerical | B | 1 | 30 | 6.6 | 0.0279 |
| Solvent to solid ratio | Numerical | C | 10 | 50 | 0.03 | 0.8685 |
| Particle size | Categorical | D | <212 μm | >500 μm | 29.0 | 0.0003 |
| Extraction type | Categorical | E | Soaking | Stirring | 0.8 | 0.3887 |
*P values with less than 0.05 (bold type) are statistically significant
After assigning particle size, temperature and time as significant factors, temperature and time were decided to include second stage of the experimental design. Although particle size was the most effective factor on phenolic extraction from pomegranate peels at this point particle size was assigned as fixed factor. It might be more efficient to use smaller sizes of particles which need handling of pomegranate peels with sophisticated sieving systems, however, this was beyond the scope of the study. Last but not least, using smaller size of particles resulted in elongation of total extraction time since sedimentation of the mixture would be more difficult. Extraction time and extraction temperature was determined as significant factors. Solvent to solid ratio was not significant which might be resulted from selected factor interval as 10–50 ml/g.
Face-centered central composite design
After screening for the important factors that affects the phenolic extraction from pomegranate peels using a fractional factorial design, a face-centered central composite design with two factors was performed in order to achieve the optimum conditions for phenolic extraction. This design was selected from a dozen of possible designs since it is a cubic design contains axial points that are situated at a distance α from the centre of the design. Alpha takes a value of ±1 in this design (Cam and Aaby 2010; Massart et al. 1997). There was a special condition in this study in terms of controlling the extraction temperature. An independent factor might be controllable and the level must be changeable according to the preset values. Since boiling point of water is 100 °C it was impossible to adjust and control the temperature of water beyond 100 °C in the extraction system of this study. Table 2 shows the coded and actual levels of experimental factors and the resulting responses, that is, total phenolics by HPLC (HPLC-TPC) as Y1 and α-glucosidase inhibition activity (%) as Y2.
Table 2.
Face-centered central composite design and experimental data (factors vs. responses)
| Run | Factors | Responses | ||
|---|---|---|---|---|
| A (Temperature, °C)* | B (Time, min)* | Y1 (Phenolics, mg/g) | Y2 (Alpha glucosidase inhibition, %) | |
| 1 | 60 (0) | 1 (−1) | 174.0 | 86.1 |
| 2 | 100 (+1) | 60 (+1) | 178.5 | 89.6 |
| 3 | 100 (+1) | 1 (−1) | 192.0 | 95.3 |
| 4 | 60 (0) | 30.5 (0) | 180.2 | 77.3 |
| 5 | 60 (0) | 60 (+1) | 178.3 | 86.4 |
| 6 | 100 (+1) | 30.5 (0) | 184.8 | 88.8 |
| 7 | 60 (0) | 30.5 (0) | 179.5 | 79.0 |
| 8 | 20 (−1) | 30.5 (0) | 156.7 | 70.6 |
| 9 | 60 (0) | 30.5 (0) | 181.7 | 79.2 |
| 10 | 60 (0) | 30.5 (0) | 175.2 | 76.6 |
| 11 | 20 (−1) | 1 (−1) | 143.0 | 75.8 |
| 12 | 60 (0) | 30.5 (0) | 174.7 | 83.1 |
| 13 | 20 (−1) | 60 (+1) | 153.9 | 76.3 |
*Values in parenthesis are the coded levels of the factors
The experiments were performed at the specified experimental points by following the run order (Table 2), and then the extracts were analyzed by HPLC for phenolics and UV–vis. spectroscopy forα-glucosidase inhibition activity determinations. The results from minimum 2 determinations were averaged and subjected to ANOVA and residual analysis of the constructed quadratic models. The data was enough to construct a second order model and no need to replicate any experimental points since residuals did not follow a pattern and scattered randomly for each response.
It should be noted that statistically significant (p < 0.01) correlation coefficient (r2 = 0.690) was found between total phenolics and α-glucosidase inhibition activity of the extracts. Due to the high correlation coefficient between total phenolics and α-glucosidase inhibition activity of the extracts we could say that phenolics play a major role in α-glucosidase inhibition mechanism.
Tables 3 and 4 show the ANOVA data and the other statistical terms to check the adequacy of the constructed models for HPLC-TPC and α-glucosidase inhibition activity results, respectively. As shown in the Tables 3 and 4, quadratic models were enough to explain the system behaviour. One important parameter to check the model is lack of fit, found as insignificant implying the fitness of the model. All model parameters for two responses were found adequate to use the models for predicting and navigating purposes within design space.
Table 3.
Analysis of variances for HPLC-phenolics
| Source | Sum of squares | df | Mean square | F value | P-value* |
|---|---|---|---|---|---|
| Model | 2165.2 | 4 | 541.3 | 42.7 | < 0.0001 |
| A-temperature | 1723.8 | 1 | 1723.8 | 135.9 | < 0.0001 |
| B-time | 0.5 | 1 | 0.5 | 0.04 | 0.8504 |
| AB | 148.8 | 1 | 148.8 | 11.7 | 0.0090 |
| A2 | 292.0 | 1 | 292.0 | 23.0 | 0.0014 |
| Residual | 101.5 | 8 | 12.7 | ||
| Lack of fit | 62.3 | 4 | 15.6 | 1.6 | 0.3319 |
| Pure error | 39.2 | 4 | 9.8 | ||
| Corrected total | 2266.6 | 12 | |||
| R 2 | 0.9552 | ||||
| Adjusted-R 2 | 0.9338 | ||||
| Predicted-R 2 | 0.8029 | ||||
| Adequate precision | 20.8690 |
*p values with less than 0.05 (bold type) are statistically significant
Table 4.
Analysis of variances for α-glucosidase inhibition activity
| Source | Sum of squares | df | Mean square | F value | P-value* |
|---|---|---|---|---|---|
| Model | 542.0 | 3 | 180.7 | 36.2 | < 0.0001 |
| A-temperature | 433.5 | 1 | 433.5 | 86.8 | < 0.0001 |
| B-time | 4.0 | 1 | 4.0 | 0.8 | 0.3940 |
| B2 | 104.5 | 1 | 104.5 | 20.9 | 0.0013 |
| Residual | 44.9 | 9 | 5.0 | ||
| Lack of fit | 19.4 | 5 | 3.9 | 0.6 | 0.7021 |
| Pure error | 25.5 | 4 | 6.4 | ||
| Corrected total | 587.0 | 12 | |||
| R 2 | 0.9234 | ||||
| Adjusted-R 2 | 0.8979 | ||||
| Predicted-R 2 | 0.8291 | ||||
| Adequate precision | 18.9630 |
*p values with than 0.05 (bold type) are statistically significant
The quadratic equations in terms of actual factors were as follows:
where A represents extraction temperature and B represent extraction time.
Second order models were constructed by taking into account model hierarchy and excluding the unimportant factors and factor interactions. Prediction for specific points and optimization are possible with the help of these equations as long as staying within design space. For optimization, independent variables were selected as in range, and the goal for responses was entered as maximize, several possible solutions were proposed by Design Expert software. Maximization of the quadratic equations was performed by desirability function method that combines all the responses into one measurement (Eren and Kaymak-Ertekin 2007), and desirability value might be between 0 and 1. The proposed optimum point with highest desirability value (0.967) was 100 °C for temperature and 1 min for extraction time.
Additional triplicate experiments were performed at optimum point to check the predicting ability and verify the models. The results from verification experiment at optimum point were 187.1 ± 7.0 for phenolics by HPLC that was predicted by the model as 190.9, while 95.3 ± 3.1 for α-glucosidase inhibition activity that was predicted as 94.2.
Extraction of phenolics at 100 °C gave the higher results among tested temperatures, the higher the temperature the higher the phenolic yield and α-glucosidase inhibition activity. One minute for extraction time was enough to extract the phenolics from pomegranate peels. An increase in the amounts of phenolics might be expected with elongated extraction time. Both for extraction temperatures of 20 and 60 °C, extraction yields increased when extraction time was extended from 1 to 60 min (Table 2). For instance, extension of extraction time from 1 to 60 min while keeping the extraction temperature at 20 °C resulted in improvement in phenolics yield from 143 to 153.9 mg/g. A similar situation was observed for the extraction time of 60 °C. However, the amount of phenolics was decreased with elongated extraction time at 100 °C. This might be due to the destructive effect of 100 °C on phenolics combined with elongated extraction time. Similar results have been noted from a study in which phenolics have been extracted from pomegranate marc with water at various temperature and time combinations, extraction rate had the highest for highest temperatures as well as phenolic extraction reached its equilibrium at extraction time of 1 min at 95 °C and stayed almost stable for additional 3 min (Qu et al. 2010). Effects of elongated extraction time at 95 °C remained unclear since no information was noted.
Comparison of drying methods and extraction methods
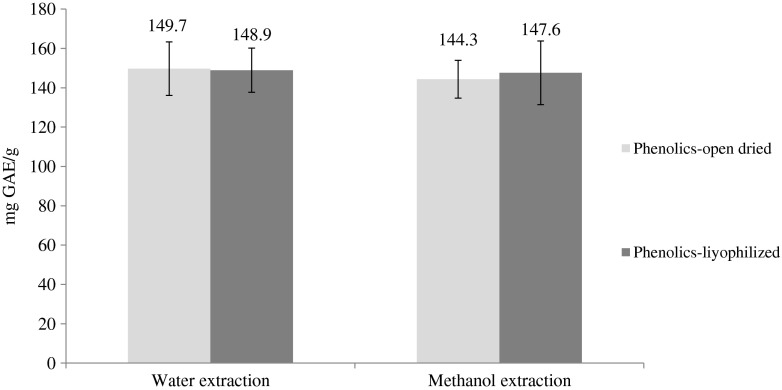
To check the efficiency of optimized phenolic extraction with water the results were compared with methanol extraction. Phenolics were also extracted from open air dried and freeze dried pomegranate peels to compare the effects of drying conditions on phenolic yields. Figure 1 shows that there is no significant difference between water extraction and methanol extraction results in terms of TPC. There were also no differences between drying methods. Therefore, there is no need to follow strict temperature conditions when drying pomegranate peels. This information is useful for large scale extraction process which might need drying huge amount of pomegranate peels.
Fig. 1.
Comparison of extraction and drying methods. Bars show mean ± S.D of three replicates, There are no differences among the results p > 0.05)
Our results, both from methanol and water extractions, are higher than those obtained by Viuda-Martos et al. (2012) who reported the total phenolic content of pomegranate peel in a co-product form in the juice extraction process as 54.84 mg/g. However, our results are lesser than the study carried out by Cam and Hisil (2010) who reported the total phenolic content of pomegranate peel as 258.2 mg/g. Such differences might be due to the cultivars of pomegranate grown in each area or pomegranate juice production practises which eventually affects the final content of pomegranate peel.
Apha amylase and alpha glucosidase inhibition potentials of pomegranate peel extracts
Pomegranate peel extracts were found as ineffective on α-amylase enzyme but strongly effective on α-glucosidase enzyme inhibition. These results were in agreement with the previous findings (Kam et al. 2012). Alpha glucosidase inhibition activity as IC50 value of pomegranate peel extract was found 5.56 ± 2.23 μg/ml. This activity might be attributable to punicalagins. Total phenolic content of the peels by HPLC was 192.0 mg/g based on dry matter. Punicalagins, ellagitannin type main phenolics of pomegranate peels, constituted more than 98 % of the total phenolics while the rest was ellagic acid and ellagic acid derivatives. Phenolics of the peel were further fractionated into anthocyanins and non-anthocyanin phenolics to check the superiority of the fractions in terms of α-glucosidase inhibition activity. Table 5 shows the IC50 values of pomegranate peel phenolic fractions. Results clearly showed the inhibitive effects of phenolics of pomegranate peels on α-glucosidase enzyme. There were no statistically significant differences among the tested fractions in α-glucosidase inhibition activity.
Table 5.
Effects of phenolics on α-amylase and α-glucosidase enzymes
| Material | IC50 (μg/ml) | |
|---|---|---|
| α-amylase inhibition | α-glucosidase inhibition* | |
| Pomegranate Peel Phenolics | Non-effective | 5.56 ± 2.23a |
| Anthocyanin fraction | Non-effective | 5.81 ± 2.08a |
| Non-anthocyanin phenolic fraction | Non-effective | 6.22 ± 1.91a |
*Values in the same column with the same superscript letters are not significantly different (p > 0.05)
Diabetes mellitus is a chronic metabolic disease characterized by hyperglycemia, resulting from insufficient or inefficient insulin secretion, with alterations in carbohydrate, protein and lipid metabolism (Shobana et al. 2009). Type I diabetes is exclusively an autoimmune disorder, insulin therapy is only a possible remedy whereas type II diabetes is mainly due to insulin resistance induced complications (Prathapan et al. 2012). Complex polysaccharides are hydrolyzed by α-amylase to oligosaccharides that are further hydrolyzed to liberate glucose by intestinal α-glucosidase before being absorbed into the intestinal epithelium and entering blood circulation (Ademiluyi and Oboh 2013; Prathapan et al. 2012). Type II diabetes is more prevalent than Type I diabetes. In fact, Type II diabetes accounts for 90 % to 95 % of diagnosed cases (Bevan 2006). An effective strategy for type II diabetes management is the strong inhibition of intestinal α-glucosidases and mild inhibition of pancreatic α-amylase (Apostolidis et al. 2007a). Inhibition of these enzymes with synthetic or natural components can be important strategies for managing type II diabetes, however, abdominal distention, flatulence, meteorism and possibly diarrhea are the main side effects of synthetic drugs, resulting from excessive inhibition of α-amylase (Apostolidis et al. 2007b). In recent years plant derived medicines have received great deal of attention compared to synthetic ones, due to their potent antioxidant activities, very less side effects and economic viability (Nampoothiri et al. 2011).
Phenolics of pomegranate peel as natural compounds have potential to use as functional ingredient in terms of α-glucosidase inhibition activity. Currently glycosidase inhibitors attract considerable attention due to their promising therapeutic potential in the treatment of diabetes (Prathapan et al. 2012). Properly optimized phenolic phytochemical enriched diets could have a role in developing less expensive complimentary strategies for type 2 diabetes and hypertension management concurrent combined with other nutritional and pharmacological strategies (Apostolidis et al. 2007b). Phenolics of pomegranate peel might be advantageous as natural inhibitors, both having inhibitive activity on α-glucosidase and non-inhibitive activity on α-amylase. Pomegranate peel phenolics with its promising α-glucosidase inhibitory activity could be used as pharmacological agent for managing type II diabetes and ingredient for producing functional foods.
Conclusion
This study clearly showed the potential use of water as extraction solvent for phenolics in pomegranate peels. For large scale operations, water would be a good choice for phenolic extraction by considering health, cost and environmental issues. Next stage after extraction would be stabilization of the extracts with suitable methods such as drying, microencapsulation etc. Antioxidant properties of pomegranate peel have been well documented but the peels showed striking α-glucosidase inhibition activity. Further in vivo studies are necessary to determine the potentials of pomegranate peels in the management of type II diabetes.
Acknowledgments
This work has been financially supported by the Scientific and Research Council of Turkey (TUBITAK) under the Project Number of 110O594.
References
- Ademiluyi AO, Oboh G. Soybean phenolic-rich extracts inhibit key-enzymes linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro. Exp Toxicol Pathol. 2013;65:305–309. doi: 10.1016/j.etp.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Apostolidis E, Kwon YI, Ghaedian R, Shetty K. Fermentation of milk and soymilk by Lactobacillus bulgaricus and Lactobacillus acidophilus enhances functionality for potential dietary management of hyperglycemia and hypertension. Food Biotechnol. 2007;21:217–236. doi: 10.1080/08905430701534032. [DOI] [Google Scholar]
- Apostolidis E, Kwon YI, Shetty K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov Food Sci Emerg Technol. 2007;8:46–54. doi: 10.1016/j.ifset.2006.06.001. [DOI] [Google Scholar]
- Aqil F, Munagala R, Vadhanam MV, Kausar H, Jeyabalan J, Sschultz DJ, Gupta RC. Anti-proliferative activity and protection against oxidative DNA damage by punicalagin isolated from pomegranate husk. Food Res Int. 2012;49:345–353. doi: 10.1016/j.foodres.2012.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfeld P. Amylases, alpha and beta. In: Colowick SP, Kaplan NO, editors. Methods in enzymology. New York: Academic; 1955. [Google Scholar]
- Bevan JL. Diabetes mellitus: a review of select ADA standards for 2006. J Nurse Pract. 2006;2:674–679. doi: 10.1016/j.nurpra.2006.07.001. [DOI] [Google Scholar]
- Box GEP, Draper NR. Response surfaces, mixtures, and ridge analyses. New Jersey: John Wiley & Sons; 2007. [Google Scholar]
- Cam M, Aaby K. Optimization of extraction of apple pomace phenolics with water by response surface methodology. J Agric Food Chem. 2010;58:9103–9111. doi: 10.1021/jf1015494. [DOI] [PubMed] [Google Scholar]
- Cam M, Hisil Y. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 2010;123:878–885. doi: 10.1016/j.foodchem.2010.05.011. [DOI] [Google Scholar]
- Cerda B, Llorach R, Ceron JJ, Espin JC, Tomas-Barberan FA. Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur J Nutr. 2003;42:18–28. doi: 10.1007/s00394-003-0396-4. [DOI] [PubMed] [Google Scholar]
- Devatkal SK, Jaiswal P, Jha SN, Bharadwaj R, Viswas KN. Antibacterial activity of aqueous extract of pomegranate peel against Pseudomonas stutzeri isolated from poultry meat. J Food Sci Technol. 2013;50:555–560. doi: 10.1007/s13197-011-0351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren I, Kaymak-Ertekin F. Optimization of osmotic dehydration of potato using response surface methodology. J Food Eng. 2007;7:344–352. doi: 10.1016/j.jfoodeng.2006.01.069. [DOI] [Google Scholar]
- Huang DJ, Ou BX, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- Kam A, Li KM, Razmovski-Naumovski V, Namni S, Shi J, Chan K, Li GQ. A comparative study on the inhibitory effects of different parts and chemical constituents of pomegranate ob α-amylase and α-glucosidase. Phytother Res. 2012 doi: 10.1002/ptr.4913. [DOI] [PubMed] [Google Scholar]
- Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109:177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Lundstedt T, Seifert E, Abramo L, Thelin B, Nystrom A, Pettersen J, Bergman R. Experimental design and optimization. Chemometr Intell Lab Syst. 1998;42:3–40. doi: 10.1016/S0169-7439(98)00065-3. [DOI] [Google Scholar]
- Malviya S, Arvind JA, Hettiarachchy N. Antioxidant and antibacterial potential of pomegranate peel extract. J Food Sci Technol. 2013 doi: 10.1007/s13197-013-0956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massart BGMV, Vandeginste BGM, Buydens LMC, De Jong S, Lewi PJ. Handbook of chemometrics and qualimetrics: part A. Amsterdam: Elsevier; 1997. [Google Scholar]
- Matsui T, Ueda T, Oki T, Sugita K, Terahara N, Matsumoto K. Alpha-glucosidase inhibitory action of natural acylated anthocyanins. 1. Survey of natural pigments with potent inhibitory activity. J Agric Food Chem. 2001;49:1948–1951. doi: 10.1021/jf001251u. [DOI] [PubMed] [Google Scholar]
- McDougall GJ, Shpiro F, Dobson P, Smith P, Blake A, Stewart D. Different polyphenolic components of soft fruits inhibit alpha-amylase and alpha-glucosidase. J Agric Food Chem. 2005;53:2760–2766. doi: 10.1021/jf0489926. [DOI] [PubMed] [Google Scholar]
- Mirsaeedghazi H, Mousavi SM, Emam-Djomeh Z, Rzaei K, Aroujalian A, Navidbakhsh M. Comparison between ultrafiltration and microfiltration in the clarification of pomegranate juice. J Food Process Eng. 2012;35:424–436. doi: 10.1111/j.1745-4530.2010.00598.x. [DOI] [Google Scholar]
- Myers RH, Montgomery DC. Response surface methodology, process and product optimization using designed experiments. 2. New York: John Wiley and Sons; 1995. [Google Scholar]
- Nampoothiri SV, Prathapan A, Cherian OL, Raghu KG, Venugopalan VV, Sundaresan A. In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2 diabetes. Food Chem Toxicol. 2011;49:125–131. doi: 10.1016/j.fct.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Nwosu F, Morris J, Lund VA, Stewart D, Ross HA, McDougall GJ. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011;126:1006–1012. doi: 10.1016/j.foodchem.2010.11.111. [DOI] [Google Scholar]
- Prathapan A, Krishna MS, Nisha VM, Sundaresan A, Raghu KG. Polyphenol rich fruit pulp of Aegle marmelos (L.) Correa exhibits nutraceutical properties to down regulate diabetic complications -An in vitro study. Food Res Int. 2012;48:690–695. doi: 10.1016/j.foodres.2012.06.008. [DOI] [Google Scholar]
- Qu WJ, Pan ZL, Ma HL. Extraction modeling and activities of antioxidants from pomegranate marc. J Food Eng. 2010;99:16–23. doi: 10.1016/j.jfoodeng.2010.01.020. [DOI] [Google Scholar]
- Ryu HW, Lee BW, Marcus JCL, Jung S, Ryu YB, Lee WS, Park KH. Polyphenols from Broussonetia papyrifera displaying potent alpha-glucosidase inhibition. J Agric Food Chem. 2010;58:202–208. doi: 10.1021/jf903068k. [DOI] [PubMed] [Google Scholar]
- Seeram N, Lee R, Hardy M, Heber D. Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Sep Purif Technol. 2005;41:49–55. doi: 10.1016/j.seppur.2004.04.003. [DOI] [Google Scholar]
- Shobana S, Sreerama YN, Malleshi NG. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: Mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009;115:1268–1273. doi: 10.1016/j.foodchem.2009.01.042. [DOI] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Oxid Antioxid. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Skrede G, Wrolstad RE, Durst RW. Changes in anthocyanins and polyphenolics during juice processing of Highbush blueberries. J Food Sci. 2000;65:357–364. doi: 10.1111/j.1365-2621.2000.tb16007.x. [DOI] [Google Scholar]
- Smith RM. Extractions with superheated water. J Chromatogr A. 2002;975:31–46. doi: 10.1016/S0021-9673(02)01225-6. [DOI] [PubMed] [Google Scholar]
- Viuda-Martos M, Perez-Alvarez JA, Sendra E, Fernandez-Lopez J. In vitro antioxidant properties of pomegranate (Punica granatum) peel powder extract obtained as coproduct in the juice extraction process. J Food Process Preserv. 2012 [Google Scholar]
- Wang Z, Pan Z, Ma H, Atungulu GG. Extract of phenolics from pomegranate peels. Open Food Sci J. 2011;5:17–25. doi: 10.2174/1874256401105010017. [DOI] [Google Scholar]
- You Q, Chen F, Wang X, Jiang Y, Lin S. Anti-diabetic activities of phenolic compounds in muscadine against alpha-glucosidase and pancreatic lipase. LWT. 2012;46:164–168. doi: 10.1016/j.lwt.2011.10.011. [DOI] [Google Scholar]