Abstract
The objective of this study was to determine vitamin C stability in fresh and freeze-dried capsicum during storage at different temperatures. Fresh capsicum stored at 20 °C showed an initial decrease in vitamin C with a minimum peak after 2 days and then increased to a maximum peak after 13 days followed by a gradual decay. In general a gradual decrease of vitamin C was observed in the cases of fresh (i.e. stored at 5, −20, −40 °C) and freeze-dried capsicum stored at all temperatures (i.e. 60 to −40 °C). The degradation kinetics of vitamin C was modeled by zero and first order reaction and rate constants were estimated. The rate constant increased with the increase in storage temperature, while it was decreased with the decrease of moisture content. At storage temperature 5 °C, first order rate constants were observed as 7.1 × 10−2, 7.7 × 10−2, and 4.3 × 10−3 day−1 in the cases of samples containing moisture contents 94, 15 and 5 g/100 g sample, respectively.
Keywords: Ascorbic acid, Capsicum, Food stability, Vitamin C, Drying
Introduction
The role of fruits and vegetables in reducing the risk of chronic diseases, like cardiovascular diseases and cancer, is well recognized. These health benefits are linked to the important components of vegetables and fruits, which include macronutrients, such as fibers and micronutrients particularly ascorbic acid (Leong and Oey 2012). While many fruits and vegetables are consumed primarily in their fresh state, some produces are also consumed at their processed state. Many vitamins are lost during handling, processing and storage. The retaining ability of vitamins under chemical, physical and/or thermal stress is called stability. Vitamin C or ascorbic acid, a water soluble vitamin, is mainly present in fresh fruits and vegetables. It is the most sensitive and destructed when a commodity is exposed to adverse handling and storage conditions (Lee and Kader 2000). Vitamin C stability in fruits and vegetables was studied during different methods of drying (Goula and Adamopoulos 2006; Marques et al. 2006, 2007; Gupta et al. 2013; Kek et al. 2013) and during storage at chilled or around room temperature (Johnston and Hale 2005; Burdurlu et al. 2006; Torregrosa et al. 2006; Klimczak et al. 2007; Majumdar et al. 2009; Gonzalez-Molina et al. 2012). The loss of vitamin C during storage was considerably lower in freeze dried powder as compared to vacuum, and tunnel dried guava powder (Verma et al. 1993). Leong and Oey (2012) determined the vitamin C stability in pepper (fresh pepper containing 10.8 mg vitamin C/g dry-solids) during heat-treatment (98 °C for 10 min), freeze-drying (48 h), and frozen-thawed (freeze at −20 °C, and thawed at 4 °C for 2 h). They observed that vitamin C content increased or decreased by 125, 90 and 99 % for thermal-treated, freeze-dried and frozen-thawed samples, respectively. The fresh green and red pepper contained vitamin C as 134 and 155 mg/100 g sample, respectively and vitamin C of fresh pepper degraded during processing and cooking (Vanderslice et al. 1990). Blanching reduced the vitamin C content, but it limits further decay during storage at frozen condition (Lee and Kader 2000). Most of the vitamin C stability during storage was performed at a specific temperature (i.e. usually around room temperature) with varied moisture content(s). Sablani et al. (2007) studied the stability of vitamin C in a commercial fortified formula food product at 23 °C with the variation of moisture contents from 4.6 to 20.6 g/100 g sample, and modeled the data with zero and first order reaction kinetics. In these types of studies, it was difficult to observe the effect of temperature on vitamin C stability at different levels of moisture contents. There is negligible work available in the literature related to the stability of vitamins as a function of storage temperature as well as moisture content. The objectives of this study were to determined vitamin C losses in fresh and freeze-dried capsicum during storage as a function of moisture content and temperature. Usually freeze-drying retained maximum level of vitamins in foods (i.e. ideal condition), thus in this study samples were prepared by freeze drying.
Materials and methods
Materials
Fresh green capsicums (bell pepper) were purchased from local vegetable market in Muscat, Oman. The experiments were performed into 3 moisture levels: one as its original state (moisture: 94 g/100 g sample) and others two were at its dried state (moisture: 15 and 5 g/100 g sample). Original moisture content of the raw capsicum used in this work was 94 g/100 g sample and fresh samples were used in order to keep its original structure. Most of the dried fruits and vegetables contain moisture 15 to 5 g/100 g sample, thus these two levels are used in this study. For fresh capsicum, four levels of temperature used (20 °C, room temperature, i.e. normal storage; 5 °C, i.e. chilled condition; −20 °C, i.e. commercial storage, and −40 °C, i.e. industrial bulk storage or below commercial storage). Higher temperatures did not use for the fresh one since its structure degraded within few hours. The temperatures used for dried states were −40, −20, 5, 20, 45, 60 °C. However in order to reduce the experimental runs, higher temperature (20, 45 and 60 °C) did not use for samples containing moisture 5 g/100 g sample, while lower temperature (−20 and −40 °C) did not use for the samples containing moisture 15 g/100 g sample. Data points of storage were selected based on the degradation rate in different samples, since time frames of degradation in dried samples were much longer as compared to the fresh one. Thus different time intervals (7–10 points) and total duration (19–155 days) were selected based on the rate of decay during different storage temperatures. Whole Capsicums were washed with tap water and stored at 20, 5, −20 and −40 °C for determining vitamin C stability. For freeze-drying, capsicums were cut into small pieces (i.e. 2 cm × 2 cm) and placed into plastic containers (50 ml). The samples were kept in a freezer at −40 °C for at least 24 h. The frozen capsicums at −40 °C were freeze-dried at 20 °C for 48 or 96 h using Edwards K4 Freeze Dryer (Corawky, Crawley, England). A vacuum of 200 Pa was used in the drying chamber while condensing plate temperature was at −65 °C. Dried capsicums were ground into powder using a KMF hammer mill (KIKA Werke, Wilmington, USA) at 6,000 rpm. Powders of 50 g were kept in air tight plastic containers and stored at 45, 20, 5, −20 and −40 °C, while air tight metal cell was used for storage at 60 °C.
Moisture and vitamin C analysis
Moisture content was determined by drying a 2 g sample in a convection oven for at least 18 h at 105 °C. Samples stored at different temperature were taken out at different time intervals and ascorbic acid contents were determined. Vitamin C content in capsicum sample was determined using a titration method with 2,6-dichloro-indophenol reagents (AOAC 1990). Two gram of freeze-dried or 8 g of fresh sample was mixed with 100 ml of 0.1 N meta-phosphoric acid. Ten ml solution was titrated with 2,6-dichloro-indophenol. The end point of titration was determined from the change of color to pink. Ascorbic acid content was expressed as mg ascorbic acid/100 g sample of fresh or dried capsicum. Three replicates were performed for each analysis.
Reaction kinetics
Degradation of vitamins is commonly modeled by zero and first order kinetics. The zero order kinetics can be expressed as:
| 1 |
where Co and C are the vitamin C concentration at the beginning of storage and at any time of storage (mg/100 g sample), t is the storage time (day), and ko is the zero order rate constant (day−1). The first order reaction kinetics can be written as:
| 2 |
where k1 is the first order rate constant (day−1). If experimental data is fitted with a linear equation with an intercept as:
| 3 |
Activation energy of the first order kinetics was estimated using Arrhenius equation:
| 4 |
where, k1 is the rate constant (day−1), R is the universal gas constant (8.314 J/g mol °C), Ea is the activation energy (J/g mole), D is the pre-exponent factor, and T is the storage temperature (K), respectively. The activation energy was estimated from the slope of linear plot (ln k1 versus 1/T).
Statistical analysis
Means of 3 replicates for each data point were calculated and presented. Significant differences between the vitamin C content in the capsicum with time were determined by ANOVA (two factors) using SAS glm procedure (SAS 2002) and p values are presented. The degradation kinetics were modeled by zero and first order using Excel. The r2 statistic was determined for both models. Activation energy was estimated from the linearized Arrhenius equations using Excel.
Results and discussion
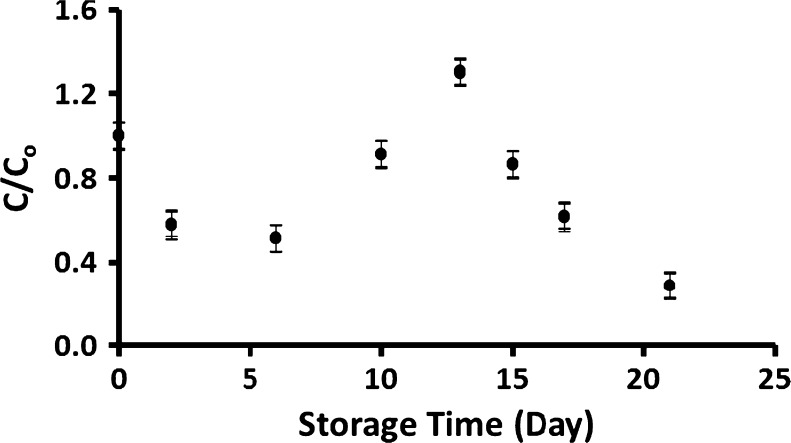
The moisture content of the fresh capsicum was 94 g/100 g fresh-capsicum. Table 1 shows the vitamin C contents as a function of storage time. Significant degradation of vitamin C was observed in all storage conditions (p < 0.05) except fresh capsicum stored at 20 °C (p > 0.55). Figure 1 shows vitamin C loss of capsicum stored at 20 °C. Initially a decreasing trend was observed with a minimum peak followed by an increase to a maximum peak after 13 days. The green color changed to yellow at its minimum peak and visual structural damage (i.e. softening) was observed. This may be due to the disruption of the cell membrane leading to the release of membrane bound phytochemicals (Lemmens et al. 2009). Some early investigators reported that ascorbic acid content increased in the immediate postharvest period and then gradually declined. For example, Carnelossi et al. (2009) reported an increase in vitamin C content in mangaba fruits after 2 days of storage at 24 °C, followed by a sharp fall.
Table 1.
Vitamin C contents in capsicum during storage as a function of moisture content and temperature
| Fresh capsicum (X w: 94 g/100 g sample) | Freeze dried capsicum (X w: 15 g/100 g sample) | Freeze dried capsicum (X w: 5 g/100 g sample) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 °C | 5 °C | −20 °C | −40 °C | 5 °C | 20 °C | 45 °C | 60 °C | 5 °C | −20 °C | −40 °C | |||||||||||
| Time (Day) | C | Time (Day) | C | Time (Day) | C | Time (Day) | C | Time (Day) | C | Time (Day) | C | Time (Day) | C | Time (Day) | C | Time (Day) | C | Time (Day) | C | Time (Day) | C |
| 0 | 68.0 | 0 | 68.0 | 0 | 41.5 | 0 | 41.5 | 0 | 78.0 | 0 | 78.0 | 0 | 78.0 | 0 | 78.0 | 0 | 110.2 | 0 | 110.2 | 0 | 110.2 |
| 2 | 39.3 | 2 | 44.3 | 4 | 41.4 | 4 | 41.4 | 1 | 70.2 | 1 | 72.2 | 1 | 66.9 | 1 | 47.3 | 5 | 106.7 | 5 | 110.4 | 5 | 107.9 |
| 6 | 35.0 | 6 | 40.0 | 8 | 34.5 | 8 | 41.4 | 5 | 60.6 | 5 | 62.4 | 5 | 50.2 | 5 | 27.7 | 13 | 104.0 | 13 | 107.4 | 13 | 107.2 |
| 10 | 62.0 | 8 | 38.4 | 16 | 29.9 | 16 | 20.8 | 6 | 59.9 | 6 | 60.6 | 6 | 47.0 | 6 | 26.5 | 19 | 103.5 | 19 | 104 | 19 | 105.9 |
| 13 | 88.6 | 10 | 37.2 | 18 | 11.4 | 18 | 25.7 | 8 | 59.0 | 8 | 57.2 | 8 | 41.1 | 8 | 21.7 | 36 | 98.4 | 36 | 99.9 | 36 | 103.5 |
| 15 | 58.8 | 13 | 30.5 | 22 | 10.0 | 22 | 15.0 | 20 | 53.6 | 20 | 42.3 | 9 | 38.8 | 9 | 20.8 | 42 | 96.3 | 42 | 98.5 | 42 | 102.3 |
| 17 | 41.9 | 15 | 23.5 | 25 | 6.8 | 25 | 14.0 | 32 | 50.9 | 32 | 34.6 | 12 | 33.3 | 12 | 19.0 | 66 | 95.5 | 66 | 97.5 | 66 | 101.4 |
| 21 | 19.5 | 17 | 19.6 | 38 | 49.9 | 38 | 30.6 | 19 | 22.8 | 19 | 17.5 | 135 | 57.6 | 135 | 61.3 | 135 | 64.4 | ||||
| 21 | 11.8 | 43 | 49.9 | 43 | 28.8 | 155 | 55.0 | 155 | 58.7 | 155 | 61.1 | ||||||||||
| 50 | 49.8 | 50 | 28.6 | ||||||||||||||||||
| p > 0.55 | p < 0.01 | p < 0.05 | p < 0.01 | p < 0.005 | p < 0.0001 | p < 0.0001 | p < 0.005 | p < 0.0001 | p < 0.001 | p < 0.001 | |||||||||||
C: Vitamin C concentration (mg/100 g sample)
X w: Moisture content (g/100 g sample)
Standard deviations of vitamin C analysis were varied from ±0.2 to ±0.8 mg/100 g sample
Fig. 1.
Concentration ratio (C/C o) of fresh capsicum stored at room temperature (20 °C) as a function of storage time
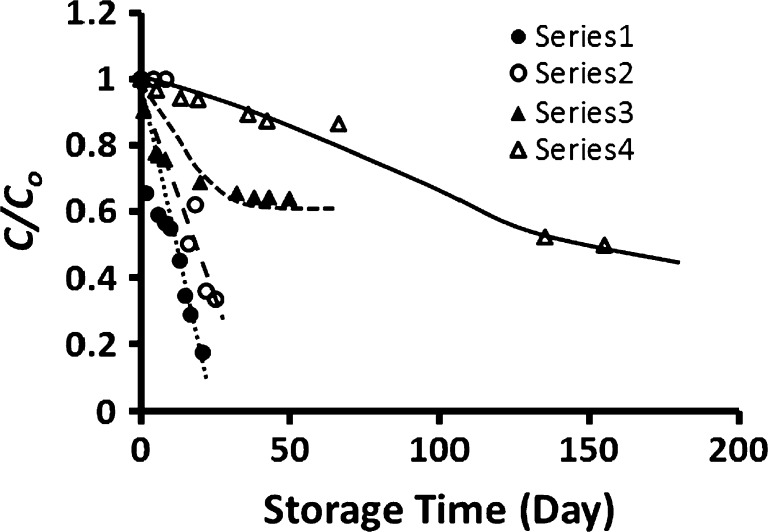
At 5 °C storage, much slower decreasing trend was observed during storage and capsicum surface was dried and wrinkled without softening (Fig. 2). However, an initial shift with very short time was observed and then followed a decreasing trend. Ascorbic acid was oxidized, thus a gradual decrease was observed during refrigerated storage (Howard et al. 1999). Similar trend was also observed in the case of mushroom (Blasco et al. 2004). However, sample stored at −20 and −40 °C showed an initial shoulder and then decreased (Fig. 2). In the case of freeze-dried capsicum (Xw: 15 g/100 g sample) stored at 5 °C, an exponential decrease (i.e. a sharp decrease followed by an equilibrium) of the concentration was observed (Fig. 2). Similar trends were also observed for the samples with moisture content 15 g/100 g sample stored at 20, 45 and 60 °C. Similar decay kinetics was also reported in the literature. Vitamin C (40 mg/100 ml) in lemon juice decreased exponentially to zero within 7 days when stored at room temperature with nitrogen atmosphere (Gonzalez-Molina et al. 2012). However in the cases of lemon juice mixed with elderberry or grape juice, vitamin C decrease reached to an equilibrium value of around 24 mg/100 ml within 7 days and remained nearly constant up to 57 days of storage. Thus the shape of the decreasing curve depended on the other factors and compositions. Freeze-dried samples containing moisture 5 g/100 g sample showed relatively linear decrease of C/Co when stored at 5, −20 and −40 °C (Fig. 2).
Fig. 2.
Selected plots of concentration ratio (C/C o) as a function of storage time. Series1: Fresh capsicum (X w: 94 g/100 g sample) stored at 5 °C, Series2: Fresh capsicum (X w: 94 g/100 g sample) stored at −40 °C as a function of storage time, Series3: Freeze-dried capsicum (X w: 15 g/100 g sample) stored at room 5 °C, Series4: Freeze-dried capsicum (X w: 5 g/100 g sample) stored at room 5 °C as a function of storage time (X w: moisture content)
Howard et al. (1999) reported that ascorbic acid concentration decreased during storage in the vegetables they examined (Broccoli, carrots, and green beans), but the rate and pattern of decline depended on the types of vegetables. For example, ascorbic acid concentration in fresh green beans was reduced rapidly, with more than 70 % disappearing after 1 week of storage. Similarly Wu et al. (1992) reported 58 % loss ascorbic acid in green beans after 3 days of storage. Howard et al. (1999) measured vitamin C degradation over long-term storage (e.g., initial versus 3 weeks storage or initial versus 1 year for frozen vegetables) and observed a zero order trend in most instances. However, fluctuations observed during initial days of refrigeration, thus zero or first order rates were not clear when complete storage period was considered. They explained this by the variability due to the plant matrix and conditions pre and postharvest, as well as the initial ascorbic acid concentration. This makes it more difficult to develop a reliable generic model for ascorbic retention during storage of refrigerated or processed vegetables. In the literature however, most of the cases vitamin losses in foods were modeled by zero and/or first order reaction kinetics (Blasco et al. 2004; Burdurlu et al. 2006; Goula and Adamopoulos 2006; Torregrosa et al. 2006; Sablani et al. 2007; Hiatt et al. 2010).
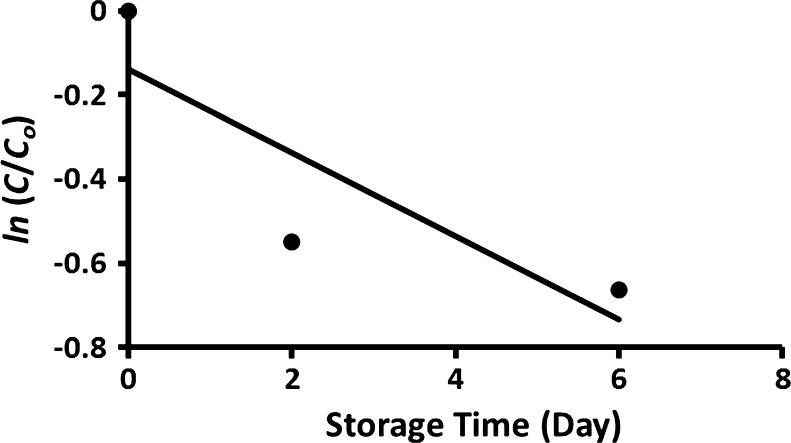
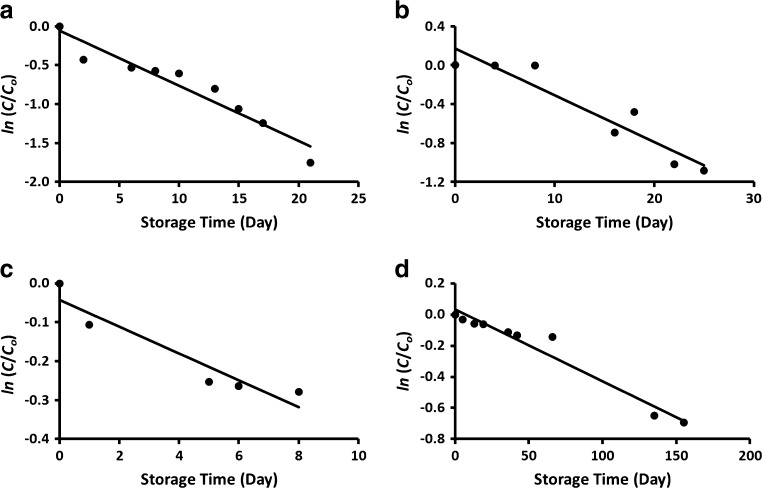
Linear plots of the ln (C/Co) against storage time are shown in the Figs. 3 and 4. Table 2 shows that at a specific moisture content, rate constant decreased with the decrease of storage temperature. At storage temperature 5 °C, the first order rate constants were 7.1 × 10−2, 7.7 × 10−2, and 4.3 × 10−3 day−1 for the moisture contents 94, 15 and 5 g/100 g sample, respectively (Table 2). In general, it was observed that the rate constant decreased with the decrease in moisture content and storage temperature. However, drying rate constants for the samples containing moisture 5 g/100 g sample showed one order of magnitude lower as compared to the samples at moisture contents 94 and 15 g/100 g sample. Similarly, Hiatt et al. (2010) observed a drastic loss of vitamin C above critical moisture content. However, exact critical moisture content must be determined with more experiments at different moisture content. In the case of formulated commercial product stored at 23 °C, the rate constants were 0.26 and 0.98 day−1, for the samples containing moisture 4.5 and 16.2 g/100 g sample, respectively (Sablani et al. 2007). These values are similar to the results obtained in this study for the samples containing moisture as 5 and 15 g/100 g sample and stored at 20 °C (Table 2). In the case of raw guava at water activity 0.97 and stored at 40 °C showed rate constant as 59.3 × 10−2 day−1. Hossain and Gottschalk (2009) studied the vitamin C stability in dried tomato and they observed rate constants as 2.3 × 10−3 and 3.2 × 10−3 day−1, for the sample contained water content 18 g/100 g sample and stored at 5 and 20 °C, respectively. These values are one order lower than those observed for capsicum with moisture 15 g/100 g sample (Table 2). Phillips et al. (2010) determined the rate constants of raw green bean and potato homogenates (prepared in liquid nitrogen) as 4.06 × 10−4 and 8.28 × 10−4 day−1, respectively when stored at −60 °C. These values were much lower compared with 4.8 × 10−2 day−1 for raw capsicum stored at −40 °C. This may be due to the temperature difference of storage used in this study, low dissolved oxygen as they used liquid nitrogen, and physico-chemical characteristic of the raw material. Hiatt et al. (2010) studied the sodium ascorbate and vitamin C stability as a function of water activity (i.e. 0.75, 0.85 and 0.98) and storage temperature (25, 35, and 40 °C). The degradation rate constant for sodium ascorbate at water activity 0.75 and 40 °C was 10.0 × 10−2 day−1, while in the case of ascorbic acid it was 4.3 × 10−2 day−1 for water activity 0.98. These values are similar to the values observed in this study. In the case of blanching of fresh pumpkin leave at 60 °C, the rate constant of vitamin C degradation was observed as 31.0 day−1 (Ariahu et al. 2011). The activation energy of fresh (moisture: 94 g/100 g sample) and freeze-dried capsicum (moisture: 5 g/100 g sample) within the temperature 5 to −40 °C were estimated as 273.0 and 0.84 J/g mole.
Fig. 3.
Plot of ln (C/C o) of fresh capsicum stored at room temperature (20 °C) as a function of storage time
Fig. 4.
Plots of ln (C/C o) as a function of storage time. a Fresh capsicum (X w: 94 g/100 g sample) stored at 5 °C, b Fresh capsicum (X w: 94 g/100 g sample) stored at −40 °C as a function of storage time, C: Freeze-dried capsicum (X w: 15 g/100 g sample) stored at room 5 °c, d Freeze-dried capsicum (X w: 5 g/100 g sample) stored at room 5 °C as a function of storage time (X w: moisture content)
Table 2.
Zero order and first order reaction rate constants for the vitamin C degradation in capsicum during storage
| Fresh capsicum (X w: 94 g/100 g sample) | Freeze dried capsicum (X w: 15 g/100 g sample) | Freeze dried capsicum (X w: 5 g/100 g sample) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | k o (day−1) | a o | r 2 | k 1 (day−1) | a 1 | r 2 | T (°C) | k o (day−1) | a o | r 2 | k 1 (day−1) | a 1 | r 2 | T (°C) | k o (day−1) | a o | r 2 | k 1 (day−1) | a 1 | r 2 |
| 20(6 ) | 4.9 | 60.4 | 0.69 | 9.9 × 10−2 | −0.14 | 0.73 | 60(8) | 6.2 | 65.1 | 0.82 | 14.8 × 10−2 | −0.19 | 0.93 | 5(155) | 3.6 × 10−1 | 110.8 | 0.97 | 4.6 × 10−3 | 0.03 | 0.96 |
| 5(21) | 2.2 | 57.6 | 0.90 | 7.1 × 10−2 | −0.05 | 0.92 | 45(19) | 4.4 | 74.4 | 0.97 | 7.7 × 10−2 | −0.04 | 0.99 | −20(155) | 3.5 × 10−1 | 112.4 | 0.97 | 4.3 × 10−3 | 0.04 | 0.96 |
| −20(25) | 1.5 | 45.4 | 0.89 | 7.4 × 10−2 | 0.26 | 0.84 | 20(20) | 2.5 | 76.2 | 0.97 | 3.8 × 10−2 | −0.02 | 0.98 | −40(155) | 3.3 × 10−1 | 113.2 | 0.95 | 3.9 × 10−3 | 0.05 | 0.93 |
| −40(25) | 1.3 | 45.5 | 0.91 | 4.8 × 10−2 | 0.17 | 0.89 | 5(8) | 2.3 | 74.8 | 0.89 | 3.4 × 10−2 | −0.04 | 0.90 | |||||||
Values in the parentheses are the maximum time (hr) for linear plot and used to fit the kinetics data
X w: Moisture content
r 2: regression coefficient
Hiatt et al. (2010) also observed that both water activity and temperature impacted vitamin C degradation, while water activity (i.e. moisture) showed a more pronounced effect. Generally vitamin C stability decreased dramatically near or above critical water activity. Further studies need to be performed with wide variations of moisture content and temperature in order to observe a complete scenario of the combined effect of moisture and temperature. In addition, other physico-chemical parameters should also be included to explain the variations in different foods. In this study, only selected moisture content of capsicum and storage conditions were considered and further works are in progress to include wide spectrum of moisture contents and storage temperature.
Conclusion
The degradation kinetics of vitamin C in fresh and freeze-dried capsicum was measured and modeled by zero and first order reaction. In general, the first order rate constants were decreased with the decrease of moisture and storage temperature. For fresh capsicum (moisture: 94 g/100 g moisture) the rate constant varied from 9.9 × 10−2 to 4.8 × 10−2 day−1 when storage temperature raised from 20 to −40 °C, respectively. In the case of freeze-dried capsicum (moisture: 5 g/100 g sample) the rate constant varied from 4.6 × 10−3 to 3.9 × 10−3 day−1 when storage temperature raised from 20 to −40 °C, respectively. The activation energy of fresh and freeze-dried capsicum within the temperature 5 to −40 °C were estimated as 273.0 and 0.84 J/g mole.
Acknowledgments
The authors would like to acknowledge the support of Sultan Qaboos University towards their research in the area of food structure and its stability.
References
- AOAC . In: Association of official analytical chemists. Official methods of analysis of the AOAC. Helrich K, editor. Arlington: AOAC; 1990. [Google Scholar]
- Ariahu CC, Abashi DK, Chinma CE. Kinetics of ascorbic acid loss during hot water blanching of fluted pumpkin (Telfairia occidentalis) leaves. J Food Sci Technol. 2011;48(4):454–459. doi: 10.1007/s13197-010-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco R, Esteve MJ, Frigola A, Rodrigo M. Ascorbic acid degradation kinetics in mushrooms in a high-temperature short-time process controlled by a thermoresistometer. Food Sci Technol. 2004;37:171–175. [Google Scholar]
- Burdurlu HS, Koca N, Karadeniz F. Degradation of vitamin C in citrus juice concentrates during storage. J Food Eng. 2006;74:211–216. doi: 10.1016/j.jfoodeng.2005.03.026. [DOI] [Google Scholar]
- Carnelossi MAG, de Sena HC, Narain N, Yaguiu P, da Silva GF. Physico-chemical quality changes in Mangaba (Hancornia speciosa gomes) fruit stored at different temperatures. Braz Arch Biol Technol. 2009;52(4):985–990. doi: 10.1590/S1516-89132009000400023. [DOI] [Google Scholar]
- Gonzalez-Molina E, Girones-Vilaplana A, Mena P, Moreno DA, Garcia-Viguera C. New beverages of lemon juice with elderberry and grape concentrates as a source of bioactive compounds. J Food Sci. 2012;77(6):C728–C733. doi: 10.1111/j.1750-3841.2012.02715.x. [DOI] [PubMed] [Google Scholar]
- Goula AM, Adamopoulos KG. Retention of ascorbic acid during drying of tomato halves and tomato pulp. Drying Technol. 2006;24:57–64. doi: 10.1080/07373930500538709. [DOI] [Google Scholar]
- Gupta MK, Sengal VK, Arora S. Optimization of drying process parameters for cauliflower drying. J Food Sci Technol. 2013;50(1):62–69. doi: 10.1007/s13197-011-0231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt AN, Taylor LS, Mauer LJ. Influence of simultaneous variations in temperature and relative humidity on chemical stability of two vitamin C forms and implications for shelf life models. J Agric Food Chem. 2010;58:3532–3540. doi: 10.1021/jf903342f. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Gottschalk K. Effect of moisture content, storage temperature and storage period on colour, ascorbic acid, lycopene and total flavonoids of dried tomato halves. Int J Food Sci Technol. 2009;44:1245–1253. doi: 10.1111/j.1365-2621.2009.01954.x. [DOI] [Google Scholar]
- Howard LA, Wong AD, Perry AK, Klein BP. β-carotene and ascorbic acid retention in fresh and processed vegetables. J Food Sci. 1999;64(5):929–936. doi: 10.1111/j.1365-2621.1999.tb15943.x. [DOI] [Google Scholar]
- Johnston CS, Hale JC. Oxidation of ascorbic acid in stored orange juice is associated with reduced plasma vitamin C concentrations and elevated lipid peroxides. J Am Diet Assoc. 2005;105:106–109. doi: 10.1016/j.jada.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Kek SP, Chin NL, Yusof YA (2013) Simultaneous time-temperature thickness suspension theoretical and statistical modeling of convective drying of guava. J Food Sci Technol (in press) [DOI] [PMC free article] [PubMed]
- Klimczak I, Maecka M, Szlachta M, Gliszczynska-Swigo A. Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. J Food Comp Anal. 2007;20:313–322. doi: 10.1016/j.jfca.2006.02.012. [DOI] [Google Scholar]
- Lee SK, Kader AA. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol Technol. 2000;20:207–220. doi: 10.1016/S0925-5214(00)00133-2. [DOI] [Google Scholar]
- Lemmens L, Van Buggenhout S, Oey I, Van Loey A, Hendrickx M. Towards a better understanding of the relationship between the beta-carotene in vitro bio-accessibility and pectin structural changes: a case study on carrots. Food Res Int. 2009;42(9):1323–1330. doi: 10.1016/j.foodres.2009.04.006. [DOI] [Google Scholar]
- Leong SY, Oey I. Effects of processing on anthocyanins, carotenoids and vitamin C in summer fruits and vegetables. Food Chem. 2012;133:1577–1587. doi: 10.1016/j.foodchem.2012.02.052. [DOI] [Google Scholar]
- Majumdar TK, Vasudish CR, Premavalli KS, Bawa AS. Development and storage stability of cucumber-litchi-lemon juice. J Food Sci Technol. 2009;46(3):269–270. [Google Scholar]
- Marques LG, Silveira AM, Freire JT. Freeze-drying characteristics of tropical fruits. Drying Technol. 2006;24:457–463. doi: 10.1080/07373930600611919. [DOI] [Google Scholar]
- Marques LG, Ferreira MC, Freire JT. Freeze-drying of acerola (Malpighia glabra L.) Chem Eng Process. 2007;46:451–457. doi: 10.1016/j.cep.2006.04.011. [DOI] [Google Scholar]
- Phillips KM, Tarrago-Trani MT, Gebhardt SE, Exler J, Patterson KY, Haytowitz DB, Pehrsson PR, Holden JM. Stability of vitamin C in frozen raw fruit and vegetable homogenates. J Food Comp Anal. 2010;23:253–259. doi: 10.1016/j.jfca.2009.08.018. [DOI] [Google Scholar]
- Sablani SS, Al-Belushi K, Al-Marhubi I, Al-Belushi R. Evaluating stability of vitamin C in fortified formula using water activity and glass transition. Int J Food Prop. 2007;10:61–71. doi: 10.1080/10942910600717284. [DOI] [Google Scholar]
- SAS . The SAS system for windows, version 9. Carry: SAS Institute; 2002. [Google Scholar]
- Torregrosa F, Esteve MJ, Frigola A, Cortes C. Ascorbic acid stability during refrigerated storage of orange–carrot juice treated by high pulsed electric field and comparison with pasteurized juice. J Food Eng. 2006;73:339–345. doi: 10.1016/j.jfoodeng.2005.01.034. [DOI] [Google Scholar]
- Vanderslice JT, Higgs DJ, Hayes JM, Block G. Ascorbic acid and dehydroascorbic acid content of foods-as eaten. J Food Comp Anal. 1990;3:105–118. doi: 10.1016/0889-1575(90)90018-H. [DOI] [Google Scholar]
- Verma M, Singh J, Kaur D, Mishra V, Rai GK (1993) Effects of various dehydration methods and storage on physicochemical properties of guava powder. J Food Sci Technol. doi:10.1007/s13197-013-1020-0
- Wu Y, Perry AK, Klein BP. Vitamin C and β-carotene in fresh and frozen green beans and broccoli in a simulated system. J Food Qual. 1992;15:87–96. doi: 10.1111/j.1745-4557.1992.tb00977.x. [DOI] [Google Scholar]