Abstract
The fish oil (FO), and fish oil powder (FOP) at 10 % of recommended daily intake (RDI) were used to make two types of fortified feta cheeses. The physicochemical, rheological and sensory properties of ripened samples at 0, 30, and 60th days of cold store (5 °C) showed that the FO samples had a faster pH reduction, higher MSNF (milk solid non-fat) increase (p < 0.05) and more pores formation. Storage (G’) and loss (G”) moduli for both samples decreased until the 30th day of cold storage and then increased until the end of the storage time but both of them were higher for FOP samples. The index of secondary lipid oxidation or thiobarbituric acid reactive substances (TBARS) of FO was lower than FOP samples. Although the polyunsaturated fatty acids of both samples were much higher than common feta cheese, their degradation in FO was less than FOP samples after storage. The sensory scores of FO were significantly higher than FOP sample (P < 0.05), and it obtained up to 70 % of overall acceptability after 30 and 60 days storage for its better hardness, texture and flavor.
Keywords: Fortification, Fish oil, Fish oil powder, Omega 3, Microstructure, Storage and loss moduli
Introduction
Lipids and essential fatty acids respectively are a source of energy and vital components of a balanced diet (Martini et al. 2009). However, there are enough scientific evidences to show that the composition model of dietary fat affects blood lipid concentration. The serum LDL (low density lipoprotein) cholesterol rises about 2 % for every 1 % increase in calories (as a percentage of total energy) obtained from saturated fatty acids. Conversely, 1 % reduction in saturated fatty acids will reduce this serum LDL cholesterol to about 2 % (Mensink and Katan 1992). The number of foods targeting the cardiovascular (CV) system has been increased noticeably during the last two decades.
When low cholesterol foods are consumed regularly in the context of a healthy diet and lifestyle, the CV system will improve in a healthy condition (Lopez-Huertas 2010). Foods supplemented with omega-3 fatty acids (especially with long-chain polyunsaturated fatty acids or LCPUFAs including docosahexaenoic acid or DHA and eicosapentaenoic acid or EPA) provide extensive nutritional and health benefits (Ruxton et al. 2004). They have obtained from marine sources and attracted much attention in the past decade (Drusch et al. 2007). The EPA and DHA will able to control lipoproteins, blood pressure, cardiac function, endothelial function, vascular reactivity and cardiac electrophysiology, and they have antiplatelet and anti-inflammatory effects (Hooper et al. 2004). Overall, omega-3 with LCPUFA has ability to prevent coronary heart disease, hypertension, type 2 diabetes, rheumatoid arthritis, Crohn’s and obstructive pulmonary diseases (Simopoulos 1999; WHO 2002). According to Kris-Etherton (1999), when 5 % saturated fatty acids is replaced with oleic acid or polyunsaturated fatty acids (PUFA), the coronary heart disease is reduced by 20–40 % mainly by less consumption of LDL-cholesterol. An increase in the intake of oleic acid may be beneficial because it limits the intake of saturated fat which is higher than recommended levels in many countries. This goal can be achieved by altering dietary patterns, such as replacing butter with olive oil, or using new technique in food processing to modify the fatty acid profile of foods naturally rich in saturated fatty acids with oleic acid (Lopez-Huertas 2010).
Various studies have recommended that the daily dose of EPA and DHA for an average person is approximately 300 mg (190–330 mg) and represents the consumption of two portions of fish per week (one oily). The amount 2–10 g of oleic acid has been suggested to use daily instead of fatty acids from milk fat. It represents only 1–4 % of the energy in a 2500 kcal diet (AHA 2006; AFSSA 2003; Health Council of the Netherlands 2006). While milk and dairy products are perceived as good sources of bioavailable calcium, milk fat contains about 70 % saturated fatty acids (mainly Myristic and Palmitic acids) and have potentials to raise total and LDL cholesterol and increase the risk of cardiovascular disease (AHA 2006). To reduce the intake of saturated fatty acids in favor of healthier fatty acids, it is a good nutritional strategy to substitute the milk fat of dairy products with a combination of oleic acid and fish oil (Lopez-Huertas 2010; Hibbeln et al. 2006).
The modern dairy plants make the popular Iranian UF-Feta cheese from ultrafiltered and pasteurized bovine milk mixed with mesophilic starter cultures and commercial microbial rennet. The minimum of 35 % (w/w) total solids, minimum 6 % protein content (w/w), about 16 % fat content and a maximum pH = 5.2 are the main characteristics of this kind of cheese (Karami et al. 2009).
Milk proteins (used as surfactants in many formulated food) are adsorbed rapidly either as individual molecules or in the form of aggregates at the new oil/water interface during emulsification (Walstra and Smulders 1997). However, they are not completely similar to each other. Whey proteins (β-lactoglobulin, α-lactalbumin, bovine serum albumin and immunoglobulin) are characterized by well-defined three-dimensional structures held together by disulphide bridges, and they are much more rigid than caseins (Kinsella 1984). It has been demonstrated that whey protein and casein can efficiently stabilize fish oil emulsions and have anti-oxidative function in an emulsion system (Ye et al. 2009).
Cumin has been used for decades for its good taste –as a spice- and medicinal purposes (Jalali-Heravi 2007). It is also used as a flavoring agent in different cheeses in Iran. So, it is a good case for masking the fishy flavor of fortified cheeses with fish oil and fish oil powder.
Martini et al. (2009) fortified 50 % reduced fat cheddar cheese with n-3 fatty acids (DHA and EPA) and observed that cheeses with low fortification levels (18 and 35 mg of DHA/EPA per serving size) did not develop a significant fishy flavor compared with the control, whereas at the highest fortification level (71 mg of DHA/EPA per serving size) the fishy flavor was significantly stronger in young cheeses. Ye et al. (2009) fortified processed cheese with fish oil and concluded that a fish oil emulsion made with a milk protein complex is a useful carrier for elevating the fortification level of omega-3 LC PUFA in this dairy product. Aryana (2007) substituted Omega-Pure (a commercial oil rich in n-3 fatty acids) with 100, 50, 25, and 0 % of the milk fat in different cheeses and found a significant difference between the cheese flavors fortified with n-3 fatty acids compared with the control cheeses (0 % DHA).
Due to the high content of LCPUFAs in liquid fish oil, it is difficult to protect them against oxidation. Microencapsulation of LCPUFAs offer the possibility for the protection and release controlling of lipophilic food ingredients and can be used for supplementation of foods almost without oxidation (Drusch et al. 2007). Microencapsulation also has been used to mask unpleasant taste in food sciences as well as to protect against light and airborne oxidation (Gibbs et al. 1999; Kolanowski et al. 2004). Microencapsulation of fish oil produces a dry powder from liquid fish oil, which can be used for enrichment of different food with omega-3 LCPUFA (Thautwein 2001; Kolanowski and Laufenberg 2006). Kolanowski et al. (2007) reported that this process has a good potential to protect the nutritional aspects of instant foods fortified with fish oil powder. Bermudez-Aguirre and Barbosa-canovas (2011) used flaxseed oil and fish oil powder to fortify cheddar and mozzarella cheeses with omega-3 and concluded that sensory scores and shelf life of the both cheeses enriched with fish oil powder were much better and more than the ones enriched with flaxseed oil. Barrow et al. (2009) compared the encapsulated and microencapsulated fish-oil and found out that both processes provide equivalent bioavailability of omega-3 fatty acids for the consumers as long as they delivered in soft-gel capsules.
The objective of this study was to evaluate the effects of storage time (0, 30 and 60 days) on micro-structural and rheological properties of low fat UF-Feta cheeses fortified with either fish oil or fish oil powder.
Materials and methods
Materials
Fresh skim retentate was supplied by Pegah-e Khorasan Dairy Co., Mashhad, Iran. Whey protein concentrate (WPC) was purchased from Milei Co., Germany. Fish oil (Ropufa 30 n-3 EPA oil) and fish oil powder (Ropufa 10 n-3 Food Powder S/SD) were supplied by DSM Co, Switzerland. The fish oil had 270 mg/g PUFA content. In fish oil powder, the fish oil is encapsulated using powder microencapsulation technology and resulted powder –according to the certificate of analysis- 95 % passes through a 80-mesh sieve. Butter oil and olive oil were purchased from Razavi Dairy Co, Mashhad, Iran and ETKA Co, Varamin, Iran, respectively. Mesophil starter cultures (R-704) with a combination of Lactococcus lactis subspecies of cremoris and Lactococcus lactis subspecies lactis were purchased from Christian Hansen Co., Denmark. Rennet (Fromase®TL) as a microbial coagulant (a product of Rhizomucor miehei) was purchased from DSM Food Specialties, Netherlands. Cumin powder was purchased from a local grocery store.
Cheese making
We previously optimized the operational parameters to fortify reduced-fat UF-Feta cheese with fish oil and fish oil powder using response surface methodology. The maximum desirability for fish oil fortified cheese samples was 66.6 % with 8 % cheese fat (5 % butter oil + 3 % olive oil), 0.4 % (w/w) fish oil (equal to 10 % of recommended daily intake for DHA + EPA), 0.7 % (w/w) WPC and 0.5 % (w/w) cumin. The maximum desirability for fish oil powder fortified cheese samples was 61.9 % with 8 % cheese fat, 1.5 % (w/w) fish oil powder (equal to 10 % of recommended daily intake for DHA + EPA), 0.54 % (w/w) WPC and 0.5 % (w/w) cumin. Both of them were best used up to 30 days. The olive oil -according to its health benefits- was constant (3 %) for all the samples.
Experimental optimized cheese samples were made at Pegah-e-Khorasan Dairy Co. (Mashhad, Iran) according to a UF cheese making method (Bylund 1995; Robinson and Tamime 1991) proposed by Tetra-Pak processing systems AB (Lund, Sweden) and two formulation mentioned above. Since the fat level varies in milk, skim retentate (by ultrafiltration) was prepared and the fat level was adjusted by adding butter oil and olive oil. First, WPC and cumin were mixed with skim retentate in glass containers and blended with an electric mixer (Bosch model MFQ-1620, Germany) for 2 min. Then butter oil, olive oil and fish oil or fish oil powder were added to the mixture and blended for another 2 min. The samples were then preheated to 45 °C and homogenized with an Ultra-turrax T25 (IKA, Germany) for 1.5 min at 20000 rpm. Then samples were pasteurized at 74 °C for 40 s and then cooled to 32 °C. A mixture of starter and rennet were added to the samples and the mixture was poured into 100 g polypropylene cheese cups and incubated at 30 °C for 20 min. After coagulation, 2.5 % salt was poured onto the parchment paper layer on top of the cheese and the container was sealed with aluminum foil. In the pre-ripening stage (30 ± 1 °C), after lowering the pH of the cheese to 4.8, the cheese samples were transferred to a cold room (5 ± 1 °C) for ripening (3 days) after which chemical, textural and microstructural analyses were performed.
Physicochemical analysis
The most important quality indicators of feta cheese including pH, milk solids-non fat (MSNF), fat, protein, peroxide value and thiobarbituric acid reactive substances (Fox et al. 1993, International Dairy Federation Standards 1991, and AOCS American Oil Chemists Society 2009) were measured at different storage time intervals. The pH of cheese samples was measured using a digital pH-meter (Knick, 766 Calimatic, NielsBohrweg, Ultrecht, Netherlands). Cheese samples were analyzed for total solids by heating to a constant weight in a vacuum oven. The fat and protein contents of cheese samples were determined respectively by the Gerber method (James 1995) and Kjeldahl method (AOAC Association of Official Analytical Chemists 1997 method 920.123). Two methods of Mortensen et al. (2002) and IDF standard 74A:1991 with some modification (as described by Drusch et al. 2007) were used to determine peroxide value of cheese lipid and it was recorded as milli-equivalents of oxygen per kilogram of cheese lipid. The Standard Method of AOCS (Official Method Cd 19–90 in 2009) was used to measure thiobarbituric acid reactive substances (TBARS) of each sample. 50 mg of each cheese sample was weighed in a 25 mL volumetric flask and its volume adjusted with 1-butanol. Then, 5 mL of the TBA reagent was added to 5 mL of the test solution and placed into a thermostatic bath at 95 °C After 120 min the test tube was removed from the bath and cooled under running tap water for about 10 min until its temperature reached to 20 °C. Then a 10 mm cuvette was used to read the absorbance of the reaction solution at 530 nm.
Uniaxial compression
A universal testing machine (Testometric, M350-10 CT, England) with some modification was used to perform uniaxial compression procedure (as described by Gunasekaran and Mehmet 2003, and also Madadlou et al. 2007). A flat plunger with a 40 mm diameter was attached to the crosshead. The cheese cubes (15 × 15 × 15 mm) were prepared and tempered to 21 °C for 4 h. Then these samples were compressed to 60 % (9 mm) of their initial height at a crosshead speed of 30 mm min−1. Then the hardness of the cheese samples was determined by the force necessary to fracture the sample which was the first break of the compression curve. Each sample was analyzed by three replicates.
Dynamic rheological measurements
The method of Madadlou et al. (2007) was used to measure frequency sweep test by using a Universal Dynamic Spectrometer (Anton Paar, Physica MCR 301, Austria) with small amplitude oscillatory shear measurements. According to the Tunick method (2000), the primary viscoelastic terms including the storage (G’) and loss moduli (G”) were determined. The measuring geometry consisted of two parallel plates with a diameter of 25 and a gap size of 1 mm (sample thickness). The required samples were cut from the center of cheese blocks at 5 °C, placed immediately in plastic bags, sealed and equilibrated at room temperature (20 ± 1 °C) for at least 4 h. Then a small piece of each cheese sample was placed on the lower plate and the upper plate was slowly moved down until the gap size of 1 mm was obtained. The extra cheese parts were trimmed off carefully with a razor blade and the sample kept for 15 min in the rheometer to relax and remove its stresses induced during sample handling. A strain sweep test at 0.1 Hz frequency was performed to obtain a linear viscoelastic range as the percentage of strain values varied from 0.01 to 2 %. In the linear region a strain of (0.02 %) was then selected and a frequency sweep test was performed from 0.1 to 10 Hz.
Microstructure
Based on the method of Drake et al. (1996) and its modifications suggested by Madadlou et al. (2007), cheese samples were prepared for scanning electron microscopy (SEM) at the 0, 30 and 60th day of storage time. After cutting the cheese blocks into 5–6 mm3 cubes (by using a sharp razor), they were immersed in 2.5 % (w/w) gluteraldehyde fixative (Merck, Darmstadt, Germany) and 1.25 % paraformaldehyde for 3 h. After two times washing of cheese cubes with 0.1 M piperizine-N, N-bis (2-ethanesulfonic acid) (PIPES) buffer (each time 10 min), they dehydrated in a graded series of ethanol (40 %, 55 %, 70 %, 85 %, 90 % and 96 %) each one for 30 min followed by three times defatting in chloroform (10 min each). After pre-cooling of the samples in refrigerator at 4 °C and they submerged in liquid nitrogen to fracture them into approximately 1 mm pieces due to quick freezing (Sepahioglu et al. 1999). These pieces were then vacuum-dried to a critical moisture level and a sputter-coater was used to cover them with gold for 6 min (Balzers, Type SCD 005, BalTec Inc., Switzerland). The prepared samples were examined with a scanning electron microscope (XL Series, model XL30, Philips, Eindhoven, The Netherlands) operated at 20 kV and the structure images of cheese samples were recorded at 1 K and 2.5 K magnification levels.
Fatty acid composition
The method of Curtis et al. (2008) was used for measuring the fatty acids quantitatively by convert the fatty acids of cheese samples into methyl ester derivatives. According to the method, the C19:0 synthetic triglyceride trinonadecanoin was used as a surrogate spike to calculate analyte recovery, and the C23:0 methyl ester tricosanoic acid was used as an internal standard. A GC/MS of Agilent Technologies 7890 (SGE Analytical Science, Germany) equipped with an ID-BPX 70 column (120 m × 0.25 mm i.d) and a flame-ionization detector was used to analyze these derivatives and identify the fatty acids profiles in each sample. The injector was operated in split mode (100:1 split ratio) and nitrogen was used as the carrier gas at a fixed linear velocity of 41.1 cm/s. The injector temperature was maintained at 250 °C, and the total running time of each sample was 38 min.
Sensory evaluation
The sensory panel was selected based on the ability of the panelists to detect differences in “fishy” flavors. Sensory characteristics of the samples including taste, odor, mouthfeel, appearance and overall acceptability were evaluated by 30 panelists from the UF cheese factory and the research and development section of Pegah-e-Khorasan Dairy Co. They were familiar with the test method used and trained in sensory evaluation.
About 15 g of each cheese sample was placed into a 50 g clear sample cup with a lid and conditioned at room temperature for 2 h before evaluation. A hedonic 9-point scale was used (1=least liked; 9=most liked). Testers were seated in sensory booths with standard lighting. For each evaluation, a sample was presented twice and the samples were presented in random order.
Statistical analysis
The cheese making was carried out in triplicate using a complete randomized design. The analysis of variance (ANOVA) was carried out by using a general linear model (GLM) of Minitab 16 software (English version, Minitab Inc., USA) to determine the differences among data means at 5 % significant level.
Results and discussion
Physicochemical characteristics
Table 1 shows the effects of storage time of low fat UF-Feta cheese fortified with powdered fish oil and liquid fish oil on its pH, milk solids non fat (MSNF), Fat, Protein and texture hardness.
Table 1.
Effect of 5 °C storage time on physicochemical properties of low fat UF-Feta cheese fortified with fish oil (FO) or fish oil powder (FOP)
| Physicochemical characteristics | Storage time (day) | |||||
|---|---|---|---|---|---|---|
| 0 | 30 | 60 | ||||
| FO | FOP | FO | FOP | FO | FOP | |
| pH | 4.68a | 4.62b | 4.47d | 4.54c | 4.47d | 4.48d |
| MSNF1 | 16.7c | 19.7a | 19.2a | 19.7a | 18.1b | 19.6a |
| Fat | 8.0d | 8.6cd | 8.7bcd | 9.0bc | 8.7bcd | 10.8a |
| Protein | 13.1ab | 12.5bc | 13.5a | 12.4c | 13.7a | 13.2a |
| Texture hardness (N) | 1.1d | 1.4cd | 2ab | 1.6bcd | 2abc | 1.8abc |
| Panel Score2 | 6.16bc | 4.5d | 6.9ab | 5.26cd | 6.16bc | 4.81d |
Means within the same row that do not share a letter are significantly different (P < 0.05)
1MSNF: Milk solids non fat
2Overall acceptability
The pH level of FO and FOP cheese samples decreased significantly from around 4.7 to 4.5 during 60 days of 5 °C storage time. Rapid acidification of cheese curd (an essential element of cheese making) is normally achieved at pH < 5 (by using a starter culture) and in-situ conversion of lactose to lactic acid. The residual lactose is fermented relatively quickly to some extent depending on the salt to moisture (S/M) ratio in the curd. Increasing S/M ratio leads to increased osmotic pressure in the aqueous phase of the cheese, causing further dehydration of both desirable and undesirable bacterial cells, killing them or inhibiting their growth. This leads to a decreased rate of lactic acid production by the starter cells, and consequently to a higher pH (Fox et al. 2000). The minerals (attached to casein micelles) increase buffering capacity of UF retentates and change the acidification kinetics of lactic acid bacteria. Also amino acids released by the proteolysis reaction cause a slight increase in pH during cheese ripening (Waagner-Nielsen 1993).
The MSNF of FO samples increased significantly until the 30th day and then decreased until 60th day of storage while the MSNF of FOP samples remained constant. At low pH (<5.2), colloidal calcium phosphate dissociates from casein micelles and progressive breakdown of submicelles into small aggregates of casein take place (Walstra et al. 1999). After that, the bound water was released and syneresis takes place. So, the MSNF of FO samples was increased. After that, casein network started to rearrange and therefore new bonds (cross-links) were formed among the peptides due to the disruption of fat globules and this caused the water to remain in the network, so the MSNF was decreased. According to using fish oil powder in FOP samples, the MSNF of them was higher than FO samples and it remained constant during the 60 days of storage.
The fat and protein content for both samples changed insignificantly during the storage time which is correlated to the MSNF of the cheese samples.
Oxidation of FO and FOP cheese samples
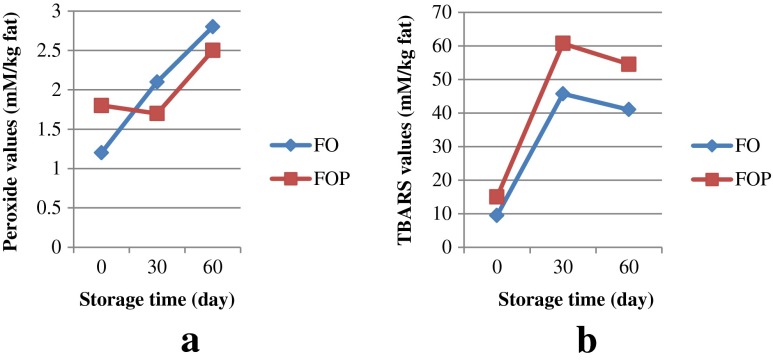
The PV of FO and FOP cheese samples increased slightly during the storage time. Intense mechanical stress and agitation caused by shear and turbulence during preparation leads to oxygen inclusion. In addition, droplet disruption by cavitations and the subsequent rearrangement of fish oil droplets during homogenization promoted the distribution of oxygen, catalysts and lipid oxidation products among the newly arranged oil droplets and may accelerate lipid oxidation (Serfert et al. 2009; Drusch et al. 2007). The presence of polyunsaturated fatty acid in cumin oil (~35 %) was another reason for increasing the PV (as it was confirmed by Jalali-Heravi et al. 2007). Since the FOP sample had a kind of barrier against oxidation of omega-3 LC PUFA, and its encapsulated fish oil coated with protein complex layer, the increasing rate of its PV value was lower than FO sample (Fig. 1(a)). Singh et al. (2009) stated that a microencapsulation of fish oil with casein/whey protein complexes not only provides a thick, interfacial coating on their droplets but also induces a strong anti-oxidative effect in the prepared emulsion. This is achieved through the metal-chelating capacity of phosphoseryl groups in casein (Bennett et al. 2000) and through free radical scavenging by the sulfhydryl groups of whey protein (Faraji et al. 2004). Ye et al. (2009) reported that there was no change in the PV results of processed cheese fortified with encapsulated fish oil and stored at 5 °C In spite of faster oxidation rate of FO cheese samples in the first stage, its PV was much lower than the standard level even after 2 months storage.
Fig. 1.
Effects of storage time on Peroxide values (a) and TBARS or Thiobarbituric acid reactive substances (b) of fish oil (FO) and fish oil powder (FOP) cheeses, (Sd = 0.01, n = 3)
Whereas PV measures the initial stages of lipid oxidation in cheese sample, TBARS quantifies the secondary lipid oxidation products which are cleaved thermally and include mostly low-molecular-weight aldehydes, ketones, alcohols, and short chain hydrocarbons (Choe and Min 2006). The development of TBARS in both samples is shown in Fig. 1(b). The TBARS of both samples increased noticeably during the 30 days after processing and then decreased slightly until the end of the storage time (60 days). However, the TBARS of the cheese samples fortified with FOP had a higher increasing rate than FO samples after 2 months of cold storage. One of the most important factors that determine the stability of dried powders is the presence of moisture (Baik et al. 2004). He reported that increasing the humidity, slightly increased the lag time of second part of oxidation in microencapsulated fish oil. Since FOP samples of this experiment had less moisture than FO samples, their TBARS were higher than FO samples of feta cheese. Ye et al. (2009) reported that in processed cheese fortified with microencapsulated fish oil, the TBRAS of samples increased very fast during 35 days of storage at 30 °C.
Rheological properties
Texture hardness
The hardness is defined as the maximum force excreted on a cheese cube to attain 70 % compression (Brennan 1980). Since the pH of FO sample decreased significantly more than FOP samples until the 30th day, therefore its hardness increased faster (Table 1). Due to the quick reduction of the pH in a cheese sample, its sub-micelles breakdown into small aggregates of casein very fast and causes more hardness in this dairy product (Fox et al. 2000). The further breakdown of casein (proteolysis), large peptides and oligopeptides into small peptides and amino acids (by the proteinases and peptidases of lactic acid bacteria) forms the secondary phase of ripening (Martinez-Cuesta et al. 2001) and cheese samples became softer in this period. Fredrick et al. (1986) found good correlation between hardness and proteolysis, thus, the softening of the cheese samples is likely a result of proteolysis. Madadlou et al. (2007) and Aminifar et al. (2010) reported the same results on traditional UF cheese.
Storage and loss moduli
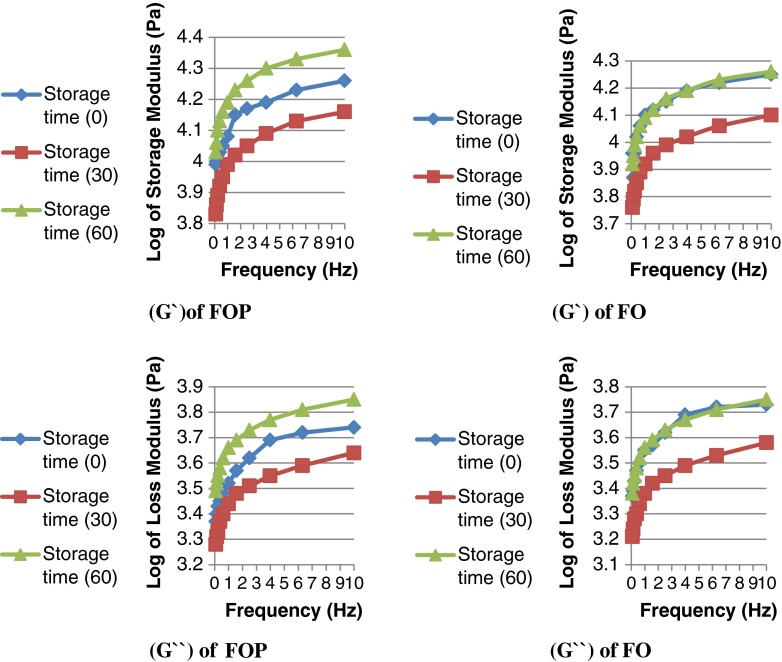
Changes in the rheological properties of FO and FOP cheese samples during the storage time based on its storage (G’) and loss (G”) moduli are shown in Fig. 2.
Fig. 2.
Effects of sweep test frequency on (G’) storage modulus (top view) and (G”) loss modulus (bottom view) of low fat UF-feta cheese fortified with fish oil powder (FOP) and fish oil (FO) at different storage times, (Sd = 0.05, n = 3)
With an increase in the storage time (3, 30 and 60 days) storage and loss moduli increased at different rates. If G’>G” the elastic behavior dominates over the viscous behavior and cheese has a gel character, however if G”>G’ the viscous behavior dominates over the elastic behavior and cheese has a liquid character (Steffe 1996). Although in two types of FO and FOP cheeses G’>G”, and both samples showed gel character, but the gel character of FO samples became very similar to fresh samples (zero day storage) after 60 days of storage and 30-day-old cheeses in both samples had the lowest storage and loss moduli throughout the frequency range studied. Figure 2 shows the changes of storage and loss moduli throughout the frequency range of 0–10 Hz. G’ modulus of FO samples increased from 5.8 to 18.5 kPa and G” increased from 1.6 to 5.7 kPa and for FOP samples G’ increased from 6.7 to 23.4 kPa and G” increased from 1.9 to 7.2 kPa in the 60 days of storage. Karami et al. (2009) reported 20–70 kPa for G’ and 7–27 for G” at 0.1–100 Hz for high fat UF-Feta cheese. It can be concluded that lower fat and higher whey protein concentration of cheese samples in this study caused a reduction in storage and loss moduli. Lopez and Dufour (2001) reported that whey protein causes high water binding capacity in the UF cheeses and results a softer texture as the ripening is continued. Sharp decrease of pH in FO samples during storage or ripening time was another reason to have a soft or elastic behavior in cheese texture. Since pH reduction of FO was higher than FOP cheese samples during 2 months of storage, its texture was softer than FOP samples. Researchers reported that the softening of cheese was dependent to its pH and the lower pH of feta cheese (4.5–4.7) was probably the main reason for its dominant elastic behavior (Wium et al. 1997 and Karoui and Dufour 2003).
Microstructure
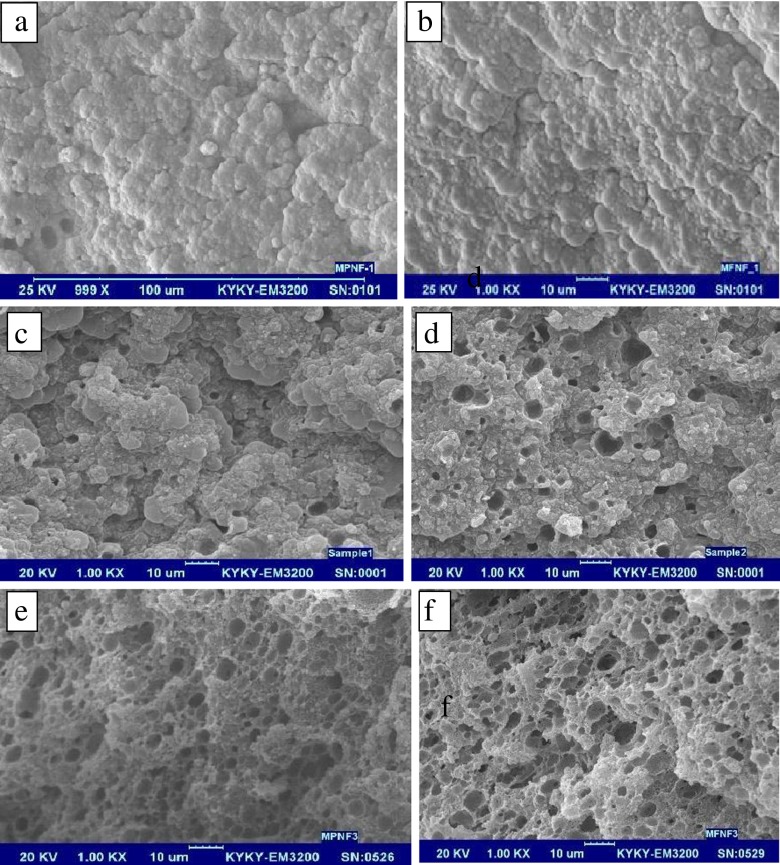
Scanning electron micrographs of cheese samples at 3, 30 and 60 days of storage time are shown in Fig. 3.
Fig. 3.
Scanning electron micrographs of fish oil fortified (FO) cheese samples during 0 (b), 30 (d), and 60 (f) days of storage time and fish oil powder (FOP) fortified cheese samples during 0 (a), 30 (c) and 60 (e) days of storage time. Magnification level is ×1000
After 3 days of ripening and at the first day of storage, the scanning electron micrographs of FO and FOP cheeses showed that they had large protein aggregates with few pores (Fig. 3(a, b)). By continuing the slow cheese fermentation during storage until the 30th day, the number of pores increased (Fig. 3(c, d)). However, the pores number of FOP samples was much less than FO samples, mainly because the fish oil powder made a more compact texture. Most probably these pores were the result of gas outlets and water drainage during storage time. According to Fox et al. (1993) during cheese fermentation, glycolysis, proteolysis and lipolysis continue in the storage time and make some holes in the casein networks. Different researchers hypothesized that the fat removal of the cheese samples was the main cause for pores creation (Lobato-Calleros et al. 2006; Madadlou, et al. 2007; Karami et al. 2009, and Aminifar et al. 2010). Formation of large and deep pores in the FO samples was the sign of proteolysis, rearrangement of protein matrix, and decreasing hardness of feta cheese texture after 60 days of storage (Fig. 3(e, f)). Lower pH of FO samples of feta cheese in this study also made more breakdown of the sub-micelles into non-linear strands of casein and finally to the aggregation of protein matrix (as confirmed by Tunick 2000). Additionally, a reduction in the week points, an increase in the cross-links among the non-linear strands of casein, and improving the elasticity of feta cheese were the results of fat globules disruption during the storage time (Wium and Qvist 1998).
Fatty acids profile
The fatty acids analysis and profile of FO and FOP cheese samples is shown on Table 2. In this study the fortified cheese samples (containing fish oil and fish oil powder) had 30 mg or 10 % of daily dose recommendations of EPA and DHA (AHA 2006; AFFSSA 2003). At the beginning of storage time, the DHA and EPA content in the FO and FOP cheese samples had 1.2 % and 1.24 % of low cheese fat (8.4 %), respectively. This is about 99 mg in 100 g cheese which is equal to 29.7 mg in one portion (30 g) of cheese. Table 2 shows 11 % reduction in omega-3 LC PUFA during 60 days storage time which can be compensated during formula preparation. A comparison between the high fat feta cheese (available in the market) and formulated low fat feta cheese fortified with fish oil or fish oil powder showed that 3 major saturated fatty acids of palmitic, stearic and myristic acid were decreased about 28.5, 26.2 and 45 %, respectively and oleic acid, EPA and DHA were increased about 60, 100 and 100 %, respectively. Martini et al. (2009) reported no change in DHA/EPA content of cheddar cheeses happened with aging, when it was fortified with fish oil. However, the bottom rows of Table 2 show that the trans and saturated fatty acids of FO samples were a little lower than the fat in FOP samples. Furthermore, the PUFA degradation in FO samples was less than FOP samples after 2 months of cold storage (Table 2).
Table 2.
Fatty acid composition of cheese fat (%) in the low fat (8.4 %) FO (fish oil) and FOP (fish oil powder) samples of feta cheese; (n = 3)
| Fatty acid (%) | Days of 5 °C storage | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 30 | 60 | |||||
| FO | FOP | FO | FOP | FO | FOP | ||
| C4:0 | Butyric acid | 3.15 ± 0.05 | 2.86 ± 0.05 | 2.95 ± 0.05 | 2.81 ± 0.05 | 2.85 ± 0.05 | 2.76 ± 0.05 |
| C6:0 | Caproic acid | 1.35 ± 0.05 | 1.27 ± 0.05 | 1.23 ± 0.05 | 1.15 ± 0.05 | 1.12 ± 0.05 | 0.98 ± 0.05 |
| C8:0 | Caprylic acid | 1.08 ± 0.02 | 0.95 ± 0.02 | 0.99 ± 0.02 | 0.76 ± 0.02 | 0.78 ± 0.02 | 0.71 ± 0.02 |
| C10:0 | Capric acid | 1.45 ± 0.05 | 2.12 ± 0.05 | 1.29 ± 0.05 | 1.96 ± 0.05 | 1.2 ± 0.05 | 1.24 ± 0.05 |
| C12:0 | Lauric acid | 3.1 ± 0.04 | 3.84 ± 0.04 | 2.99 ± 0.04 | 3.57 ± 0.04 | 2.79 ± 0.04 | 3.3 ± 0.04 |
| C14:0 | Myristic acid | 7.2 ± 0.05 | 6.85 ± 0.05 | 7.12 ± 0.05 | 6.72 ± 0.05 | 7.05 ± 0.05 | 6.56 ± 0.05 |
| C14:1 | Myristoleic acid | 1.09 ± 0.02 | 1.07 ± 0.02 | 1.07 ± 0.02 | 1.05 ± 0.02 | 0.84 ± 0.02 | 0.7 ± 0.02 |
| C15:0 | Pentadecanoic acid | 0.91 ± 0.01 | 1.04 ± 0.01 | 0.85 ± 0.01 | 0.84 ± 0.01 | 0.71 ± 0.01 | 0.8 ± 0.01 |
| C16:0 | Palmitic acid | 22.35 ± 0.05 | 22.38 ± 0.05 | 22.1 ± 0.05 | 22.21 ± 0.05 | 21.95 ± 0.05 | 22.13 ± 0.05 |
| C16:1 | Palmitoleic acid | 2.29 ± 0.05 | 2.09 ± 0.05 | 2.2 ± 0.05 | 1.69 ± 0.05 | 2.09 ± 0.05 | 1.56 ± 0.05 |
| C17:0 | Margaric acid | 0.57 ± 0.02 | 0.52 ± 0.02 | 0.47 ± 0.02 | 0.49 ± 0.02 | 0.36 ± 0.02 | 0.37 ± 0.02 |
| C18:0 | Stearic acid | 9.21 ± 0.05 | 8.68 ± 0.05 | 8.18 ± 0.05 | 8.36 ± 0.05 | 7.35 ± 0.05 | 8.27 ± 0.05 |
| C18:1t | Elaidic acid | 1.72 ± 0.01 | 1.85 ± 0.01 | 1.62 ± 0.01 | 1.78 ± 0.01 | 1.3 ± 0.01 | 1.63 ± 0.01 |
| C18:1c | Oleic acid | 37.41 ± 0.05 | 36.95 ± 0.05 | 37.03 ± 0.05 | 36.72 ± 0.05 | 36.92 ± 0.05 | 36.53 ± 0.05 |
| C18:2 | Linoleic acid | 4.97 ± 0.02 | 4.42 ± 0.02 | 4.75 ± 0.02 | 3.97 ± 0.02 | 4.74 ± 0.02 | 3.8 ± 0.02 |
| C18:3 | Linolenic acid | 0.55 ± 0.01 | 0.53 ± 0.01 | 0.49 ± 0.01 | 0.51 ± 0.01 | 0.48 ± 0.01 | 0.43 ± 0.01 |
| C20:1 | Gadoleic acid | 0.36 ± 0.05 | 0.47 ± 0.05 | 0.32 ± 0.05 | 0.43 ± 0.05 | 0.11 ± 0.05 | 0.38 ± 0.05 |
| C20:5 | Eicosapentaenoic acid (EPA) | 0.75 ± 0.01 | 0.77 ± 0.01 | 0.72 ± 0.01 | 0.72 ± 0.01 | 0.69 ± 0.01 | 0.67 ± 0.01 |
| C22:6 | Docosahexaenoic acid (DHA) | 0.45 ± 0.01 | 0.48 ± 0.01 | 0.39 ± 0.01 | 0.42 ± 0.01 | 0.36 ± 0.01 | 0.39 ± 0.01 |
| Total TFA (%) a | 1.72 | 1.85 | 1.62 | 1.78 | 1.30 | 1.63 | |
| Total SFA (%) b | 50.37 | 50.51 | 48.17 | 48.87 | 46.16 | 47.12 | |
| PUFA (DHA+EPA) c | 1.2 | 1.25 | 1.11 | 1.14 | 1.05 | 1.06 | |
aTrans fatty acids
bSaturated fatty acids
cPoly unsaturated fatty acids (Docosahexaenoic acid and eicosapentaenoic acid)
Sensory evaluation
The sensory panel scores (overall acceptability) of FO sample were significantly higher (P < 0.05) than FOP sample of fortified feta cheese after 30 and 60 days of cold storage (Table 1). Although the fishy flavor significantly decreases the sensory quality of fish oil and foods containing fish oil (Ye et al. 2009), and it is not easy to mask this most sensitive flavor in the foods fortified with fish oil (as reported by Kolanowski et al. 2007), the usage of cumin powder helped us to cover this flavor in feta cheese fortified with fish oil considerably. Ye et al. (2009) did not detect significant difference in the sensory perceptions of processed cheeses containing a low level of fish oil (5 g kg−1) and the similar sample with no fish oil. Kolanowski et al. (2007) showed that in semi solid dairy products (like yoghurt) and low fat cream, the fish oil level should be restricted between 1 and 5 g/kg. However, when the fat content becomes higher, the usage level of fish oil can be increased too. In our study, no fishy off-flavor, oxidized and rancid flavors in both samples were detected most probably because of masking properties of cumin and low levels of fish oil (4 g/kg) and fish oil powder (15 g/Kg) which represents 10 % of recommended daily intakes of EPA and DHA. Overall acceptability was maximum for FO and FOP cheese samples at 30th day of storage, but the color of FOP samples changed to yellow during the second month of storage and the panelists did not like the appearance of the FOP samples in comparison with FO samples. Conversely, the panelists gave up to 70 % of maximum desirability score to the hardness, texture and flavor of FO samples most likely because it did not have the coating materials of FOP, and containing WPC as a consisting agent, and fine cumin powder as a deodorizer and masking agent of fortified feta cheese.
Conclusion
This research showed that it is possible to fortify low fat (only 8.4 %) ultra-filtrated feta cheese with fish oil (at the level of 4 g/kg) oils containing omega-3 LC PUFA and also olive oil. The cumin seed powder had a good potential to mask successfully the fishy flavor of fish oil as an ingredient of enriched feta cheese. ANOVA results showed that the pH, MSNF, fat and protein of feta cheeses fortified with fish oil (FO) and fish oil powder (FOP) were significantly (P < 0.05) different during 0, 30 and 60 days of cold storage. Storage and loss moduli decreased until the 30th day of storage for both samples and then increased until the end of the storage time, but for FOP samples both moduli were higher than FO samples. The TBARS values (or the sign of secondary oxidation) in feta cheese samples of FO were lower than FOP but its PV was higher than FOP samples after 2 months storage. The important unsaturated fatty acids (oleic acid, EPA and DHA) and main saturated fatty acids (palmitic, stearic and myristic) in FO and FOP samples of feta cheese were significantly higher and lower than usual (unfortified) feta cheese, respectively. However, the PUFA degradation of FO samples was lesser than FOP samples after 60 days of cold storage. Additionally, the sensory properties and overall acceptability of FO were higher than FOP samples of feta cheese mainly due to its improving texture and taste and also stable color during storage.
Overall, the resulting FO sample of feta cheese had 10 % of daily requirements of DHA and EPA (beside 18 % MSNF) and also a high acceptance and desirability (~70 %) among its consumers.
Acknowledgments
The authors would like to appreciate The University of Tehran and Iran Dairy Industries Co. especially Pegah-e-Khorasan, for their financial support and technical assistance (physicochemical analysis). They also express their thanks to Akbarieh Co. and Mr. Dolatkhah-Nezhad for supplying the fish oil and fish oil powder and also Testa Laboratory (Dr. Beheshti) for performing the analytical tests.
References
- AFFSA (2003) Acides Gras de la Famille Oméga 3 et Système Cardiovasculaire: Intérêtnutritionnel et allegations. AgenceFrancaise de Sécurité Sanitaire des Aliments. http://www.anses.fr/Documents/NUT-Ra-omega3.pdf (accessed 17 September 2012)
- AHA Diet and lifestyle recommendations revision: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- Aminifar M, Hamedi M, Emam-Djomeh Z, Mehdinia A. Microstructural, compositional and textural properties during ripening of Lighvan cheese, A traditional raw sheep cheese. J Texture Stud. 2010;41:579–593. doi: 10.1111/j.1745-4603.2010.00244.x. [DOI] [Google Scholar]
- AOAC (Association of Official Analytical Chemists) (1997) Official methods of analysis (16th ed., 3rd rev. method 920.123), Arlington, VA, USA
- AOCS (American Oil Chemists Society) (2009) 2- Thiobarbituric acid value, direct method, official method Cd 19–90, 2710 S. Boulder, Urbana, IL 61802–6996 USA
- Aryana KJ. Cheddar cheese manufactured with oil high in omega-3 fatty acids. Milchwissenschaft. 2007;62:167–170. [Google Scholar]
- Baik MY, Suhendro EL, Nawar WW, McClements DJ, Decker EA, Chinachoti P. Effect of antioxidants and humidity on the oxidative stability of microencapsulated fish oil. JAOCS. 2004;81(4):355–360. [Google Scholar]
- Barrow CJ, Nolan C, Holub BJ (2009) Bioequivalence of encapsulated and microencapsulated fish-oil supplementation. J Funct Foods I: 38–43
- Bennett T, Desmond A, Harrington M. The effect of high intakes of casein and casein phosphopeptide on calcium absorption in the rat. Br J Nutr. 2000;83:673–680. doi: 10.1017/S0007114500000854. [DOI] [PubMed] [Google Scholar]
- Bermudez-Aguirre D, Barbosa-Canovas GV. Quality of selected cheeses fortified with vegetable and animal sources of omega-3. LWT-Food Sci Technol. 2011;44(7):1577–1584. doi: 10.1016/j.lwt.2011.01.023. [DOI] [Google Scholar]
- Brennan JG. Food texture measurements. In: King RD, editor. Developments in food analysis techniques. London: Applied Science Publisher; 1980. pp. 41–45. [Google Scholar]
- Bylund G (1995) Dairy processing handbook. Tetra Pak processing systems AB, S-221 86 Lund, Sweden, pp 326–328
- Choe E, Min DB. Mechanisms and factors for edible oil oxidation. Compr Rev Food Sci Food Safety. 2006;5:169–186. doi: 10.1111/j.1541-4337.2006.00009.x. [DOI] [Google Scholar]
- Curtis JM, Berrigan N, Dauphinee P. The determination of n-3 fatty acid levels in Food Products containing microencapsulated fish oil using the one-step extraction method. J Am Oil Chem Soc. 2008;85:297–305. doi: 10.1007/s11746-008-1194-1. [DOI] [Google Scholar]
- Drake MA, Herrett W, Boylston TD, Swanson BG. Lecithin improves texture of reduced fat cheeses. J Food Sci. 1996;61:639–642. doi: 10.1111/j.1365-2621.1996.tb13176.x. [DOI] [Google Scholar]
- Drusch S, Serfert Y, Scampicchio M, Schmidt-Hansberg B, Schwaz K. Impact of physicochemical characteristics on the oxidative stability of fish oil microencapsulated by spray-drying. J Agric Food Chem. 2007;55(26):11044–11051. doi: 10.1021/jf072536a. [DOI] [PubMed] [Google Scholar]
- Faraji H, McClements DJ, Decker EA. Role of continuous phase protein on the oxidative stability of fish oil-in-water emulsions. J Agric Food Chem. 2004;52:4558–4564. doi: 10.1021/jf035346i. [DOI] [PubMed] [Google Scholar]
- Fox PF, McSweeney PLH, Cogan TM, Guinee TP. Biochemistry of cheese ripening. In: Fox PF, editor. Cheese: chemistry. Chapman: Physics and Microbiology; 1993. pp. 389–438. [Google Scholar]
- Fox PF, McSweeney PLH, Cogan TM, Guinee TP. Fundamentals of cheese science. Maryland: Aspen Publishers Inc; 2000. [Google Scholar]
- Fredrick IA, Atson JW, Nottingham SM, Dulley J. The effect of neutral fungal protease in cheddar cheese ripening. N Z J Dairy Sci Technol. 1986;43:35–39. [Google Scholar]
- Gibbs BF, Kermasha S, Alli I, Mullingan CN. Encapsulation in the food industry: a review. Int J Food Sci Nutr. 1999;50:213–224. doi: 10.1080/096374899101256. [DOI] [PubMed] [Google Scholar]
- Gunasekaran S, Mehmet Ak M. Cheese rheology and texture. Florida: CRC press; 2003. [Google Scholar]
- Health Council of the Netherlands (2006) Guidelines for a Healthy Diet. Health Council of the Netherlands Publication No. 2006/21E, The Hague, Netherlands
- Hibbeln JR, Nieminen LRG, Blasbalg TL, Riggs JA, Lands WEM. Healthy intakes of n-3 and n-6 fatty acids: Estimations considering worldwide diversity. Am J Clin Nutr. 2006;83:1483s–1493s. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- Hooper L, Harrison RA, Summerbell CD, Moore H, Worthington HV, Ness A, Capps N, Davey Smith G, Riemersma R, Ebrahim S. Omega 3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database Syst Rev. 2004;4:CD 003177. doi: 10.1002/14651858.CD003177.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDF (International Dairy Federation) (1991) Anhydrous milk fat, Determination of peroxide value. In: International IDF Standards Sect. 74A:1991, Square Vergot 41, Brussels, Belgium
- Jalali-Heravi M, Zekavat B, Sereshti H. Use of gas chromatography–mass spectrometry combined with resolution methods to characterize the essential oil components of Iranian cumin and caraway. J Chromatogr A. 2007;1143(1–2):215–226. doi: 10.1016/j.chroma.2007.01.042. [DOI] [PubMed] [Google Scholar]
- James CS. Analytical chemistry of foods. Glasgow: Blackie Academic and professional; 1995. [Google Scholar]
- Karami M, Ehsani MR, Mousavi SM, Rezaei K, Safari M. Changes in the rheological properties of Iranian UF-feta cheese during ripening. Food Chem. 2009;112(3):539–544. doi: 10.1016/j.foodchem.2008.06.003. [DOI] [Google Scholar]
- Karoui R, Dufour E. Dynamic testing rheology and fluorescence spectroscopy investigations of surface to centre differences in ripened soft cheeses. Int Dairy J. 2003;13:973–985. doi: 10.1016/S0958-6946(03)00121-3. [DOI] [Google Scholar]
- Kinsella JE. Milk proteins: Physicochemical and functional properties. Crit Rev Food Sci Nutr. 1984;21:197–262. doi: 10.1080/10408398409527401. [DOI] [PubMed] [Google Scholar]
- Kolanowski W, Laufenberg G. Enrichment of food products with polyunsaturated fatty acids by fish oil addition. Eur Food Res Technol. 2006;222:472–477. doi: 10.1007/s00217-005-0089-8. [DOI] [Google Scholar]
- Kolanowski W, Laufenberg G, Kunz B. Fish oil stabilisation by microencapsulation with modified cellulose. Int J Food Sci Nutr. 2004;55:333–343. doi: 10.1080/09637480410001725157. [DOI] [PubMed] [Google Scholar]
- Kolanowski W, Jaworska D, Weißbrodt J. Importance of instrumental and sensory analysis in the assessment of oxidative deterioration of omega-3 long-chain polyunsaturated fatty acid-rich foods. J Sci Food Agric. 2007;87(2):181–191. doi: 10.1002/jsfa.2733. [DOI] [Google Scholar]
- Kris-Etherton PM. Monounsaturated fatty acids and risk of cardiovascular disease. Circulation. 1999;100:1253–1258. doi: 10.1161/01.CIR.100.11.1253. [DOI] [PubMed] [Google Scholar]
- Lobato-Calleros C, Rodriguez E, Sandoval-Castilla O, Vernon-Carter EJ, Alvarez-Ramirez J. Reduced-fat white fresh cheese-like products obtained from W1/o/W2 multiple emulsions: Viscoelastic and high-resolution image analyses. Food Res Int. 2006;39:678–685. doi: 10.1016/j.foodres.2006.01.006. [DOI] [Google Scholar]
- Lopez C, Dufour E. The composition of the milk fat globule surface alters the structural characteristics of the coagulum. J Colloid Interface Sci. 2001;233:241–249. doi: 10.1006/jcis.2000.7255. [DOI] [PubMed] [Google Scholar]
- Lopez-Huertas E. Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks: A review of intervention studies. Pharmacol Res. 2010;61:200–207. doi: 10.1016/j.phrs.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Madadlou A, Khosrowshahi-Asl A, Mousavi ME, Farmani J. The influence of brine concentration on chemical composition and texture of Iranian white cheese. J Food Eng. 2007;81:330–335. doi: 10.1016/j.jfoodeng.2006.11.010. [DOI] [Google Scholar]
- Martinez-Cuesta MC, De Palencia PF, Requena T, Pelaez C. Enzymatic ability of Lactobacillus caseisubsp. caseiIFPL 731 for flavor development in cheese. Int Dairy J. 2001;11:577–585. doi: 10.1016/S0958-6946(01)00046-2. [DOI] [Google Scholar]
- Martini S, Thurgood JE, Brothersen C, Ward R, McMahon DJ. Fortification of reduced-fat Cheddar cheese with n-3 fatty acids: Effect on off-flavor generation. J Dairy Sci. 2009;92(5):1876–1884. doi: 10.3168/jds.2008-1871. [DOI] [PubMed] [Google Scholar]
- Mensink RP, Katan MB. Effects of dietary fatty acids on serum lipids and lipoproteins: A meta-analysis of 27 trials. Arterioscler Thromb Vasc Biol. 1992;12:911–919. doi: 10.1161/01.ATV.12.8.911. [DOI] [PubMed] [Google Scholar]
- Mortensen G, Sorensen J, Stapelfeldt H. Comparison of peroxide value methods used for semihard cheeses. J Agric Food Chem. 2002;50:5007–5011. doi: 10.1021/jf0200220. [DOI] [PubMed] [Google Scholar]
- Robinson RK, Tamime AY (1991) Feta and related cheeses. Ellis Horwood Limited, Market Cross House, Chichester, West Sussex, PO19 1EB, England
- Ruxton CHS, Reed SC, Simpson MJA, Millington KJ. The health benefits of omega-3 polyunsaturated fatty acids: A review of the evidence. J Hum Nutr Diet. 2004;17(5):449–459. doi: 10.1111/j.1365-277X.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- Sepahioglu O, Alvarez V, Solano-Lopez C. Structure, physico-chemical and sensory properties of Feta cheese made with tapioca starch and lecithin as fat mimetics. Int Dairy J. 1999;9:783–789. doi: 10.1016/S0958-6946(99)00150-8. [DOI] [Google Scholar]
- Serfert Y, Drusch S, Schwarz K. Chemical stabilisation of oils rich in long-chain polyunsaturated fatty acids during homogenisation, microencapsulation and storage. Food Chem. 2009;113(4):1106–1112. doi: 10.1016/j.foodchem.2008.08.079. [DOI] [Google Scholar]
- Simopoulos AP. Essential fatty acids in health and chronic disease. Am J Clin Nutr. 1999;70:560–569. doi: 10.1093/ajcn/70.3.560s. [DOI] [PubMed] [Google Scholar]
- Singh H, Zhu X-Q, Ye A (2009) Lipid encapsulation. US patent 20090029017
- Steffe JF (1996) Rheological methods in food process engineering. Freeman press, East lansing, USA
- Thautwein EA. N-3 fatty acids–physiological and technical aspects for their use in food. Eur J Lipid Sci Technol. 2001;103:45–55. doi: 10.1002/1438-9312(200101)103:1<45::AID-EJLT45>3.0.CO;2-9. [DOI] [Google Scholar]
- Tunick MH. Rheology of dairy foods. J Dairy Sci. 2000;83:1892–1898. doi: 10.3168/jds.S0022-0302(00)75062-4. [DOI] [PubMed] [Google Scholar]
- Waagner-Nielsen E. North European varieties of cheese. In: Fox PF, editor. Cheese: chemistry. London: Physics and Microbiology; 1993. pp. 389–438. [Google Scholar]
- Walstra P, Smulders I. Making emulsions and foams: an overview. In: Dickinson E, Bergenstahl B, editors. Food colloids: proteins, lipids and polysaccharides. London: The Royal Society of Chemistry; 1997. pp. 367–381. [Google Scholar]
- Walstra P, Geurts TJ, Noomen A, Jellema A, Van Boekel MAJS. Dairy technology. Principles of milk properties and processes. 270 Madison Avenue: Marcel Decker Inc; 1999. [Google Scholar]
- WHO . Diet nutrition and the prevention of chronic diseases: Report of a WHO/FAO joint expert consultation, technical report 916. Geneva: World Health Organization; 2002. [Google Scholar]
- Wium H, Qvist KB. Rheological properties of Uf-Feta cheese determined by uniaxial compression and dynamic testing. J Texture Stud. 1998;28:435–454. doi: 10.1111/j.1745-4603.1997.tb00127.x. [DOI] [Google Scholar]
- Wium H, Gross M, Qvist K. Uniaxial compression of UF-Feta cheese related to sensory texture analysis. J Texture Stud. 1997;28:455–476. doi: 10.1111/j.1745-4603.1997.tb00128.x. [DOI] [Google Scholar]
- Ye A, Cui J, Taneja A, Zhu X, Singh H. Evaluation of processed cheese fortified with fish oil emulsion. Food Res Int. 2009;42(8):1093–1098. doi: 10.1016/j.foodres.2009.05.006. [DOI] [Google Scholar]