Abstract
Cauliflower is a rich source of proteins, carbohydrates, vitamins and minerals and also a very important vegetable having maximum availability in tropical climate from November to February which causes glut in the market and consequently producers do not get remunerative prices. The partially blanched cauliflower pieces of 3–4 cm long having stalk upto 2 cm in length are steeped in different levels of sodium chloride (NaCl, 2–6 %) and acetic acid (1–2 %) along with 350 ppm sulphur dioxide (SO2) using modified response surface methodology (RSM). The cauliflower samples steeped in 4 % NaCl, 1 % acetic acid and 350 ppm SO2 were rated best with maximum mean overall acceptability (OAA) score (6.90) and minimum mean preference (6.25) to the experimental run consisting of 6 % NaCl, 2 % acetic acid and 350 ppm SO2 was noted after 120 days of storage. There has been sharp decrease in pH values after 15 days of storage in all the experimental run of preserved cauliflower samples. The maximum mean decrease in hardness values (15.20–0.55 g.cm) was obtained in steeped cauliflower samples consisting of 6 % NaCl, 2 % acetic acid and 350 ppm SO2 during storage for 120 days at room temperature. The decrease in extent of browning, ascorbic acid and total carotenoids content in cauliflower samples was reported in all the experimental runs during 120 days of storage at room temperature. The optimum concentration for maximum desirability in the preservation of cauliflower consisted of 3.5 % sodium chloride and 1.1 % acetic acid and 350 ppm SO2.
Keywords: Steeping preservation, Cauliflower, RSM, Hurdle concept, Shelf life studies
The post harvest losses of horticultural produce are enormous which is estimated to be as high as 30 % with monetary loss of about 7.29 US billion dollar per annum (Chandra and Kar 2004). The shelf life of perishable vegetables is very low and post harvest losses in vegetables such as brinjal, cauliflower and chilli were reported to be of higher level (Jayanthi 2005). There are various physical methods such as thermal processing (pasteurization, sterilization, aseptic packaging), storage at low temperature(refrigeration, freezing, dehydro-freezing), removal of water(concentration, dehydration) and irradiation (UV or ioninzing radiation) are employed for increasing the shelf life of fruits and vegetables (Girdharilal et al. 1986). These physical methods cause substantial losses of water soluble vitamins and volatile flavours from fruits and vegetables and reflects the sensory properties of fruits and vegetables. The chemical methods include addition of acid, salting or brining and addition of chemical preservatives such as sodium benzoate and potassium metabisulphite for extending the shelf life of vegetables (Girdharilal et al. 1986).
Fruits and vegetables are important sources of essential nutrients such as vitamins and minerals for human consumption. These fruits and vegetables are also rich sources of phytochemicals which acts in preventing various chronic diseases such as reducing cardiovascular diseases, reduction towards the risk of prostate and cervical cancer, blood cholesterol levels etc (Karpagapandi et al. 2006). In view of good functional qualities, the large quantities of fruits and vegetables perish during glut season due to poor post harvest management and inadequate processing facilities. The suitable steeping preservation methods may be helpful in harnessing the useful characteristics of vegetables during lean season.
Among the various methods of preservation, drying is one of the oldest methods of food preservation (Lima et al. 2002). Drying not only facilitates reduction in the bulk of fresh and easy transport because of reduced weight and volume but also increases availability of food throughout the year at reasonable price. However, drying causes irreversible structural damage to the cellular structure of food, whereby rehydration of the dehydrated product is adversely affected (Gupta et al. 2011).
Hurdle technology involves the use of several preservation techniques in combination. This reduces the extensive use of one preservation technique producing the lower impact on sensory quality (Ahn et al. 2005). The method of steeping preservation of vegetables with hurdle concept, minimizes the damage to the sensory properties along with the synergistic action is exploited for food preservation (Leistner 1985). Baby corn can effectively be preserved by the combination of 6 % salt and 0.75 % acetic acid with respect to acceptable organoleptic quality during storage (Aggarwal and Kaur 2010). Citric acid treatment in combination with gamma radiation and modified atmospheric packaging is employed as hurdles for control of microorganisms and extending the shelf life of minimally processed French beans (Gupta et al. 2012). Hurdle technology is referred as very effective alternative for ensuring microbial quality and safety of minimally processed fruits and vegetables and is also recommended for processed fruits and vegetables (Shashidhar et al. 2007).
Cauliflower (Brassica oleracea var. ‘Botrytis’) is a very important vegetables having maximum availability in tropical climate from the month of November to February, which causes glut on the market and consequently producers do not get remunerative prices. Cauliflower is produced on an area of 0.348 million hectare with the total production of 6.57 million tones with the productivity of 18.9 t/ha (Indian Horticulture Database 2010). Fresh cauliflower can be stored for 2–4 weeks at storage temperature of 0 °C and Relative Humidity (RH) of 95–98 % and post harvest losses are estimated as 49 % in India (Indian Horticulture Database 2010 and Mudgal and Pandey 2007). Furthermore, cauliflower is a rich source of proteins, carbohydrates, vitamins and minerals (Bose et al. 1993). The preservation of cauliflower in native state can provide nutritional security to the large section of populations. The low cost preservation technique with extended shelf life is of great significance in India without changing the form of vegetables. The surplus production during the peak season may preserve the bulk amount of cauliflower which can suitably be utilized during the offseason for the manufacture of cauliflower based curry and preparation of cauliflower pickle. Thus the present investigation has been carried out to preserve cauliflower by different hurdles of sodium chloride, acetic acid and potassium metabisulphite for longer period of preservation with multivariate statistical techniques such as RSM to assess the sensory and physico-chemical properties of steeped cauliflower. In view of two independent variables and interaction studies, the modified RSM has been carried out with repetition of the levels for effective optimization process.
Materials and methods
The white compact heads of cauliflower variety ‘Snowball’ were obtained from vegetable research farm of Indian Institute of Vegetable Research, Varanasi. After sorting and cutting of stalk, the cauliflower heads were washed thoroughly in water and were cut into 3–4 cm long pieces, each piece having a stalk up to 2 cm in length followed by blanching in boiling water. Preliminary trials on blanching confirmed that 1 min of blanching in boiling water caused complete inactivation of enzymes and it resulted in softening of blanched cauliflower pieces after 15 days of storage in steeped solution. Thus, cauliflower pieces (500 g) tied in muslin cloth were blanched in boiling (100 °C) water (1,500 ml) for 30 s to inactivate partially catalase and peroxidase enzymes.
Standardization of levels of NaCl concentration (A) and acetic acid concentration (B): The different levels of NaCl (2–6 %, w/v) and acetic acid concentration (1–2 %, v/v) along with 350 ppm SO2 using modified RSM of D6 Hoke’s response surface design was adopted in the experimental design using 2 variables at 3 levels were standardized as per the following details:
| Process variables | Coded values | ||
| −1 | 0 | +1 | |
| Sodium chloride (%, w/v) | 2.0 | 4.0 | 6.0 |
| Acetic acid (%, v/v) | 1.0 | 1.5 | 2.0 |
| Treatment | Sodium chloride (%) | Acetic acid (%) | SO2 (ppm) |
| T1 | 2 | 1 | 350 |
| T2 | 4 | 1 | 350 |
| T3 | 6 | 1 | 350 |
| T4 | 2 | 2 | 350 |
| T5 | 4 | 2 | 350 |
| T6 | 6 | 2 | 350 |
| T7 | 2 | 1 | 350 |
| T8 | 4 | 1 | 350 |
| T9 | 6 | 1 | 350 |
| T10 | 2 | 2 | 350 |
| T11 | 4 | 2 | 350 |
| T12 | 6 | 2 | 350 |
| T13 | 2 | 1 | 350 |
The response of each treatment was judged on the basis of sensory and physico-chemical properties in cauliflower samples. Cauliflower samples (250 g) of each treatment were filled in wide mouth screw capped glass containers of 1 L capacity and tightly capped, labeled and stored at room temperature of 27–32 °C for 120 days.
Sensory evaluation: Cauliflower samples after definite period of steeping were washed thoroughly for half an hour and were evaluated by a panel of 10 trained judges for flavour, body and texture, colour and appearance and OAA on 9-point Hedonic scale (Lawless and Haymann 1998).
Analytical techniques: The total soluble solids (TSS) in cauliflower samples were estimated by hand refractometer and the values were expressed as oBrix at 20 °C. The ascorbic acid content in cauliflower samples was estimated by 2,6 dichlorindophenol dye as per the method described by Ranganna (1997). The pH values in cauliflower samples were measured using a micropro- cessor digital pH meter after addition of 20 ml distilled water in 10 g suspension in pestle mortar. The acidity of the cauliflower samples was estimated by titrating with 0.1 N NaOH solution using phenolphathelin indicator and was expressed in terms of % citric acid as per the method described in IS (1960). The extent of browning in cauliflower samples was determined by alcohol extraction method (Klin and Nagy 1988). The salt content in steeping preserved cauliflower samples was carried out by silver nitrate titration method (Ranganna 1997). The hardness values in cauliflower samples was estimated with the help of texture analyzer (Texture Expert Exceed, Stable Micro System, Godalming, UK) using blade set with knife of 50 kg load cell having pre-test speed of 2.0 mm/s, test speed of 2.0 mm/s and post test speed of 10.0 mm/s.
Statistical analysis: All the treatments were replicated thrice and data were analysed statistically using completely randomized design (Snedecor and Cochran 1967).
Results and discussion
Sensory score
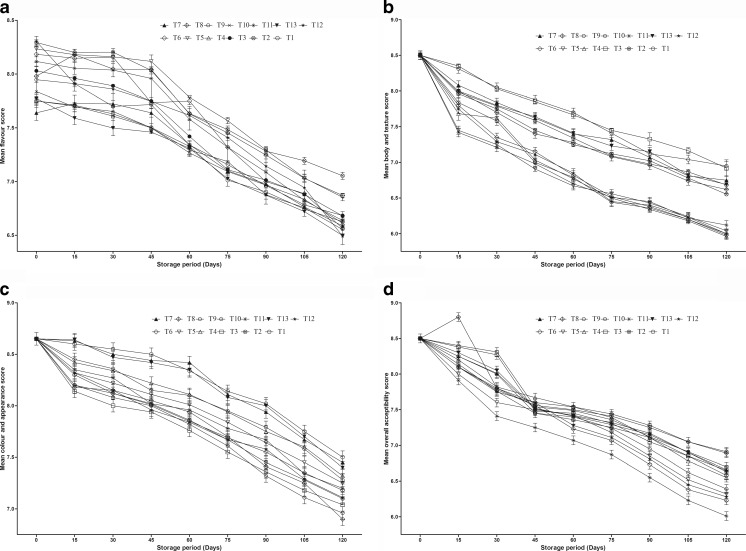
The results of sodium chloride concentration, acetic acid concentration and preservative potassium metabisulphite on sensory score in steeped cauliflower during storage at room temperature are described in Fig. 1a–d. The second order polynominal models were carried out for the shelf stable steeped cauliflower. Maximum flavour score (8.3) was obtained for treatments 2 and 8 both of which contained 4 % NaCl, 1 % acetic acid and 350 ppm of SO2 after 15 days of storage. Minimum flavour score (6.7) was reported after 120 days of storage in the treatment 10 which had 2 % NaCl, 2 % acetic acid and 350 ppm of SO2. All the treatments, storage period and interaction of treatments and storage period had shown significant effect (P < 0.5, CD: T 0.160, I 0.175 and TxI 0.393) on flavour score in steeped cauliflower. The body and texture sensory score declined in steeped cauliflower during 120 days of storage in all the treatments. The maximum body and texture score (8.3) was obtained for the treatments 3 and 9 having 6 % NaCl, 1 % acetic acid and 350 ppm SO2 which was decreased to 15.9 % and 16.4 % during storage for 120 days. The decrease in body and texture sensory score can be due to the effect of 2 % acetic acid which caused softening of cauliflower pieces during storage. Cocci et al. (2006) reported that citric acid concentration of 10 g L−1 could lead to softening of texture of apple slices due to acid hydrolysis of pectic substances. However, minimum body and texture score (7.4) was attained in the treatment 5 and 11 having 4 % NaCl, 2 % acetic acid and 350 ppm SO2 after 15 days of steeping which was reduced to 18.9 % and 16.4 % after 120 days of storage. The treatments containing higher levels of NaCl (4–6 %), 1 % acetic acid and 350 ppm SO2 were rated superior in texture. Similar results were obtained by Rodriguez et al. (1983) towards the suitability of cauliflower cultivars for canning. The effects of different treatments, storage period and interaction of treatments and storage period had also exhibited significant effect (P < 0.05, CD: T 0.110, I 0.121, TxI 0.270) on body and texture sensory score in steeped cauliflower during 120 days of storage. The maximum preference for colour and appearance score (8.6) was recorded for the treatments 1, 7 and 13 which had 2 % NaCl, 1 % acetic acid and 350 ppm SO2 after 15 days of storage while minimum colour and appearance score (7.0) was obtained in the treatments 3 and 9 after 120 days of storage which had 6 % NaCl, 1 % acetic acid and 350 ppm of SO2. The higher colour and appearance score in steeped cauliflower for the treatments 1, 7 and 13 can be reflected due to suitable combinations of acid, salt and KMS which resulted in maintaining white colour of cauliflower head. Similarly Barwal et al. (2005) also reported the decrease in colour and appearance score in steeped cauliflower in different levels of NaCl (5–15 %) and maximum (7.3) colour and appearance score was recorded in cauliflower samples containing 15 % NaCl + 0.2 % KMS after 180 days of storage. Furthermore, the improvement in colour of steeped cauliflower in NaCl and potassium metabisulphite (KMS) over fully control cauliflower is attributed due to cumulative effect of blanching and KMS (Srivastava and Sulebele 1975). Different treatments in steeping preservation of cauliflower, storage period and interaction of treatments and storage period had significant effect (P < 0.05, CD: T 0.159, I 0.175, TxI 0.391) on colour and appearance score of steeped cauliflower during storage. Maximum OAA score (8.4) was obtained in the treatments 3 and 9 having 6 % NaCl, 1 % acetic acid and 350 ppm SO2 while minimum OAA score (8.0 and 7.9) was judged for the treatments 5 and 11 which contained 4 % NaCl, 2 % acetic acid and 350 ppm SO2 after 15 days of storage. However, maximum OAA preference (6.9) was recorded for treatments 2 and 8 and minimum preference (6.2 and 6.3) was reported in the treatments 6 and 12 at the end of 120 days in steeped cauliflower samples. The higher OAA score in steeped cauliflower is obtained with higher level of NaCl (6 %) and lower level (1 %) of acetic acid. Sinha and Chandra (2012) reported that hurdle concept for preservation of cauliflower remained acceptable for 180 days at refrigerated storage temperature (5–7 °C) on good OAA score in 8 % salt, 0.3 % citric acid, 300 ppm potassium metabisulphite and 300 ppm sodium benzoate. The authors further reported that there had been significant differences between OAA scores of treated cauliflower pieces with the combinations of additives of 8–12 % salt solution, 300–500 ppm potassium metabisulphite and 100–300 ppm sodium benzoate due to combination of preservatives and days of storage while there had been no significant effects of storage temperatures at ambient temperature (30–37 °C) and refrigerated storage temperature (5–7 °C). The effect of different treatments, storage period and interaction of treatments and storage period had significant effect (P < 0.5, CD: T 0.094, I 0.103, TxI 0.232) on OAA score of steeped cauliflower during storage.
Fig. 1.
Changes in mean sensory scores of steeping preserved cauliflower during storage
ANOVA studies had shown that sodium chloride and acetic acid level had resulted in significant (P < 0.05) effect on flavour and body and texture sensory score, respectively whereas both variables had shown significant (P < 0.05) effect for colour and appearance and overall acceptability score in steeping preservation of cauliflower during storage. Acetic acid concentration had negative effect on flavour and body and texture score. Sodium chloride concentration had negative response towards colour and appearance score during steeping preservation of cauliflower. The calculated adequate precision value was 6.34, 5.16, 9.16 and 16.20 for flavour, body and texture, colour and appearance and overall acceptability score, respectively which was greater than 4.0. A ratio greater than 4.0 is desirable which indicates that model can be used to navigate the design space. The correlation coefficient values for flavour, body and texture, colour and appearance and OAA score in steeped cauliflower during storage was 0.60, 0.73, 0.86 and 0.96, respectively. The model thus developed with coded variables is as follows:
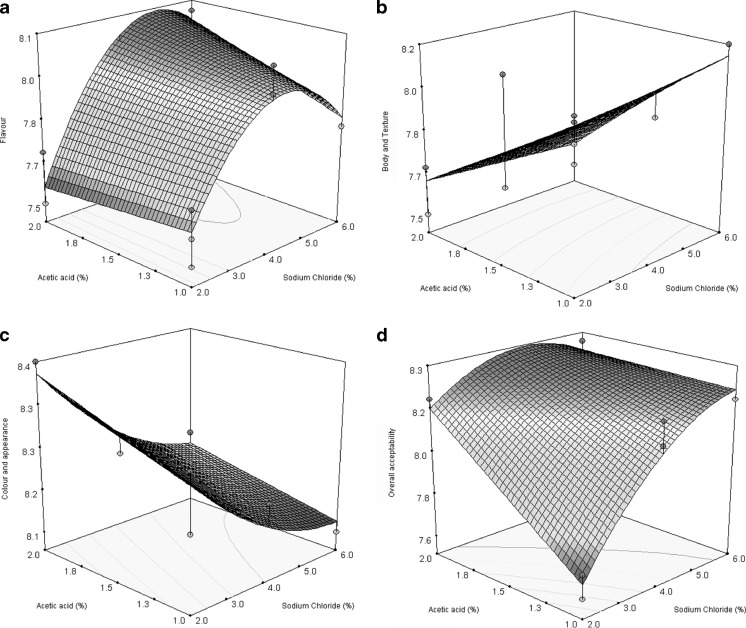
The flavour score in steeped cauliflower had shown decreasing trend with acetic acid concentration (1–2 %) and sodium chloride concentration (2–6 %). The body and texture score was also decreased with acetic acid concentration (1–2 %) and the score is increased with sodium chloride concentration (1–2 %). It could be reported that the increase in acetic acid concentration had resulted in softening of the tissues of cauliflower and increase in sodium chloride concentration had also shown hardening the tissues of cauliflower. The colour and appearance score also increased with acetic acid concentration (1–2 %). The increase in colour and appearance score in steeped cauliflower is reported due to more whitening. Initially the OAA score in steeped cauliflower increased with sodium chloride concentration (2–6 %) and acetic acid concentration of 1–2 % (Fig. 2a, b, c and d).
Fig. 2.
Response surface plot of sensory scores of cauliflower
Physico-chemical properties
The effect of sodium chloride and acetic acid along with SO2 treatment on physico-chemical properties on steeped cauliflower is presented in Tables 1, 2, 3 and 4. It is evident from Table 1 that there has been sharp decrease in pH values after 15 days of storage in all the steeped cauliflower samples. The sudden decrease in pH values in cauliflower samples of all the experimental runs after 15 days of steeping can be attributed due to adsorption of acetic acid in the cell walls of cauliflower samples which afterwards remained saturated. The minimum mean decrease in pH (6.30–3.25) was obtained for the treatments 3 and 9 having 6 % sodium chloride, 1 % acetic acid and 350 ppm SO2 and maximum mean decrease in pH (6.30–2.75) was reflected in the treatments 5 and 11 with 4 % sodium chloride, 2 % acetic acid and 350 ppm SO2 after 120 days of storage at room temperature (Table 1). Our findings for decrease in pH are conformity with the observations recorded by Sinha and Chandra (2012).
Table 1.
Effect of hurdle treatments on changes in pH and acidity values (% citric cid) in cauliflower samples at different storage periods
| Storage period (days) | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | T12 | T13 | Mean | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 0 | 6.30 | 6.30 | 6.30 | 6.30 | 6.30 | 6.30 | 6.30 | 6.30 | 6.30 | 6.30 | 6.30 | 6.30 | 6.30 | 6.30 ± 0.0 |
| 15 | 3.75 | 3.68 | 3.57 | 3.43 | 3.08 | 3.31 | 3.73 | 3.69 | 3.56 | 3.41 | 3.11 | 3.28 | 3.74 | 3.49 ± 0.24 | |
| 30 | 3.68 | 3.60 | 3.48 | 3.30 | 3.00 | 3.23 | 3.67 | 3.61 | 3.44 | 3.27 | 3.10 | 3.20 | 3.65 | 3.40 ± 0.23 | |
| 45 | 3.40 | 3.56 | 3.68 | 3.21 | 3.11 | 3.18 | 3.37 | 3.54 | 3.65 | 3.16 | 3.05 | 3.15 | 3.38 | 3.34 ± 0.21 | |
| 60 | 3.34 | 3.50 | 3.62 | 3.16 | 3.07 | 3.14 | 3.29 | 3.48 | 3.60 | 3.11 | 3.00 | 3.09 | 3.31 | 3.29 ± 0.21 | |
| 75 | 3.30 | 3.41 | 3.49 | 3.12 | 3.00 | 3.06 | 3.24 | 3.42 | 3.51 | 3.05 | 2.94 | 3.03 | 3.21 | 3.21 ± 0.20 | |
| 90 | 3.21 | 3.33 | 3.41 | 3.05 | 2.92 | 2.94 | 3.20 | 3.35 | 3.43 | 2.95 | 2.88 | 2.92 | 3.15 | 3.13 ± 0.20 | |
| 105 | 3.11 | 3.24 | 3.32 | 2.91 | 2.84 | 2.88 | 3.12 | 3.22 | 3.31 | 2.88 | 2.81 | 2.87 | 3.06 | 3.04 ± 0.19 | |
| 120 | 3.04 | 3.14 | 3.26 | 2.84 | 2.78 | 2.80 | 3.02 | 3.12 | 3.24 | 2.81 | 2.72 | 2.79 | 2.96 | 2.96 ± 0.19 | |
| Mean ± SD n = 3 | 3.68 ± 1.05 | 3.75 ± 0.97 | 3.79 ± 0.95 | 3.48 ± 1.07 | 3.34 ± 1.11 | 3.4 ± 1.09 | 3.66 ± 1.02 | 3.75 ± 0.97 | 3.7 ± 0.95 | 3.44 ± 1.09 | 3.32 ± 1.12 | 3.4 ± 1.10 | 3.6 ± 1.03 | ||
| P < 0.05, CD: T 0.078, I 0.085, TxI = 0.192 | |||||||||||||||
| Titratable acidity | 0 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 ± 0.0 |
| 15 | 0.34 | 0.37 | 0.45 | 0.58 | 0.47 | 0.69 | 0.32 | 0.35 | 0.46 | 0.59 | 0.45 | 0.71 | 0.33 | 0.47 ± 0.13 | |
| 30 | 0.45 | 0.44 | 0.47 | 0.59 | 0.49 | 0.71 | 0.42 | 0.41 | 0.48 | 0.61 | 0.47 | 0.73 | 0.44 | 0.52 ± 0.11 | |
| 45 | 0.50 | 0.48 | 0.51 | 0.62 | 0.53 | 0.74 | 0.51 | 0.50 | 0.53 | 0.64 | 0.52 | 0.76 | 0.51 | 0.57 ± 0.09 | |
| 60 | 0.57 | 0.52 | 0.55 | 0.66 | 0.58 | 0.77 | 0.56 | 0.54 | 0.57 | 0.68 | 0.60 | 0.79 | 0.56 | 0.61 ± 0.09 | |
| 75 | 0.61 | 0.56 | 0.59 | 0.68 | 0.61 | 0.79 | 0.60 | 0.58 | 0.60 | 0.71 | 0.64 | 0.83 | 0.60 | 0.65 ± 0.08 | |
| 90 | 0.66 | 0.68 | 0.67 | 0.78 | 0.68 | 0.83 | 0.65 | 0.67 | 0.68 | 0.77 | 0.69 | 0.87 | 0.67 | 0.72 ± 0.07 | |
| 105 | 0.68 | 0.72 | 0.71 | 0.83 | 0.72 | 0.87 | 0.68 | 0.71 | 0.71 | 0.82 | 0.72 | 0.89 | 0.69 | 0.75 ± 0.07 | |
| 120 | 0.71 | 0.74 | 0.75 | 0.86 | 0.75 | 0.89 | 0.72 | 0.75 | 0.74 | 0.87 | 0.76 | 0.92 | 0.72 | 0.78 ± 0.07 | |
| Mean ± SD n = 3 | 0.52 ± 0.19 | 0.51 ± 0.20 | 0.54 ± 0.19 | 0.64 ± 0.22 | 0.55 ± 0.19 | 0.7 ± 0.23 | 0.5 ± 0.19 | 0.51 ± 0.20 | 0.54 ± 0.19 | 0.65 ± 0.22 | 0.55 ± 0.20 | 0.74 ± 0.24 | 0.52 ± 0.19 | ||
| P < 0.05, CD: T 0.070 I 0.0775, TxI = 0.173 | |||||||||||||||
Table 2.
Effect of hurdle treatments on changes in hardness (g.cm) and extent of browning (OD at 440 nm) in cauliflower samples at different storage periods
| Storage period (days) | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | T12 | T13 | Mean | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hardness | 0 | 15.20 | 15.20 | 15.20 | 15.20 | 15.20 | 15.20 | 15.20 | 15.20 | 15.20 | 15.20 | 15.20 | 15.20 | 15.20 | 15.20 ± 0.00 |
| 15 | 10.50 | 13.40 | 13.70 | 8.50 | 9.60 | 9.80 | 10.70 | 13.30 | 13.60 | 8.40 | 9.50 | 9.90 | 10.80 | 10.90 ± 1.94 | |
| 30 | 8.70 | 11.80 | 12.40 | 6.60 | 7.80 | 7.90 | 8.80 | 11.60 | 12.30 | 6.50 | 7.50 | 7.60 | 8.60 | 9.08 ± 2.16 | |
| 45 | 6.80 | 11.20 | 11.00 | 6.20 | 4.60 | 6.40 | 6.60 | 11.10 | 11.10 | 6.20 | 4.40 | 6.10 | 6.50 | 7.55 ± 2.56 | |
| 60 | 4.00 | 9.10 | 9.20 | 6.10 | 3.20 | 3.50 | 4.10 | 9.20 | 9.00 | 6.00 | 3.30 | 3.40 | 4.20 | 5.71 ± 2.53 | |
| 75 | 3.80 | 8.70 | 8.90 | 3.50 | 2.60 | 2.70 | 3.70 | 8.60 | 8.60 | 3.40 | 2.20 | 2.80 | 3.70 | 4.85 ± 2.71 | |
| 90 | 3.60 | 7.60 | 7.80 | 2.60 | 1.80 | 1.70 | 3.40 | 7.50 | 7.70 | 2.30 | 1.60 | 1.50 | 3.30 | 4.03 ± 2.60 | |
| 105 | 2.70 | 6.50 | 6.70 | 1.70 | 1.10 | 0.90 | 2.60 | 6.40 | 6.60 | 1.60 | 1.00 | 0.70 | 2.50 | 3.15 ± 2.44 | |
| 120 | 1.80 | 6.20 | 6.40 | 0.90 | 0.90 | 0.60 | 1.70 | 6.10 | 6.20 | 0.70 | 0.80 | 0.50 | 1.60 | 2.64 ± 2.52 | |
| Mean ± SD n = 3 | 6.34 ± 4.4 | 9.97 ± 3.13 | 10.14 ± 3.12 | 5.70 ± 4.37 | 5.20 ± 4.80 | 5.41 ± 4.88 | 6.31 ± 4.47 | 9.89 ± 3.13 | 10.02 ± 3.18 | 5.59 ± 4.43 | 5.05 ± 4.83 | 5.30 ± 4.92 | 6.27 ± 4.49 | ||
| P < 0.05, CD: T 2.06,I 1.33, TxI = 4.56 | |||||||||||||||
| Extent of browning | 0 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 ± 0.00 |
| 15 | 0.07 | 0.07 | 0.06 | 0.04 | 0.04 | 0.05 | 0.07 | 0.07 | 0.06 | 0.05 | 0.04 | 0.05 | 0.07 | 0.06 ± 0.01 | |
| 30 | 0.05 | 0.05 | 0.05 | 0.04 | 0.03 | 0.04 | 0.05 | 0.05 | 0.05 | 0.04 | 0.03 | 0.04 | 0.05 | 0.04 ± 0.01 | |
| 45 | 0.04 | 0.03 | 0.04 | 0.03 | 0.03 | 0.04 | 0.04 | 0.03 | 0.04 | 0.03 | 0.03 | 0.04 | 0.04 | 0.04 ± 0.01 | |
| 60 | 0.03 | 0.03 | 0.04 | 0.03 | 0.03 | 0.04 | 0.03 | 0.03 | 0.04 | 0.03 | 0.03 | 0.04 | 0.04 | 0.03 ± 0.01 | |
| 75 | 0.03 | 0.03 | 0.04 | 0.03 | 0.03 | 0.04 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.04 | 0.03 | 0.03 ± 0.02 | |
| 90 | 0.03 | 0.02 | 0.03 | 0.03 | 0.03 | 0.04 | 0.03 | 0.02 | 0.03 | 0.03 | 0.03 | 0.04 | 0.03 | 0.03 ± 0.02 | |
| 105 | 0.03 | 0.02 | 0.03 | 0.02 | 0.04 | 0.04 | 0.02 | 0.02 | 0.03 | 0.02 | 0.04 | 0.04 | 0.02 | 0.03 ± 0.01 | |
| 120 | 0.02 | 0.02 | 0.03 | 0.02 | 0.04 | 0.04 | 0.02 | 0.02 | 0.03 | 0.02 | 0.04 | 0.04 | 0.02 | 0.03 ± 0.01 | |
| Mean ± SD n = 3 | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.02 | ||
| P < 0.05, CD: T 0.0054, I 0.0049, TxI = 0.0117 | |||||||||||||||
Table 3.
Effect of hurdle treatments on changes in total soluble solids (%) and salt content (%) in cauliflower samples at different storage periods
| Total soluble solids | Storage period (days) | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | T12 | T13 | Mean ± S D |
| 0 | 3.40 | 3.40 | 3.40 | 3.40 | 3.40 | 3.40 | 3.40 | 3.40 | 3.40 | 3.40 | 3.40 | 3.40 | 3.40 | 3.40 ± 0.0 | |
| 15 | 10.60 | 6.10 | 8.33 | 10.90 | 7.10 | 9.33 | 10.90 | 6.20 | 8.45 | 10.80 | 7. 00 | 9.20 | 10.80 | 8.90 ± 1.85 | |
| 30 | 9.30 | 5.05 | 7.27 | 9.06 | 5.90 | 7.75 | 9.10 | 4.95 | 7.10 | 9.15 | 5.80 | 7.30 | 9.20 | 7.46 ± 1.64 | |
| 45 | 7.52 | 4. 00 | 6.20 | 7.23 | 4.70 | 6.16 | 7.30 | 3.95 | 6.10 | 7.20 | 4.65 | 6.10 | 7.20 | 6.02 ± 1.30 | |
| 60 | 7.05 | 3.60 | 5.76 | 6.72 | 4.08 | 5.40 | 7. 00 | 3.50 | 5.65 | 6.65 | 4. 00 | 5.35 | 6.95 | 5.52 ± 1.34 | |
| 75 | 6.45 | 3.31 | 5.33 | 6.20 | 3.46 | 4.63 | 6.70 | 3.25 | 5.25 | 6.15 | 3.40 | 4.55 | 6.65 | 5.03 ± 1.35 | |
| 90 | 6.51 | 3.14 | 4.82 | 6.05 | 3.35 | 4.51 | 6.45 | 3.20 | 4.75 | 6. 00 | 3.25 | 4.45 | 6.41 | 4.84 ± 1.33 | |
| 105 | 6.35 | 2.96 | 4.30 | 5.90 | 3.20 | 4.40 | 6.20 | 3. 00 | 4.25 | 5.95 | 3.15 | 4.31 | 6.14 | 4.62 ± 1.33 | |
| 120 | 5.85 | 2.40 | 3.90 | 5.76 | 2.43 | 4.30 | 5.96 | 2.44 | 3.95 | 5.80 | 2.35 | 4.15 | 5.90 | 4.25 ± 1.49 | |
| Mean ± SD n = 3 | 7.00 ± 1.94 | 3.77 ± 1.08 | 5.48 ± 1.51 | 6.80 ± 2.01 | 4.18 ± 1.48 | 5.54 ± 1.90 | 7.00 ± 2.09 | 3.77 ± 1.15 | 5.43 ± 1.60 | 6.79 ± 2.12 | 4.11 ± 1.47 | 5.42 ± 1.73 | 6.96 ± 2.08 | ||
| P < 0.05, CD: T 2.06,I 1.33, TxI = 4.56 | |||||||||||||||
| Salt content | Storage period (days) | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | T12 | T13 | Mean |
| 0 | 2.00 | 4.00 | 6.00 | 2.00 | 4.00 | 6.00 | 2.00 | 4.00 | 6.00 | 2.00 | 4.00 | 6.00 | 2. 00 | 4.00 ± 1.71 | |
| 15 | 1.93 | 3.75 | 5.54 | 1.95 | 3.64 | 5.22 | 1.92 | 3.68 | 5.50 | 1.81 | 3.68 | 5.33 | 1.96 | 3.66 ± 1.49 | |
| 30 | 1.86 | 3.50 | 5.08 | 1.90 | 3.28 | 4.44 | 1.84 | 3.36 | 5. 00 | 1.62 | 3.36 | 4.66 | 1.92 | 3.33 ± 1.29 | |
| 45 | 1.79 | 3.25 | 4.62 | 1.85 | 2.92 | 3.66 | 1.76 | 3.04 | 4.50 | 1.43 | 3.04 | 3.99 | 1.88 | 2.99 ± 1.09 | |
| 60 | 1.72 | 3. 00 | 4.16 | 1.80 | 2.56 | 2.88 | 1.68 | 2.72 | 4. 00 | 1.24 | 2.72 | 3.32 | 1.84 | 2.65 ± 0.92 | |
| 75 | 1.65 | 2.75 | 3.70 | 1.75 | 2.20 | 2.10 | 1.60 | 2.40 | 3.50 | 1.05 | 2.40 | 2.65 | 1.80 | 2.31 ± 0.77 | |
| 90 | 1.58 | 2.50 | 3.24 | 1.70 | 1.84 | 1.32 | 1.52 | 2.08 | 3. 00 | 0.86 | 2.08 | 1.98 | 1.76 | 1.98 ± 0.68 | |
| 105 | 1.51 | 2.25 | 2.78 | 1.65 | 1.48 | 0.54 | 1.44 | 1.76 | 2.50 | 0.67 | 1.76 | 1.31 | 1.72 | 1.64 ± 0.66 | |
| 120 | 1.41 | 2.05 | 2.45 | 1.52 | 1.25 | 0.36 | 1.34 | 1.61 | 2.26 | 0.45 | 1.35 | 1.01 | 1.55 | 1.42 ± 0.64 | |
| Mean ± SD n = 3 | 1.72 ± 0.20 | 3.01 ± 0.68 | 4.17 ± 1.24 | 1.79 ± 0.15 | 2.57 ± 0.96 | 2.95 ± 2.03 | 1.68 ± 0.22 | 2.74 ± 0.85 | 4.03 ± 1.32 | 1.24 ± 0.53 | 2.71 ± 0.89 | 3.36 ± 1.77 | 3.36 ± 1.77 | ||
| P < 0.05, CD: T 0.0756, I 0.082, TxI = 0.1854 | |||||||||||||||
Table 4.
Effect of hurdle treatments on changes in ascorbic acid (mg/100 g) and total carotenoids (mg/100 g) in cauliflower samples at different storage periods
| Ascorbic acid | Storage period (days) | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | T12 | T13 | Mean |
| 0 | 24.50 | 24.50 | 24.50 | 24.50 | 24.50 | 24.50 | 24.50 | 24.50 | 24.50 | 24.50 | 24.50 | 24.50 | 24.50 | 24.50 ± 0.00 | |
| 15 | 14.50 | 14.90 | 14.00 | 14.70 | 12.90 | 12.50 | 14.40 | 14.70 | 13.80 | 14.50 | 12.80 | 12.10 | 14.10 | 13.80 ± 0.90 | |
| 30 | 13.90 | 14.50 | 13.90 | 13.80 | 11.60 | 11.80 | 13.80 | 14.40 | 13.50 | 13.70 | 11.60 | 11.70 | 13.50 | 13.20 ± 1.10 | |
| 45 | 13.30 | 14.20 | 13.80 | 13.00 | 10.20 | 11.10 | 13.20 | 14.10 | 13.10 | 12.70 | 10.20 | 11.00 | 13.10 | 12.50 ± 1.40 | |
| 60 | 11.40 | 13.40 | 13.20 | 12.20 | 9.50 | 10.10 | 11.20 | 13.20 | 12.90 | 11.80 | 9.40 | 10.00 | 11.20 | 11.50 ± 1.40 | |
| 75 | 9.40 | 12.60 | 12.70 | 11.40 | 8.70 | 9.10 | 9.30 | 12.40 | 12.30 | 11.30 | 8.60 | 9.00 | 9.30 | 10.50 ± 1.70 | |
| 90 | 7.90 | 10.50 | 10.80 | 9.60 | 8.00 | 7.70 | 7.80 | 10.30 | 10.50 | 9.50 | 7.90 | 7.50 | 7.70 | 8.90 ± 1.30 | |
| 105 | 6.40 | 8.50 | 9.00 | 7.90 | 7.30 | 6.30 | 6.30 | 8.30 | 8.60 | 7.80 | 7.20 | 6.10 | 6.30 | 7.40 ± 1.00 | |
| 120 | 5.10 | 6.20 | 6.60 | 5.70 | 5.90 | 5.60 | 5.20 | 6.00 | 6.40 | 5.50 | 5.70 | 5.40 | 5.00 | 5.70 ± 0.50 | |
| Mean | 11.80 ± 5.80 | 13.20 ± 4.20 | 13.20 ± 5.00 | 12.50 ± 5.30 | 10.90 ± 5.50 | 11.00 ± 5.60 | 11.70 ± 5.80 | 13.10 ± 5.20 | 12.80 ± 4.70 | 12.40 ± 5.40 | 10.90 ± 5.60 | 10.80 ± 5.70 | 11.60 ± 5.80 | ||
| P < 0.05, CD: T 0.178, I 0.195, TxI = 0.438 | |||||||||||||||
| Total carotenoids | Storage period (days) | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | T12 | T13 | Mean ± S D |
| 0 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 ± 0.0 | |
| 15 | 0.09 | 0.12 | 0.10 | 0.09 | 0.12 | 0.11 | 0.08 | 0.12 | 0.09 | 0.08 | 0.11 | 0.10 | 0.08 | 0.10 ± 0.02 | |
| 30 | 0.08 | 0.12 | 0.09 | 0.08 | 0.12 | 0.10 | 0.08 | 0.12 | 0.09 | 0.08 | 0.11 | 0.09 | 0.08 | 0.09 ± 0.02 | |
| 45 | 0.08 | 0.12 | 0.08 | 0.08 | 0.11 | 0.09 | 0.07 | 0.12 | 0.08 | 0.07 | 0.10 | 0.09 | 0.07 | 0.09 ± 0.02 | |
| 60 | 0.07 | 0.11 | 0.08 | 0.07 | 0.10 | 0.08 | 0.07 | 0.10 | 0.08 | 0.07 | 0.09 | 0.08 | 0.06 | 0.08 ± 0.02 | |
| 75 | 0.06 | 0.11 | 0.07 | 0.06 | 0.09 | 0.07 | 0.05 | 0.09 | 0.07 | 0.05 | 0.08 | 0.07 | 0.06 | 0.07 ± 0.02 | |
| 90 | 0.05 | 0.10 | 0.07 | 0.05 | 0.08 | 0.07 | 0.05 | 0.09 | 0.06 | 0.04 | 0.07 | 0.06 | 0.06 | 0.07 ± 0.02 | |
| 105 | 0.05 | 0.10 | 0.06 | 0.05 | 0.08 | 0.05 | 0.04 | 0.08 | 0.05 | 0.04 | 0.07 | 0.06 | 0.05 | 0.06 ± 0.02 | |
| 120 | 0.03 | 0.09 | 0.05 | 0.04 | 0.06 | 0.04 | 0.03 | 0.07 | 0.04 | 0.03 | 0.06 | 0.06 | 0.03 | 0.05 ± 0.02 | |
| Mean ± SD n = 3 | 0.07 ± 0.03 | 0.11 ± 0.01 | 0.08 ± 0.02 | 0.07 ± 0.03 | 0.10 ± 0.02 | 0.08 ± 0.03 | 0.07 ± 0.03 | 0.10 ± 0.02 | 0.08 ± 0.02 | 0.06 ± 0.03 | 0.09 ± 0.02 | 0.08 ± 0.02 | 0.07 ± 0.03 | ||
| P < 0.05, CD: T 0.0117, I 0.012, TxI = 0.0287 | |||||||||||||||
The concentration of acetic acid played a significant role in increasing the acidity in steeped cauliflower samples during storage. Maximum mean increase in acidity (0.12–0.70 % acidity as acetic acid) was obtained after 15 days in steeped cauliflower samples of the treatments of 6 and 12 having 6 % sodium chloride, 2 % acetic acid and 350 ppm SO2 which further increased to the mean increase of 0.9 % as acetic acid after 120 days of storage and minimum mean increase in acidity from 0.12 to 0.72 % acidity in treatments of 1, 7 and 13 with 2 % sodium chloride, 1 % acetic acid and 350 ppm SO2 (Table 1).
The hardness values in all the steeped cauliflower samples decreased during storage in different treatments (Table 2). However, there has been minimum decrease in mean hardness values from 15.20 to 6.22 g. cm in the treatments of 2, 3, 8 and 9 with 4–6 % sodium chloride, 1 % acetic acid and 350 ppm SO2 and maximum mean decrease in hardness values (15.20–0.55 g.cm) in steeped cauliflower samples in the treatments of 6 and 12 having 6 % sodium chloride, 2 % acetic acid and 350 ppm SO2 in steeped cauliflower samples during storage for 120 days at room temperature. The higher hardness values in the treatments of 2, 3, 8 and 9 can be attributed due to higher salt content of 4–6 % sodium chloride and lower level (1 %) of acetic acid which caused toughening in cauliflower samples and the maximum softening in steeped cauliflower samples of the treatments of 6 and 12 can be related due to higher level (2 %) of acetic acid during storage for 120 days at room temperature (Table 2). Gupta et al. (2012) also proposed that depolymerization of pectin and other cell wall components such as cellulose and hemicellulose in the presence of higher citric acid concentration could lead to decreased firmness and softening of plant tissues.
Data on extent of browning relates decrease in the extent of browning of steeped cauliflower samples in all the treatments which reflects more whitening in cauliflower curds due to acetic acid (Table 2). Minimum decrease in extent of browning (0.08–0.04) was reported in the treatments of 5, 6, 11 and 12 with 6 % NaCl, 2 % acetic acid and 350 ppm SO2 and maximum decrease (0.08–0.02) was obtained in the treatments of 1, 2, 4, 7, 8, 10 and 13 with 4 % sodium chloride, 1 % acetic acid and 350 ppm SO2 during storage for 120 days at room temperature (Table 2).
The mean changes in TSS levels in steeped cauliflower samples during storage in different treatments are described in Table 3. There has been sudden increase in TSS levels in steeped cauliflower samples during steeping for 15 days in all the treatments. The increase in TSS levels in steeped cauliflower samples can be attributed due to increase in sodium chloride concentration from 2 to 6 % in different experimental runs which contributed in increasing the TSS levels in cauliflower samples during storage for 15 days. Gupta et al. (1992) reported that the increase in TSS in red chillies might be attributed to the loss of moisture and breakdown of sugars and starches. However, the TSS level showed decreasing trend in all the treatments beyond 15 days of storage at room temperature. The decrease in TSS level beyond 15 days of storage can be pointed out due to dissolution of cellulose and pectic substances in 1–2 % acetic acid solution during steeping preservation of cauliflower samples during storage. The maximum mean TSS (5.9 %) was observed in steeped cauliflower samples in the treatments 1, 7 and 13 having 2 % sodium chloride, 1 % acetic acid and 350 ppm SO2 and minimum mean in TSS (2.39 %) in the treatments of 5 and 11 having 4 % sodium chloride, 2 % acetic acid and 350 ppm SO2 after120 days of storage at room temperature (Table 3).
The NaCl content in steeped solution of cauliflower samples decreased in all the treatments during entire storage period of 120 days (Table 3). Maximum decrease in salt content (11.2–13 %) was analysed in the treatments of 6 and 12 having 6 % NaCl, 2 % acetic acid and 350 ppm SO2 after 15 days of storage which was further decreased to 83.2–94 % after 120 days of storage. Barwal et al. (2005) also reported the decrease in the salt content of the steeping solution during preservation of cauliflower samples in 5–15 % salt solution, 1 % citric acid and 0.2 % KMS. The decrease in sodium chloride in steeped cauliflower samples during storage is attributed due to incrassation of salt in vegetable pieces and subsequently the decrease of salt content in steeping solution (Pruthi et al. 1980; Gupta et al. 1992).
The ascorbic acid content in steeped cauliflower samples decreased with advancement of storage period (Table 4). The maximum and minimum mean decrease in ascorbic acid was recorded after 15 days of storage were 49.9 % and 39.8 % in the treatments of 6 and 12 and 2, 4 and 8, respectively which decreased to mean values of 77.5 % and 76.3 % after 120 days of storage, respectively. The decrease in ascorbic acid is attributed due to loss of ascorbic acid due to leaching as a result of steeping solution of NaCl (4–6 %) and 1–2 % acetic acid. The maximum decrease in ascorbic acid may be related due to higher concentration (2 %) acetic acid in the formulation of 6 and 12. Similar decrease in ascorbic acid content in steeped cauliflower samples during storage for 180 days was also recorded by Sinha and Chandra (2012).
The changes in mean total carotenoids content showed declining trend in steeped cauliflower during storage in all the treatments. Minimum mean decrease (33.3 %) in total carotenoids content was obtained in the treatments of T2 and T8 having 4 % NaCl, 1 % acetic acid and 350 ppm SO2 while maximum mean decrease (75.0 %) in total carotenoids content in steeped cauliflower was reported in the treatments of T1, T7, T10 and T13 (Table 4). The higher concentration of acetic acid (1–2 %) may be responsible for decrease in carotenoids content in steeped cauliflower during storage.
Optimization In order to optimize the sodium chloride content and acetic acid to preserve the blanched cauliflower under steeping preservation, the process variable consisted of NaCl content (2–6 %), acetic acid (1–2 %) and 350 ppm SO2 for increasing the shelf life of cauliflower. The maximum desirability was obtained with 3.5 % NaCl, 1.1 % acetic acid and 350 ppm SO2.
Conclusion
The low cost processing technology of steeping preservation can be very well adopted at rural level to prevent huge post harvest losses in horticultural produce. The blanched cauliflower at 100 °C for 30 s can suitably be preserved in steeping solution consisting of 4 % NaCl, 1 % acetic acid and 350 ppm SO2 for extending the shelf life for 4 months at room temperature.
Contributor Information
Sudhir Singh, Phone: +91-0542-2635236, FAX: +91-05443-229007, Email: sudhiriivr@gmail.com.
Ashutosh Rai, Email: ashutoshraiiivr@gmail.com.
References
- Aggarwal P, Kaur R. Steeping preservation of baby corn. Int J Veg Sci. 2010;16:103–117. doi: 10.1080/19315260903299212. [DOI] [Google Scholar]
- Ahn HJ, Kim JH, Kim JK, Kim DH, Yook HS, Byun MW. Combined effects of irradiation and modified atmosphere packaging on minimally processed Chinese cabbage (Brassica rapa L.) Food Chem. 2005;89:589–597. doi: 10.1016/j.foodchem.2004.03.029. [DOI] [Google Scholar]
- Barwal VS, Rakesh S, Rajinder S. Preservation of cauliflower by hurdle technology. J Food Sci Technol. 2005;42:26–31. [Google Scholar]
- Bose TK, Som MG, Kabir J (1993) Cauliflower. In: Vegetable Crops in India. Naya Prokash, Calcutta, pp 152–169
- Chandra P, Kar A (2004) Post harvest processing for developed India. International seminar on emerging technologies in agricultural and food engineering, IIT, Kharagpur, India, December 14–17, 2004
- Cocci E, Rocculi P, Romani S, Rosa MD. Changes in nutritional properties of minimally processed apples during storage. Post Harvest Biol Technol. 2006;39:265–271. doi: 10.1016/j.postharvbio.2005.12.001. [DOI] [Google Scholar]
- Girdharilal G, Siddappa S, Tandon GL. Preservation of fruits and vegetables. New Delhi: ICAR; 1986. [Google Scholar]
- Gupta AK, Tomar MC, Singh UB, Singh S. Steeping preservation of red chillies for the preparation of stuffed chilly pickle. Indian Food Packer. 1992;46:47–52. [Google Scholar]
- Gupta MK, Sehgal VK, Sadhana A. Optimization of drying process parameters for cauliflower drying. J Food Sci Technol. 2011 doi: 10.1007/s13197-011-0231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Chatterjee S, Vaishnav J, Kumar V, Variyar PS, Sharma A. Hurdle technology for shelf stable minimally processed french beans (Phaseolus vulgaris): a response surface methodology approach. LWT Food Sci Technol. 2012;48:182–189. doi: 10.1016/j.lwt.2012.03.010. [DOI] [Google Scholar]
- Indian Horticulture Database . In: Facts and figures. Ministry of Agriculture. Mistry NC, Singh B, Gandhi CP, editors. Gurgaon: Govt of India; 2010. p. 4. [Google Scholar]
- IS 1479 . Methods of test for dairy industry. Part I. Rapid examination of milk. New Delhi: Indian Standards Institution; 1960. [Google Scholar]
- Jayanthi M. Innovative solution to extent the shelf life of fruits. Process Food Ind. 2005;9:37–38. [Google Scholar]
- Karpagapandi L, Kalaiselvan A, Selvi J, Arokiamary S. Phytochemicals a good source to eat plenty of vegetables and fruits. Process Food Ind. 2006;9:22–25. [Google Scholar]
- Klin M, Nagy S. An improved method to determine non-enzymatic browning in citrus juices. J Agri Food Chem. 1988;36(6):1271–1274. doi: 10.1021/jf00084a036. [DOI] [Google Scholar]
- Lawless HJ, Haymann H. Consumer field test and questionnaire design. In: Champan H, editor. Sensory evaluation of food. New York: CRC Press; 1998. pp. 480–518. [Google Scholar]
- Leistner L. Hurdle technology applied to meat products of the shelf stable product and intermediate moisture food types. In: Simatos D, Multon JL, editors. Properties of water in foods in relation to quality and stability. T. Dordrecht: Martinus Nijhor; 1985. pp. 309–329. [Google Scholar]
- Lima AGB, Queiroz MR, Nebra SA. Simultaneous moisture transport and shrinkage during drying of solids and ellipsoidal configuration. Chem Eng J. 2002;86:85–93. doi: 10.1016/S1385-8947(01)00276-5. [DOI] [Google Scholar]
- Mudgal VD, Pandey VK (2007) Dehydration characteristics of cauliflower. Int J Food Eng 3(6): Article 6. doi: 10.2202/1556-3758.1278
- Pruthi JS, Saxena AK, Manan JS. Studies on the determination of optimum condition of preservation of fresh vegetables in acidified sulphited brine for subsequent use in Indian style curries etc. Indian Food Packer. 1980;34:9–16. [Google Scholar]
- Ranganna S. Handbook of analysis and quality control for fruit and vegetable products. New Delhi: TataMcGraw hill Publication Co Ltd; 1997. [Google Scholar]
- Rodriguez R, Agarwal PC, Saha NK. Physico-chemical characteristics of some cauliflower cultivars and their suitability for canning. Indian Food Packer. 1983;37:67–73. [Google Scholar]
- Shashidhar R, Dhokane V, Hajare S, Sharma A, Bandekar JR. Effectiveness of radiation processing for elimination of Salmonella typhimurium from minimally processed pineapple (Ananas comosus Merr.) J Food Sci. 2007;72:98–101. doi: 10.1111/j.1750-3841.2007.00300.x. [DOI] [PubMed] [Google Scholar]
- Sinha J, Chandra R. Microbial, sensory and nutritional properties of cauliflower preserved by hurdle technology. Int J Curr Res Rev. 2012;4:74–80. [Google Scholar]
- Snedecor GW, Cochran WG. Statistical methods. New Delhi: East West Press; 1967. [Google Scholar]
- Srivastava GK, Sulebele GA. Dehydration of cauliflower: effect of pretreatments on rehydration characteristics. Indian Food Packer. 1975;29:5–10. [Google Scholar]