Abstract
Polyphenols was extracted with subcritical water from the sea buckthorn seed residue (after oil recovery), and the extraction parameters were optimized using response surface methodology (RSM). The independent processing variables were extraction temperature, extraction time and the ratio of water to solid. The optimal extraction parameters for the extracts with highest ABTS radical scavenging activity were 120 °C, 36 min and the water to solid ratio of 20, and the maximize antioxidant capacity value was 32.42 mmol Trolox equivalent (TE)/100 g. Under the optimal conditions, the yield of total phenolics, total flavonoids and proanthocyanidins was 36.62 mg gallic acid equivalents (GAE)/g, 19.98 mg rutin equivalent (RE)/g and 10.76 mg catechin equivalents (CE)/g, respectively.
Keywords: Sea buckthorn, Polyphenols, Flavonoids, Antioxidant, Response surface methodology
Introduction
Sea buckthorn (Hippophaë rhamnoides L.), a hardy bush, belongs to the Elaeagnaceae family and naturally distributed over Asia and Europe (Guliyev et al. 2004). The berries of sea buckthorn consist of many nutrients and bioactive compounds, such as polyphenols, flavonoids, sterols/terpenes, etc., which can be widely used in food and medicine and health protection. These compounds possess biological and therapeutic activities, including antioxidant (Chauhan et al. 2007; Ting et al. 2011), antimicrobial (Chauhan et al. 2007), anticancer, antitumor (Ferguson et al. 2004; Hakimuddin et al. 2004; Nijveldt et al. 2001; Zhang et al. 2004; Spencer et al. 2004), cardiovascular-protective (Basu et al. 2007), gastrohelcosis-protective (Xing et al. 2002) acitivities, etc. Several pharmaceutical preparations of sea buckthorn have been clinically used to treat radiation damage, burns, oral inflammation and gastric ulcers in China (Chauhan et al. 2007). Since sea buckthorn berries have multiple benefits for human health, they are increasingly recognized as food material in the recent years.
Great attention has been paid to the natural bioactive compounds from plant origin. Food waste such as fruit peels, seeds and pomace producing from food industry may contain substantial amounts of valuable natural antioxidants (Rösch et al. 2004). The residue of sea buckthorn seed after oil recovery was usually discarded or just used as fodder. Phenolic compounds in sea buckthorn have been shown to exhibit in vitro antioxidant properties and are suggested to be primarily responsible to the health benefits (Fan and Ding 2006).
In recent years, subcritical water extraction (SWE) has been developed as a new extraction technique. It was considered to be a promising and environmental friendly technique with the advantages of short extraction time, high-efficiency and low energy-consumption. It has been used for extracting organic pollutants in environmental samples and active ingredients from traditional medicinal plants (Hawthorne et al. 1994; Jimenez-Carmona et al. 1997; Latawiec and Reid 2010). Many factors such as extraction temperature, extraction time, particle size, solid to solvent ratio, extraction pressure, the type of entrainers, etc. have significant effects on the extraction yield (Smith 2002; Ramos et al. 2002; Guo et al. 2009; Luque-Rodríguez et al. 2006; He et al. 2012). Temperature is a key parameter of the extraction process. With the increase of temperature, the water dielectric constant, viscosity, surface tension decrease significantly, but the molecular diffusion rate is increased (Smith 2002; Uematsu and Franck 1980). Pressure has been reported to play no role other than to keep the extraction solvent liquid at the high-temperature used, for the steam is corrosive and it can damage the equipment (Ramos et al. 2002). With the increase of pressure, the efficiency of the targeted extract does not change significantly (Jimenez-Carmona et al. 1999).
Response surface method (RSM) is a reasonable statistical method to find the optimal process parameters through the analysis of the regression equation, with the aim of solving the problem of a multivariate by using the functional relationship between multiple quadratic regression equation and response factors. RSM has became an effective method with the advantages of reducing costs, optimizing the processing condition, and widely used in agriculture, biotechnology, food, chemical and other fields (Ballard et al. 2009; Karacabey and Mazza 2010).
The objective of this study was to extract the antioxidant polyphenols from the sea buckthorn seed residue by SWE, and to investigate the effect of extraction temperature, extraction time, the ratio of water to solid on the yield of total phenolics (TP), total flavonoids (TF), proanthocyanidins (PC) and ABTS (2,2′-azinobis-(3-ethylbenzothiazolin-6-sulfoni acid)) radical scavenging activity of the extracts. The extraction parameters were optimized by RSM, in which the sea buckthorn seed residue extract has the highest ABTS radical scavenging activity.
Materials and methods
Materials
Folin–Ciocalteu’s phenol reagent, ABTS, vanillin, gallic acid, rutin and catechins were purchased from Sigma-Aldrich (St. Louis, MO, USA). All the solvents (analytical grade) were purchased from Beijing Chemical Co. (Beijing, China).
Preparation of samples
The sea buckthorn seed residue was provided by Rui Bao Food Co., Ltd. (Beijing, China) with moisture content of 5.10 %. The sea buckthorn seed oil has been extracted by supercritical carbon dioxide extraction with extraction temperature at 55 °C and pressure at 30 MPa for 3 h. The residue was crushed into fine powder and then the powder was defatted again by Soxhlet extraction with n-hexane. The oil recovery in the residue was 2.1–2.2 %. The defatted powder was packaged and stored in dark at room temperature until used.
Conventional extraction
The defatted residue (2 g) was extracted respectively with 60 mL of water, methanol and ethanol in the shaker incubator at 45 °C for 1 h. The solid–liquid mixture was filtered by vacuum filter and then the solid was extracted again under the same condition. The extracts were merged and then centrifuged for 20 min at the rate of 4,200 rpm. The supernatant was selected and vaporized to dry under vacuum at ≤ 40 °C. The extracts were then dissolved in methanol and kept at −18 °C for analysis.
Preliminary extraction experiments
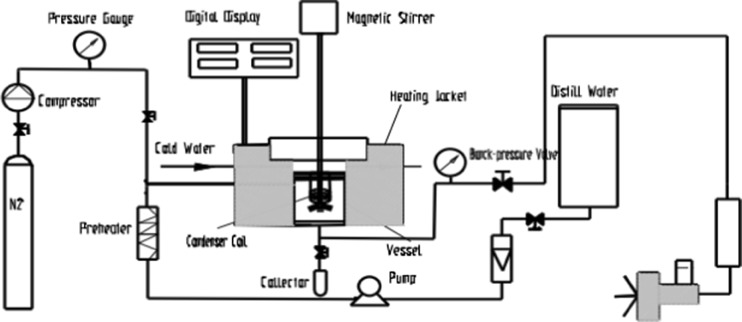
The objective of the preliminary experiments was to choose the reasonable parameters (temperature, time and the water to solid ratio) for the extraction of polyphenols from the sea buckthorn seed residue. The SWE was carried out by an equipment with a monitor of pressure and temperature (Model CWYF-2, Nantong, China), and its schematic diagram was shown in Fig. 1. The detailed extraction process was described as follows: loading designed amount of residue powder into the extraction chamber, setting the pump at a certain flow rate to inject the fixed volume of deionized water into the chamber, extracting at the fixed temperature for several minutes. At the end of the process, the extract was collected and then centrifuged for 20 min at the rate of 4,200 rpm. The supernatant was selected and vaporized to dry under vacuum at ≤ 40 °C. The extracts were then dissolved in methanol and kept at −18 °C for analysis.
Fig. 1.
The schematic diagram of subcritical water extraction
Experimental design for the response surface procedure
The RSM used a three-factor and central composite design for the optimization of process variables at 5 levels with 20 runs, including six replicates at the central point. The independent processing variables were extraction temperature (X1), extraction time (X2), and the water to solid ratio (X3). The levels of the independent parameters were based on the preliminary experimental results. The range and levels of independent variables were presented in their coded forms in Table 1. The experimental data were fitted to quadratic polynomial models, which expressed the yield of TP (Y1), TF (Y2), PC (Y3) and ABTS-TEAC (Trolox equivalent antioxidant capacity) (Y4) as a function of the independent variables as follows:
where Yn represents the response variables, a0 is a constant, ai, aii and aij are the linear, quadratic and interactive coefficients, respectively. Xi and Xj are the levels of the independent variables. The coefficients of the response surface equation were estimated by using Design-Expert 8.0.5.2. The test of statistical significance was based on the total error criteria with a confidence level of 95 % (p < 0.05).
Table 1.
Central composite design setting in the original and coded forms of the independent variables (X 1, X 2, X 3) and experimental results of TP (Y 1), TF (Y 2), PC (Y 3) and ABTS-TEAC(Y 4)
| Runa number | Temperature (X 1) (°C) | Time (X 2) (min) | Water to solid ratio (X 3) (v/m) | TP (Y 1) (mg GAE/g) | TF (Y 2) (mg RE/g) | PC (Y 3) (mg CE/g) | ABTS-TEAC (Y 4) (mmol TE/100 g) |
|---|---|---|---|---|---|---|---|
| 1 | 80 (−2.0)b | 45 (0.0) | 30 (0.0) | 23.37 | 12.96 | 5.73 | 15.71 |
| 2 | 100 (−1.0) | 60 (1.0) | 40 (1.0) | 18.85 | 9.56 | 4.62 | 15.52 |
| 3 | 120 (0.0) | 45 (0.0) | 30 (0.0) | 35.10 | 18.98 | 9.15 | 31.49 |
| 4 | 140 (1.0) | 30 (−1.0) | 40(1.0) | 20.92 | 10.71 | 5.16 | 18.33 |
| 5 | 100 (−1.0) | 60 (1.0) | 20(−1.0) | 28.89 | 17.12 | 9.13 | 23.90 |
| 6 | 120 (0.0) | 45 (0.0) | 30 (0.0) | 28.02 | 16.12 | 8.04 | 25.07 |
| 7 | 120 (0.0) | 45 (0.0) | 10(−2.0) | 41.10 | 23.70 | 14.36 | 37.71 |
| 8 | 120 (0.0) | 45 (0.0) | 30 (0.0) | 27.24 | 15.17 | 7.59 | 24.09 |
| 9 | 100 (−1.0) | 30 (−1.0) | 40(1.0) | 22.64 | 13.22 | 6.51 | 13.22 |
| 10 | 100 (−1.0) | 30 (−1.0) | 20 (−1.0) | 34.16 | 18.35 | 9.96 | 25.64 |
| 11 | 120 (0.0) | 45 (0.0) | 30 (0.0) | 28.97 | 16.48 | 8.18 | 26.53 |
| 12 | 140 (1.0) | 60 (1.0) | 40(1.0) | 19.06 | 8.48 | 3.66 | 14.49 |
| 13 | 120 (0.0) | 45 (0.0) | 30 (0.0) | 30.02 | 17.07 | 8.49 | 28.33 |
| 14 | 140 (1.0) | 60 (1.0) | 20 (−1.0) | 21.21 | 9.93 | 5.17 | 16.90 |
| 15 | 160 (2.0) | 45 (0.0) | 30 (0.0) | 28.33 | 12.44 | 5.13 | 21.64 |
| 16 | 120 (0.0) | 75 (2.0) | 30 (0.0) | 11.64 | 5.90 | 3.20 | 9.61 |
| 17 | 120 (0.0) | 15 (−2.0) | 30 (0.0) | 24.97 | 13.39 | 7.63 | 25.06 |
| 18 | 120 (0.0) | 45 (0.0) | 50 (2.0) | 14.74 | 7.79 | 3.80 | 11.95 |
| 19 | 120 (0.0) | 45 (0.0) | 30 (0.0) | 33.44 | 18.66 | 9.84 | 31.28 |
| 20 | 140 (1.0) | 30 (−1.0) | 20 (−1.0) | 32.35 | 15.03 | 7.15 | 24.65 |
aNon-randomised
bValues in parenthesis are the coded forms of variables in the experimental design
Quantitative analysis
Total phenolics content
The total phenolics content was measured by Folin–Ciocalteu method as described by Gong et al. (2012) with slightly modifications. Briefly, 0.5 mL of appropriately diluted sample was mixed with 2.5 mL of Folin–Ciocalteu reagent (0.2 mol/L), and 2.0 mL of sodium carbonate (7.5 g/100 mL) was added 5 min later. Then the mixture was kept at room temperature for 2 h after fully shaken. The absorbance of the mixture was measured at 760 nm against a reagent blank (0.5 mL deionized water instead of the sample) with a UV-visible spectrophotometer (Shimadzu UV-1800, Kyoto, Japan). The total phenolics content were calculated by the standard curve of gallic acid (GA) and expressed as gallic acid equivalents (GAE mg/g).
Total flavonoids content
The total flavonoids content was determined by the aluminium chloride colorimetric method of Siddhuraju and Becker (2003). Briefly, 0.5 mL of sample was placed in a 10 mL graduated test tube, and then 3.5 mL of deionized water and 0.3 mL sodium nitrite (5 g/100 mL) were added and mixed. 3 mL of aluminium chloride solution (1 g/100 mL) was added after 5 min. 6 min later, 2 mL sodium hydroxide solution (4 g/100 mL) was added, and the total volume was made up to 10 mL with deionized water and fully shaked for 20 s. After 15 min the absorbance was measured against a blank at 510 nm (0.5 mL deionized water instead of the sample). The total flavonoids content was calculated by the standard curve of rutin and expressed as rutin equivalent (RE mg/g).
Proanthocyanidins content
The proanthocyanidins content was determined by the method of Li et al. (2006) with slightly modifications. Briefly, 0.5 mL of appropriately diluted sample was mixed with 3 mL of vanillin (4 g/100 mL, methanol as solvent), and 1.5 mL of concentrated hydrochloric acid was added later. Then the mixture was kept at room temperature for 15 min after fully shaken. The absorbance of the mixture was measured at 505 nm against a reagent blank (0.5 mL deionized water instead of the sample). The proanthocyanidins content was calculated by the standard curve of catechin equivalent and expressed as catechin equivalents (CE mg/g).
ABTS radical-scavenging activity
The capacity of the extracts to scavenge ABTS·+ was carried out according to the method of Pellegrini et al. (2001). The ABTS·+ was prepared by mixing an ABTS stock solution (7 mmol/L in water) with 2.45 mmol/L potassium persulfate. This mixture was kept still for 12–16 h in the dark at room temperature. The ABTS·+ working solution was obtained by diluting the stock solution in water to an absorbance of 0.7 ± 0.02 at 734 nm. 1 mL of the appropriately diluted sample was added to 3 mL of ABTS·+ working solution and mixed thoroughly. The reaction mixture was kept at room temperature in the dark for 1 h, and the absorbance was recorded at 734 nm. A reagent control was measured by the same way. The antioxidant activity of the extracts was calculated from the dose–response curves of Trolox and the results were expressed as Trolox equivalent antioxidant capacity (TEAC) values (mmol TE/100 g) in this study.
Data analysis
All measurements were performed in triplicate. Data and figures of the preliminary experiments were carried out by Microsoft Excel. Analysis of variance (ANOVA) of the results of the RSM test was analyzed using Design-Expert 8.0.5.2.
Results and discussion
Conventional extraction
To study the influence of different solvent on the extraction of TP, TF and PC from the sea buckthorn seed residue with conventional extraction method, water, methanol and ethanol were used as solvent in the experiment. The results were shown in Table 2. According to Table 2, it could be easily found that methanol was the optimal solvent to extract polyphenols from the sea buckthorn seed residue, and the contents of TP, TF and PC were 46.30 ± 0.25 mg GAE/g, 27.53 ± 0.12 mg RE/g and 25.65 ± 0.24 mg CE/g, respectively, while the extracts with water contained the lowest values of TP, TF and PC. Those could be attributed to the polar of the solvent. Ethanol has been commonly applied to extract polyphenols from natural sources: they give quite high yield of total extract even though they are not highly selective for phenols. Water at room temperature is a very polar solvent, thus intermediate or low polarity compounds could not be extracted according to the principle of dissolution in the similar material structure.
Table 2.
TP, TF and PC of sea buckthorn seed residue extracts obtained by conventional extraction and SWE methods
| Factors | TP (mg GAE/g) | TF (mg RE/g) | PC (mg CE/g) | ||
|---|---|---|---|---|---|
| Conventional extraction | Water | 8.72 ± 0.29 | 3.28 ± 0.05 | 3.07 ± 0.12 | |
| Methanol | 46.30 ± 0.25 | 27.53 ± 0.12 | 25.65 ± 0.24 | ||
| Ethanol | 35.60 ± 0.50 | 17.45 ± 0.19 | 21.31 ± 0.51 | ||
| SWE extraction | Temperature (°C) | 80 | 33.17 ± 0.30 | 19.35 ± 0.47 | 12.87 ± 0.35 |
| 100 | 40.06 ± 0.46 | 23.85 ± 0.38 | 14.71 ± 0.14 | ||
| 120 | 44.51 ± 0.44 | 26.25 ± 0.93 | 14.81 ± 0.24 | ||
| 140 | 33.46 ± 0.22 | 19.04 ± 0.71 | 8.79 ± 0.10 | ||
| 160 | 15.84 ± 0.40 | 7.65 ± 0.10 | 1.81 ± 0.04 | ||
| 180 | 11.24 ± 0.24 | 3.84 ± 0.08 | 1.20 ± 0.01 | ||
| Time (min) | 15 | 17.36 ± 0.37 | 8.97 ± 0.04 | 6.79 ± 0.06 | |
| 30 | 27.69 ± 0.12 | 17.61 ± 0.11 | 11.85 ± 0.10 | ||
| 45 | 36.45 ± 0.32 | 19.79 ± 0.25 | 13.82 ± 0.32 | ||
| 60 | 25.18 ± 0.46 | 16.12 ± 0.19 | 10.10 ± 0.13 | ||
| 75 | 10.37 ± 0.06 | 5.06 ± 0.12 | 3.29 ± 0.06 | ||
| 90 | 6.04 ± 0.75 | 1.75 ± 0.03 | 1.65 ± 0.02 | ||
| Water to solid ratio | 10 | 33.71 ± 0.64 | 20.38 ± 0.18 | 13.53 ± 0.24 | |
| 20 | 37.39 ± 0.09 | 22.91 ± 0.13 | 17.98 ± 0.17 | ||
| 30 | 41.04 ± 0.31 | 25.62 ± 0.11 | 19.30 ± 0.31 | ||
| 40 | 31.53 ± 0.10 | 19.31 ± 0.31 | 15.44 ± 0.23 | ||
| 50 | 19.87 ± 0.02 | 12.18 ± 0.08 | 8.74 ± 0.05 |
Preliminary extraction experiments
The effect of extraction temperature
The effect of extraction temperature on the yield of TP, TF and PC from the sea buckthorn seed residue was carried out respectively at 80–180 °C for 45 min with the water to solid ratio of 20. During the processing, the extraction pressure was fixed at 6 MPa and the rotation speed of magnetic stirrer was kept at 300 rpm.
Temperature is the key factor during the SWE. With the increase of temperature, the solubility of polyphenols and the mass transfer coefficient between matrix and media also increased (Al-Farsi and Lee 2008). Silva et al. (2007) and Durling et al. (2007) found that heat can increase the extraction yield of polyphenols. As shown in Table 2, the effect of temperature on the yield of TP, TF and PC was significant (p < 0.05). With the increase of extraction temperature, the yield of TP, TF and PC increased slowly at first, and then decreased rapidly. At the temperature of 120 °C, the yield of TP, TF and PC reached the peak value 44.51 mg GAE/g, 26.25 mg RE/g and 14.81 mg CE/g, respectively. Nevertheless, the yield of PC at 100 °C (14.71 mg CE/g) and 120 °C (14.81 mg CE/g) had no significant difference. It was accorded with the results García-Marino et al. (2006) reported that the content of catechin, epicatechin dimers and flavanol increased when the extraction temperature was increased from 100 °C to 150 °C, but the change is not obvious.
Plant polyphenols commonly combine with proteins, alkaloids, polysaccharides, anthocyanins and other substances in the plant tissue. There are many combination ways between polyphenols and protein, such as hydrogen bond, hydrophobic bond and the synergism of them. In the sea buckthorn seed, the combination also exists. The combination of polyphenols and protein hindered the dissolution of polyphenols into the medium. High temperature can damage the linkage between polyphenols and protein/polysaccharide, so polyphenols can be released from the tissue. In addition, the increase of extraction temperature can damage the cell wall of plant to increase the cell’s penetration ability, inducing the polyphenols dissolving in medium largely (Thoo et al. 2010). Therefore, the yield of polyphenols in the extracts increased in the range of 80–120 °C. However, some polyphenols, which are thermal instability, can degrade under the high temperature. Additionally, some polyphenols may polymerize and reduce the free polyphenols in the extracts, resulting in the low yield of polyphenols. Therefore, when the extraction temperature is higher than 120 °C, the yield of polyphenols declined rapidly in the extract, indicating that the dagradation of polyphenols might be the major effect factor. When the temperature is higher than 160 °C, the caramelization of the powder became seriously in the experiment. In brief, the best extraction temperature for the highest yield of polyphenols is 120 °C.
The effect of extraction time
The impact of extraction time on the yield of TP, TF and PC from the sea buckthorn seed residue was investigated at the time interval 15–90 min with the water to solid ratio of 20. Herein, extraction temperature of 120 °C, extraction pressure of 6 MPa and the rotation speed of magnetic stirrer of 300 rpm were fixed during the extraction process. As described in Table 2, the yield of TP, TF and PC increased at first and then decreased with the extension of the extraction time. When the extraction time reached 45 min, the yield of TP, TF and PC was 36.45 mg GAE/g, 19.79 mg RE/g and 13.82 mg CE/g, respectively. During the period of 15–45 min, the yield of polyphenols increased with the extension of extraction time. Because of the time extension, the powder particle fully contacts with the subcritical water and the dissolution rate of polyphenols also increased. With the extraction time lengthened enough, the solution of polyphenols reached the equilibrium state, and the polyphenols couldn’t be dissolved in the water any more. In addition, some heatlabile polyphenols would degrade and polymerize under the high temperature conditions. Thus when the extraction time was longer than 45 min, the yield of polyphenols decreased.
The effect of the water to solid ratio
The influence of the water to solid ratio on the yield of TP, TF and PC from the sea buckthorn seed residue was carried out at the water to solid ratio of 10–50 (v/m) for 45 min, respectively. Herein, extraction temperature of 120 °C, extraction pressure of 6 MPa and the rotation speed of magnetic stirrer of 300 rpm were fixed during the extraction process.
Table 2 shows that the yield of TP, TF and PC increased firstly and then decreased rapidly with the increase of the water to solid ratio. When the ratio reached 30, the yield of TP, TF and PC was 41.04 mg GAE/g, 25.63 mg RE/g and 19.30 mg CE/g, respectively. When the water to solid ratio was low, the water was limited and the powder was just immersed, resulting in the lower extraction efficiency. When the ratio of water to solid was higher (> 30), the yield of polyphenols decreased. It is accorded with the result that Yao et al. (2007) achieved but different from the result that Al-Farsi and Lee (2008) and Silva et al. (2007) reported. Therefore, the water to solid ratio of 30 was preferred as the appropriate ratio.
Comparing SWE method with the conventional extraction method, it could be concluded that at the proper condition, SWE is not only an environmental friendly processing technology but also a high efficient method for the extraction of polyphenols from sea buckthorn seed residue.
Optimization of the extraction process by RSM
Model fitting
Twenty experimental trials were performed and the trials were carried out in random order as required in many design procedures. The content of TP (Y1), TF (Y2) and PC (Y3) in the sea buckthorn seed residue extracts obtained from all the experiments and the ABTS-TEAC (Y4) of them were shown in Table 1. The software generated four regression equations which demonstrated the empirical relationship between the response values and extraction parameters of extraction temperature (X1), extraction time (X2) and the water to solid ratio (X3), and the results of the analysis of variance, goodness of fit, and the adequacy of the models were summarized in Table 3. The corresponding coefficients of determination (R2) values of the models were 0.8936, 0.8914, 0.8852 and 0.8455 for TP, TF, PC and ABTS-TEAC, respectively. There was no significance in the lack of fit (p > 0.05) in each of the four models, which indicated that the models could be used to predict the responses.
Table 3.
Regression coefficients and analysis of the model for three response variables
| Coefficient | Coefficients estimated | |||
|---|---|---|---|---|
| TP (Y 1) | TF (Y 2) | PC (Y 3) | ABTS-TEAC (Y 4) | |
| a 0 | −1.4021 | −16.3452 | −8.4656 | −71.3632 |
| Linear | ||||
| a 1 | 0.6609 | 0.5798 | 0.4043 | 1.5628 |
| a 2 | 0.9728** | 0.7773** | 0.3285* | 1.3923* |
| a 3 | −1.1217*** | −0.5954*** | −0.5580*** | −1.0968*** |
| Quadratic | ||||
| a 11 | −0.0031 | −0.0030* | −0.0021** | −0.0064** |
| a 22 | −0.0139*** | −0.0087*** | −0.0038** | −0.0129** |
| a 33 | −0.0072 | −0.0042 | 0.0007 | −0.0102 |
| Interaction | ||||
| a 12 | −0.0016 | −0.0010 | −0.0003 | −0.0051 |
| a 13 | 0.0050 | 0.0043 | 0.0028 | 0.0075 |
| a 23 | 0.0090 | 0.0037 | −0.0005 | 0.0066 |
| lack of fit | 0.4234 | 0.1475 | 0.0862 | 0.1923 |
| R 2 | 0.8937 | 0.8912 | 0.8853 | 0.8455 |
| Adj-R 2 | 0.7980 | 0.7932 | 0.7821 | 0.7065 |
*p < 0.05, **p < 0.01, ***p < 0.001
a 0 is a constant, a i, a ii and a ij are the linear, quadratic and interactive coefficients, respectively
Analysis of extraction parameters for polyphenols and ABTS radical scavenging activity
The effect of extraction temperature, extraction time, the water to solid ratio and their interactions on the yield of TP, TF and PC and the ABTS-TEAC of the extracts were shown in Fig. 2.
Fig. 2.
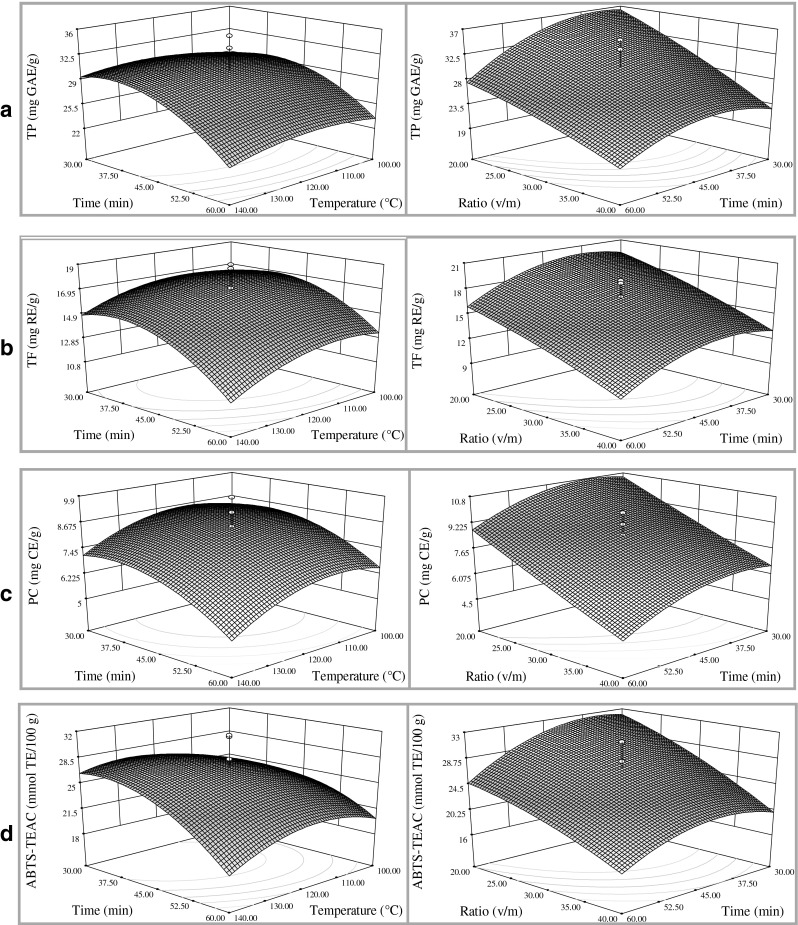
Response surface plots for the effect of extraction temperature, extraction time and the water to solid ratio on the yield of TP, TF, PC from sea buckthorn seed residue and the ABTS-TEAC of the extracts
In Fig. 2a, the 3D response surface plot of the yield of TP was introduced as the function of the interactions within the parameters. As found from Table 3 and Fig. 2a, the extraction temperature generated no significant effect on the yield of TP (p > 0.05, Table 3). The effect of extraction time on the yield of TP was unconspicuous from 30 to 45 min, but it showed a significant negative linear effect from 45 to 60 min. When the temperature was fixed at 120 °C and the water to solid ratio was lower, the downtrend was more rapidly. The quadratic item of X22 also indicated a extremely significant effect on the yield of TP (p < 0.01, Table 3). The water to solid ratio had a extremely significant negative linear effect on the yield of TP at the ratio of water to solid from 20 to 40 (p < 0.001, Table 3). There was no significant effect on the interactions. These showed that the yield of TP was mainly affected by the extraction time and the water to solid ratio.
The effect of the extraction parameters on the yield of TF and their interactions were shown in Fig. 2b. As show in Table 3 and Fig. 2b, the extraction temperature had no significant linear effect on the yield of TF (p > 0.05), but the quadratic item of X12 generated significant influence (p < 0.05, Table 3). In the range of temperature from 100 to 120 °C, the yield of TF expressed no significant change. However, when the temperature was higher (> 120 °C), the yield of TF decreased with the increase of the temperature, which indicated the extracts might contain heat sensitive compounds. The influence of the extraction time and the water to solid ratio on the yield of TF was similar to the extraction of TP. These showed that the yield of TF was mainly dominated by the extraction time and the water to solid ratio.
The influence of extraction parameters on the yield of proanthocyanidins was shown in Fig. 2c. As found from Table 3 and Fig. 2c, the effect of the parameters on the proanthocyanidins extraction yield was very similar to the TF extraction process. The extraction temperature also had no significant linear effect (p > 0.05) but significant quadratic influence on the yield of PC (p < 0.05, Table 3). When the temperature was higher (> 120 °C), the yield of PC decreased more rapidly with the increase of the temperature than the TF extraction. Extraction time had a significant linear and quadratic effect on the yield of proanthocyanidins from 45 to 60 min (p < 0.05). The ratio of water to solid expressed extremely significant negative linear effect on the yield of proanthocyanidins (p < 0.001, Table 3). These indicated that the yield of proanthocyanidins was also mainly affected by the extraction time and the water to solid ratio.
The impact of the extraction parameters on the ABTS-TEAC of the extracts was displayed in Fig. 2d. Extraction temperature showed uncertain influence on the response value. When the ratio of water to solid was fixed at 30 and the extraction time was lower than 45 min, the ABTS-TEAC of the extracts increased rapidly from 100 to 120 °C and then nearly kept stable. However, when the extraction time was longer than 45 min, the response value increased slowly at first and then dropped sharply. Extraction time displayed significant linear effect (p < 0.05) and quadratic item influence (p < 0.01, Table 3). While the extraction temperature was fixed at 120 °C, extending the extraction time from 30 to 45 min could get slow increase of ABTS-TEAC values, but longer time extraction would obtain extracts with lower antioxidant activity. Ratio of the water to solid showed extremely significant negative linear effect on the ABTS-TEAC of the extracts (p < 0.001, Table 3).
Analyzing the changes among the 3D response surface plots in Fig. 2, it could be found that the variation trend of ABTS-TEAC of the extracts was similar to the changes of the content of TP, TF and PC. Thus, correlation analysis was used to gain a more knowledge of the relationship between the content of polyphenols and the ABTS radical activity of the extracts. The results showed that the ABTS-TEAC of the extracts was highly correlated with the content of TP, TF and PC, and the correlation coefficients were 0.9072, 0.9171 and 0.8797, which was accorded with the results Fan and Ding (2006) reported.
Optimization of extraction parameters
Optimization process was carried out to determine the optimum value of the ABTS-TEAC using the Design-Expert 8.0.5.2 software, for obtaining extracts with high antioxidant capacity would be one of the main purposes of the extraction study. Accordingly, the optimum extraction parameters and the highest value of ABTS-TEAC were established, and the optimal extraction parameters were predicted to be: extraction temperature 120 °C, extraction time 36 min and the water to solid ratio of 20, and the maximize ABTS-TEAC value was 32.42 mmol TE/100 g. Under the optimal conditions, the yield of TP, TF and PC were 36.62 mg GAE/g, 19.98 mg RE/g and 10.76 mg CE/g, respectively.
Conclusions
In the present study, RSM was applied successfully for the optimization of SWE conditions to obtain the sea buckthorn seed residues extract with the maximum ABTS radical scavenging activity. Results demonstrated that extraction time and the water to solid ratio were the major factors which affected the extraction yield of polyphenols from sea buckthorn seed residues and the ABTS radical scavenging activity of the extracts, while extraction temperature expressed as a crucial factor. The antioxidant of the sea buckthorn seed residues extracts was highly correlated with the content of the polyphenols.
References
- Al-Farsi MA, Lee CY. Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem. 2008;108:977–985. doi: 10.1016/j.foodchem.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Ballard TS, Mallikarjunan P, Zhou K, O’Keefe SF. Optimizing the extraction of phenolic antioxidants from peanut skins using response surface methodology. J Agric Food Chem. 2009;57:3064–3072. doi: 10.1021/jf8030925. [DOI] [PubMed] [Google Scholar]
- Basu M, Prasad R, Jayamurthy P, Pal K, Arumughan C, Sawhney RC. Anti-atherogenic effects of sea buckthorn (Hippophaea rhamnoides) seed oil. Phytomedicine. 2007;14:770–777. doi: 10.1016/j.phymed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Chauhan AS, Negi PS, Ramteke RS. Antioxidant and antibacterial activities of aqueous extract of Seabuckthorn (Hippophae rhamnoides L.) seeds. Fitoterapia. 2007;78:590–592. doi: 10.1016/j.fitote.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Durling NE, Catchpole OJ, Grey JB, Webby RF, Mitchell KA, Foo LY, Perry NB. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol-water mixtures. Food Chem. 2007;101:1417–1424. doi: 10.1016/j.foodchem.2006.03.050. [DOI] [Google Scholar]
- Fan JL, Ding XL. Antioxidant activity and phenolic components of Sea Buckthorn seed extracts. Nat Prod Res Dev. 2006;18:529–534. [Google Scholar]
- Ferguson PJ, Kurowska E, Freeman DJ, Chambers AF, Koropatnick DJ. A flavonoid fraction from cranberry extract inhibits proliferation of human tumor cell lines. J Nutr. 2004;134:1529–1535. doi: 10.1093/jn/134.6.1529. [DOI] [PubMed] [Google Scholar]
- García-Marino M, Rivas-Gonzalo JC, Ibáñez E, García-Moreno C. Recovery of catechins and proanthocyanidins from winery by-products using subcritical water extraction. Anal Chim Acta. 2006;563:44–50. doi: 10.1016/j.aca.2005.10.054. [DOI] [Google Scholar]
- Gong Y, Hou Z, Gao Y, Xue Y, Liu X, Liu G. Optimization of extraction parameters of bioactive components from defatted marigold (Tagetes erecta L.) residue using response surface methodology. Food Bioprod Process. 2012;90:9–16. doi: 10.1016/j.fbp.2010.12.004. [DOI] [Google Scholar]
- Guliyev VB, Gul M, Yildirim A (2004) Hippophae rhamnoides L.: chromatographic methods to determine chemical composition, use in traditional medicine and pharmacological effects. J Chromatogr B 812:291–307 [DOI] [PubMed]
- Guo J, Xing X, Kong L, Qiu T, Yang R. Extraction of essential oil from dried Zanthoxylum Bungeanum Maxim by subcritical water. Chem Bioeng. 2009;26:18–21. [Google Scholar]
- Hakimuddin F, Paliyath G, Meckling K. Selective cytotoxicity of a red grape wine flavonoid fraction against MCF-7 cells. Breast Cancer Res Treat. 2004;85:65–79. doi: 10.1023/B:BREA.0000021048.52430.c0. [DOI] [PubMed] [Google Scholar]
- Hawthorne SB, Yang Y, Miller DJ. Extraction of organic pollutants from environmental solids with sub- and supercritical water. Anal Chem. 1994;66:2912–2920. doi: 10.1021/ac00090a019. [DOI] [Google Scholar]
- He L, Zhang X, Xu H, Xu C, Yuan F, Knez Z, Novak Z, Gao Y. Subcritical water extraction of phenolic compounds from pomegranate (Punica granatum L.) seed residues and investigation into their antioxidant capacities by HPLC-ABTS·+ assay. Food Bioprod Process. 2012;90:215–223. doi: 10.1016/j.fbp.2011.03.003. [DOI] [Google Scholar]
- Jimenez-Carmona MM, Manclús JJ, Montoya A, Luque de Castro MD. Sub-and supercritical fluid extraction of trichloropyridinol from soil prior to immunoassay. J Chromatogr A. 1997;785:329–336. doi: 10.1016/S0021-9673(97)00576-1. [DOI] [PubMed] [Google Scholar]
- Jimenez-Carmona MM, Ubera JL, Luque de Castro MD. Comparison of continuous subcritical water extraction and hydrodistillation of marjoram essential oil. J Chromatogr A. 1999;855:625–632. doi: 10.1016/S0021-9673(99)00703-7. [DOI] [PubMed] [Google Scholar]
- Karacabey E, Mazza G. Optimisation of antioxidant activity of grape cane extracts using response surface methodology. Food Chem. 2010;119:343–348. doi: 10.1016/j.foodchem.2009.06.029. [DOI] [Google Scholar]
- Latawiec AE, Reid BJ. Sequential extraction of polycyclic aromatic hydrocarbons using subcritical water. Chemosphere. 2010;78:1042–1048. doi: 10.1016/j.chemosphere.2009.11.029. [DOI] [PubMed] [Google Scholar]
- Li Y, Guo C, Yang J, Wei J, Xu J, Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006;96:254–260. doi: 10.1016/j.foodchem.2005.02.033. [DOI] [Google Scholar]
- Luque-Rodríguez JM, Pérez-Juan P, Luque De Castro MD. Extraction of polyphenols from vine shoots of Vitis vinifera by superheated ethanol-water mixtures. J Agric Food Chem. 2006;54:8775–8781. doi: 10.1021/jf061855j. [DOI] [PubMed] [Google Scholar]
- Nijveldt RJ, Van Nood E, Van Hoorn DEC, Boelens PG, Van Norren K, Van Leeuwen PAM. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- Pellegrini N, Visioli F, Buratti S, Brighenti F. Direct analysis of total antioxidant activity of olive oil and studies on the influence of heating. J Agric Food Chem. 2001;49:2532–2538. doi: 10.1021/jf001418j. [DOI] [PubMed] [Google Scholar]
- Ramos L, Kristenson EM, Brinkman UAT. Current use of pressurised liquid extraction and subcritical water extraction in environmental analysis. J Chromatogr A. 2002;975:3–29. doi: 10.1016/S0021-9673(02)01336-5. [DOI] [PubMed] [Google Scholar]
- Rösch D, Krumbein A, Mügge C, Kroh LW. Structural investigations of flavonol glycosides from Sea Buckthorn (Hippophaë rhamnoides) pomace by NMR spectroscopy and HPLC-ESI-MSn. J Agric Food Chem. 2004;52:4039–4046. doi: 10.1021/jf0306791. [DOI] [PubMed] [Google Scholar]
- Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem. 2003;51:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Silva EM, Rogez H, Larondelle Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep Purif Technol. 2007;55:381–387. doi: 10.1016/j.seppur.2007.01.008. [DOI] [Google Scholar]
- Smith RM. Extractions with superheated water. J Chromatogr A. 2002;975:31–46. doi: 10.1016/S0021-9673(02)01225-6. [DOI] [PubMed] [Google Scholar]
- Spencer JPE, Abd El Mohsen MM, Rice-Evans C. Cellular uptake and metabolism of flavonoids and their metabolites: implications for their bioactivity. Arch Biochem Biophys. 2004;423:148–161. doi: 10.1016/j.abb.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Thoo YY, Ho SK, Liang JY, Ho CW, Tan CP. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia) Food Chem. 2010;120:290–295. doi: 10.1016/j.foodchem.2009.09.064. [DOI] [Google Scholar]
- Ting HC, Hsu YW, Tsai CF, Lu FJ, Chou MC, Chen WK. The in vitro and in vivo antioxidant properties of sea buckthorn (Hippophae rhamnoides L.) seed oil. Food Chem. 2011;125:652–659. doi: 10.1016/j.foodchem.2010.09.057. [DOI] [Google Scholar]
- Uematsu M, Franck EU. Static dielectric constant of water and steam. J Phys Chem Ref Data. 1980;9:1291–1306. doi: 10.1063/1.555632. [DOI] [Google Scholar]
- Xing J, Yang B, Dong Y, Wang B, Wang J, Kallio HP. Effects of sea buckthorn (Hippophae rhamnoides L.) seed and pulp oils on experimental models of gastric ulcer in rats. Fitoterapia. 2002;73:644–650. doi: 10.1016/S0367-326X(02)00221-6. [DOI] [PubMed] [Google Scholar]
- Yao YZ, Zuo JP, Wang ZH. The extraction of polyphenols by ethanol in red peanut. China Oils Fats. 2007;32:51–53. [Google Scholar]
- Zhang S, Yang X, Morris ME. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol Pharmacol. 2004;65:1208–1216. doi: 10.1124/mol.65.5.1208. [DOI] [PubMed] [Google Scholar]