Abstract
Carotenoids are increasingly drawing the attention of researchers as a major natural food color due to their inherent nutritional characteristics and the implicated possible role in prevention and protection against degenerative diseases. In this report, we review the role of red pepper as a source for natural carotenoids. The composition of the carotenoids in red pepper and the application of different methodologies for their analysis were discussed in this report. The stability of red pepper carotenoids during post-harvest processing and storage is also reviewed. This review highlights the potential of red pepper carotenoids as a source of natural food colors and also discusses the need for a standardized approach for the analysis and reporting of composition of carotenoids in plant products and designing model systems for stability studies.
Keywords: Carotenoids, Capsicum annum, Red pepper, Natural food color, Color stability
Introduction
Color and appearance create the first impression and greatly influence the acceptability of food; hence the development of food items with attractive color and appearance is an important goal in food industry. Color is added to food to replace the color lost during processing, to enhance the color already present, to minimize batch to batch variations and to color otherwise uncolored. Food colors can be classified as natural colors, nature-identical colors, synthetic colors and inorganic colors (Henry 1996). Now days, food producers pay more attention towards colors and additives of natural origin, since many artificial colors and additives have been shown to impart negative health effects. The increased demand for natural food additives fuels the research to offer more natural ways to color and preserve foods. Research on natural pigments focuses in finding new pigments and new sources for known pigments, optimizing the conditions for the enhanced pigment synthesis and improved recovery, minimizing the detrimental effects of food processing and storage on pigments, and developing delivery systems suitable for different types of food matrices and to offer improved stability to the pigments during processing and storage. Natural colors include both the natural pigments and certain pigments prepared by the modification of natural compounds such as caramel, vegetable carbon, Cu-chlorophyllin etc. Based on the chemical structure, natural pigments are grouped as tetrapyrrols (e.g. chlorophyll) tetraterpenoids (e.g. carotenoids), flavonoids (e.g. anthocyanins), anthraquinones (e.g. carmine, lac etc.) and betains. Chlorophylls and carotenoids are the most abundant pigments in nature and have a major role in photosynthesis, the fundamental process of life in earth.
Carotenoids
Carotenoids are lipophilic yellow-orange-red pigments found in photosynthetic plants, algae and microorganisms. Animals are not able to synthesis carotenoids denova, so their presence is due to dietary intake. Apart from coloration these pigments are important for plant and animal health as they play a special role in protecting tissues from light and oxygen. Changes in the content and structure of carotenoids in plants can also be considered as markers of environmental damages. Carotenoids are subgroup of isoprenoid compounds and currently comprise more than 700 characterized structures. Since 1980 around 7500 papers have been published about carotenoids in various research areas of chemistry, physics, food, biology and medicine as its significance stretches form natural coloration to profound physiological effects. Carotenoids are commercially exploited as food colorants and feed additives and are being used in pharmaceutical, nutraceutical and cosmaceutical products.
The vast majority of carotenoids are derived from the linear tetraterpen phytene (C40), as eight isoprenoid units join head to tail pattern except at the centre to form 40-carbon carotenoid back bone. The pigment character of carotenoids, for which they are best known, is imparted to the colorless basal phytene structure by introducing additional double bonds in conjugation. Cyclic or acyclic end groups terminate the polyene chain thus formed. A number of modifications in this linear conjugated backbone by cyclases, hydroxylases, ketolases and other enzymes give rise to the wide spectrum of carotenoid diversity from simple hydrocarbon carotenes to xanthophylls. Xanthophylls have oxygen in terminal rings in the form of hydroxy (e.g. zeaxanthine, lutein etc.), keto (e.g. astaxanthin, canthaxanthin etc.) or cyclic ether (violaxanthin, fucoxanthin etc.) groups. No heteroatom other than oxygen is so far been known to be present in natural carotenoids. Certain bacteria synthesize carotenoids with C30 (e.g. staphyloxanthine from Staphylococus aureus) (Pelz et al. 2005) and C50 (decaprenoxanthin from Corynebacterium glutanicum) (Krubasik et al. 2001) carbon skeletons. Carotenoids with aromatic rings are occasionally found in certain marine sponges, mycobacteria and cyanobacteria (Graham and Bryant 2008). Similarly monocyclic carotenoids such as torulene occur rarely in certain yeasts (Frengova and Beshkova 2009).
Based on the cyclic or open nature of end groups, carotenoids are classified as acyclic i.e., both end groups are open (e.g. lycopene), mono cyclic i.e., one end group closed (e.g. γ carotene) and bicyclic i.e., both end groups closed (e.g. β-carotene). Allenic (e.g. neoxanthin) and acetylenic (e.g. dehydroapocarotenoid) carotenoids are also found in nature. Based on their biological significance, they are also classified as primary and secondary carotenoids. Primary carotenoids are directly involved in the photosynthesis e.g. β-carotene, zeaxanthin, lutein etc. Secondary carotenioids such as lycopene, α-carotene, capsanthin etc. do not have direct role in photosynthesis. Most of the natural carotenoids are chiral with one to six chiral centers. In general all trans isomers are more stable than cis-trans and all cis isomers (Khoo et al. 2011). Interactions with light, heat, oxidants and other reactive chemical species alter the isomerisation pattern of carotenoids and thus impart significant changes in their properties. Hydroxycarotenoids in fruits and vegetables are predominantly found as esterified with fatty acids. Variations in number and position of hydroxyl groups in carotenoids coupled with the availability of variety of fatty acids in nature results in a wide spectrum of carotenoid esters. In some fruits during ripening degree of esterification increases. Esterification is believed to provide high stability to carotenoids against thermo, photo and enzymatic oxidation reactions.
In higher plants carotenoids are seen in plastids, particularly chloroplasts of photosynthetic tissues and chromoplasts of flowers and fruits. They occur mostly as free forms in leaves and as esterified forms in other tissues. The carotenoid composition of fruits and vegetables varies with variety; maturation and ripening, agro-climatic conditions etc. Carotenoids in human diet are primarily derived from leafy vegetables; yellow orange fruits and some vegetable oils. Many algae and fungi are being utilized as commercial source of carotenoids. β-carotene, one of the major carotene in diet finds in a variety of orange, yellow and green fruits and vegetables. Particularly, carrot, red chilli, paprika, palm oil, sea buckthorn berries (Ong and Tee. 1992; Ranjith et al. 2006, Khoo et al. 2011) etc. are the major sources of β-carotene. The best-known sources of lycopene are tomatoes, watermelons, guava and pink grape fruit. The fungus Blakslea trispora is used for the synthetic production of lycopene, Lycopene provides an orange shade in solution and is more prone to oxidation than β-carotene. Carotenes such as phytoene, phytofluene, γ-carotene, δ-carotene are also seen in small amounts along with α and β-carotenes in plants. Annatto, the carotenoid pigment extracted from the seeds of a topical tree Bixa orellana, being used to color dairy products contains bixin and norbixin as the major carotenoids. Lutein, the xanthophyll extracted from Marigold (Tagetes erecta) flowers is being used to impart yellow color to egg yolks and skin of broilers. The dried stigma of saffron (Crocus sativus) being used as a colorant contains a water-soluble carotenoid crocetin as the major pigment. The high cost of saffron limits its application as a colorant.
A number of physiological benefits have been proposed for Carotenoids. β-carotene along with α-carotene and β-cryptoxanthin are sources of provitamin A. Observational studies describing the inverse relationship between human chronic disease incidents and intake of high carotenoid containing diets are available. Carotenoids have been shown to reduce oxidative stress (Edge et al. 1997), inhibit cancer cells (Maoka et al. 2001; Tang et al. 2005) and offer protection from cardiovascular diseases (Voutilainen et al. 2006), macular degeneration and cataract (Gale et al. 2003). The bioavailabilty of carotenoids is influenced by dietary factors including type and amount of carotenoid consumed and nature of food to which carotenoids are incorporated and post-harvest processing and storage and cooking practices of food items (Pugliesea et al. 2013).
Carotenoids in red pepper
The red pepper, ‘Capsicum’ belonging to Solanaceae family has been used since ancient times to impart red color and pungency to foodstuffs. The genus Capsicum consists of approximately 22 wild species and 5 domesticated species viz; C. annuum, C. baccatum, C. chinense, C. frutescens, and C. pubescens. Capsicum is endemic to western hemisphere. C. Annuum cultivars are the most commercially cultivated pepper in worldwide. Fruits of capsicum can vary tremendously in color, shape, and size both between and within the species. Ripe pepper fruits belonging to different varieties display a range of colors from white to deep red. The intensity of red color and degree of pungency are valued as major quality parameters in paprika trade. The amount of capsaicin in hot peppers also varies significantly between varieties, and is measured in Scoville heat units (SHU). The world’s current hottest known pepper as rated in SHU is the Trinidad Moruga Scorpion, which has been measured at over 2,000,000. Both ground pepper (paprika) and oleoresins are being used to modify color and flavor of soups, stews, sausage, cheese, snacks, salad dressing, souces, pizza, confectionaries, beverages etc. The annual production of paprika and paprika oleoresins was 60,000 and 1400 t respectively. Though a very large number of capsicum varieties exist, only a few have been studied in detail.
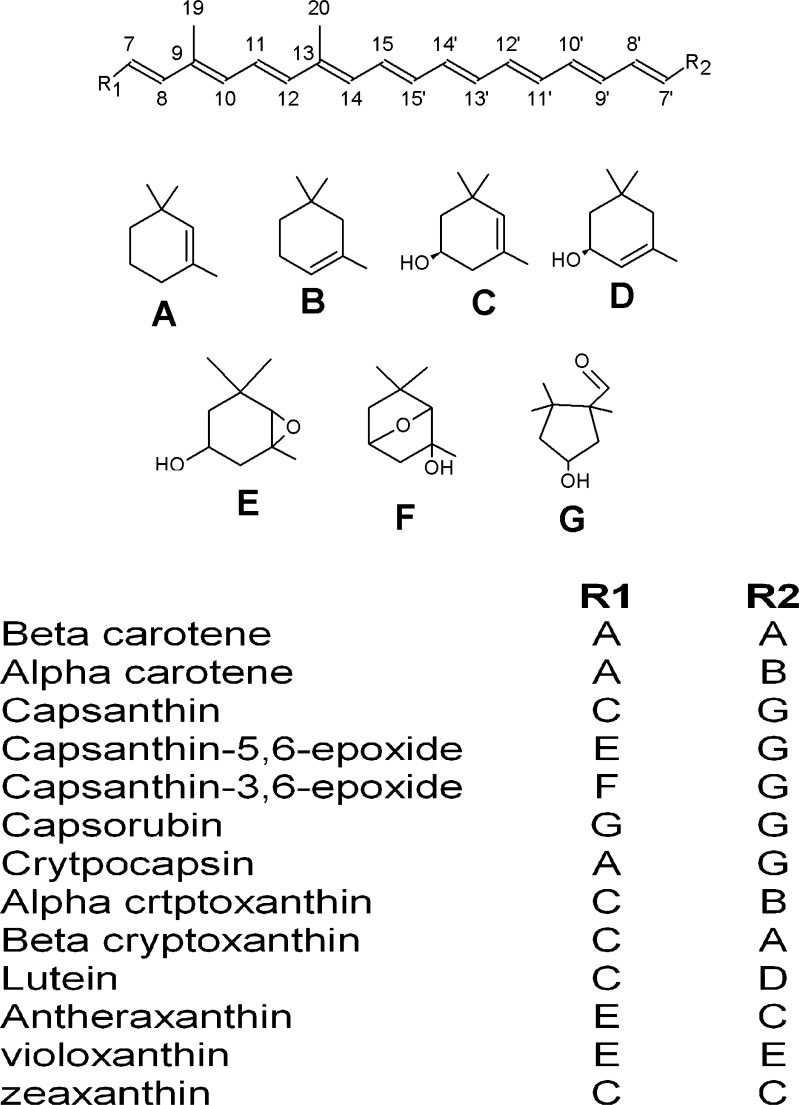
The red color of capsicum are imparted by carotenoids with more than 50 identified structures (Almela et al. 1991; Cervantes-Paz et al. 2014; Collera-Zúñiga et al. 2005; Giuffrida et al. 2013; Guil-Guerrero et al. 2006; Howard et al. 2000). The reports on the content of major carotenoids in red pepper is summarized in Table 1. Carotenoids content in the reports varies from 0.1 to 3.2 g/100 g dry weight with marked difference in composition. The unique keto carotenoids capsanthin, capsorubin and cryptocapsin impart brilliant red color to ripen chilly pods, while the yellow orange color is from β-carotene, zeaxanthin, violaxanthin and β-cryptoxanthin (Fig. 1). Most of the xanthophylls in red pepper occur as esters with C12, C14 and C18 fatty acids whereas green pepper extracts comprised mostly of free carotenoids (Breithaupt and Schwack 2000; Cervantes-Paz et al. 2014; Giuffrida et al. 2013;). The carotenoids such as capsanthin and capsorubin with κ-ring as end groups were previously reported to be characteristic of capsicum species (Deli and Molnar, 2002). Capsanthin contributes 30–70 % of carotenoids in most of the varieties and cultivars. The proportions of capsanthin and capsorubin increase in the advanced stages of ripening (Deli et al. 1996). Significant proportions of capsanthin-5,6-epoxide, capsanthin-3,6-epoxide, cucurbitaxanthin A, cucurbitaxanthin B, violaxanthin, antheraxanthin, capsanthone, neoxanthin, lutein etc. have also been reported in red pepper (Almela et al. 1991; Minguez-Mosquera and Hornero-Mendez 1993; Deli et al. 1996; Hornero-Méndez and Mínguez-Mosquera. 1998; Hornero-Méndez et al. 2000).
Table 1.
Composition of major carotenoids in red pepper
| Red pepper (unit of concentration) | Carotenoids | Total | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antheraxanthin | Neoxanthin | Violaxanthin | Capsorubin | Capsanthin | Lutein | Zeaxanthin | Cryptoxanthin | α-carotene | β-carotene | Cryptocapsin | Capsanthin 5,6 & 3,6 epoxides | |||
| C. annuum var Jalapeno (a) | 58 | Nd | 12 | 13 | 750 | nd | 104 | 42 | nd | 77 | – | 8 | 1065 | Cervantes-Paz et al., 2014 |
| C. Chinense (7 varieties) (b) | nd–9.9 | – | – | – | nd–63.3 | nd–48.3 | nd–13.4 | nd–3.3 | nd–10.4 | nd–19.1 | nd–10.3 | – | – | Giuffrida et al. 2013 |
| C. annuum (3 varieties) (b) | nd–2.8 | – | – | – | nd–71.8 | nd–0.4 | 1.1–4.8 | nd–2.9 | nd | nd–10.8 | nd–1.1 | – | – | |
| C. frutescens (b) | 1.5 | – | 55.0 | ND | 11.0 | 0.2 | ND | ND | 2.2 | – | – | |||
| C. annum hybrids | ||||||||||||||
| F1 Amanda (c) | – | – | 31 | – | nd | 8 | – | – | – | 2 | – | – | 41 | De Azevedo-Meleiro and Rodriguez-Amaya 2009 |
| F1 Magali (c) | – | – | 7 | 33 | 7 | – | – | – | 6 | – | 53 | |||
| C annum cv. AlevaNK (b) | 1.8 | – | 3.0 | 6.6 | 45.8 | – | 6.8 | – | – | 5.5 | – | – | 69.5 | Kevresan et al. 2009 |
| C. annuum 5Cv (a) | – | – | 50–101 | 26–67 | 769–1270 | – | 213–410 | 113–165 | – | 69–124 | – | – | 1440–2390 | Topuz and Ozdemir 2007 |
| C. annuum cv. Almuden (c) | – | – | 185–265 | 130–255 | 1053–1825 | – | 211–238 | 165–315 | – | 85–295 | – | – | 1829–3231 | Pérez-López et al. 2007 |
| C. annuum (10 varieties) (a) | nd–77 | 4–105 | 2–333 | nd–40 | nd–185 | 5–802 | nd–184 | nd–86 | nd–39 | 5.8–319 | nd–97 | – | 50–1796 | Guil-Guerrero et al. 2006 |
| C. annuum (3 varieties) (d) | Tr–7.1 | nd–2.2 | 13.2–22.0 | 0.35–4.2 | 19.8–22.6 | nd–0.9 | 3.6–7.2 | 6.8–14.9 | 1.3–3.0 | 14.9–20.9 | 0.2–10.0 | nd–13.0 | 6.76–7.52 | Collera-Zúñiga et al. 2005 |
| C. annuum cv. Jaranda (a) | 185 | – | 234 | 218 | 2211 | – | 640 | 575 | – | 656 | – | 69 | 4788 | Perez-Galvez and Minguez-Mosquera 2004 |
| C. annuum cv. Vergasa (e) | 1.1 | – | 2.3 | 3.0 | 26.2 | – | 4.0 | 1.5 | – | 4.3 | – | 0.9 | 45.6 | Marín et al. 2004 |
| C. annuum, cv. Turkish (a) | – | – | 392 | 382 | 4006 | – | 1022 | 430 | – | 360 | – | – | 7142 | Topuz and Ozdemir. 2003 |
| C. annuum (4 cultivars) (f) | – | – | – | – | nd–7443 | nd–955 | 293–956 | 131–973 | nd–2127 | 337–800 | – | – | 4146–32406 | Howard et al. 2000 |
| C. chinense (2 cultivars) (f) | – | – | – | – | 984–6754 | nd | 15–470 | nd–353 | nd–222 | 2–861 | – | – | 1001–8660 | |
| C. frutescens (f) | – | – | – | – | 14434 | ND | 1958 | 414 | 1252 | 1187 | – | – | 19245 | |
| C. annuum var. Km 622 (a) | – | – | 1008 | 1129 | 3404 | 381 | – | 166 | – | 1120 | 773 | – | 10160 | Markus et al. 1999 |
| C.aanuum var. longum ceratoid cv. Szentesi Kosszarvu paprika (g) | 1.6 | Nd | 2.0 | 2.6 | 31.8 | ND | 0.8 | 6.3 | 0.2 | 9.5 | 0.8 | 3.6 | 995 | Deli et al. 1996 |
| C. annuum (7 varieties) (h) | 0.1–0.7 | 0.1–0.8 | 0.1–0.6 | 0.1–2.0 | 2.9–5.7 | nd–0.4 | 0.3–0.6 | 0.2–0.5 | – | 0.3–1.6 | – | 0.1–0.7 | 4.2–13.6 | Almela et al. 1991 |
Units. a: mg/kg or μg/g in dry weight basis, b: % area in chromatogram, c: mg/kg or μg/g in fresh weight basis (or not specified), d: relative % of individual carotenoids & total carotenoids in mg/100 g in dry weight basis, e: mg/100 g in fresh weight basis, f: μg/100 g in dry weight basis, g: relative peak height of individual carotenoids to canthaxanthin in the chromatogram & total in mg/kg in dry weight basis, h: mg/g in dry weight basis
Fig. 1.
Structure of major carotenoids in red pepper
Peppers are a rich source of vitamin A and C, phenolic compounds and micro and macro elements (Guil-Guerrero et al. 2006). Red pepper preparations are suggested for the treatment of gastro-intestinal problems, skin diseases and arthritis, for healing wounds and as a blood purifier traditionally (Govindarajan and Sathyanarayana 1991). Experimental evidences for the potential of red pepper to reduce oxidation stress (Cervantes-Paz et al. 2014), inflammation (Spiller et al. 2008), pain (Group 1991), fat intake and body weight (Kawabata et al. 2006) and to control dyspepsia (Bortolotti et al. 2002) are available. The purported health benefits of peppers have been partially attributed to their carotenoids content. Carotenoids bearing κ-ring as end groups have been shown to posses strong reactive oxygen scavenging potential (Maoka et al. 2001). Capsanthin was shown to be among bioavailable carotenoids (Pugliesea et al. 2013).
Analysis of red pepper carotenoids
The wide structural diversity coupled with possible isomeric forms and derivatives make the analysis of carotenoids a difficult task. Reports on the method of analysis of red pepper carotenoids are summarized in Table 2. The general steps of carotenoid analysis include; sample preparation, extraction, purification, saponification, separation, detection and quantification. The instability associated with the characteristic conjugated double bond structure of carotenoids requisites the incorporation of control measures to minimize the possible loss of carotenoids during the course of analysis (Oliver and Palou 2000; de Quiros and Costa 2006). The precautions include carrying out the laboratory operations in dimmed, yellow or red light and performing sample preparation, extraction, evaporation, and saponification steps in presence of antioxidants under a protective nitrogen or argon environment at a temperature below 40 °C and storing the samples/extracts in inert atmosphere at temperatures around −20 °C (Ladislav et al. 2005).
Table 2.
Analysis of carotenoids in red pepper
| Reference | (A) Extraction (B) Purification (C) Saponification |
Analytical Instrument | Stationary phase | Mobile phase1 |
|---|---|---|---|---|
| Cervantes-Paz et al. 2014 | (A) CaCO3-MeOH, acetone, hexane (C) 40 % KOH in MeOH |
HPLC-DAD-APCI-TOF | C30, 3 μm, 4.6 × 250 mm | Water-MeOH-MTBE (G) |
| Giuffrida et al. 2013 | (A) acetone-NaHCO3
(B) DEE-10 % NaCl |
HPLC-DAD-APCI-MS | C30, 5 μm, 3.0 × 250 mm | A: MeOH-MTBE-water (82:16:2) B: MeOH-MTBE-water (10:88:2) (G) |
| Cacciola et al. 2012 | (A) MeOH-EA-petroleum ether (1:1:1) | LC X LC-PDA, LCMS (APCI)-IT-TOF | (1) Cyano, 5 μm, 10 × 250 mm | (1). A: n-hexane, B: n-hexane/butylacetate/acetone (80:15:5) (G) |
| (2) C18, 2.7 μm, 30 × 4.6 mm | (2). A: water/ACN (10:90), B: IPA (G) | |||
| Kevresan et al. 2009 | (A) acetone (B) DEE-10 % NaCl |
HPLC-DAD | C18, 5 μm, 3.0 × 250 mm | A: acetone-water (3:1) B: acetone-MeOH (3:1) (G) |
| de Azevedo-Meleiro and Rodriguez-Amaya 2009 | (A) acetone (C) 10 % KOH |
HPLC-DAD, HPLC-MS | C18, 3 μm, 3.0 × 150 mm | 0.05 % triethylamine in ACN/MEOH/EA, gradient |
| Pérez-López et al. 2007 | (A) acetone (B) DEE-10 % NaCl (C) 20 % KOH in MeOH |
HPLC-DAD | C30, 5 μm, 4.6 × 250 mm | A: MeOH/(MTBE)/H2O (81:15:4) B: MTBE/MeOH (91:9) (G) |
| Topuz and Ozdemir 2007 | (A) acetone (B) DEE-10 % NaCl (C) 10 % KOH in MeOH |
HPLC-UV | C18, 5 μm, 4.0 × 250 mm | acetone/water (G) |
| Guil-Guerrero et al. 2006 | (A) acetone (B) DEE-water (C) KOH in MeOH |
HPLC-MS | C30, 5 μm, 3.0 × 250 mm | A: MeOH, B: MTBE (G) |
| Daood and Blanc. 2005 | (A) MeOH-acetone-DCM (2:2:1) | HPLC-PDA | C18, 3 μm, 4.6 × 250 mm | A: Water-MeOH (9:91), B: MeOH-ACN-IPA (10:35:55), C: MeOH (G) |
| Collera-Zúñiga et al. 2005 | (A) acetone (B) DEE-10 % NaCl (C) 20 % KOH in MeOH |
HPLC-UV | μ-porasil 125 A0, 3.9 × 150 mm | hexane/acetone (G) |
| C18, 5 μm, 3.9 × 150 mm | water/MeOH (G) | |||
| Kim et al. 2004 | (A) acetone (B) DEE-10 % NaCl (C) 30 % KOH in MeOH |
HPLC-UV HPLC-APCI-MS |
C18, 10 μm, 3.0 × 200 mm | ACN-MeOH (95:5) (I) |
| HPLC-APCI-MS | C18, 10 μm, 3.0 × 200 mm | A: MeOH-MTBE-water (81:15:4) B: MeOH-MTBE-water (6:90:4) (G) |
||
| Marín et al. 2004 | (A) acetone (B) DEE-10 % NaCl (C) 20 % KOH in MeOH |
HPLC-DAD | C18, 5 μm, 4 × 250 mm | – |
| Perez-Galvez and Minguez-Mosquera 2004 | (A) acetone (B) DEE-10 % NaCl (C) 20 % KOH in MeOH |
HPLC-DAD | C18, 5 μm, 4.0 × 250 mm | Acetone/water (G) |
| Breithaupt and Schwack. 2000 | (A) MeOH-EA-light petroleum (1:1:1) (C) 0.5 KOH in MeOH |
LC-DAD-MS | C30, 5 μm, 4.0 × 250 mm | A: MeOH-MTBE-water (81:15:4) B: MeOH-MTBE-water (6:90:4) (G) |
| Cserhati et al. 2000 | (A) acteone | HPLC-DAD | C18, 5 μm, 4 × 150 mm | A: water, B: MeOH-ACN (8:2) (G) |
| Howard et al. 2000 | (A) acetone (B) DEE-10 % NaCl (C) KOH in MeOH |
HPLC-DAD | - | A: ACN-0.05 M Amm. acetate in MeOH-DCM-triethylamine (75:20:5:0.05) with 0.1 % BHT B: MeOH with 0.1 % BHT (G) |
| Hornero-Méndez et al. 2000 | (A) acetone (B) DEE-10 % NaCl (C) 20 % KOH in MeOH |
HPLC-DAD | C18, 5 μm, 4.6 × 250 mm | A: acetone, B: water (G) |
| Kosa et al. 2001 | (A) acetone | HPLC-DAD | C18, 5 μm, 2 × 150 mm | A: MeOH-ACN (8:2), B: water (G) |
| Illés et al. 1999 | (A) (i) Super critical CO2 (ii) DEE-acetone-MeOH (2:1:1) | HPLC-UV | C18, 5 μm, 4.6 × 250 mm | ACN-IPA-MeOH (2:1.75:1.25) (I) |
| Jaren-Galan et al. 1999 | (A) Super critical CO2
(C) 15 % KOH in MeOH |
HPLC-DAD | C18, 5 μm, 4.6 × 250 mm | A: acetone, B: MeOH-Water (1:1) (G) |
| Weissenberg et al. 1997 | (A) DEE, MeOH (C) 5 % KOH in MeOH |
HPLC-UV | C18, 5 μm, 4 × 250 mm; C18, 4 μm, 4 × 125 mm | ACN-IPA-EA (80:10:10) (I) |
| Deli et al. 1996 | (A) MeOH-DEE (C) 30 % KOH in MeOH |
HPLC-DAD | C18, 6 μm, 4.6 × 250 mm | A: 12 % water-MeOH, B: MeOH C: 50 % acetone-MeOH (G) |
Abbreviations: MeOH methanol, DEE diethyl ether, DCM dichloromethane, EA ethyl acetate, ACN acetonitrile, MTBE methyl tertiary butyl ether, I isocratic, G gradient
Extraction and pretreatment of samples
Carotenoids are lipophilic compounds and their distribution is linked to the lipid profile of plants. In mature capsicum fruits, carotenoids are localized in the pods. The best solvents for the extraction of carotenoids are chloroform, dichloromethane and tetrahydrofuran in which their solubility may attain 1000 to 10,000 mg/L. Less polar organic solvents such as hexane, petroleum ether, diethyl ether, dichloromethane, acetone, ethyl acetate, isoproponol, methanol etc. either alone or in mixtures, preferably in chilled form have been used to extract pepper carotenoids (Table 2). Better extractability from high moisture content samples may be achieved by extracting with hydrophilic solvents such as methanol, ethanol, acetone etc. to remove moisture followed by lesser polar solvents (Markus et al. 1999; Ranjith et al. 2006). The polar compounds co extracted with the carotenoids are traditionally removed by partitioning with water or aqueous salt solutions (Cacciola et al. 2012; Giuffrida et al. 2013; Guil-Guerrero et al. 2006). The homogenization and extraction of samples are to be carried out in inert atmosphere preferably in presence of antioxidants. Now days increased concern on the polluting and toxicological effects of petroleum solvents makes agro industries to substitute petroleum solvents for alternative less toxic solvents, particularly in products for human consumption, with the first option being alcohols.
The traditional extraction techniques such as soxhlet extraction, homogenization and sonication are often replaced by microwave assisted extraction (MAE) and supercritical fluid extraction (SFE) techniques. MAE shortens extraction time, reduces solvent consumption and improves extraction yield. Efficiency and selectivity of MAE depends on the dielectric constant of extraction media (Kiss et al. 2000). SFE has also been described as a desirable alternative to solvent extraction of carotenoids as it enhances the speed and efficiency of extraction and eliminates concentration steps (Turner et al. 2001). Supercritical carbon dioxide (SC–CO2) is the most commonly used extraction agent because of its non-toxicity, chemical inertness, and low cost and easy availability. In addition, CO2 presents a low critical temperature value (Tc 31.8 °C), making it ideal for extraction of thermally labile compounds like carotenoids. SFE methods particularly using SC–CO2 for the carotenoid extraction from fruits and vegetables including capsicum have been described by many authors (Illés et al. 1999; Uquiche et al. 2004; Arimboor et al. 2006). In SC–CO2 extraction, larger extraction volume, higher pressure, lower particle size and the use of ethanol or acetone as co-solvents have been reported to yield more pigments from red pepper (Jaren-Galan et al. 1999; Ambrogi et al. 2002; Uquiche et al. 2004; Tepic et al. 2009). It has also been shown that the proportion of red carotenoids such as capsorubin, capsanthin and zeaxanthin increased with the increase in extraction pressure (Jaren-Galan et al. 1999). On line monitoring of SC–CO2 extraction of paprika carotenoids using near infrared-visible detector showed that the ratio of β-carotene + free xanthophylls to total carotenoids in the recovered oleoresin diminished from nearly 50 % at the beginning down to 10 % at the end of extraction (Ambrogi et al. 2002). Tepic et al. (2009) reported that the content of carotenoids in SC–CO2 extracts was lower than that in traditional hexane extracts. Gnayfeed et al. (2001) has shown that sub critical propanol was superior to SC–CO2 in extracting carotenoids particularly diesters of xanthophylls and tocopherols but less efficient in the extraction of capsaicinoids. Richins et al. (2010) has shown that the oleoresin prepared with SC–CO2 containing 20 % ethanol as modifier from high pungent chillies could be precipitated in 76 % ethanol/water (v/v) to prepare red pepper pigments with low pungency. It is recommended to add antioxidants in SC–CO2 extraction to minimize the possible degradation of carotenoids by the traces of oxygen present in CO2 (Cocero et al. 2000).
The presence of the pungent principles, capsaicinoids in oleoresins of hot pepper is a serious limitation for its application as a food colorant and restricts the exploitation of a large numbers of high yielding pungent red chilli varieties for the colorant. Efforts to improve the methods to prepare non-pungent oleoresins from pungent capsicum fruits by the selective removal of capsaicinoids are still progressing. Interestingly capsaicin being a therapeutic agent particularly a chemo protector against mutogenesis and tumerogenesis is considered as a valuable byproduct of pepper color extraction. It has been reported that fractionation with 70 % methanol considerably reduced pungency of hexane extract and recovered 87 % of carotenoids (Balakrishnan and Verghese 1997). Santamaria et al. (2000) showed that direct extraction of capsicum floor with ethanol (96 % v/v) recovered 73 % carotenoids along with 80 % capsaicinoids, whereas when the flour was extracted with ethanol (96 %, v/v) after extracting with 30 % ethanol (v/v), the recovery of carotenoids and capsaicinoids became 70 and 26 % respectively. It has also been shown that pretreatment of the flour with enzymes those break cell wall enhanced the recovery of carotenoids by 11 %. Pretreatment of capsicum powder with 10–30 % sodium hydroxide and subsequent extraction with ethanol has been reported to yield pungent free red pepper colorant (Chen and Wu 2009).
Hydroxycarotenoids in red pepper predominantly occur as esterified with fatty acids, hence saponification of the extracts with aqueous or alcoholic solutions of KOH is frequently employed prior to chromatographic analysis to simplify the carotenoid profile (Kim et al. 2004; Pérez-López et al. 2007; de Azevedo-Meleiro and Rodriguez-Amaya 2009; Cervantes-Paz et al. 2014). Lower temperature, inert atmosphere and presence of antioxidants have been suggested to reduce the carotenoid loss during saponification (Oliver and Palou 2000; Ladislav et al. 2005). Since physicochemical and biological properties of carotenoids are largely controlled by their stereochemistry and nature of derivatistion, interest to fingerprint isomeric and natural derivatised forms of carotenoids has grown in recent years.
Application of solid phase extraction (SPE) technique using different types of sorbents has been demonstrated for the enrichment and purification of carotenoids (de Quiros and Costa 2006). Shen et al. (2009) evaluated the carotenoid retention capacity of different SPE cartridges and found that C18 and C30 SPE cartridges had good retention capacity for lutein and β-carotene, whereas Diol SPE was suitable for the purification of lutein only. The gentiobiose imprinted polymer (Gent-MIP) has been reported to enable selective extraction of crocin with a high recovery (84 %) from saffron (Mohajeri et al. 2010). In general SPE is a rapid and simple purification method for carotenoids and results in high recovery, accuracy and precision. SPE requires small amount of sample and consumes small amounts of organic solvents.
Separation, detection and quantification
The diversity and inherent instability restrict the commercial availability of carotenoid reference standards to certain common and widespread carotenoids. The purity of the available carotenoids is to be verified and to be purified if necessary prior to use as analytical standards. For other carotenoids the best sources are to be extracted and purified. Both column and thin layer chromatographic techniques are being used for the purification of carotenoids (Oliver and Palou 2000; Ladislav et al. 2005). Preparative HPLC techniques permit rapid, reliable and reproducible isolation and purification of carotenoids (Tan 1988; Scalia and Francis 1989; Mazzei and d’Avila 2003). High speed counter current chromatography has also been demonstrated as a effective tool for the preparation and purification of carotenoids from plant sources (Aman et al. 2005; Chen et al. 2005; Tsao and Yang 2006).
Uv-visible spectrophotometric quantification is the primary tool for carotenoid analysis, in which total absorbance of the extract is measured at a wavelength specific to the most abundant carotenoid in the sample or β-carotene (Ranjith et al. 2006). ASTA recommends absorption of 1 % red pepper acetone extract at 460 nm to compare the color quality of pepper products (AOAC Official Method 971.26) Differential wavelength technique has also been demonstrated as a an effective tool for the easy determination of red pepper carotenoids (Hornero-Mendez and Minguez-Mosquera 2001). Traditional gas chromatographic techniques are not suitable for the carotenoid analysis, because of their inherent instability and non-volatility.
Now days HPLC is turned as the most acceptable technique for carotenoid separation and analysis. RP-HPLC with both isocratic and gradient mobile phase is frequently used for the analysis of carotenoids (Breithaupt and Schwack 2000; Schweiggert et al. 2005b). However reports demonstrating the application of normal phase HPLC for the separation of carotenoids are also seen in literature (Collera-Zúñiga et al. 2005; Cacciola et al. 2012). Though most of the RP HPLC methods use C18 column for separation of pepper carotenoids (Kim et al. 2004; Marín et al. 2004; Perez-Galvez and Minguez-Mosquera 2004; Topuz and Ozdemir 2007; de Azevedo-Meleiro and Rodriguez-Amaya 2009; Kevresan et al. 2009), it seldom shows adequate ability to separate the isomeric and esterified form of carotenoids. Polymeric C30 column offers better resolution of carotenoid isomers (Strohschein et al. 1999; Cervantes-Paz et al. 2014; Giuffrida et al. 2013). Reports on the application of polymeric C30 columns for the profiling of pepper carotenoids in their natural form (Cervantes-Paz et al. 2014; Giuffrida et al. 2013) and after saponification (Guil-Guerrero et al. 2006; Pérez-López et al. 2007; Cervantes-Paz et al. 2014) are available. Application of comprehensive normal-phase × reversed-phase liquid chromatography system for the separation of red pepper carotenoids was recently demonsatrated by Cacciola et al. (2012). Chiral stationary phase have also been used for the resolution of isomeric carotenoids from food and biological samples (Khachik et al. 2002; Grewe et al. 2007). Mostly, RP chromatography with both isocratic and gradient mobile phases is being used to profile carotenoid esters. Addition of antioxidants such as BHT and low column temperature are recommended to prevent the decomposition of carotenoids during HPLC separation.
HPLC with DAD or Uv-visible detectors are extensively used for pepper carotenoid analysis (Table 2). The ability of DAD detectors to provide absorption spectra of analytes has been shown to be useful in differentiating the carotenoids in a limited way (Cserhati et al. 2000; Pérez-López et al. 2007; Kevresan et al. 2009). Simultaneous determination of carotenoids and red sudan dyes using HPLC-DAD has been reported by Daood and Biacs 2005. The ability of LC-MS techniques to provide more details on the structure of analytes makes it more preferable for the analysis of carotenoids and their metabolites. Both ESI (Guaratini et al. 2004; Carmona et al. 2006) and APCI (Schweiggert et al. 2005a; Giuffrida et al. 2013; Cervantes-Paz et al. 2014) interfaces have been described for carotenoid analysis. The sensitivity of ESI-MS detection is at least two orders of magnitude higher than that of UV-visible detection. APCI has a broader linear dynamic range than ESI (over at least three orders of magnitude). The efficacy of MS technique to detect isotope labeled carotenoids extents its application to bioavailabilty and metabolic studies. LC-MS technique has also been demonstrated as an effective tool for the analysis of pepper carotenoids both in free and esterified forms (Tables 1 and 2). UV-Visible and mass spectral data obtained from HPLC-DAD-MS and chemical reactions have been used to differentiate carotenoids in red and yellow pepper (de Azevedo-Meleiro and Rodriguez-Amaya 2009, Giuffrida et al. 2013). Perez-Galvez et al. (2005) have used HPLC-DAD-MS to profile thermal degradation products of paprika carotenoids. Independent of the mobile phase composition, similar pattern of chromatographic separation of pepper carotenoid esters are obtained in RP chromatography. The RP chromatogram can be divided into four zones, first zone representing free carotenoids, second zone to monoesters, third zone to isomers of carotenoids and finally fourth zone to diesterified carotenoids (Schweiggert et al. 2005a; 2007; Giuffrida et al. 2013).
Though, NMR (Dachtler et al. 2000), electrochemical (Ferruzzi et al. 1998) and thermal lens spectrometry (Navas and Jimenez 2003) detectors have also been demonstrated as suitable for carotenoid analysis, their application in food analysis is limited due to the increased cost of instrumentation and tedious sample preparation procedures. The other sensitive techniques such as Raman spectroscopy (Bernstein et al. 2002; Hammond and Wooten 2004), MALDI (Guaratini et al. 2004) and particle beam electron capture negative ion MS (Careri et al. 1999) find their application for carotenoid analysis mostly in biological samples.
Analytical data on the carotenoid composition of red pepper is divergent. Most of the reports identify capsanthin as the major carotenoid in red pepper (Table 1) whereas, lutein (Heinonen et al. 1989; Guil-Guerrero et al. 2006) and violoxanthin (de Azevedo-Meleiro and Rodriguez-Amaya 2009; Matus et al. 1991) are the principal carotenoid in some varieties. Varying proportions of violaxanthin, zeaxanthin, lutein, β-carotene and capsorubin have been reported as the other major carotenoids in red pepper (Table 1). The wide variation in the carotenoid composition in same species in different reports may be attributed to the variations in agro climatic and post-harvest conditions and/or limitations in analytical methods. Many of the published papers reported carotenoid composition based on chromatographic retention data and the data on composition is restricted to the available reference compounds. Identification based merely on the retention data may be misleading for a class of compounds like carotenoids as they give close elution pattern in most of the available chromatographic methods. Polymeric C30 stationary phase have proven advantage over C18 stationary phase in resolving close eluting carotenoids (Cervantes-Paz et al. 2014; Giuffrida et al. 2013; Pérez-López et al. 2007). A combined approach based on chromatographic data, absorption and mass spectral data and chemical tests has been suggested as more effective tool for carotenoid analysis (de Azevedo-Meleiro and Rodriguez-Amaya 2009). Structurally close compounds may have significant variations in MS responses; though they are not much differed in their UV/PDA responses. This restricts the usage of reference standards for the quantitation of other structurally related compounds in MS analysis, which is usually practiced in HPLC-UV/PDA. Since the availability of pure standards of carotenoids and their natural derivatives is very much limited, HPLC-PDA-MS configuration could be used for the analysis in which the identity of compounds can be confirmed from Mass and UV-Visible spectral data and quantification can be performed using the DAD data against the available structurally related compounds (Arimboor and Arumughan 2012, Giuffrida et al. 2013). The nature of matrix greatly influence the response of analytes in MS by suppressing or enhancing analyte ion intensities, hence the effects of different matrices and sample preparation methods on the MS response of carotenoids have to be evaluated and necessary corrections have to be incorporated to the analytical results if MS response is using for quantitation.
Stability of red pepper carotenoids
Electron rich conjugated polyene chain makes carotenoids more susceptible to the reactions with electrophilic reagents. Carotenoids in their natural environment, when they are incorporated to lipoproteins or membranes are relatively well protected. However if they are isolated and transformed into solution, they are more prone to isomerisation and oxidative degradation reactions. Knowledge on the mechanism of carotenoid degradation reactions and the effects of such reactions and products on color, odor, appearance and overall acceptability of food products is essential for the management of carotenoids as food colorants. Oxidation contributes to the major degradation loss of carotenoids during processing and storage. Both light and heat influence the stability of carotenoids through radical mediated oxidation reactions. Availability of oxygen, lower pH and presence of pro-oxidants, catalytic metals, oxidative enzymes and unsaturated lipids in food enhance the carotenoid degradation; whereas reduced water activity and presence of antioxidants have protective roles (Boon et al. 2010; Henry et al. 2000; Konovalova et al. 2001; Gao and Kispert 2003). The conditions that favor oxidation reactions generally found to favor the isomerization of naturally predominant all-trans carotenoids to their cis conformations (Boon et al. 2010; Gao et al. 1996; Gao and Kispert 2003). Isomerization of carotenoids influences their stability as well as bioavailability and physiological properties (Khoo et al. 2011). β-carotene is more susceptible to isomerisation than lycopene (Abushita et al. 2000). β-carotene in its trans form has been shown to be more bioactive than their common cis isomers, whereas lycopene have been reported to be more bioavailable in its cis form. The higher bioavailability of lycopene from processed tomatoes than from fresh tomatoes has been attributed to the isomerization of lycopene that taken place during processing (van Hof et al. 2000). Contents of carotenoids in plant tissues are controlled by many factors and may undergo changes not only in living plants but also during post-harvest processing and storage. Processing operations of plant foods usually rupture plant cells where carotenoids have been safely stored and expose carotenoids to oxidative enzymes and other degrading agents. At the same time it may be beneficial as the disruption of food matrices may facilitate the liberation and solubilisation of carotenoids resulting enhanced bioavailability (Giuffrida et al. 2013; Pugliesea et al. 2013).
Storage
Storage conditions crucially affect the carotenoid content of crops and finished products. Considerable loss of carotenoids from red pepper, pepper powder and paprika during post-harvest storage has been reported by Schweiggert et al. (2007). Carotenoid loss during storage is more for paprika than red pepper and illumination enhances the loss in both samples. Topuz (2008) reported that the color degradation of paprika follows a first order kinetics with higher rates at enhanced temperature and water activity during storage. Topuz and Ozdemir (2003) have shown that 10 months storage at ambient temperature results in 42.1 % reduction in capsanthin content in paprika. The sealed flexible vacuum–hermetic (80–100 mmHg) storage has been shown to have substantial advantages over traditional storage methods in the preservation of quality characteristics such as colour, pungency, and aflatoxin content of Turkish Red Chilli Peppers with 10 % moisture level for longer storage periods (Duman 2010). The loss of carotenoids in paprika samples stored under industrial controlled conditions (4 °C, 70 % RH) is minimum (Perez-Galvez et al. 2009). In general low temperature, oxygen deficiency, decreased humidity and absence of light were found to reduce the carotenoid degradation during the storage of crops.
Drying
Naturally convective dried paprika samples have retained more carotenoid pigments than the samples subjected to hot air oven, refractive window and freeze drying (Topuz et al. 2011; Topuz et al. 2009). Evaluation of the effects of chemical pretreatments on the color stability of red peppers during drying shows that dipping red peppers in a solution of 2 % ethyloleate + 0.2 % NaOH + 0.4 % K2CO3 at 60 °C helped to retain best color during drying. The high moisture levels (>80 %) of fresh red pepper fruits allow the fruits to have longer metabolic activity during drying at mild temperatures and therefore yield dried products with equal or more carotenoid content than the raw materials (Minguez-Mosquera et al. 2000). ASTA color values of Korean red pepper powders have been shown to be reduced by 50 % after 42 days sunlight exposure (Kim et al. 2002). Carotenoids in chilli pericarp discs exposed to the light have decreased rapidly than their counter parts kept in dark and ascorbic acid suppressed the carotenoid loss during the drying (Jung and Lee 2006). A sharp increase in the levels of MDA and changes in electrolyte leakage when carotenoids have exhausted indicates their photoprotective and antioxidants roles in plant cells (Jung et al. 2006). During spray drying of paprika oleoresins carotenoid retention in the microcapsules increases as inlet temperature increases from 160 to 200 °C and yellow fraction is more stable than the red fraction at all temperatures tested. Maximal stability for carotenoid oxidation was found at aw’s of 0.274 and 0.710 for microcapsules prepared with GA and SPI respectively (Rascon et al. 2010).
Processing
Short term mild heat treatments such as blanching (70–105 °C) and pasteurization (60–95 °C) usually enhance the storage life of food products by inactivating enzymes and vegetative microorganisms. Blanching generally helps to decrease the loss of carotenoids during storage by reducing the enzyme activities (Negi and Roy 2000a). However, reports indicating the loss of carotenoids during blanching are also available (Negi and Roy 2000b). Pasteurization of canned red pepper pickles containing 2 % NaCl and 2 % acetic acid at 83 °C for 5.2 min has been shown as the optimum condition to minimize the carotenoid loss (Guerra-vargas et al. 2001). Thermal treatments at 80, 90 and 100 °C for 5 and 10 min reduce carotenoids by 25–34 and 20–53 % in red pepper and paprika respectively. Mono and diesterified carotenoids are more stable during processing than their non-esterified counter parts (Schweiggert et al. 2007). HPLC-APCI-MS and EI-MS analysis of paprika oleoresins after high temperature treatment revealed 9,10,11,12,13,14,19,20-octnor-capsorubin, 9,10,11,12,13,14,19,20-octnor-5, 6-epoxide-capsanthin and 9,10,11,12,13,14,19,20-octnor-capsanthin and its isomers as the major thermal degradation products of pepper carotenoids (Perez-Galvez et al. 2005). It has been shown that degree of unsaturation of lipid media did not have significant effect on the rate of degradation and trans to cis isomerisation of carotenoids during non-oxygenated autoxidation of paprika oleoresin (Perez-Galvez and Minguez-Mosquera 2004). An isokinetic temperature for the thermal degradation of chilli carotenoids has been postulated by Perez-Galvez et al. 2000, above which, degradation of red carotenoids is more favored and below which degradation is preferentially towards yellow carotenoids. Domestic cooking of vegetables also results in the loss of carotenoids. Pressure-cooking has been shown to retain more carotenoids than open vessel cooking (Gayathri et al. 2004). Addition of antioxidants spices like turmeric and acidulants reduces the loss of carotenoids during cooking. The highest γ-irradiation dosage 10 kGy causes 11.1 % capsanthin reduction in paprika (Topuz and Ozdemir 2003).
Product storage
Studies on the storage stability of red pepper carotenoids as colorants in finished products are rather limited. The degree of redness imparted to meat products with paprika has been reported to be stable during the shelf life, whereas pasteurization of paprika alters the color stability and addition of natural antioxidants helps to maintain the color levels of the products through out the shelf life (Gomez et al. 2008). In general carotenoid retention can be improved by the closed storage at lower temperature and reduced water and cationic metal activities in absence of light and oxygen.
Conclusion
Red pepper is one of the oldest, popular and economically important natural coloring and flavoring agent with an average annual production of 2.5 million tones. Red pepper pods are abundant with diverse carotenoids, and their composition largely depends on the varieties and geo-climatic conditions of cultivation. However reports on the detailed carotenoid composition of red pepper varieties in respect to their agro-climatic conditions are limited. In practice, it is very important to take into account the carotenoid instability to establish processing conditions for its coloring applications. Wide variations in the samples and model assay systems used to evaluate the storage and processing stability of red pepper carotenoids in the available reports made the assessment of stability data difficult. Processing and storage practices may change the nature and properties of plant matrices resulting in variations in the liberation of carotenoids during extraction for analysis, which may even show increase in carotenoid contents during processing and storage. Interaction of components in food matrices may enhance or inhibit the degradation kinetics of carotenoids. Therefore properly designed blank experiments to accommodate these variations are to be incorporated in the studies. The comparison of analytical results is not informative unless a well validated analytical method is used and the contents are expressed in dry weight basis or moisture content is specified if reported in wet basis. These inconsistencies emphasize the need for standardized protocols for sample preparation, extraction, and analysis and reporting of results and for the development of model systems for stability studies of carotenoids suitable for the wide variety of raw and finished products. Detailed studies on the stability of red pepper carotenoids in different products following well defined and standardized protocols and using well designed model systems are to be conducted in order to asses the potential of pepper carotenoids as food colorants. On the other hand, it has been clearly established that technology development has introduced new methodologies or processes to avoid the intrinsic oxidative and acidic instability and solubility problems of carotenoids.
References
- Abushita AA, Daood HG, Biacs PA. Change in carotenoids and antioxidant vitamins in tomato as a function of varietal and technological factors. J Agric Food Chem. 2000;48(6):2075–2081. doi: 10.1021/jf990715p. [DOI] [PubMed] [Google Scholar]
- Almela L, Jos M, Lopez-Roca CME, Alcdzart MD. Carotenoid composition of new cultivars of red pepper for paprika. J Agric Food Chem. 1991;39:1606–1609. [Google Scholar]
- Aman R, Carle R, Jr C, Beifuss U, Schieber A. Isolation of carotenoids from plant materials and dietary supplements by high-speed counter-current chromatography. J Chromatogr A. 2005;1074:99–105. doi: 10.1016/j.chroma.2005.03.055. [DOI] [PubMed] [Google Scholar]
- Ambrogi A, Cardarelli DA, Eggers R. Fractional extraction of paprika using supercritical carbon dioxide and on-line determination of carotenoids. J Food Sci. 2002;67(9):3236–3241. [Google Scholar]
- AOAC Official Method 971.26. Colour (extracatble) in spices, spectrophotometric method Section 43.1.02, 1980.
- Arimboor R, Arumughan C. HPLC-DAD-MS/MS profiling of antioxidant flavonoid glycosides in sea buckthorn (Hippophae rhamnoides L.) seeds. Int J Food Sci Nutr. 2012;63(6):730–738. doi: 10.3109/09637486.2011.652075. [DOI] [PubMed] [Google Scholar]
- Arimboor R, Venugopalan VV, Sarinkumar K, Arumughan C, Sawhney RC. Integrated processing of fresh Indian sea buckthorn (Hippophae rhamnoides) berries and chemical evaluation of products. J Sci Food Agric. 2006;86(14):2345–2353. [Google Scholar]
- Balakrishnan KV, Verghese J. Studies on the recovery of pungency-free colour matter from Indian capsicum extracts. Acta Aliment. 1997;26:9–21. [Google Scholar]
- Bernstein PS, Zhao D-Y, Wintch SW, Ermakov IV, McClane RW, Gellermann W. Resonance Raman measurement of macular carotenoids in normal subjects and in age-related macular degeneration patients. Ophthalmology. 2002;109(10):1780–1787. doi: 10.1016/s0161-6420(02)01173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon CS, McClements DJ, Weiss J, Decker EA. Factors influencing the chemical stability of carotenoids in foods. Crit Rev Food Sci Nutr. 2010;50(6):515–532. doi: 10.1080/10408390802565889. [DOI] [PubMed] [Google Scholar]
- Bortolotti M, Coccia G, Grossi G, Miglioli M. The treatment of functional dyspepsia with red pepper. Aliment Pharmacol Ther. 2002;16:1075–1082. doi: 10.1046/j.1365-2036.2002.01280.x. [DOI] [PubMed] [Google Scholar]
- Breithaupt DE, Schwack W. Determination of free and bound carotenoids in paprika (Capsicum annuum L.) by LC/MS. Eur Food Res Technol. 2000;211(1):52–55. [Google Scholar]
- Cacciola F, Donatoc P, Giuffridad D, Torreb G, Dugob P, Mondellob L. Ultra high pressure in the second dimension of a comprehensive two-dimensional liquid chromatographic system for carotenoid separation in red chili peppers. J Chromatogr A. 2012;1255:244–251. doi: 10.1016/j.chroma.2012.06.076. [DOI] [PubMed] [Google Scholar]
- Careri M, Lombardi P, Mucchino C, Cantoni E. Use of eluent modifiers for liquid chromatography particle-beam electron-capture negative-ion mass spectrometry of carotenoids. Rapid Commun Mass Spectrom. 1999;13(2):118–124. [Google Scholar]
- Carmona M, Zalacain A, Sanchez AM, Novella JL, Alonso GL. Crocetin esters, picrocrocin and its related compounds present in crocus sativus stigmas and gardenia jasminoides fruits tentative identification of seven new compounds by lC-ESI-MS. J Agric Food Chem. 2006;54(3):973–979. doi: 10.1021/jf052297w. [DOI] [PubMed] [Google Scholar]
- Cervantes-Paz B, Yahia EM, Ornelas-Paz JJ, Victoria-Campos CI, Ibarra-Junquera V, Pérez-Martínez JD, Escalante-Minakata P. Antioxidant activity and content of chlorophylls and carotenoids in raw and heat-processed Jalapeño peppers at intermediate stages of ripening. Food Chem. 2014;146:188–196. doi: 10.1016/j.foodchem.2013.09.060. [DOI] [PubMed] [Google Scholar]
- Chen D, Wu Z. Study on extraction and purification process of capsicum red pigment. J Agric Sci. 2009;1(2):94–100. [Google Scholar]
- Chen F, Li H-B, Wong RN-S, Ji B, Jiang Y. Isolation and purification of the bioactive carotenoid zeaxanthin from the microalga Microcystis aeruginosa by high-speed counter-current chromatography. J Chromatogr A. 2005;1064(2):183–186. doi: 10.1016/j.chroma.2004.12.065. [DOI] [PubMed] [Google Scholar]
- Cocero MJ, Gonzalez S, Perez S, Alonso E. Supercritical extraction of unsaturated products. Degradation of β-carotene in supercritical extraction processes. J Supercrit Fluids. 2000;19(1):39–44. [Google Scholar]
- Collera-Zúñiga O, Jiménez FG, Gordillo RM. Comparative study of carotenoid composition in three mexican varieties of Capsicum annuum L. Food Chem. 2005;90(1–2):109–114. [Google Scholar]
- Cserhati T, Forgacs E, Morais MH, Mota T, Ramos A. Separation and quantitation of colour pigments of chili powder (Capsicum frutescens) by high-performance liquid chromatography-diode array detection. J Chromatogr A. 2000;896(1–2):69–73. doi: 10.1016/s0021-9673(00)00580-x. [DOI] [PubMed] [Google Scholar]
- Dachtler M, Glaser T, Kohler K, Albert K. Combined HPLC-MS and HPLC-NMR on-line coupling for the separation and determination of lutein and zeaxanthin stereoisomers in spinach and in retina. Anal Chem. 2000;73(3):667–674. doi: 10.1021/ac000635g. [DOI] [PubMed] [Google Scholar]
- Daood HG, Biacs Pt A. Simultaneous determination of sudan dyes and carotenoids in red pepper and tomato products by HPLC. J Chromatogr Sci. 2005;43(9):461–465. doi: 10.1093/chromsci/43.9.461. [DOI] [PubMed] [Google Scholar]
- de Azevedo-Meleiro CH, Rodriguez-Amaya DB. Qualitative and quantitative differences in the carotenoid composition of yellow and red peppers determined by HPLC-DAD-MS. J Sep Sci. 2009;32(21):3652–3658. doi: 10.1002/jssc.200900311. [DOI] [PubMed] [Google Scholar]
- de Quiros AR-B, Costa HS. Analysis of carotenoids in vegetable and plasma samples: a review. J Food Compos Anal. 2006;19(2–3):97–111. [Google Scholar]
- Deli J, Molnar P. Paprika carotenoids: analysis, isolation, structure elucidation. Curr Org Chem. 2002;6:1197–1219. [Google Scholar]
- Deli J, Matus Z, Tóth G. Carotenoid composition in the fruits of Capsicum annuum cv. Szentesi Kosszarvú during ripening. J Agric Food Chem. 1996;44:711–716. [Google Scholar]
- Duman AD. Storage of red chili pepper under hermetically sealed or vacuum conditions for preservation of its quality and prevention of mycotoxin occurrence. J Stored Prod Res. 2010;46(3):155–160. [Google Scholar]
- Edge R, McGarvey DJ, Truscott TG. The carotenoids as anti-oxidants: a review. J Photochem Photobiol B Biol. 1997;41(3):189–200. doi: 10.1016/s1011-1344(97)00092-4. [DOI] [PubMed] [Google Scholar]
- Ferruzzi MG, Sander LC, Rock CL, Schwartz SJ. Carotenoid determination in biological microsamples using liquid chromatography with a coulometric electrochemical array detector. Anal Biochem. 1998;256(1):74–81. doi: 10.1006/abio.1997.2484. [DOI] [PubMed] [Google Scholar]
- Frengova G, Beshkova D. Carotenoids from Rhodotorula and Phaffia yeasts of biotechnological importance. J Ind Microbiol Biotechnol. 2009;36(2):163–180. doi: 10.1007/s10295-008-0492-9. [DOI] [PubMed] [Google Scholar]
- Gale CR, Hall NF, Phillips DIW, Martyn CN. Lutein and zeaxanthin status and risk of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2003;44(6):2461–2465. doi: 10.1167/iovs.02-0929. [DOI] [PubMed] [Google Scholar]
- Gao Y, Kispert LD. Reaction of carotenoids and ferric chloride: equilibria, isomerization, and products. J Phys Chem B. 2003;107(22):5333–5338. [Google Scholar]
- Gao G, Wei CC, Jeevarajan AS, Kispert LD. Geometrical isomerization of carotenoids mediated by cation radical/dication formation. J Phys Chem. 1996;100(13):5362–5366. [Google Scholar]
- Gayathri GN, Platel K, Prakash J, Srinivasan K. Influence of antioxidant spices on the retention of β-carotene in vegetables during domestic cooking processes. Food Chem. 2004;84(1):35–43. [Google Scholar]
- Giuffrida D, Dugo P, Torre G, Bignardi C, Cavazza A, Corradini C, Dugo G. Characterization of 12 Capsicum varieties by evaluation of their carotenoid profile and pungency determination. Food Chem. 2013;140(4):794–802. doi: 10.1016/j.foodchem.2012.09.060. [DOI] [PubMed] [Google Scholar]
- Gnayfeed MH, Daood HG, Illes V, Biacs PA. Supercritical CO2 and subcritical propane extraction of pungent paprika and quantification of carotenoids, tocopherols, and capsaicinoids. J Agric Food Chem. 2001;49:2761–2766. doi: 10.1021/jf001292q. [DOI] [PubMed] [Google Scholar]
- Gomez R, Alvarez-Orti M, Pardo JE. Influence of the paprika type on redness loss in red line meat products. Meat Sci. 2008;80(3):823–828. doi: 10.1016/j.meatsci.2008.03.031. [DOI] [PubMed] [Google Scholar]
- Govindarajan VS, Sathyanarayana MN. Capsicum—production, technology, chemistry, and quality. Part V. Impact on physiology, pharmacology, nutrition, and metabolism; structure, pungency, pain, and desensitization sequences. Crit Rev Food Sci Nutr. 1991;29:435–474. doi: 10.1080/10408399109527536. [DOI] [PubMed] [Google Scholar]
- Graham JE, Bryant DA. The biosynthetic pathway for synechoxanthin, an aromatic carotenoid synthesized by the euryhaline, unicellular Cyanobacterium Synechococcus sp. Strain PCC 7002. J Bacteriol. 2008;190(24):7966–7974. doi: 10.1128/JB.00985-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe C, Menge S, Griehl C. Enantioselective separation of all-E-astaxanthin and its determination in microbial sources. J Chromatogr A. 2007;1166(1–2):97–100. doi: 10.1016/j.chroma.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Group TSC Treatment of painful diabetic neuropathy with topical capsaicin. A multicenter, double-blind, vehicle-controlled study. Arch Intern Med. 1991;151:2225–2229. doi: 10.1001/archinte.151.11.2225. [DOI] [PubMed] [Google Scholar]
- Guaratini T, Vessecchi RL, Lavarda FC, Maia Campos PMBG, Naal Z, Gates PJ, Lopes NP. New chemical evidence for the ability to generate radical molecular ions of polyenes from ESI and HR-MALDI mass spectrometry. Analyst. 2004;129(12):1223–1226. doi: 10.1039/b412154f. [DOI] [PubMed] [Google Scholar]
- Guerra-vargas M, Jaramillo-flores ME, Dorantes-alvarez L, Hernández-sánchez H. Carotenoid retention in canned pickled jalapeño peppers and carrots as affected by sodium chloride, acetic acid, and pasteurization. J Food Sci. 2001;66(4):620–626. [Google Scholar]
- Guil-Guerrero JL, Martínez-Guirado C, del Mar Rebolloso-Fuentes M, Carrique-Pérez A. Nutrient composition and antioxidant activity of 10 pepper (Capsicum annuun) varieties. Eur Food Res Technol. 2006;224(1):1–9. [Google Scholar]
- Hammond BR, Jr, Wooten BR. Validity issues with the in vivo measurement of skin carotenoids using Raman Spectroscopy. J Investig Dermatol. 2004;122(2):544–546. doi: 10.1046/j.0022-202X.2004.22219.x. [DOI] [PubMed] [Google Scholar]
- Heinonen MI, Ollilainen V, Linkola EK, Varo PT, Koivistoinen PE. Carotenoids in Finnish foods: vegetables, fruits, and berries. J Agric Food Chem. 1989;37(3):655–659. [Google Scholar]
- Henry BS (1996) Natural food colorants. In: Hendry GAF, Houghton JD (Eds) Natural food colorants, 2nd edn. Blackie. pp 40–79
- Henry LK, Puspitasari-Nienaber NL, Jaren-Galan M, van Breemen RB, Catignani GL, Schwartz SJ. Effects of ozone and oxygen on the degradation of carotenoids in an aqueous model system. J Agric Food Chem. 2000;48(10):5008–5013. doi: 10.1021/jf000503o. [DOI] [PubMed] [Google Scholar]
- Hornero-Méndez D, Mínguez-Mosquera MI. Isolation and identification of the carotenoid capsolutein from capsicum annuum as cucurbitaxanthin A. J Agric Food Chem. 1998;46(10):4087–4090. [Google Scholar]
- Hornero-Mendez D, Minguez-Mosquera MI. Rapid spectrophotometric determination of red and yellow isochromic carotenoid fractions in paprika and red pepper oleoresins. J Agric Food Chem. 2001;49(8):3584–3588. doi: 10.1021/jf010400l. [DOI] [PubMed] [Google Scholar]
- Hornero-Méndez D, de Guevara RG-L, Mínguez-Mosquera MI. Carotenoid biosynthesis changes in five red pepper (Capsicum annuum L.) cultivars during ripening. Cultivar selection for breeding. J Agric Food Chem. 2000;48:3857–3864. doi: 10.1021/jf991020r. [DOI] [PubMed] [Google Scholar]
- Howard LR, Talcott ST, Brenes CH, Villalon B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J Agric Food Chem. 2000;48(5):1713–1720. doi: 10.1021/jf990916t. [DOI] [PubMed] [Google Scholar]
- Illés V, Daood HG, Biacs PA, Gnayfeed MH, Mészáros B. Supercritical CO2 and subcritical propane extraction of spice red pepper oil with special regard to carotenoid and tocopherol content. J Chromatogr Sci. 1999;37:345–352. [Google Scholar]
- Jaren-Galan M, Nienaber U, Schwartz SJ. Paprika (Capsicum annuum) oleoresin extraction with supercritical carbon dioxide. J Agric Food Chem. 1999;47(9):3558–3564. doi: 10.1021/jf9900985. [DOI] [PubMed] [Google Scholar]
- Jung JW, Lee, Seung Koo Photobleaching, light-induced discolouration in red peppers. J Sci Food Agric. 2006;86(14):2296–2301. [Google Scholar]
- Kawabata F, Inoue N, Yazawa S, Kawada T, Inoue K, Fushiki T. Effects of CH-19 sweet, a non-pungent cultivar of red pepper in decreasing the body weight and suppressing body fat accumulation by sympathetic nerve activation in humans. Biosci Biotechnol Biochem. 2006;70(12):2824–2835. doi: 10.1271/bbb.60206. [DOI] [PubMed] [Google Scholar]
- Kevresan ZS, Mandic AP, Kuhajda KN, Sakac MB. Carotenoid content in fresh and dry pepper (Capsicum annuum L) fruits for paprika production. Food Process Qual Saf. 2009;1–2:21–27. [Google Scholar]
- Khachik F, de Moura FF, Zhao D-Y, Aebischer C-P, Bernstein PS. Transformations of selected carotenoids in plasma, liver, and ocular tissues of humans and in nonprimate animal models. Invest Ophthalmol Vis Sci. 2002;43(11):3383–3392. [PubMed] [Google Scholar]
- Khoo H-E, Prasad KN, Kong K-W, Jiang Y, Ismail A. Carotenoids and their isomers: color pigments in fruits and vegetables. Molecules. 2011;16:1710–1738. doi: 10.3390/molecules16021710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Park JB, Hwang IK. Quality attributes of various varieties of Korean red pepper powders (Capsicum annuum L.) and color stability during sunlight exposure. J Food Sci. 2002;67(8):2957–2961. [Google Scholar]
- Kim S, Park JB, Hwang IK. Composition of main carotenoids in Korean red pepper (capsicum annuum, L) and changes of pigment stability during the drying and storage process. J Food Sci. 2004;69(1):FCT39–FCT44. [Google Scholar]
- Kiss GAC, Forgacs E, Cserhati T, Mota T, Morais H, Ramos A. Optimisation of the microwave-assisted extraction of pigments from paprika (Capsicum annuum L.) powders. J Chromatogr A. 2000;889(1–2):41–49. doi: 10.1016/s0021-9673(00)00440-4. [DOI] [PubMed] [Google Scholar]
- Konovalova TA, Gao Y, Schad R, Kispert LD, Saylor CA, Brunel L-C. Photooxidation of carotenoids in mesoporous mcm-41, ni-mcm-41 and al-mcm-41 molecular sieves. J Phys Chem B. 2001;105(31):7459–7464. [Google Scholar]
- Kosa A, Cserhati T, Forgacs E, Morais H, Mota T, Ramos AC. Profiling of colour pigments of chili powders of different origin by high-performance liquid chromatography. J Chromatogr A. 2001;915(1–2):149–154. doi: 10.1016/s0021-9673(01)00640-9. [DOI] [PubMed] [Google Scholar]
- Krubasik P, Kobayashi M, Sandmann G. Expression and functional analysis of a gene cluster involved in the synthesis of decaprenoxanthin reveals the mechanisms for C50 carotenoid formation. Eur J Biochem. 2001;268(13):3702–3708. doi: 10.1046/j.1432-1327.2001.02275.x. [DOI] [PubMed] [Google Scholar]
- Ladislav F, Vera P, Karel S, Karel V. Reliability of carotenoid analyses: a review. Curr Anal Chem. 2005;1(1):93–102. [Google Scholar]
- Maoka T, Mochida K, Kozuka M, Ito Y, Fujiwara Y, Hashimoto K, Enjo F, Ogata M, Nobukuni Y, Tokuda H, Nishino H. Cancer chemopreventive activity of carotenoids in the fruits of red paprika Capsicum annuum L. Cancer Lett. 2001;172(2):103–109. doi: 10.1016/s0304-3835(01)00635-8. [DOI] [PubMed] [Google Scholar]
- Marín A, Ferreres F, Tomás-Barberán FA, Gil MI. Characterization and quantitation of antioxidant constituents of sweet pepper (Capsicum annuum L.) J Agric Food Chem. 2004;52(12):3861–3869. doi: 10.1021/jf0497915. [DOI] [PubMed] [Google Scholar]
- Markus F, Daood HG, Kapitany J, Biacs PA. Change in the carotenoid and antioxidant content of spice red pepper (paprika) as a function of ripening and some technological factors. J Agric Food Chem. 1999;47(1):100–107. doi: 10.1021/jf980485z. [DOI] [PubMed] [Google Scholar]
- Matus Z, Deli J, Szabolcs J. Carotenoid composition of yellow pepper during ripening: isolation of.beta.-cryptoxanthin 5,6-epoxide. J Agric Food Chem. 1991;39(11):1907–1914. [Google Scholar]
- Mazzei JL, d’Avila LA. Chromatographic models as tools for scale up of isolation of natural products by semi-preparative HPLC. J Liq Chromatogr Relat Technol. 2003;26(2):177–193. [Google Scholar]
- Minguez-Mosquera MI, Hornero-Mendez D. Separation and quantification of the carotenoid pigments in red peppers (Capsicum annuum L.), paprika, and oleoresin by reversed-phase HPLC. J Agric Food Chem. 1993;41(10):1616–1620. [Google Scholar]
- Minguez-Mosquera MI, Perez-Galvez A, Garrido-Fernandez J. Carotenoid content of the varieties jaranda and jariza (Capsicum annuum L.) and response during the industrial slow drying and grinding steps in paprika processing. J Agric Food Chem. 2000;48(7):2972–2976. doi: 10.1021/jf9908143. [DOI] [PubMed] [Google Scholar]
- Mohajeri SA, Hosseinzadeh H, Keyhanfar F, Aghamohammadian J. Extraction of crocin from saffron (Crocus sativus) using molecularly imprinted polymer solid-phase extraction. J Sep Sci. 2010;33(15):2302–2309. doi: 10.1002/jssc.201000183. [DOI] [PubMed] [Google Scholar]
- Navas MJ, Jimenez AM. Thermal lens spectrometry as analytical tool. Crit Rev Anal Chem. 2003;33(2):77–88. [Google Scholar]
- Negi PS, Roy SK. Effect of blanching and drying methods on β-carotene, ascorbic acid and chlorophyll retention of leafy vegetables. LWT Food Sci Technol. 2000;33(4):295–298. [Google Scholar]
- Negi PS, Roy SK. Effect of low-cost storage and packaging on quality and nutritive value of fresh and dehydrated carrots. J Sci Food Agric. 2000;80(15):2169–2175. [Google Scholar]
- Oliver J, Palou A. Chromatographic determination of carotenoids in foods. J Chromatogr A. 2000;881(1–2):543–555. doi: 10.1016/s0021-9673(00)00329-0. [DOI] [PubMed] [Google Scholar]
- Ong ASH, Tee ES. Natural sources of carotenoids from plants and oils. Methods Enzymol. 1992;213:142–167. [Google Scholar]
- Pelz A, Wieland K-P, Putzbach K, Hentschel P, Albert K, Gotz F. Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. J Biol Chem. 2005;37:32493–32498. doi: 10.1074/jbc.M505070200. [DOI] [PubMed] [Google Scholar]
- Perez-Galvez A, Minguez-Mosquera MI. Degradation, under non-oxygen-mediated autooxidation, of carotenoid profile present in paprika oleoresins with lipid substrates of different fatty acid composition. J Agric Food Chem. 2004;52(3):632–637. doi: 10.1021/jf0351063. [DOI] [PubMed] [Google Scholar]
- Perez-Galvez A, Jaren-Galan M, Minguez-Mosquera MI. Effect of high-temperature degradative processes on ketocarotenoids present in paprika oleoresins. J Agric Food Chem. 2000;48(7):2966–2971. doi: 10.1021/jf0000979. [DOI] [PubMed] [Google Scholar]
- Perez-Galvez A, Rios JJ, Minguez-Mosquera MI. Thermal degradation products formed from carotenoids during a heat-induced degradation process of paprika oleoresins (Capsicum annuum L.) J Agric Food Chem. 2005;53(12):4820–4826. doi: 10.1021/jf050119x. [DOI] [PubMed] [Google Scholar]
- Perez-Galvez A, Hornero-Mendez D, Minguez-Mosquera MI. Stability of paprika without supplementary antioxidants during storage under industrial controlled conditions. J Agric Food Chem. 2009;57(11):4718–4723. doi: 10.1021/jf804058m. [DOI] [PubMed] [Google Scholar]
- Pérez-López AJ, Núñez-Delicado E, López-Nicolas JM, Amor FMD, Carbonell-Barrachina AA. Effects of agricultural practices on color, carotenoids composition, and minerals contents of sweet peppers, cv. almuden. J Agric Food Chem. 2007;55:8158–8164. doi: 10.1021/jf071534n. [DOI] [PubMed] [Google Scholar]
- Pugliesea A, Loizzoa MR, Tundis R, O’Callaghanb Y, Galvinb K, Menichini F, O’Brienb N. The effect of domestic processing on the content and bioaccessibility of carotenoids from chili peppers (Capsicum species) Food Chem. 2013;141:2606–2613. doi: 10.1016/j.foodchem.2013.05.046. [DOI] [PubMed] [Google Scholar]
- Ranjith A, Kumar K, Venugopalan V, Arumughan C, Sawhney R, Singh V. Fatty acids, tocols, and carotenoids in pulp oil of three sea buckthorn species (Hippophae rhamnoides, H. salicifolia and H. tibetana) grown in the Indian Himalayas. J Am Oil Chem Soc. 2006;83(4):359–364. [Google Scholar]
- Rascon MP, Beristain CI, Garcia HS, Salgado MA. Carotenoid retention and storage stability of spray-dried encapsulated paprika oleoresin using gum Arabic and soy protein isolate as wall materials. LWT Food Sci Technol. 2010;44(2):549–557. [Google Scholar]
- Richins RD, Hernandez L, Dungan B, Hambly S, Holguin FO, O’Connell MA. A green extraction protocol to recover red pigments from hot capsicum fruit. HortSci. 2010;45(7):1084–1087. [Google Scholar]
- Santamaria RI, Reyes-Duarte MD, Barzana E, Fernando D, Gama FM, Mota M, Lopez-Munguia A. Selective enzyme-mediated extraction of capsaicinoids and carotenoids from chili guajillo puya (Capsicum annuum l.) using ethanol as solvent. J Agric Food Chem. 2000;48(7):3063–3067. doi: 10.1021/jf991242p. [DOI] [PubMed] [Google Scholar]
- Scalia S, Francis G. Preparative scale reversed-phase HPLC method for simultaneous separation of carotenoids and carotenoid esters. Chromatographia. 1989;28(3):129–132. [Google Scholar]
- Schweiggert U, Kammerer DR, Carle R, Schieber A. Characterization of carotenoids and carotenoid esters in red pepper pods (Capsicum annuum L.) by high-performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Rapid Commun Mass Spectrom. 2005;19(18):2617–2628. doi: 10.1002/rcm.2104. [DOI] [PubMed] [Google Scholar]
- Schweiggert U, Mix K, Schieber A, Carle R. An innovative process for the production of spices through immediate thermal treatment of the plant material. Innov Food Sci Emerg Technol. 2005;6(2):143–153. [Google Scholar]
- Schweiggert U, Kurz C, Schieber A, Carle R. Effects of processing and storage on the stability of free and esterified carotenoids of red peppers (Capsicum annuum L.) and hot chilli peppers (Capsicum frutescens L.) Eur Food Res Technol. 2007;225(2):261–270. [Google Scholar]
- Shen Y, Hu Y, Huang K, Yin S, Chen B, Yao S. Solid-phase extraction of carotenoids. J Chromatogr A. 2009;1216(30):5763–5768. doi: 10.1016/j.chroma.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Spiller F, Alves MK, Vieira SM, Carvalho TA, Leite CE, Lunardelli A, Poloni JA, Cunha FQ, de Oliveira JR. Anti-inflammatory effects of red pepper (Capsicum baccatum) on carrageenan- and antigen-induced inflammation. J Pharm Pharm. 2008;60:473–478. doi: 10.1211/jpp.60.4.0010. [DOI] [PubMed] [Google Scholar]
- Strohschein S, Pursch M, Albert K. Hyphenation of high performance liquid chromatography with nuclear magnetic resonance spectroscopy for the characterization of β-carotene isomers employing a C30 stationary phase. J Pharm Biomed Anal. 1999;21(3):669–677. doi: 10.1016/s0731-7085(99)00164-8. [DOI] [PubMed] [Google Scholar]
- Tan B. Analytical and preparative chromatography of tomato paste carotenoids. J Food Sci. 1988;53(3):954–959. [Google Scholar]
- Tang L, Jin T, Zeng X, Wang J-S. Lycopene inhibits the growth of human androgen-independent prostate cancer cells in vitro and in BALB/c Nude Mice. J Nutr. 2005;135(2):287–290. doi: 10.1093/jn/135.2.287. [DOI] [PubMed] [Google Scholar]
- Tepic A, Zekovic Z, Kravic S, Mandic A. Pigment content and fatty acid composition of paprika oleoresins obtained by conventional and supercritical carbon dioxide extraction. CyTA J Food. 2009;7(2):95–102. [Google Scholar]
- Topuz A. A novel approach for color degradation kinetics of paprika as a function of water activity. LWT Food Sci Technol. 2008;41(9):1672–1677. [Google Scholar]
- Topuz A, Ozdemir F. Influences of γ-irradiation and storage on the carotenoids of sun-dried and dehydrated paprika. J Agric Food Chem. 2003;51(17):4972–4977. doi: 10.1021/jf034177z. [DOI] [PubMed] [Google Scholar]
- Topuz A, Ozdemir F. Assessment of carotenoids, capsaicinoids and ascorbic acid composition of some selected pepper cultivars (Capsicum annuum L.) grown in Turkey. J Food Compos Anal. 2007;20:596–602. [Google Scholar]
- Topuz A, Feng H, Kushad M. The effect of drying method and storage on color characteristics of paprika. LWT Food Sci Technol. 2009;42(10):1667–1673. [Google Scholar]
- Topuz A, Dincer C, Ozdemir KS, Feng H, Kushad M. Influence of different drying methods on carotenoids and capsaicinoids of paprika (Cv., Jalapeno) Food Chem. 2011;129(3):860–865. doi: 10.1016/j.foodchem.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Tsao R, Yang R. Lutein in selected Canadian crops and agri-food processing by-products and purification by high-speed counter-current chromatography. J Chromatogr A. 2006;1112(1–2):202–208. doi: 10.1016/j.chroma.2005.09.088. [DOI] [PubMed] [Google Scholar]
- Turner C, King JW, Mathiasson L. Supercritical fluid extraction and chromatography for fat-soluble vitamin analysis. J Chromatogr A. 2001;936(1–2):215–237. doi: 10.1016/s0021-9673(01)01082-2. [DOI] [PubMed] [Google Scholar]
- Uquiche E, del Valle JM, Ortiz J. Supercritical carbon dioxide extraction of red pepper (Capsicum annuum L.) oleoresin. J Food Eng. 2004;65(1):55–66. [Google Scholar]
- van Hof KH, de Boer BCJ, Tijburg LBM, Lucius BRHM, Zijp I, West CE, Hautvast JGAJ, Weststrate JA. Carotenoid bioavailability in humans from tomatoes processed in different ways determined from the carotenoid response in the triglyceride-rich lipoprotein fraction of plasma after a single consumption and in plasma after four days of consumption. J Nutr. 2000;130(5):1189–1196. doi: 10.1093/jn/130.5.1189. [DOI] [PubMed] [Google Scholar]
- Voutilainen S, Nurmi T, Mursu J, Rissanen TH. Carotenoids and cardiovascular health. Am J Clin Nutr. 2006;83(6):1265–1271. doi: 10.1093/ajcn/83.6.1265. [DOI] [PubMed] [Google Scholar]
- Weissenberg M, Schaeffler I, Menagem E, Barzilai M, Levy A. Isocratic non-aqueous reversed-phase high-performance liquid chromatographic separation of capsanthin and capsorubin in red peppers (Capsicum annuum L.), paprika and oleoresin. J Chromatogr A. 1997;757(1–2):89–95. doi: 10.1016/s0021-9673(96)00665-6. [DOI] [PubMed] [Google Scholar]