Abstract
Astaxanthin has been used as a colorant and antioxidant with excellent results, its application and stability in food matrices to human consumption has been little studied. The aim of this work was the incorporation of astaxanthin oleoresin to milks with different fat content, simulating the red-orange color that can impart apricot fruit. For astaxanthin determination by HPLC, a methodology was implemented for its extraction from the food matrix, followed by saponification with KOH. Milk samples were stored (5 ± 2 °C) and stability of color and astaxanthin content were determined by colorimetry and high performance liquid chromatography each 24 h for a week. Pigment degradation followed first-order kinetic with a constant degradation of 0.259 day−1 and 0.104 day−1, in whole and semi-skimmed milk, respectively. Chromaticity coordinates L*, a*, b* for different types of milk showed a low dispersion of their values during the storage time, indicating high stability of astaxanthin within the matrix.
Keywords: Milks, Astaxanthin, HPLC, Colours properties, Storage
Introduction
The current trend in the food industry for human consumption is to favor the use of natural colorants instead of those produced by chemical synthesis, mostly due to the demand made by people from different countries in regards to the toxicological aspects that are inherent and their potential carcinogenic, teratogenic and other behavior disorders, especially in children, which are sufficiently supported by studies conducted by public or private companies related to the health of consumers.
In most countries, the use of food additives (including colorants) is governed by strict regulation. The legislation specifies which colorants may be used, the source(s) of the colorant, the purity of the colorant, to which foods the colorant may be added, and at what level the colorant may be added to a specific food. However, although the list of synthetic colorants is shrinking every day, a few are still being used, which are preferred by industries that add them to their formulations with purposes of cost and stability in foods (Mortensen 2006).
The dairy industry is no exception to this situation, as many of their products need to be partially or fully colored to: a) enhanced color display is required to show sensory quality as attractive as possible, with positive impact on the consumer, b) overlap, cover up or simply work on the tintorial power displayed by the product due to degradative problems occurred in the production process; c) diversify the color palette of their products, making additions that contribute to form pleasing combinations.
If the above is complemented by the addition of natural origin colorant which also has antioxidant properties, then we can say “this additive has a plus in his favor and the consumer will choose it over another similar product which only has color features”.
Several investigations have been made in recent years regarding the use of natural colorants in liquid (milk) and semisolid (yoghurt) dairy products, which includes studying aspects of stability in terms heat, pH and light, as well as coupling effects of the complex structures of lipid – protein interactions, dispersibility and color deficiencies (Mendi et al. 2000; Jing and Giusti 2005; Chattopadhyay et al. 2008; Kang et al. 2010; Aberoumand 2011).
The green unicellular freshwater alga, Haematococcus pluvialis, is a potent producer of astaxanthin (Czygan 1968; Borowitzka et al. 1991; Boussiba and Vonshak 1991; Grung et al. 1992). Astaxanthin or 3,3’-dihydroxy-β-β’-carotene-4-4’-dione, one of many xanthophyll carotenoid pigments, a highly unsaturated molecule that decomposes easily when being exposed to heat, light and oxygen (Christophersen et al. 1991; Jorgensen et al. 1992; Nielsen et al. 1996; Zhao et al. 2006).
Due to its outstanding antioxidant activity astaxanthin has been attributed with extraordinary potential for protecting the organism against a wide range of ailments such as cardiovascular problems, different types of cancer and some diseases of the immunological system (Quan and Turner 2009). Much work has also been focused on the use of natural sources of astaxanthin as an alternative to the synthetic pigment which currently covers most of the world’s markets (Higuera-Ciapara et al. 2006).
Astaxanthin has low aqueous solubility and limited solubility in common dietary oils, mainly in those type of long chain triglycerides, and low bioavailability as other nutritional functional lipid compounds (Chen et al. 2007). The bioavailability is directly related to the solubility of the bioactive compounds, a slow dissolution or solubilization of nutrients lipids functional compounds based on aqueous systems, causes a low absorption rate and consequently a low bioavailability. For this reason, colloidal delivery systems can be used as efficient alternative of low bioavailability problems of water insoluble bioactive compounds (Tan and Nakajima 2005). In these sense Anarjan et al. (2012); Anarjan and Tan (2013) achieved astaxanthin colloidal particles using solvent-diffusion technique in the presence of different food grade surface active compounds and their mixture, could produce the colloidal particles with more desirable physicochemical and biological characteristics. It was confirmed that the incorporation of astaxanthin in colloidal systems could efficiently improve its bioavailability.
There are far few direct applications in food products processed for human consumption. The applications has been directed to feed (Lorenz and Cysewski 2000; Gouveia et al. 2003; Carranco et al. 2003; Forsberg and Guttormsen 2006; Ytrestøyl and Bjerkeng 2007; Choubert et al. 2009) Moreover, human consumption of astaxanthin is in the form nutraceutical capsules, taking into account its antioxidant power (Spolaore et al. 2006).
The state agency by the U.S. Food and Drug Administration (FDA) was the first to analyze the microalgae Haematococcus pluvialis, as source of astaxanthin for human nutrition, complement as BioAstin® marketed in 1999 without objection and was allowed to be sold in the USA as a human dietary supplement (Capelli 2007). BioAstin has recommended amount of natural astaxanthin per serving 12 mg and serving size of 4 capsules. However, there is good data to indicate a single 10-mg dose can persist in the blood for 24 h and a 100-mg dose for 76 h (Coral-Hinostroza et al. 2004). Doses as low as 1 mg can significantly increase blood levels when taken once daily for four weeks (Miyazawa et al. 2011). Astaxanthin’s bioavailability is substantially affected by meal timing and by smoking. A single 48-mg dose was much better absorbed when taken just after a meal than on an empty stomach, and was about 40-percent less bioavailable in subjects who smoked (Okada et al. 2009). It is always best to take Astaxanthin with fatty food, or in combination with a supplement that is also fatty such as fish oil or krill oil. The product is commonly available in 4 mg softgels. One 4 mg dose of natural astaxanthin per day is more than sufficient (Moerck 2012).
The first steps towards the use of astaxanthin in lipid-protein matrices have already been started in dairy foods, liquids and semi-liquids, such as flavored milks and yogurts; for instance: Immunoglobulin-rich milk (Lignell and Inborr 2002); aggregates of astaxanthin from 1 to 20 ppm in dairy products (Lüddecke et al. 2004); dairy products in generally (Rădulescu et al. 2007); milks with different fats contained as well as plain and diet yoghurt (Cerezal et al. 2010).
The aim was directed to make the determinations of the content of astaxanthin in milks with different fats contained, simulating apricot (Prunus armeniaca L.) color, in cold storage during 7 days (T = 5 ± 2 °C), and therefore by high performance liquid chromatography (HPLC), and the measurement of its chromaticity coordinates using the color space CIEL*a*b*. With both measures we study the stability that holds the pigment and through them, determining the color stability of astaxanthin in the different lipid-protein matrices.
Materials and methods
Materials
Skimmed milk (SM) (3.0 g protein, 0.1 g fat, and 4.7 g carbohydrate per 100 mL serving), whole milk (WM) (3.0 g protein, 3.1 g fat, and 5.0 g carbohydrate per 100 mL serving) and semi-skimmed milk (SSM) (3.0 g protein, 1.5 g fat, and 5.0 g carbohydrate per 100 mL serving) were purchased from a local market in the Antofagasta city.
At present, there is no apricot-flavored milk from local market of the Antofagasta city, so we decided to simulate the color of apricot samples from traditional apricot yoghurt with 34.0 g kg−1 of total fat. These samples were purchased in local market of four different companies, taking into account the sample “control” of those having a coloration faithfully reproduce the apricot color, since it is known that the color of apricot yoghurt between different companies varies appreciably.
Oleoresin samples of natural astaxanthin complex, extracted from the biomass of Haematococcus pluvialis by means of CO2 supercritical extraction process with a concentration of 40.0 g kg−1 astaxanthin was kindly delivered by the Company Atacama Bio Natural Products Inc. (Iquique, Chile).
Potassium hydroxide, sodium chloride, dimethyl sulfoxide (DMSO) and citric acid, all PA grade, and acetone, methanol, water, and hexane, all HPLC grade, were purchased from Merck Co. (Chile). Astaxanthin standard 955.0 g kg−1 was obtained from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Refined white sugar was purchased from a local market in the Antofagasta city.
Milks pigmentation with astaxanthin
The traditional colored and flavored cow milk, sold in markets, have added sugar in values ranging between 50 and 80 g kg-1. In cow’s milk, the caseins account for about 80 % of total proteins, ie, 25 to 28 g per liter of milk and precipitated at pH 4.6, which is its isoelectric point (Walsh and Brown 2000). In previous research has been established that astaxanthin oleoresin is stable and well dispersed by reducing of milk pH to 5.2, near the isoelectric point (Cerezal et al. 2010, 2011). Therefore, the development set for 100 mL of milk, regardless of fat content, was the addition of 7.7 g of sugar and 0.27 g of citric acid.
The control yoghurt sample was selected according to color preference established by a panel of untrained judges among fifteen students of Food Engineering Career, University of Antofagasta with product knowledge and sensory evaluation techniques, using a simple selection criteria among the four apricot yogurt samples acquired in the local market. Then, its parameters L* (measures the sample’s lightness), a* measures redness (+a* = red, −a* = green) and b* measures yellowness (+b* = yellow, −b* = blue) were registered with the purpose of coloring milks with astaxanthin to achieve the apricot color desired. Color determinations were done following the operating instructions provided by the manufacturer, Hunter Colour Instruments (Model ColorFlex spectrocolorimeter, Hunterlab Associates Laboratory, Inc., Virginia, USA), the recommended illuminant/observer: D65/10°, using the method of measurement for translucent semi-solid samples.
Storage
Preparation of whole, skimmed and semi-skimmed milk samples.
For each type of milk, 300 mL were added to the quantities of sugar (7.7 %) and citric acid (0.27 %) formulation according to previously established.
They were mixed with an electric mixer at a maximum speed of 1050 ± 25 min−1 during 5 min. Then 22.1 mg astaxanthin oleoresin was added and stirred for 1 min in the same conditions. Considering that the concentration of astaxanthin in the oleoresin is 4.0 %, would be adding 0.884 mg astaxanthin in 300 mL of milk. A portion of 250 mL would be equal to 0.74 mg of astaxanthin.
At the end of mixing (without apparent bubbles), the glass container of 30 mL was filled with twist-off caps, which were previously marked and wrapped in aluminium foil. The storage of the samples was made in a domestic refrigerator 60 L at T = 5 ± 2 º C.
Colorimetric readings and astaxanthin analysis by HPLC were made on a daily basis from 0 to 7 days in triplicate for each of the three types of milk studied.
Color analysis
An analysis of color in time for the three types of milk subject to assessment by observing the variations of L*, a* and b* and color differences (ΔE*) was performed. For the determination of ΔE* following equation was used.
Where L*o, a*o, and b*o were the values of the samples at zero time (this time zero was taken after two hours of concluded process) and L*i, a*i, and b*i were the measured values of each sample in time (each 24-hours period by seven days).
Astaxanthin analysis by HPLC
HPLC was conducted on Hitachi liquid chromatograph model 7100 equipped with three pumps and a UV–Vis detector. The pigment extracted and hydrolyzed was analyzed (20 μL aliquots) using a C18 column (250 × 4.6 mm; 5 μm) at 25 °C. The mobile phase consisted of mixture of solvent A (acetone), solvent B (methanol) and solvent C (water). For the analysis of free astaxanthin and astaxanthin esters, the following elution procedure was used: 60:23:17 A:B:C (V/V) for 2 min; linear gradient at 60:30:10 A:B:C (V/V) for 4 min and then maintained for 2 min. The mobile phase was pumped at a flow rate of 1 mL min−1 and the response was detected at 474 nm. The identification of astaxanthin was accomplished by comparison of retention times with reference standards and co-chromatography with added standards. Freshly prepared standard solutions (1 to 40 ppm of astaxanthin) were injected into the HPLC system for producing the standard curve of astaxanthin. Calculation of the astaxanthin concentration in the samples was done using the standard curve.
Sample preparation for hydrolysis
Known amounts of milk pigmented with astaxanthin oleoresin (4 g) were mixed with 2 mL DMSO and 3 mL of acetone, 2 mL brine solution and 2 mL n-hexane in a 15 mL conical tube. The mixture was shaked with vortex for 15 s and the tube was centrifuged at 2147 × g for 3 min. The hexane layer containing the carotenoids mixture was removed with a pipette to a clean tube containing 1.0 g of anhydrous sodium sulfate. Extractions were repeated with 2 mL hexane until the extract was clear. The hexane layer was transferred to a 50 mL 24/40 flask, the sodium sulfate crystals were washed with a small amount of hexane until all the carotenoids were recovered and the solvent was eliminated at 40 °C to dryness with a rotary evaporator. It was re-dissolved into 10 mL of acetone. The acetone extract was adjusted so that the absorbency at 474 nm ranged between 0.8–1.2, required for good accuracy in measurement of spectrophotometer, and 3 mL of aliquots of this extract were used for alkaline hydrolysis. Analysis was carried out in triplicate and recovery test was done for each milk sample.
Alkaline hydrolysis
Three milliliters of the extract was transferred to a test tube to eliminate the acetone using rotary evaporator at 40 º C and contents were dissolved in 2 mL methanol. Alkaline hydrolysis was carried out using the modified method reported by Cerezal et al. (2010); Yuan and Chen (1999). Following, 0.1 mL KOH 10 g kg−1 was added and mixed in the vortex for 1 min. Astaxanthin esters were hydrolyzed at room temperature under N2 in darkness for 18.0 h. The methanol phase was extracted with n-hexane and the organic phase was washed with brine solution until alkaline-free at pH = 7. The hexane extract was dried with sodium sulfate anhydrous, and the solvent was removed by flushing nitrogen gas. It was then re-dissolved into 3 mL acetone and analyzed by HPLC.
Kinetics parameters of astaxanthin degradation
The degradation of astaxanthin was found to follow a first-order kinetic reaction (Niamnuy et al. 2008; Raj 2010). Suppose that at the beginning (0 days) the concentration of astaxanthin was C0 and for any time the amount of astaxanthin was C, according to: C = C0 exp (−kt); the logarithms were taken on both sides of equation ln C = ln C0 – kt. The equation was rearranged as ln (C/C0) = − kt. A plot of ln (C/C0) versus t was constructed to determine k values and correlation coefficients.
Statistical analysis
Experimental results were expressed as mean of triplicate + standard deviation. The statistical evaluation of the results was performed using common statistical and the spreadsheet software Microsoft Office Excel 2003. Comparison of variance was carried out using one-way analysis of variance (ANOVA) followed up by Duncan’s Multiple Range tests for the determination of statistically different groups. Differences were considered significant when p < 0.05.
Results and discussions
Milk’s pigmentation with astaxanthin
In order to match color of natural apricot yoghurt acquired in the market in the three types of milk, it was necessary to add 7.2 ± 0.1 mg astaxanthin oleoresin in 100 mL of milk. Because the amount < 75 mg kg−1, the effect on texture and viscosity are negligible.
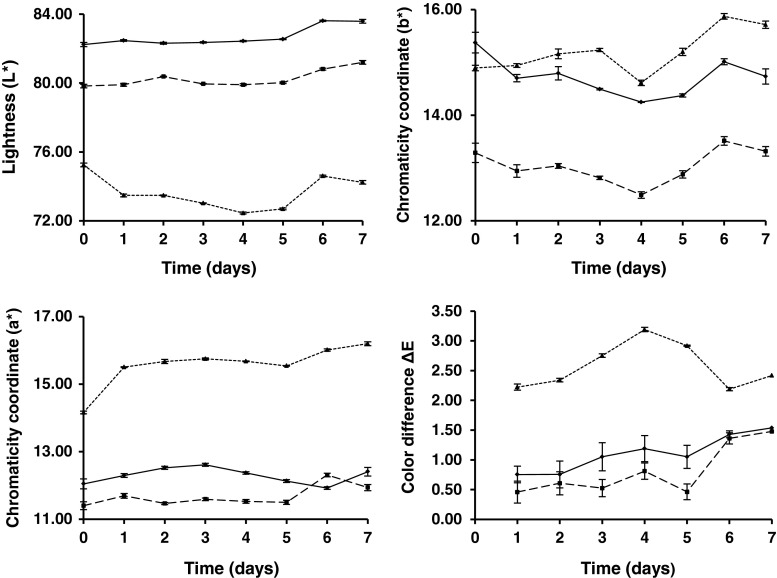
Parameter values (L*, a* and b*) for milk pigmented with astaxanthin oleoresin as shown in Fig. 1. These values of L*, a* and b* to zero time, determined by instrumental evaluation with spectrocolorimeter in SM, SSM and WM, compared with the control sample values of yoghurt L* = 81.40 ± 0.03; a* = 11.81 ± 0.03 and b* = 16.89 ± 0.01, were statistically significant different (p < 0.05) between all for all parameters; however, in SM and SSM, ΔE* values were more than 3.0 that can be easily detected by the naked human eye (Hong et al. 2004). The only product that met this condition was WM and this difference with the yoghurt apricot control sample was not appreciated at first glance by the human eye ΔE* = 1.77.
Fig. 1.
Changes in instrumental color values of skimmed ( ), semi-skimmed
), semi-skimmed  ) and whole (
) and whole ( ) milk, pigmented with astaxanthin oleoresin to simulate apricot color (n = 3)
) milk, pigmented with astaxanthin oleoresin to simulate apricot color (n = 3)
Despite these differences in the chromaticity coordinates between SM and SSM with control yogurt, determined by measuring instrumental with the spectrocolorimeter; where ΔE* = 6.88 and ΔE* = 3.95, respectively, the judges understood that color answered to the desired expectations and taken as definitive criteria regarding the choice of color.
Color stability of the milks during storage
Figure 1 show the variations of the color coordinates L*, a* and b* as well as ΔE* obtained during the storage period of 7 days for WM, SSM and SM with added sugar and citric acid to adjust to pH 5.2.
The WM sample had the highest values of L* and intermediate values of a* and b* (Fig. 1), compared with SSM and SM. Moreover, it was observed that the values of L* were stable from 0 to 5 days for WM and SSM and increased slightly to 7 days. However, the SM had a slightly decreasing values of L* to 5 days, increasing at 6 days and falling relatively little down until 7 days. Despite the above changes, the movements of the values of L* was not observed to have a drastic behavior and ΔL* between 7 days and 0 days did not exceed 1.38 for all 3 types of milk. Apparently, the fat content of milk encouraged a decrease in the value of L* between them and when this content was extremely tiny, as is the case of SM, not only the L* was smaller, but there was a slight destabilization in the values of L* to the end of 7 days storage.
Something similar to what happened in Fig. 1 with L* occurred for chromatic coordinate a* for the two types of higher fat milk, WM and SSM, which showed very slight variations, practically constant from 0 to 7 days. In addition, WM decreased very little in 6 days and increase at 7 days, a behavior that happened in reverse in SSM, which increased slightly at 6 days and decreased at 7 days. Moreover, SM increased significantly at 1 day, remains constant until 5 days and then continues on a slight rise at 6 to 7 days. Regardless of these changes for WM and SSM movements of the values of a* no dramatic behavior and that the Δa* between 7 days and 0 days did not exceed 0.54, as opposed to SM where the Δa* between the beginning and the end of storage is 2.04.
The SM, with lower lipid content had a better benefit of the component a* that is the chromatic coordinate which provides the red color, as opposite to the other two milks, WM and SSM, which were slightly affected by the higher fat content, a situation that needs to be corroborated by further studies of spatial networks between the astaxanthin molecule, its isomers and correspondence between different types of fatty acids present. For longer than 7 days storage should be performed a more detailed study of the stability of the isomers of astaxanthin.
The behavior of the chromatic coordinate b* (Fig. 1), indicated that the highest value at 0 days belonged to WM followed closely the SM and finally the SSM. Thereafter, the values of b* (in the yellow zone to be +) of the three milks had slight fluctuations throughout the storage time (7 days) however, these changes were not drastic behavior and Δb* between 7 days and 0 days to WM, SSM and SM were: 0.62, 0.03 and 0.82, respectively. We assume that the apparently lower lipid content also benefits the component b* since at the end of storage at 7 days, the SM is the one with the highest value in 15.71. Just as it happened for a*, this situation needs to be confirmed.
The ΔE* values (Fig. 1) were very low for WM and SSM, with ΔE* = 0.73 and ΔE* = 0.46, respectively, and despite their variations and upward trends ΔE* (1.53) did not exceed for 7 days. This trend did not remain the same for SM, which started with a value of ΔE* = 2.22 at 1 day and concluded with ΔE* = 2.42 at 7 days. However, regardless of the high fluctuations of SM in that period, the difference between 7 days with 1 day was only 0.2 whereas, this difference was 0.8 for WM and 1.02 for SSM. It is well known that ∆E* values less than 3.0 cannot be easily detected by the naked human eye (Hong et al. 2004).
The color achieved, because of the use astaxanthin pigment in this study, suggests that this colorant from natural sources, can be employed in liquid dairy products (milk) and semi-liquids (yoghurt), just as similar applications have been made. This may be due to the protection afforded by the lipid-protein matrix of the milk.
The high stability of astaxanthin pigment within the protein-lipid matrix of milks found in this study contrasts with the statement as a generality for others authors, on storage studies, who pointed out that the colors from natural sources tend to lose tinctorial strength or disappear with time (Krammerer et al. 2007) which means that a greater weight of plant material would be needed directly for use or for extraction of the natural dye than of the synthetic dye in order to obtain the same depth of colour. Incorporation of natural colorants to food systems faces different challenges such as their relatively low stability to processing and storage conditions, and the presence of undesirable odor or flavor characteristics (Wallace and Giusti 2008).
All this indicates that astaxanthin maintained stability throughout the refrigerated storage time for three types of milk without significant changes in any of the three color coordinates. Values of ΔE* ≥ 5.0 were not obtained at any moment during storage. This is a threshold value used by other researchers to indicate the onset of instability (Obón et al. 2009).
Astaxanthin analysis
Application of natural pigments in food products is growing up due to its obvious beneficial properties. Carotenoids have been used as food coloring additives in processed food and use of HPLC techniques to evaluate their color stability in matrix foods is scarce. There are a few reports about recovery yields when HPLC analysis is used to evaluate carotenoids. HPLC analysis of lutein incorporated into frankfurter-type sausages showed that net amounts recovered in the micellar phase was 290–340 g L−1 and 610–680 g L−1 for low-fat and high-fat content, respectively (Granado-Lorencio et al. 2010).
A multi-method by HPLC was developed for the determination of the carotenoid food additives (CFA) norbixin, bixin, capsanthin, lutein, canthaxanthin, β-apo-8′-carotenal, β-apo-8′-carotenoic acid ethyl ester, β-carotene, and lycopene in processed food using an improved accelerated solvent extraction method (Breithaupt 2004). Average recoveries of all the analytes ranged from 910 to 996 g L−1 excepted for norbixin with a value of 674 g L−1. In this study, astaxanthin recovery values of 525 ± 9.0, 505 ± 6.0 and 501 ± 1.0 g L−1 for SSM, WM and SM were obtained respectively. Recovery efficiency depends on many factors, some of them are the composition of the matrix and the sample preparation method (Kimura and Rodriguez-Amaya 1999), in this research the low recovery can be due to the complex matrix composition (lipid – protein interactions) and the laborious preparation (sample preparation for alkalyne hydrolysis) and clean processes for milky products (addition of additives in the milks: sucrose, citric acid and astaxanthin, as well as product handling and process aspects such as: mixing, filling and sealing of the container).
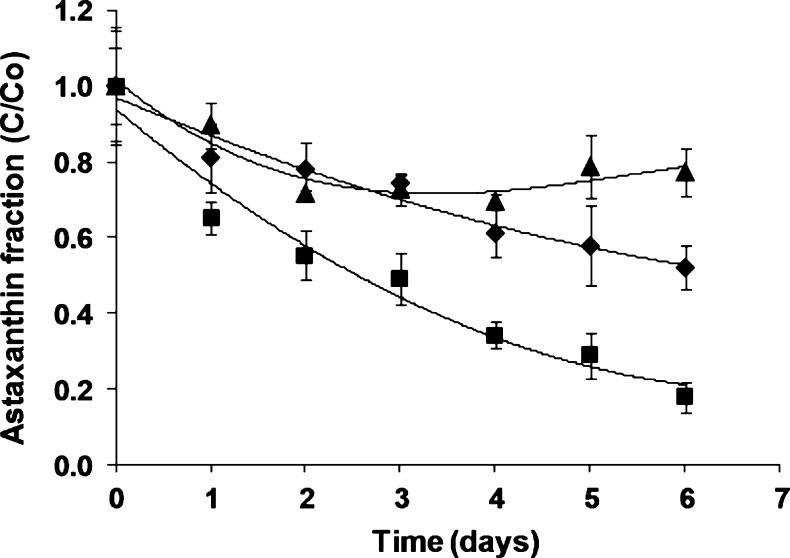
Changes of astaxanthin concentration during storage for pigmented milk with different fat-content are showed in Fig. 2. Pigment retention rates were inversely proportional to the fat amount and there are significant differences in the retained pigment fraction (p < 0.05) with values 770, 520 and 170 g L−1 for SM, SSM and WM, respectively at 6 days of storage. The highest astaxanthin reduction was visible at 24 h for all samples, for SM, SSM and WM of 10 %, 18 % and 35 %, respectively but remains constant for SM for 6 days. This fact could be explained due hydrophobic character of astaxanthin esters and fat acids present in oleoresin added to milk samples; the oily matrix could make pigment diffusion more difficult from that lipid phase for the formation of micelles (Pérez-Gálvez and Mínguez-Mosquera 2005; Anarjan et al. 2012). In the case of SM where the astaxanthin can interact more easily with milk proteins to produce stable micelles it remains in the product for more time. Also, interaction with milk proteins stabilizes carotenoids probably due to free radical scavenging by sulfhydryl and nonsulfhydryl amino acids (Ribeiro et al. 2003).
Fig. 2.
Astaxanthin retention during storage for whole (■), semi-skimmed (♦) and skimmed (▲) pigmented milk. Values plotted are the means of triplicate ± standard deviations
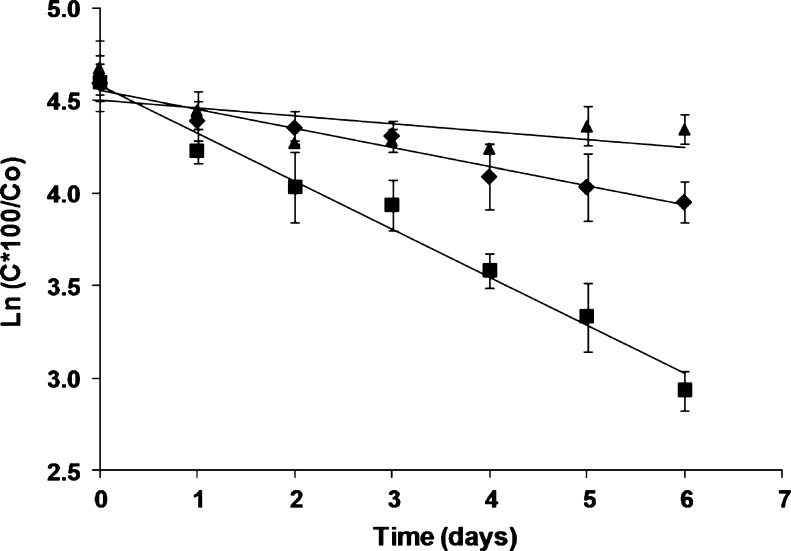
It has been described in multiple works that the degradation of natural pigments in nourishing matrices follows a first-order kinetic reaction (Pacheco-Palencia et al. 2007; Wang and Xu 2007). Figure 3 shows the degradation trend of astaxanthin in milk products with different fat-content applying a model for first-order kinetics, as it is possible to observe, pigment degradation followed first-order kinetic with a constant degradation of 0.259 day−1 and 0.104 day−1 and a coefficient of determination of R2 = 0.979 and R2 = 0.960 in WM and SSM respectively and their corresponding half-life times calculated from straight-line slope for each sample were 2.7 and 6.7 days. There is a proportional relation between astaxanthin degradation rates and fat-content in the milks. However, the linear form did not provide good fit for all data, as in the case of SM.
Fig. 3.
First-order kinetic reaction for astaxanthin during storage for whole (■), semi-skimmed (♦) and skimmed (▲) pigmented milk. Values plotted are the means of triplicate ± standard deviations
Conclusions
From the results of this study, it is concluded that it is possible to use astaxanthin oleoresin for simulating the apricot color in skimmed, semi-skimmed and whole milk in the cold storage time during 7 days without significant changes in any of the three color coordinates L*, a* and b*; Values of ΔE* ≥ 5.0 were not obtained at any moment in the storage. Moreover, pigment retention rates were inversely proportional to the fat amount and there are significant differences in the retained pigment fraction for skimmed, semi-skimmed and whole milks respectively. pigment degradation followed first-order kinetic for whole and semi-skimmed milk, but did not have the same behavior for skimmed milk.
Acknowledgments
This study was supported by the National Commission for Scientific and Technological Research (CONICYT) of Chile, through the financing of Convention Attraction of Human Capital Advanced Foreigner in his mode of short stay (MEC) (Folio No. 80095006; years 2010–2011) as well as Project Codigue N°. 2009–091 (years 2010–2011) belonging to the bilateral program of scientific collaboration between the National Council of Science and Technology (CONACYT) of Mexico and CONICYT of Chile. Similarly the University of Antofagasta through the Food Department and Research Department provided all reagents and equipment used. The Company Atacama Bionatural Products Inc. also provided pigment astaxanthin used in this study.
References
- Aberoumand A. A review article on edible pigments properties and sources as natural biocolorants in foodstuff and food industry. World J Dairy Food Sci. 2011;6:71–78. [Google Scholar]
- Anarjan N, Tan CP, Nehdi IA, Ling TC. Colloidal astaxanthin: preparation, characterisation and bioavailability evaluation. Food Chem. 2012;135:1303–1309. doi: 10.1016/j.foodchem.2012.05.091. [DOI] [PubMed] [Google Scholar]
- Anarjan N, Tan CP. Developing a three component stabilizer system for producing astaxanthin nanodispersions. Food Hydrocolloid. 2013;30:437–447. doi: 10.1016/j.foodhyd.2012.07.002. [DOI] [Google Scholar]
- Borowitzka MA, Huisman JM, Osborn A. Culture of the astaxanthin-producing green alga Haematococcus pluvialis: I. Effect of nutrients on growth and cell type. J Appl Phycol. 1991;3:295–304. doi: 10.1007/BF02392882. [DOI] [Google Scholar]
- Boussiba S, Vonshak A. Astaxanthin accumulation in the green alga Haematococcus pluvialis. Plant Cell Physiol. 1991;32:1077–1082. [Google Scholar]
- Breithaupt D. Simultaneous HPLC determination of carotenoids used as food coloring additives: applicability of accelerated solvent extraction. Food Chem. 2004;86:449–456. doi: 10.1016/j.foodchem.2003.10.027. [DOI] [Google Scholar]
- Capelli B (2007) ASTAXANTHIN: Natural Astaxanthin; King of the Carotenoids. Published by Cyanotech Corporation, Kailua-Kona, HI. USA
- Carranco ME, Calvo C, Arellano L, Pérez-Gil F, Ávila E, Fuente B. Inserting Heads Shrimp Flour (Penaeus sp.) in Rations to Laying Hens. Effect of Red Pigment Concentration on the yolk and Quality Egg. Interciencia. 2003;28:328–333. [Google Scholar]
- Cerezal P, Barragán BE, Palma J, Ortiz C (2010) Yogurt Pigmentation with Astaxanthin and Determination of its Stability by Liquid Chromatography High Performance. IV International Congress on Food Science and Biotechnology in Developing Countries. Nov. 29th – Dec, 3rd - Veracruz, Mexico
- Cerezal P, Barragán BE, Palma J, Ortiz C (2011) Use of Astaxanthin for Pigmentation of Milks and its Stability by a Short Period of Storage. VIII Iberoamerican Congress in Food Engineering (CIBIA-8). 23 to 26 October; Lima, Peru
- Chattopadhyay P, Chatterjee S, Sen SK. Biotechnological potential of natural food grade biocolorants. Afr J Biotechnol. 2008;7:2972–2985. [Google Scholar]
- Chen X, Chen R, Guo Z, Li C, Li P. The preparation and stability of the inclusion complex of astaxanthin with b-cyclodextrin. Food Chem. 2007;101:1580–1584. doi: 10.1016/j.foodchem.2006.04.020. [DOI] [Google Scholar]
- Choubert G, Cravedi JP, Laurentie M. Effect of alternate distribution of astaxanthin on rainbow trout (Oncorhynchus mykiss) muscle pigmentation. Aquaculture. 2009;286:100–104. doi: 10.1016/j.aquaculture.2008.09.001. [DOI] [Google Scholar]
- Christophersen AG, Jun H, Jorgensen K, Skibsted LH. Photobleaching of astaxanthin and canthaxanthin—quantum-yields dependence of solvent, temperature, and wavelength of irradiation in relation to packaging and storage of carotenoid pigmented salmonoids. Z Lebensm Unters F A. 1991;192:433–439. doi: 10.1007/BF01193143. [DOI] [PubMed] [Google Scholar]
- Coral-Hinostroza GN, Ytrestoyl T, Ruyter B, Bjerkeng B. Plasma appearance of unesterified astaxantin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3’R/S isomers of astaxanthin fatty acyl diesters. Comp Biochem Phys C. 2004;139:99–110. doi: 10.1016/j.cbpc.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Czygan FC. Zum Vorkommen von Crustaxanthin (3,3V,4,4V-Tetraoxi-h-carotin) und Phoenicopteron (4-Oxo-acarotin) in Aplanosporen von Haematococcus pluvialis Flotow em. Wille. Flora A. 1968;159:339–345. [Google Scholar]
- Forsberg OI, Guttormsen AG. A pigmentation model for farmed Atlantic salmon: nonlinear regression analysis of published experimental data. Aquaculture. 2006;253:415–420. doi: 10.1016/j.aquaculture.2005.09.004. [DOI] [Google Scholar]
- Gouveia L, Rema P, Pereira O, Empis J. Colouring ornamental fish (Cyprinus carpio and Carassius auratus) with microalgal biomass. Aquacult Nutr. 2003;9:123–129. doi: 10.1046/j.1365-2095.2003.00233.x. [DOI] [Google Scholar]
- Granado-Lorencio F, López-López I, Herrero-Barbudo C, Blanco-Navarro I, Cofrades S, Pérez-Sacristán B, Delgado-Pando G, Jiménez-Colmenero F. Lutein-enriched frankfurter-type products: physicochemical characteristics and lutein in vitro bioaccessibility. Food Chem. 2010;120:741–748. doi: 10.1016/j.foodchem.2009.11.005. [DOI] [Google Scholar]
- Grung M, D’Souza FML, Borowitzka M, Liaaen-Jensen S. Algal carotenoids 51. Secondary carotenoids 2. Haematococcus pluvialis aplanospores as a source of (3S, 3’S)- astaxanthin esters. J Appl Phycol. 1992;4:165–171. doi: 10.1007/BF02442465. [DOI] [Google Scholar]
- Higuera-Ciapara I, Valenzuela LF, Goycoolea FM. Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr. 2006;46:185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- Hong SI, Han JH, Krochta JM. Optical and surface properties of whey protein isolate coatings on plastic films as influenced by substrate, protein concentration, and plasticizer type. J Appl Polym Sci. 2004;92:335–343. doi: 10.1002/app.20007. [DOI] [Google Scholar]
- Jing P, Giusti M. Characterization of Anthocyanin-Rich Waste from Purple Corncobs (Zea mays L.) and Its Application to Color Milk. J Agric Food Chem. 2005;53:8775–8781. doi: 10.1021/jf051247o. [DOI] [PubMed] [Google Scholar]
- Jorgensen K, Stapelfeldt H, Skibsted LH. Fluorescence of carotenoids. Effect of oxygenation and cis/trans isomerization. Chem Phys Lett. 1992;190:514–519. doi: 10.1016/0009-2614(92)85183-B. [DOI] [Google Scholar]
- Kang EJ, Campbell RE, Bastian E, Drake MA. Annatto usage and bleaching in dairy foods. J Dairy Sci. 2010;93:3891–3901. doi: 10.3168/jds.2010-3190. [DOI] [PubMed] [Google Scholar]
- Kimura M, Rodriguez-Amaya DB. Source for errors in the quantitative analysis of food carotenoids by HPLC. Arch Latinoam Nutr. 1999;49:58–66. [PubMed] [Google Scholar]
- Krammerer D, Schillmoller S, Maier O, Schieber A, Reinhold C. Colour stability of canned strawberries using black carrot and elderberry juice concentrates as natural colorants. Eur Food Res Technol. 2007;224:667–669. doi: 10.1007/s00217-006-0356-3. [DOI] [Google Scholar]
- Lignell A, Inborr J. Immunoglobulin-rich milk, production and use thereof. US Patent. 2002;6475547:B1. [Google Scholar]
- Lorenz RT, Cysewski GR. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000;18:160–167. doi: 10.1016/S0167-7799(00)01433-5. [DOI] [PubMed] [Google Scholar]
- Lüddecke E, Auweter H, Schweikert L. Use of carotenoid aggregates as colorants. US Patent. 2004;6827941:B1. [Google Scholar]
- Mendi SD, Peter B, Kamga T. The effects of pH and heat treatments processing on the stability of natural food colours used in dairy products. J Food Tech Afr. 2000;5:59–61. [Google Scholar]
- Miyazawa T, Nakagawa K, Kimura F, Satoh A, Miyazawa T. Plasma carotenoid concentrations before and after supplementation with astaxanthin in middle-aged and senior subjects. Biosci Biotechnol Biochem. 2011;75:1856–1858. doi: 10.1271/bbb.110368. [DOI] [PubMed] [Google Scholar]
- Moerck RE (2012) Valensa Introduces New ‘Body Ready’ Astaxanthin Formulation for Optimal Absorption www.ereleases.com/pr/valensa; accessed on 12.10.2012
- Mortensen A. Carotenoids and other pigments as natural Colorants. Pure Appl Chem. 2006;78:1477–1491. doi: 10.1351/pac200678081477. [DOI] [Google Scholar]
- Niamnuy C, Devahastin S, Soponronnarit S, Raghavan GSV. Kinetics of astaxanthin degradation and color changes of dried shrimp during storage. J Food Eng. 2008;87:591–600. doi: 10.1016/j.jfoodeng.2008.01.013. [DOI] [Google Scholar]
- Nielsen BR, Mortensen A, Jorgensen K, Skibsted LH. Singlet versus triplet reactivity in photodegradation of C40 carotenoids. J Agric Food Chem. 1996;44:2106–2113. doi: 10.1021/jf9508007. [DOI] [Google Scholar]
- Obón JM, Castellar MR, Alacid M, Fernández-López JA. Production of a red–purple food colorant from Opuntia stricta fruits by spray drying and its application in food model systems. J Food Eng. 2009;90:471–479. doi: 10.1016/j.jfoodeng.2008.07.013. [DOI] [Google Scholar]
- Okada Y, Ishikura M, Maoka T. Bioavailability of astaxanthin in Haematococcus algal extract: the effects of timing of diet and smoking habits. Biosci Biotechnol Biochem. 2009;73:1928–1932. doi: 10.1271/bbb.90078. [DOI] [PubMed] [Google Scholar]
- Pacheco-Palencia LA, Hawken P, Talcott ST. Juice matrix composition and ascorbic acid fortification effects on the phytochemical, antioxidant and pigment stability of acai (Euterpe oleracea Mart.) Food Chem. 2007;105:28–35. doi: 10.1016/j.foodchem.2007.03.027. [DOI] [Google Scholar]
- Pérez-Gálvez A, Mínguez-Mosquera MI. Esterification of xanthophylls and its effect on chemical behavior and bioavailability of carotenoids in the human. Nutr Res. 2005;25:631–640. doi: 10.1016/j.nutres.2005.07.002. [DOI] [Google Scholar]
- Quan C, Turner C. Extraction of astaxanthin from shrimp waste using pressurized hot ethanol. Chromatographia. 2009;70:247–251. doi: 10.1365/s10337-009-1113-0. [DOI] [Google Scholar]
- Rădulescu G, Mocanu E, Caraene G, Ciuhu I, Mănăilă N, Săvoiu G, Eremia CM, Dumitru RI. Studies concerning the obtainment of astaxanthin, an important natural pigment used in cosmetic, food and pharmaceutical industries. Lucrari Stiintifice: Zootehnie si Biotehnologii. 2007;40:158–162. [Google Scholar]
- Raj G. Chemical kinetics. 8. Meerut: Krishna Prakashan Media P Ltd; GOEL Publishing House; 2010. [Google Scholar]
- Ribeiro HS, Ax K, Schubert H. Stability of lycopene emulsions in food systems. J Food Sci. 2003;68:2730–2734. doi: 10.1111/j.1365-2621.2003.tb05796.x. [DOI] [Google Scholar]
- Spolaore P, Joannis-Cassan C, Duran E, Isambert A. Commercial applications of microalgae. J Biosci Bioeng. 2006;101:201–211. doi: 10.1263/jbb.101.87. [DOI] [PubMed] [Google Scholar]
- Tan CP, Nakajima M. β-Carotene nanodispersions: preparation, characterization and stability evaluation. Food Chem. 2005;92:661–671. doi: 10.1016/j.foodchem.2004.08.044. [DOI] [Google Scholar]
- Wallace TC, Giusti MM. Determination of color, pigment, and phenolic stability in yogurt systems colored with nonacylated anthocyanins from Berberis boliviana L. as compared to other natural/synthetic colorants. J Food Sci. 2008;73:241–248. doi: 10.1111/j.1750-3841.2008.00706.x. [DOI] [PubMed] [Google Scholar]
- Walsh MK, Brown RJ. Use of amino acid analysis for estimating the individual concentrations of proteins in mixtures. J Chromatogr A. 2000;891:355–360. doi: 10.1016/S0021-9673(00)00649-X. [DOI] [PubMed] [Google Scholar]
- Wang WD, Xu SY. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J Food Eng. 2007;82:271–275. doi: 10.1016/j.jfoodeng.2007.01.018. [DOI] [Google Scholar]
- Ytrestøyl TK, Bjerkeng B. Dose response in uptake and deposition of intraperitoneally administered astaxanthin in Atlantic salmon (Salmo salar L.) and Atlantic cod (Gadus morhua L.) Aquaculture. 2007;263:179–191. doi: 10.1016/j.aquaculture.2006.10.021. [DOI] [Google Scholar]
- Yuan JP, Chen F. Hydrolysis kinetics of astaxanthin esters and stability of astaxanthin of Haematococcus pluvialis during saponification. J Agric Food Chem. 1999;47:31–35. doi: 10.1021/jf980465x. [DOI] [PubMed] [Google Scholar]
- Zhao L, Zhao G, Chen F, Wang Z, Wu J, Hu X. Different effects of microwave and ultrasound on the stability of (all-E)-astaxanthin. J Agric Food Chem. 2006;54:8346–8351. doi: 10.1021/jf061876d. [DOI] [PubMed] [Google Scholar]