Abstract
In this work, chemical and biological characteristics of two types of Thai fermented shrimp paste, Kapi Ta Dam and Kapi Ta Deang, at different fermentation periods and their raw materials were investigated. Kapi had low water activity and high proteins with high glutamic acid and lysine. Both Kapis, which had different sources, showed similar characteristics. The number of lactic acid bacteria in the products increased during the early stages of fermentation. Free α-amino acid contents in the products increased with the fermentation time. The water extracts from Kapi products showed strong antioxidative activities against ABTS+ radical, and ACE inhibitory activity but they did not exhibit antimicrobial activity against Staphylococcus aureus, Bacillus cereus, Escherichia coli and Salmonella Typhimurium. Biological activities in Kapi could be developed by fermentation process, enzymatic hydrolysis of proteins and non-enzymatic browning reactions. Kapi could, thus, serve as a potential source of natural bioactive substances.
Keywords: Fermented shrimp paste, Antioxidative activity, Antimicrobial activity, Angiotensin I-converting enzyme inhibitory activity
Introduction
Fermentation plays an important role in food markets. Fermented foods represent, on an average, one-third of total food consumption (Tamang and Kailaspathy 2010). They have unique flavors, aromas and textures characteristics of which are primarily due to protein and lipid degradation by autolytic and bacterial enzymes during fermentation period (Cha and Cadwallader 1995). Furthermore, fermentation process can greatly enhance the nutritional and functional properties of a food as well as extend its shelf-life by salt addition and acid and ethanol production. The activity of proteolytic enzymes present in the raw material of fermented products is considered to be a primary factor of protein hydrolysis. The proteolytic products such as amino acids, peptides and their decomposition products are formed during fermentation process (Kiesvaara 1975). Fermented food products are, therefore, good sources of peptides and amino acids. Many peptides released from food proteins exhibit biological activities, such as antimicrobial properties, blood-pressure lowering effects, cholesterol-lowering ability, antithrombotic and antioxidative activities (Hartmann and Meisel 2007).
Thai traditional fermented shrimp paste is commonly known as Kapi. It is widely used as a condiment in many Thai cuisines, in particular chili pastes, curry pastes, and sauces. Kapi is traditionally prepared from shrimp or mysid shrimp mixed with salt at the ratio of 3–5:1 and then sun-dried to decrease the moisture content, and finally it is homogenized to obtain a homogenous product (paste). During ripening period, it is compacted and allowed to ferment for 2–6 months to develop desirable and unique flavors and aromas. In Thailand, Kapi can be classified as two distinct types: Kapi Ta Dam (black paste) and Kapi Ta Deang (red paste) obtained from mangrove canals and seagrass beds, respectively. Only one species of mysid shrimp, Mesopodopsis orientalis, found in mangrove canals of the Andaman Sea is used solely for production of Kapi Ta Dam. In contrast, three different species of shrimps from the genus Acetes; Acetes indicus, Acetes japonicas, and Acetes crythraeus, found in seagrass beds in the Andaman Sea are used for production of Kapi Ta Deang (Pengchumrus and Upanoi 2005).
Biological activities of enzymatically hydrolyzed shrimp products, shrimp waste and fermented shrimp products have been previously reported. Binsan et al. (2008) reported antioxidative activity in Mungoong, an extract paste from the cephalothorax of white shrimp (Litopenaeus vannamei), towards 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonate) (ABTS+) and 2,2-diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl (DPPH) radicals as well as ferric reducing activity power (FRAP). Furthermore, they found that distilled water was the best potential solvent extracting antioxidant compounds from Mungoong among all solvents tested. The extract of a Philippine salt-fermented shrimp paste by 85 % ethanol exhibited high radical scavenging activity against DPPH radical and peroxidation of methyl linoleate (Peralta et al. 2005). Moreover, Peralta et al. (2008) found that the antioxidative activities of 80 % ethanol extract derived from the Philippine salt-fermented shrimp paste against DPPH radical, hydrogen peroxide and lipid peroxidation were increased through the prolonged fermentation time. Faithong et al. (2010) reported antioxidative activity of Thai traditional fermented shrimp and krill products including Jaloo, Koong-Som and Kapi against DPPH and ABTS+ radicals and FRAP. They found that a water-soluble fraction from Kapi showed the strongest antioxidative activity amongst soluble fractions from all products. In addition, a cholesterol lowering effect of salted and fermented small shrimp (Acetes japonicas) extract was also observed in high cholesterol-diet induced hypercholesterolemic animal models (Seok et al. 2004). Although, these investigations revealed antioxidative activity in the fermented shrimp paste products but other biological activities, such as antimicrobial and angiotensin I-converting enzyme (ACE) inhibitory activities, have not been previously reported. Therefore, this work aimed at investigating in vitro biological activities, including antioxidative, antimicrobial and ACE inhibitory activities, of two Kapi types of Thailand. Chemical and microbiological characteristics including biological activities of both products during the prolonged fermentation period were also monitored.
Materials and methods
Materials
Raw materials of Kapi Ta Dam (M. orientalis, referred to B) and Kapi Ta Deang (Acetes sp., referred to R) were harvested during October to November 2009. The initial products of Kapi Ta Dam and Kapi Ta Deang were 3-d and 7-d fermented raw materials (referred to Kp-B0 and Kp-R0, respectively). The raw materials and initial products were kindly provided by three local factories at Khao Pra Thong, Kuraburi, PhangNga, Thailand. The fermented shrimp paste products were obtained by further ferment Kp-B0 and Kp-R0 by incubation at 30 °C for 2, 4, and 6 months (referred to Kp-B2/4/6 and Kp-R2/4/6). All samples were stored at −20 °C until required.
Cultures and reagents
Gram-positive bacteria; Staphylococcus aureus ATCC25923 and Bacillus cereus TISTR687, and Gram-negative bacteria; Escherichia coli ATCC25922 and Salmonella Typhimurium DSM20017, were obtained from the Department of Microbiology, Faculty of Science, KMUTT and the Microbiological Resources Centre, Thailand Institute of Scientific and Technological Research (TISTR), respectively. All bacteria were maintained on a nutrient agar (NA) slants in glycerol at 4 °C. Chemicals and reagents used in antioxidative assays were 2,2-diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), ascorbic acid, absolute ethanol, potassium ferric cyanide, and ferric chloride, were analytical grade purchased from Sigma-Aldrich (St. Louis, MO, USA) and Fischer Scientific (Loughborough, UK). Amino acid standards were analytical grade and purchased from Fluka Biochemika (Tokyo, Japan). Other chemicals and reagents were analytical grade.
Analysis of chemical composition
Moisture, fat, protein, ash, and fiber contents and pH of the raw materials, the initial products, and the fermented products at month 6 were analyzed by the standard methods (AOAC 1995). The total carbohydrate content in the samples was determined by difference. Salt content of the samples were determined by Volhard method (AOAC 1995). Water activity of each sample was measured at 25 °C using a water activity analyzer (Aw SPRINT TH-500, Kerres GmbH, Backnang, Germany).
Microbiological analysis
Numbers of microorganisms present in the raw materials, the initial products, and the fermented products at months 2, 4, and 6 were determined by the total plate count method using pour plate technique on plate count agars (PCA; Himedia Laboratories, Mumbai, India). Enumeration of lactic acid bacteria (LAB) in the samples was conducted using De Man Rogosa and Sharpe (MRS) agar (Merck, Darmstadt, Germany) containing 0.5 % (w/v) CaCO3. One gram of each sample was aseptically mixed with 9-mL sterile normal saline solution (0.85 % w/v, NaCl). Appropriate ten-fold dilutions of each sample were prepared in sterile normal saline before transferring to a sterile petri dish overlaid with the appropriate media. The incubation was carried out at 30 °C for 48 h for total plate count and at 37 °C for 72 h for LAB count.
Determination of amino acids
Total amino acid contents of the initial products and the fermented products at month 6 were determined by HPLC equipped with a fluorescence detector (Agilent Technologies, Santa Clara, CA, USA). Each sample was hydrolyzed by 6 M HCl at 110 °C for 24 h under a vacuum condition (AOAC 1995). The analytical column was a reversed phase C18 column (Novo-Pak, 150 × 3.9 mm i.d. and 4 μm particle size, Waters, Milford, MA, USA). For peak identification and quantification of amino acids, amino acid standards with 2.5 mM L-α-amino-n-butyric acid (Sigma-Aldrich) as an internal standard were used.
Sample preparation
Sample extracts of both Kapi raw materials, the initial products, and the fermented products were prepared by mixing the samples with distilled water (pH 7.0) in the ratio of 1:5 (w/v). The mixtures were homogenized and shaken at 150 rpm on an orbital shaker for 1 h at 30 °C. The mixtures were then centrifuged at 8,400 × g for 10 min at 4 °C to remove undesired debris. The supernatants were collected and adjusted to pH 7.0 using 1 M HCl.
To obtain desalted extracts, the extracts were dialyzed with a CelluSep H1 dialysis bag with 1 kDa molecular weight cut-off (MWCO) (Membrane Filtration Products, Seguin, TX, USA) against 20-time sample volume of distilled water pH 7.0 ± 0.2 for 24 h at 30 °C. The sample and dialyzed extracts were analyzed for soluble protein content by Lowry’s method (Lowry et al. 1951) using bovine serum albumin (BSA) at concentrations of 0–100 μg/mL as a standard.
Determination of protease activity
Protease activity was analyzed according to the method of Sarath et al. (1990). The assay monitored amount of tyrosine liberated along with other amino acids and peptides from a substrate, casein, by the action of protease in the sample. Free tyrosine formed a complex with Folin’s reagent and produced blue colored chromophore which was measured at 660 nm using a Varian Cary 50 UV–vis spectrophotometer (Agilent Technologies). One unit of enzyme was defined as the amount of enzyme required to liberate 1 mg of tyrosine per minute under the assay condition.
Determination of free amino acids
Free amino acids in the extracts were monitored as free α-amino acid groups as previously described by Adler-Nissen (1979). This assay measured orange colored chromophore of primary amino group of free amino acids reacted with 2,4,6-trinitrobenzenesulfonic acid (TNBS) at 420 nm using the Varian Cary 50 UV–vis spectrophotometer (Agilent Technologies). Leucine at concentrations of 0–500 μg/mL was used as a standard. The results were expressed as mg leucine/g sample.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
The dialyzed extracts were further investigated for the protein patterns by SDS-PAGE with a 15 % running gel and a 4 % stacking gel, according to the method described by Laemmli (1970). The sample with 100 μg protein and a pre-stained broad range molecular weight protein ladder (7.1–209 kDa) (Bio-Rad Laboratories, Hercules, CA, USA) were loaded to the gel and run at 80 V (10 mA) for 2 h. The separated proteins were fixed and stained using 0.1 % (w/v) Coomassie Brilliant Blue R-250 in 40 % methanol and 10 % acetic acid for 30 min and destained using 50 % methanol and 7.5 % acetic acid for 1 h, followed by 50 % methanol and 7.5 % acetic acid for 16 h. The molecular weight of separated proteins was determined by the relative mobility (Rf) value of the protein standard molecular weight.
Antioxidative assays
The sample and dialyzed extracts of both Kapi raw materials, the initial products and the fermented products, prepared at a protein concentration of 1 mg/mL, were analyzed for antioxidative activity against three different assays. The results were expressed as either the percentage of scavenging activity or reducing power compared to 10 mM ascorbic acid used as a positive control.
DPPH radical scavenging assay
DPPH radical scavenging activities in the sample and dialyzed extracts were determined using the method previously described (van Amsterdam et al. 1992). The samples were tested against 10 μM ethanolic DPPH• solution in a 96-well plate. In the presence of an antioxidant, a decrease in the absorbance of DPPH• at 517 nm was monitored after incubation in dark at 37 °C for 30 min. The activity was calculated and reported as a percentage of scavenging activity.
ABTS+ radical scavenging assay
ABTS+ radical scavenging activities in the sample and dialyzed extracts were measured by the previously described method (Re et al. 1999). The ABTS•+ solution was firstly prepared by mixing 7 mM ABTS with 2.45 mM potassium persulfate. The radical solution was then diluted to the absorbance of 0.70 ± 0.02 at 734 nm by 5 mM phosphate buffer saline pH 7.4 after incubation in dark at 30 °C for 16 h. The sample was tested with the radical solution in a 96-well plate. The blue color of ABTS•+ was monitored at 734 nm after incubation in dark at 30 °C for 6 min. The results reported as a percentage of scavenging activity.
Reducing power assay
The reducing powers of the sample and dialyzed extracts were determined by the method of Oyaizu (1986). The assay was based on the reduction of ferric ion (Fe3+) of ferric cyanide complex to ferrous ion (Fe2+) in the presence of antioxidants in the tested samples. To monitor the concentration of Fe2+ generated, the solution was measured the formation of Perl’s Prussian blue colored chromophore at 700 nm in a 96-well plate. The activity was calculated and reported as a percentage of reducing power.
Antimicrobial assay
Antimicrobial assay was conducted using an agar well diffusion method as described by Kuete et al. (2006). The tested strains were inoculated in Mueller Hinton Broth (MHB; Oxoid, Cambridge, UK) and incubated at 30 °C for 16 h. The cultures at a cell concentration of 1.5 × 108 cfu/mL were spread on a petri dish overlaid with Mueller Hinton Agar (MHA; Oxoid). The extracts were filtered through a 0.45 μm sterile nylon membrane filter before loading to an agar well, prepared by a cork borer no. 2, at a volume of 15 μL. The plates were incubated at 37 °C for 24 h. Chloramphenicol at 100 μg/mL was used as a positive control. The zones of inhibition were recorded.
ACE inhibitory assay
ACE inhibitory assay was carried outby using the ACE Kit - WST developed by Dojindo laboratories (Kumamoto, Japan). The dialyzed extracts were tested at the different concentrations of soluble protein content to determine the ACE inhibitory activity based on the colorimetric formazan product developed and measured at 450 nm. The results were expressed as a soluble protein concentration in the sample that gave 50 % ACE inhibitory activity denoted by IC50.
Statistical analysis
All data was analyzed for analysis of variance (ANOVA) using SPSS software version 11.5 (SPSS, Illinois, USA). Significance of means was determined using the Duncan’s Multiple Range Test (DMRT) at a 95 % significant difference (p < 0.05).
Results and discussions
Chemical composition and characteristics
The proximate composition of Kapi Ta Dam and Kapi Ta Deang raw materials, the initial products, and the fermented products at month 6 is shown in Table 1. Kapi products had high salt content ranging from 13 to 17 g/100 g sample since salt was added for product preservation by controlling spoilage and proliferation of pathogenic microorganisms. Raw materials of both Kapi types (B and R) showed higher water activity (aw) than their initial products (Kp-B0 and Kp-R0) which had aw in the range of 0.63 to 0.66. This dramatic decrease in aw of both Kapi products was due to salt addition to the products. A gradual decrease in pH from slightly basic to neutral during Kapi fermentation was observed. This is possibly due to production of organic acid by lactic acid bacteria (LAB) such as Pediococcus halophilus associated with Kapi fermentation (Phithakpol 1993; Tanasupawat and Visessanguan 2008). The results indicated that the production process could preserve Kapi and extend its shelf-life by the reduction of water activity. The fermented Kapis (Kp-B6 and Kp-R6) had higher protein, fat, ash, and carbohydrate than their raw materials (Table 1) since water was released from the raw materials after salt addition and sun drying. Chemical composition of both Kapis was not significantly different. It was similar to that of Kapis made from other locations in Thailand as previously reported (Faithong et al. 2010; Phithakpol 1993). It is noted that Kapi is a high protein product with high salt. It therefore becomes a nutritious and important ingredient in the Thai cuisines.
Table 1.
Characteristics of raw materials (B and R), the initial Kapi products (Kp-B0 and Kp-R0) and the fermented products at month 6 (Kp-B6 and Kp-R6)
| Parameters | B | Kp-B0 | Kp-B6 | R | Kp-R0 | Kp-R6 |
|---|---|---|---|---|---|---|
| aw * | 0.98–0.99 | 0.65–0.66 | 0.62–0.63 | 0.97–0.98 | 0.63–0.64 | 0.62–0.63 |
| pH | 7.9–8.0 | 7.3–7.4 | 7.2–7.3 | 7.8–8.0 | 7.7–7.8 | 7.2–7.4 |
| Salt (g/100 g) | 1.6 ± 0.2C | 17.0 ± 0.1A | 13.0 ± 0.1B | 1.7 ± 0.0c | 17.1 ± 0.0a | 14.7 ± 0.2b |
| Protein (g/100 g) | 15.9 ± 0.0C | 23.0 ± 0.6B | 29.9 ± 0.2A | 18.9 ± 0.3c | 28.7 ± 0.1a | 27.0 ± 0.4b |
| Fat (g/100 g) | 0.2 ± 0.0C | 0.4 ± 0.0B | 2.1 ± 0.0A | 0.2 ± 0.0c | 0.4 ± 0.0b | 2.9 ± 0.0a |
| Fiber (g/100 g) | 3.1 ± 0.2A | 2.7 ± 0.0A | 1.2 ± 0.0B | 3.2 ± 0.1a | 2.3 ± 0.0b | 1.3 ± 0.1c |
| Ash (g/100 g) | 15.5 ± 0.1C | 39.6 ± 0.0A | 35.1 ± 0.3B | 15.0 ± 0.2b | 40.4 ± 0.1a | 38.7 ± 0.0a |
| Carbohydrate (g/100 g) | 15.4 | 21.4 | 18.4 | 15.3 | 16.4 | 16.5 |
*aw was analyzed at 25 °C
Values with different uppercase and lowercase letters are significantly different within each group at p < 0.05
Amino acid profile
Total amino acids of both fermented Kapi products (Kp-B6 and Kp-R6) compared to the initial products (Kp-B0 and Kp-R0) are shown in Table 2. The amino acid profiles of both fermented products did not differ significantly. After 6 months of fermentation, most essential amino acids of Kapi Ta Dam sample (Kp-B6) increased slightly, while the amino acids of Kapi Ta Deang (Kp-R6) decreased. Lysine, which is a limiting amino acid in cereals, and leucine were found to be predominant essential amino acids in both products. Major non-essential amino acids of both Kapis were glutamic acid and aspartic acid which were similar to Korean salt-fermented shrimp sauces (Kim et al. 2003) and fermented blue mussel sauce (Jung et al. 2004). However, Kapi products in this study had lower amino acid constituents than the Korean sauces. A high amount of those non-essential amino acids; glutamic acid, aspartic acid, and glycine, was reported as a taste attribute in fish and shellfish products (Jung et al. 2004).
Table 2.
Amino acid profiles of the initial Kapi products (Kp-B0 and Kp-R0) and the fermented products at month 6 (Kp-B6 and Kp-R6)
| Amino acid (mg/100 mg) | Kp-B0 | Kp-B6 | Kp-R0 | Kp-R6 |
|---|---|---|---|---|
| Essential amino acid | ||||
| Histidine | 0.37 | 0.26 | 0.46 | 0.26 |
| Threonine | 0.93 | 0.78 | 1.00 | 0.78 |
| Tyrosine | 0.64 | 0.85 | 0.81 | 0.77 |
| Valine | 1.09 | 1.35 | 1.34 | 1.08 |
| Lysine | 1.76 | 2.19 | 2.05 | 1.86 |
| Isoleucine | 0.95 | 1.15 | 1.14 | 1.05 |
| Leucine | 1.69 | 1.92 | 1.99 | 1.84 |
| Phenylalanine | 0.94 | 1.04 | 1.09 | 0.92 |
| Non-essential amino acid | ||||
| Aspartic acid | 2.30 | 2.55 | 2.24 | 2.31 |
| Serine | 0.78 | 0.42 | 0.70 | 0.53 |
| Glutamic acid | 4.30 | 4.10 | 4.14 | 3.96 |
| Glycine | 1.12 | 1.62 | 1.78 | 1.32 |
| Arginine | 1.16 | 0.76 | 1.05 | 0.91 |
| Alanine | 1.48 | 1.96 | 1.99 | 1.77 |
| Proline | 0.89 | 1.02 | 1.08 | 0.99 |
| Total | 20.40 | 21.97 | 22.86 | 20.35 |
Microorganism profiles
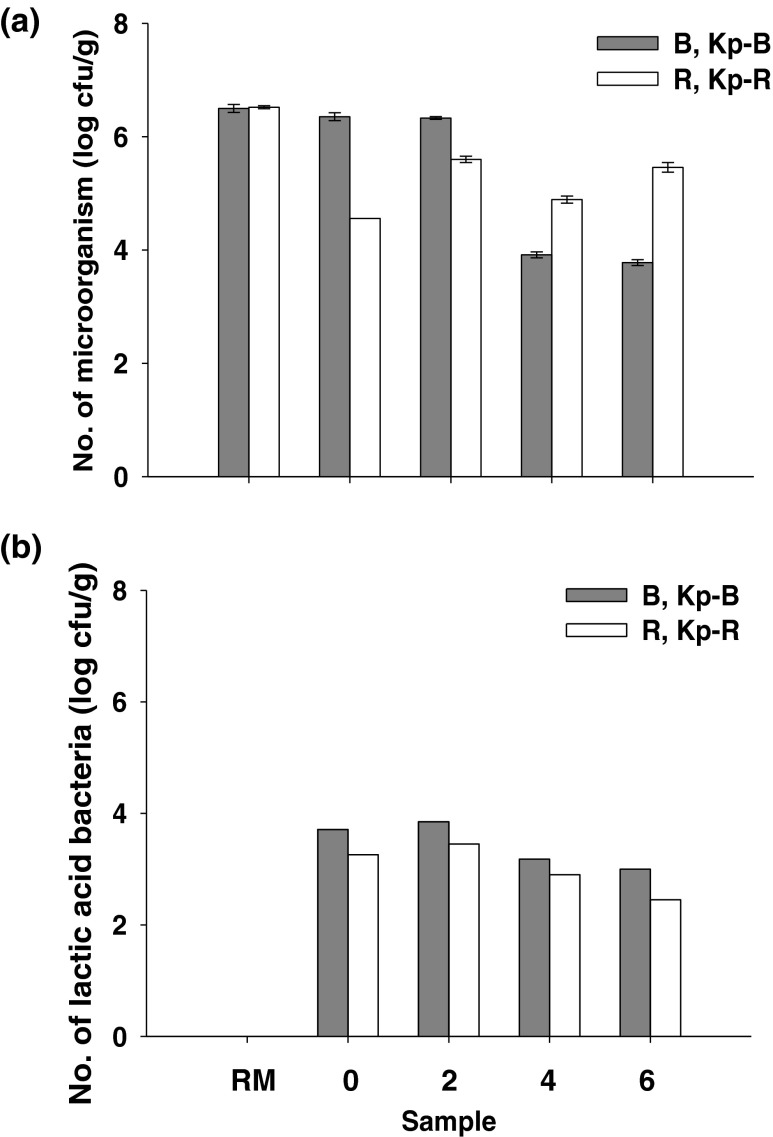
Total microbial numbers in all samples are shown in Fig. 1a. The numbers in both initial products and the fermented products were lower than those of their raw materials. During Kapi fermentation, microorganisms associated with Kp-B4 and Kp-B6 were found to decrease drastically to 3.9 and 3.8 log cfu/g sample, respectively. The numbers presented in Kapi Ta Deang were, however, the highest at the 2nd month of fermentation of 5.6 log cfu/g sample. The high salt content in the products markedly inhibited the growth of other microbial strains, especially spoilage and pathogenic bacteria, and promoted the growth of microorganisms contributing to Kapi fermentation.
Fig. 1.
Total microorganisms (a) and lactic acid bacteria (b) in Kapi raw materials (RM; B and R), the initial products (Kp-B0 and Kp-R0), and the fermented products from months 2 to 6 (Kp-B2/4/6 and Kp-R2/4/6)
LAB were found in all samples except the raw materials as shown in Fig. 1b. Since the raw materials were fresh and rich in normal flora, under aerobic condition, this could overcome the growth of LAB cells in the raw materials. However, after the production process by mixing the raw materials with sea salt and fermented for several days, LAB were observed in the initial products (Fig. 1b). Numbers of LAB gradually decreased after the 2nd month of fermentation to 2.50-2.95 log cfu/g sample at the end of fermentation. Numbers of halophilic LAB in fish sauce were also reported in a range of 2–4 log cfu/mL (Udomsil et al. 2010). However, another previous work reported a higher LAB numbers of 4–6 log cfu/g in the Indonesian shrimp paste called Terasi (Kobayashi et al. 2003). Bacterial strains isolated from seafood fermented products including Kapi were reported to be Tetragenococcus halophillus, Tetragenococcus muriaticus, Tetragenococcus sp., Virgibacillus sp., Lentinbacillus sp., and Salinicoccus sp. (Kobayashi et al. 2003; Tanasupawat and Visessanguan 2008).
Protease activity and free α-amino acid
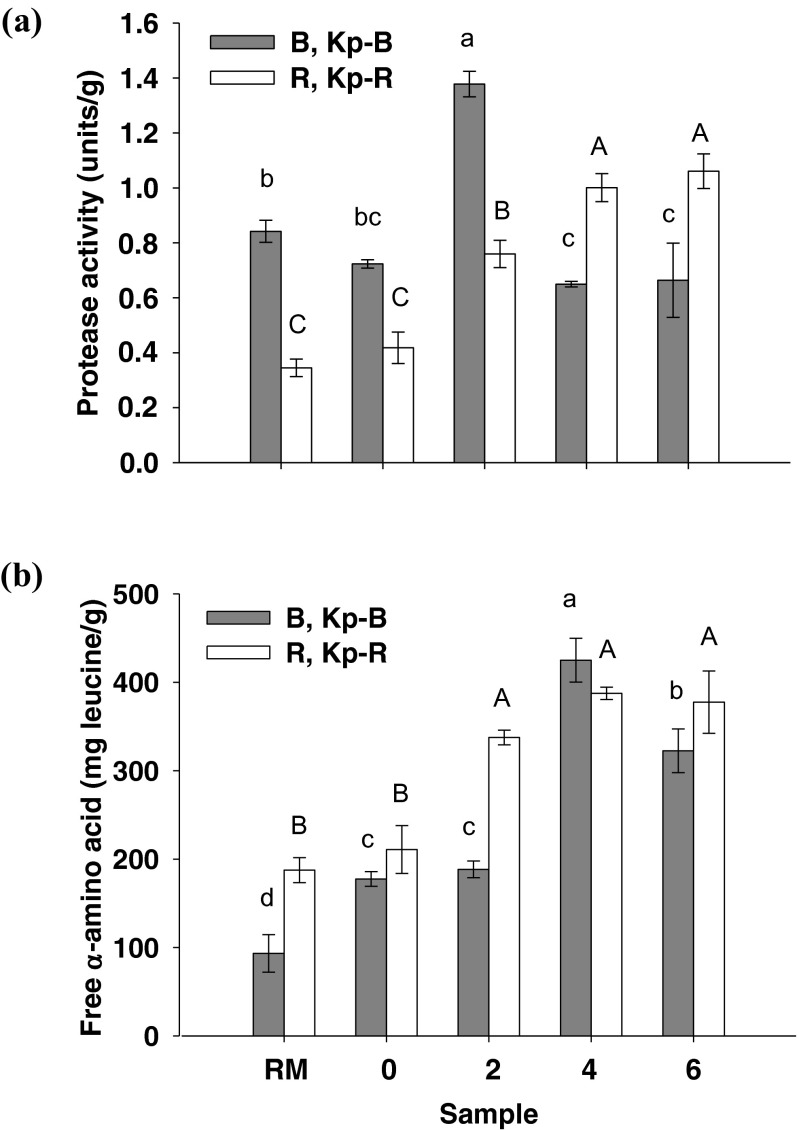
Figure 2a shows protease activitiesin the extracts of both Kapi raw materials, the initial products, and the fermented Kapi products. Protease activity of Kapi Ta Deang samples increased with increasing fermentation and was the highest at the end of fermentation (1.1 units/g). An increment of protease activity would be due to increasing of microbial protease production in the sample during fermentation (Udomsil et al. 2010). Protease activity of Kapi Ta Dam was the highest at the 2nd month of fermentation (1.4 units/g). Protease found in the samples was derived from digestive enzymes in the raw material and bacteria involving in fermentation. Most digestive enzymes in crustaceans were found to be serine protease and metalloprotease (Garcia-Carreño et al. 1994). Cysteine protease and serine protease in crude extract from muscle and hepatopancreas of fresh water prawns (Macrobrachium rosenbergii) acted as a predominant protease (Sriket et al. 2011). In addition, T. halophillus isolated from fish sauce has been reported to possess intracellular amino peptidase activity, especially alanylaminopeptidase activity (Udomsil et al. 2010). These enzymes convert peptides and oligopeptides to amino acids which serve as a precursor of flavor formation (Peralta et al. 2005).
Fig. 2.
Protease activity (a) and free α-amino acid content (b) in the sample extracts of Kapi raw materials (RM; B and R), the initial products (Kp-B0 and Kp-R0), and the fermented Kapi products from months 2 to 6 (Kp-B2/4/6 and Kp-R2/4/6). Different lower case and upper case letters are significantly different within each group at p < 0.05
Protease degrades protein in Kapi and produces short chain peptides and free amino acids. Free α-amino acid contents in the extracts of Kapi raw materials, the initial products, and the fermented products are shown in Fig. 2b. Free α-amino acid contents of Kapi Ta Deang increased with increasing fermentation time corresponding to protease activity. The highest free α-amino acid contents were found at month 4 in both Kapis (425 and 388 mg leucine/g). However, no significant difference (p < 0.05) in free α-amino acids in the fermented samples of months 2, 4 and 6 was observed. Different levels of protease activity and free α-amino acids in the samples are likely due to differences in raw material origin, production process of each Kapi type, and microorganisms involved in the fermentation process.
SDS-PAGE
Protein patterns of the raw materials and Kapi samples at different fermentation periods desalted by 1 kDa dialysis bags were visualized on 15 % SDS-PAGE. According to the sample preparation method, myosin heavy chain (MHC) could not be extracted. The band at 200 kDa could not be seen in all samples. High intensity protein bands at 49–80 kDa and 29–40 kDa with low intensity protein bands at lower 20 kDa were observed in both raw materials (data not shown). However, a lower intensity of high molecular weight protein bands of 47–80 kDa in fermented Kapi Ta Dam and Kapi Ta Deang was noted. This may be as a result of the degradation of microfibrillar proteins by proteases. Fermentation time also resulted in higher intensity of low molecular weight protein bands as revealed by an increment of lower 20 kDa band intensity. However, the actin bands at 42 kDa were still observed throughout the prolonged fermentation of both Kapi samples. These results are in agreement with previous studies that reported actin as the most resistant protein to be degraded by either endogenous or microbial proteinases (Eakpetch et al. 2008). The proteolysis in Kapi could be influenced by several factors such as indigenous microorganisms, digestive proteases in the raw material, endogenous proteases produced by microorganisms, and fermentation conditions.
Antioxidative activity
The antioxidative activities of the sample extracts and dialyzed extracts of the raw materials, the initial products, and the fermented Kapi products on three different assays were shown in Fig. 3. DPPH• scavenging activity was found in a range of 12–44 % in both dialyzed extracts (Fig. 3a and b). It was found to be the lowest among three antioxidative assays, since this scavenging assay is normally tested in an ethanolic system which has remarkable limitation for hydrophilic antioxidants. In contrast, ABTS•+ can be solubilized in an aqueous system as well as organic solution, the scavenging activity of both hydrophilic and hydrophobic compounds can thus be successfully assessed (Re et al. 1999). The results showed that the activities of both Kapi extracts against ABTS•+ were similar (71–80 %) and did not alter during fermentation period (Fig. 3c and d). However, ABTS•+ scavenging activities of the dialyzed Kapi Ta Deang samples increased from 38 to 78 % throughout the prolonged fermentation (Fig. 3d). In terms of reducing power, the activities of the dialyzed Kapi Ta Deang samples were in the range of 38 to 61 % and were higher than those of the non-dialyzed extracts (Fig. 3f). An increase in reducing power of the dialyzed Kapi Ta Deang samples through the prolonged fermentation was also observed.
Fig. 3.
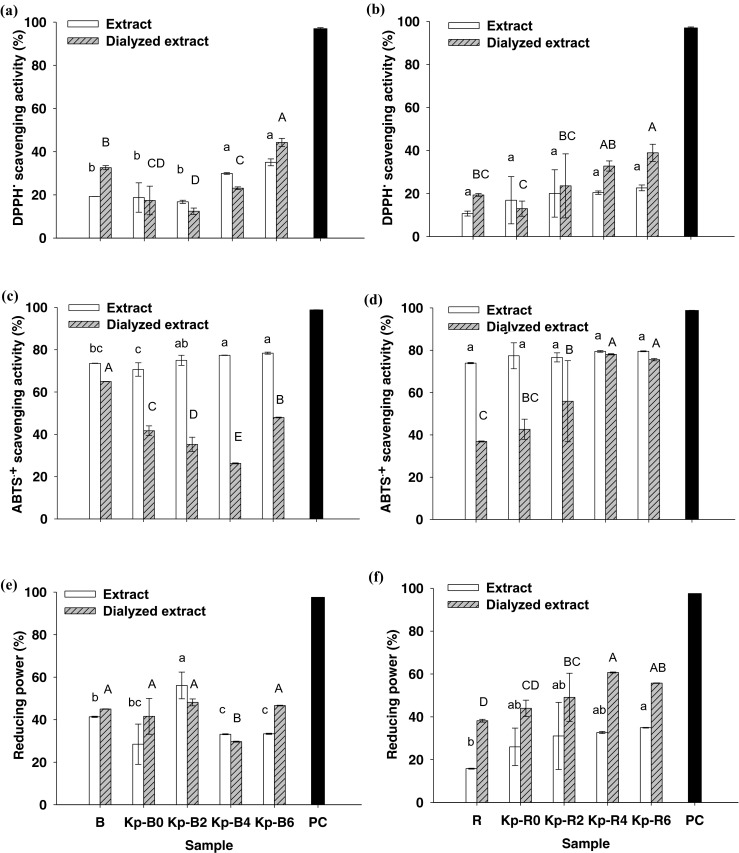
Antioxidative activities of the sample extracts and dialyzed extracts of Kapi raw materials (B and R), the initial products (Kp-B0 and Kp-R0), and the fermented Kapi products from months 2 to 6 (Kp-B2/4/6 and Kp-R2/4/6) at 1 mg protein/mL and 10 mM ascorbic acid as a positive control (PC) against DPPH• (a, b), ABTS•+ (c, d) and reducing power (e, f). Different lower case and upper case letters are significantly different within each group at p < 0.05
It was noted that ABTS•+ scavenging activities of the both Kapi extracts were higher than those of the dialyzed samples, possibly due to the loss of compounds associated with electron-donating ability to the ABTS•+ during the desalting step. On the other hand, the compounds associated with reducing power could be maintained in the desalting step, while small molecules acted as inhibitors in this assay would be removed, and thus resulting in higher reducing power of the dialyzed extracts compared to the non-dialyzed extracts.
For both Kapi types, it was observed that antioxidative activities of Kapi Ta Dam (Fig. 3a, c, and e) were not related to fermentation time, however, those of Kapi Ta Deang (Fig. 3b, d, and f) significantly increased with increasing fermentation time. This may be due to the differences in the components in their raw materials, their production process and loss of either activators or inhibitors of bioactive compounds during dialysis.
Antioxidative activity associated with fermented marine products has been previously reported. Philippine salt-fermented shrimp pastes showed DPPH• scavenging activity in the range of 24.3–61.5 % in 80 % ethanolic extract (Peralta et al. 2008). Squid miso prepared with Aspergillus oryzae-inoculated koji exhibited almost 90 % DPPH• scavenging activity and showed rapid development of reducing power at an early stage of fermentation (Giri et al. 2011). Fermented blue mussel sauce and its antioxidant peptide of 962 Da tested at 0.2 mg/mL could reduce the intensity of spin adducts of DPPH• by 22.6 % and 72 %, respectively (Rajapakse et al. 2005). In addition, Thai traditional fermented shrimp and krill products including Jaloo, Koong-Som, and Kapi were reported to possess DPPH• and ABTS•+ scavenging activities as well as reducing power ranging from 4.01 to 12.1 μmol TE/g protein, 58.6–345 μmol TE/g protein and 17.0–48.2 μmol TE/g protein, respectively (Faithong et al. 2010). Bioactive components in shrimp and its shell and fermented shrimp biowastes such as proteins, carotenoids, flavonoids, and flavonoid glucosides were also reported to be responsible for antioxidative activities (Li et al. 1994; Sachindra et al. 2007). The Maillard reaction products and intermediates formed in Mungoong extract paste and the Philippine salt-fermented shrimp paste during fermentation exhibited antioxidative activities against DPPH• and ABTS•+, ferric reducing, hydrogen peroxide, and methyl linoleate peroxidation (Binsan et al. 2008; Peralta et al. 2008).
Antimicrobial activity
All sample extracts were tested for antimicrobial activity against Gram-positive (S. aureus and B. cereus) and Gram-negative (E. coli and S. Typhimurium) bacteria. In this experiment, the extracts were tested at the protein concentrations ranging from 10.3 to 16.4 mg/mL. The results showed that no antimicrobial activities were observed in all samples. It is indicated either those concentrations were not effective against tested strains or antimicrobial substances were not present in Kapi raw materials and products. However, pediocins, antimicrobial agents, produced by Tetragenococcus spp. isolated from various fermented food products such as miso, soy sauce, and fermented meat products were reported (Papagianni and Anastasiadou 2009).
ACE inhibitory activity
In this study, it is highlighted that ACE inhibitory activities were found in Thai fermented shrimp pastes. The strongest activity of both Kapi products at month 6 was observed at the IC50 of approximately 7–8 μg/mL (Table 3). Interestingly, the activities increased with fermentation time starting from 32.91 to 7.36 μg/mL for fermented Kapi Ta Dam and from 34.08 to 7.95 μg/mL for fermented Kapi Ta Deang. These results indicated that ACE inhibitors were developed during the fermentation process. The hydrolyzed proteins in Kapi could be responsible for ACE inhibitory activity. It is noted that Kapi products had much higher ACE inhibitory activity than fermented oyster sauce (Je et al. 2005a) and fermented blue mussel sauce (Je et al. 2005b) which their activities were found at IC50 of 2.45 and 1.01 mg/mL, respectively. Fermented seafood products such as marine shrimp (Acetes chinensis) fermented by Lactobacillus fermentum SM 605have been reported to possess ACE inhibitory activity at IC50 of 2.2–5.5 μM (Ichimura et al. 2003). Bioactive peptide derived from fermented blue mussel sauce, Glu-Val-Met-Ala-Gly-Asn-Leu-Tyr-Pro-Gly, responsible for ACE inhibitory activities had IC50 of 19.3 μg/mL (Je et al. 2005b).
Table 3.
ACE inhibitory activities of the dialyzed extracts of Kapi Ta Dam and Kapi Ta Deang raw materials (B and R), the initial Kapi products (Kp-B0 and Kp-R0) and the fermented products (Kp-B2/4/6 and Kp-R2/4/6)
| Dialyzed | IC50 |
|---|---|
| extract | (μg/mL) |
| B | 27.65 |
| Kp-B0 | 32.91 |
| Kp-B2 | 23.59 |
| Kp-B4 | 13.36 |
| Kp-B6 | 7.36 |
| R | 50.39 |
| Kp-R0 | 34.08 |
| Kp-R2 | 12.28 |
| Kp-R4 | 10.20 |
| Kp-R6 | 7.95 |
Conclusions
In conclusion, a Thai traditional fermented shrimp paste (Kapi) is a high protein fermented product with strong antioxidative and ACE inhibitory activities. Type of raw material and fermentation process affected the antioxidative activities. Moreover, prolonged fermentation could improve both the antioxidative and ACE inhibitory activities of Kapi. Therefore, Kapi could serve as a natural source of antioxidant and antihypertensive agents. It not only provides nutritional benefits but also would promote consumer health as a functional food.
Acknowledgments
The authors are grateful to the Thailand Research Fund (TRF) and King Mongkut’s University of Technology Thonburi (KMUTT) for a PhD scholarship of Miss Thanyaporn Kleekayai under the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0133/2552). We thank to the Asian Core Program (ACP), “Capacity Building and Development of Microbial Potential and Fermentation Technology towards New Era”, of Japan Society for the Promotion of Science (JSPS) for the support of research cooperation and the Royal Thai Government for the financial support through research budget of KMUTT. We would finally like to thank Miss Elizabeth Finnegan and Dr. Celia Conessa from University of Limerick, Ireland, for editing and proofing our manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- Adler-Nissen J. Determination of the degree if hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J Agric Food Chem. 1979;27(6):1256–1262. doi: 10.1021/jf60226a042. [DOI] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis. 16. Virginia: AOAC International; 1995. [Google Scholar]
- Binsan W, Benjakul S, Visessanguan W, Roytrakul S, Tanaka M, Kishimura H. Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei) Food Chem. 2008;106(1):185–193. doi: 10.1016/j.foodchem.2007.05.065. [DOI] [Google Scholar]
- Cha YJ, Cadwallader KR. Volatile components in salt-fermented fish and shrimp pastes. J Food Sci. 1995;60(1):19–24. doi: 10.1111/j.1365-2621.1995.tb05597.x. [DOI] [Google Scholar]
- Eakpetch P, Benjakul S, Visessanguan W, Kijroongrojana K. Autolysis of Pacific white shrimp (Litopenaeousvannamei) meat: characterization and the effect of protein additive. J Food Sci. 2008;73(2):95–103. doi: 10.1111/j.1750-3841.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- Faithong N, Benjakul S, Phatcharat S, Binsan W. Chemical composition and antioxidative activity of Thai traditional fermented shrimp and krill products. Food Chem. 2010;119(1):133–140. doi: 10.1016/j.foodchem.2009.06.056. [DOI] [Google Scholar]
- Garcia-Carreño FL, Hernandez-Cortes MP, Haard NF. Enzyme with peptidase and proteinase activity from the digestive systems of a freshwater and a marine decapod. J Agric Food Chem. 1994;42:1456–1461. doi: 10.1021/jf00043a013. [DOI] [Google Scholar]
- Giri A, Osako K, Okamoto A, Okazaki E, Ohshima T. Antioxidative properties of aqueous and aroma extracts of squid miso prepared with Aspergillus oryzae-inoculated koji. Food Res Int. 2011;44:317–325. doi: 10.1016/j.foodres.2010.10.013. [DOI] [Google Scholar]
- Hartmann R, Meisel H. Food-derived peptides with biological activity: from research to food applications. Curr Opin Biotechnol. 2007;18:163–169. doi: 10.1016/j.copbio.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Ichimura T, Hu J, Aita DQ, Maruyama S. Angiotensin I-converting enzyme inhibitory activity and insulin secretion stimulative activity of fermented fish sauce. J Biosci Bioeng. 2003;96:496–499. doi: 10.1016/S1389-1723(03)70138-8. [DOI] [PubMed] [Google Scholar]
- Je JY, Park JY, Jung WK, Park PJ, Kim SK. Isolation of angiotensin I converting enzyme (ACE) inhibitor from fermented oyster sauce, Crassostrea gigas. Food Chem. 2005;90:809–814. doi: 10.1016/j.foodchem.2004.05.028. [DOI] [Google Scholar]
- Je JY, Park PJ, Byun HG, Jung WK, Kim SK. Angiotensin I converting enzyme (ACE) inhibitory peptide derived from the sauce of fermented blue mussel, Mytilus edulis. Bioresour Technol. 2005;96:1624–1629. doi: 10.1016/j.biortech.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Jung WK, Rajapakse N, Kim SK. Antioxidative activity of low molecular weight peptide derived from the sauce of fermented blue mussel, Mytilus edulis. Eur Food Res Technol. 2004;220:535–539. doi: 10.1007/s00217-004-1074-3. [DOI] [Google Scholar]
- Kiesvaara M (1975) On the soluble nitrogen fraction of barrel-salted herring and semi-preserves during ripening. Publication No. 10, Technical Research Center of Finland, Helsinki, Finland
- Kim JS, Shahidi F, Heu MS. Characterization of salt-fermented sauces from shrimp processing by products. J Agric Food Chem. 2003;51:784–792. doi: 10.1021/jf020710j. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kajiwara M, Wahyuni M, Kitakado T, Hamada-Sato N, Imada C, Watanabe E. Isolation and characterization of halophilic lactic acid bacteria isolated from “terasi” shrimp paste: a traditional fermented seafood products in Indonesia. J Gen Appl Microbiol. 2003;49(5):279–286. doi: 10.2323/jgam.49.279. [DOI] [PubMed] [Google Scholar]
- Kuete V, Tangmouo JG, Penlap Beng V, Ngounou FN, Lontsi D (2006) Antimicrobial activity of the methanolic extract from the stem bark of Tridesmostemon omphalocarpoides (Sapotaceae). J Ethnopharmacol 104:5–11 [DOI] [PubMed]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li SJ, Seymour TA, Morrissey MT. Isolation of a natural antioxidant from shrimp waste. In: Shahidi F, editor. Natural antioxidants and their uses in foods. Washington DC: American Chemical Society; 1994. pp. 283–295. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- Oyaizu M. Studies on products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–314. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Papagianni M, Anastasiadou S (2009) Pediocins: the bacteriocins of Pediococci. Sources, production, properties and applications. Microb Cell Fac 8(3). doi:10.1186/1475-2859-8-3 [DOI] [PMC free article] [PubMed]
- Pengchumrus W, Upanoi T (2005) Species and distribution of Acetes shrimps in seagrass beds and mangrove canals in the Andaman sea. Technical Paper No. 12/2005, Phuket Marine Biological Center, Department of Marine and Coastal Resources, Ministry of Natural Resources and Environment, Phuket, Thailand
- Peralta E, Hatate H, Watanabe D, Kawabe D, Murata H, Hama Y, Tanaka R. Antioxidative activity of Philippine salt-fermented shrimp paste and variation of its contents during fermentation. J Oleo Sci. 2005;54:553–558. doi: 10.5650/jos.54.553. [DOI] [Google Scholar]
- Peralta E, Hatate H, Kawabe D, Kuwahara R, Wakamatsu S, Murata H. Improving antioxidant activity and nutritional components of Philippine salt-fermented shrimp paste through prolonged fermentation. Food Chem. 2008;11(1):72–77. doi: 10.1016/j.foodchem.2008.03.042. [DOI] [Google Scholar]
- Phithakpol B. Fish fermentation technology in Thailand. In: Lee CH, Steinkraus KH, Reilly PJA, editors. Fish fermentation technology. Seoul: United Nations University Press; 1993. pp. 155–166. [Google Scholar]
- Rajapakse N, Mendis E, Jung WK, Je JY, Kim SK. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res Int. 2005;38:175–182. doi: 10.1016/j.foodres.2004.10.002. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9/10):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Sachindra NM, Sato E, Maeda H, Hosokawa M, Niwano Y, Kohno M, Miyashita K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J Agric Food Chem. 2007;55:8516–8522. doi: 10.1021/jf071848a. [DOI] [PubMed] [Google Scholar]
- Sarath G, de la Motte RS, Wagner FW. Protease assay methods. In: Beynon RJ, editor. Proteolytic enzymes: a practical approach. Oxford: IRL Press; 1990. pp. 25–54. [Google Scholar]
- Seok SH, Park JH, Cho SA, Choi SA, Park JH. Cholesterol lowering effect of SG-GN3, the extract of salted and fermented shrimps, Acetes japonicas, in Triton WR-1339 or high cholesterol-diet induced hypercholesterolemic rats. J Ethnopharmacol. 2004;91:231–235. doi: 10.1016/j.jep.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Sriket C, Benjakul S, Visessanguan W. Characterisation of proteolytic enzymes from muscle and hepatopancreas of fresh water prawn (Macrobrashium rosenbergii) J Sci Food Agric. 2011;91:52–59. doi: 10.1002/jsfa.4145. [DOI] [PubMed] [Google Scholar]
- Tamang JP, Kailaspathy K. Fermented foods and beverages of the world. Florida: CRC Press; 2010. [Google Scholar]
- Tanasupawat S, Visessanguan W. Thai fermented foods. In: Farnworth ER, editor. Handbook of fermented functional foods. 2. Florida: CRC Press; 2008. pp. 495–512. [Google Scholar]
- Udomsil N, Rodtong S, Tanasupawat S, Yongsawatdigul J. Proteinase-producing halophilic lactic acid bacteria isolated from fish sauce fermentation and their ability to produce volatile compounds. Int J Food Microbiol. 2010;141(3):186–194. doi: 10.1016/j.ijfoodmicro.2010.05.016. [DOI] [PubMed] [Google Scholar]
- van Amsterdam FT, Roveri A, Maiorino M, Ratti E, Ursini F. Lacidipine: a dihydropyridine calcium antagonist with antioxidant activity. Free Radic Biol Med. 1992;12:183–187. doi: 10.1016/0891-5849(92)90025-C. [DOI] [PubMed] [Google Scholar]