Abstract
Purpose
Data suggest that activity of p38 MAPK and Tie2 kinase are dysregulated in MDS and may be targets for novel therapies. A Phase 1 study of ARRY-614, an oral dual inhibitor of p38 MAPK and Tie2, was conducted in patients with low or intermediate-1 International Prognostic Scoring System risk MDS to evaluate safety, pharmacokinetics (PK), pharmacodynamics (PD), and preliminary responses by IWG 2006 criteria.
Experimental Design
Forty-five patients received ARRY-614 either QD or BID in dose escalation (400, 600, 900 or 1200 mg QD; 200 or 300 mg BID) or expansion cohorts.
Results
The 300 mg BID schedule was not tolerated, and a maximum tolerated dose was not reached for QD dosing. Treatment-related adverse events were primarily grade 1–2, with the most common being rash, diarrhea, dry skin, fatigue and anorexia. Inter-patient PK variability was high, although exposure was sufficient to achieve reduction in p38 MAPK activation in bone marrow and in the levels of circulating biomarkers. Disease responses were observed in 14 of 44 (32%) evaluable patients, 13 (93%) of whom had previously been treated with a hypomethylating agent. Responses were observed in all lineages, with 5 patients experiencing bilineage responses. Three of 25 RBC transfusion-dependent (TD) patients achieved transfusion independence (TI) and 5 of 7 platelet TD patients achieved TI.
Conclusions
ARRY-614 was well tolerated and has sufficient activity to warrant further evaluation in this patient population. We recommend 1200 mg QD as the optimal dose for further study.
INTRODUCTION
The myelodysplastic syndromes (MDS) are a heterogeneous group of bone marrow stem cell disorders characterized by ineffective hematopoiesis and increased risk of transformation to acute myeloid leukemia (AML)(1). Several classification systems have evolved to estimate prognosis, one of which is the International Prognostic Scoring System (IPSS)(2) and the more recent revised IPSS (IPSS-R)(3). A majority of newly diagnosed patients (60–70%) are classified as having “lower-risk” disease (IPSS low or intermediate-1 risk(4)).
Treatment of patients with lower-risk MDS is focused on addressing cytopenias and improvements in quality of life. Common interventions include transfusions, growth factors and antimicrobials (5, 6). Treatment with a disease-modifying agent is initiated when progressive cytopenias and/or transfusion dependence develop(7). Three disease-modifying agents (azacitidine(8), decitabine(9) and lenalidomide(10)) have been approved for patients with MDS. Although the introduction of hypomethylating agents (HMAs) represented a significant addition to the MDS treatment armamentarium, they are not curative therapies(11, 12). Following the failure of HMAs, no existing therapies have prospectively demonstrated substantial activity in patients with lower-risk MDS. Mortality in patients with lower-risk MDS is more commonly attributed to the consequences of bone marrow failure, with infection being the leading cause of death in patients with lower-risk MDS, followed by hemorrhage and transformation to AML(2, 13–15). These findings underscore the importance of addressing neutrophil and platelet cytopenias and providing a therapeutic option to lower-risk patients with MDS whose disease has relapsed following standard therapies.
The molecular mechanisms underlying MDS pathophysiology are unclear, but emerging data support a role for both p38 mitogen-activating protein kinase (p38 MAPK) and Tie2 receptor tyrosine kinase (Tie2)(16). The p38 MAPK family comprises a group of protein serine/threonine kinases that modulate the function of many cellular processes. The canonical functions of the p38 MAPK family are to control cytokine biosynthesis and the cellular response to stress, particularly hypoxia-related or oxidative stress(17). MDS are characterized by increased oxidative stress and high myelosuppressive cytokine production in the bone marrow, resulting in aberrant progenitor apoptosis, a hallmark of the disease(18–21). The outcome of this loss of progenitors is ineffective hematopoiesis, leading to peripheral cytopenias. Abnormal activation of innate immune signaling pathways and activation of p38 MAPK have recently been implicated in the pathophysiology of MDS(22–24). Tie2 plays a poorly characterized role in hematopoiesis. However, expression of Tie2 and its ligands are aberrant in MDS, and this dysregulation has been correlated with poor prognosis in MDS(25, 26).
ARRY-614 is a potent, oral, small molecule inhibitor of p38 MAPK and Tie2 (27). In an in vivo murine model of acute inflammation, ARRY-614 inhibits the production of the proinflammatory cytokines tumor necrosis factor alpha (TNFα) and interleukin 6 (IL-6) in response to lipopolysaccharide (LPS) or staphyloccus enterotoxin A(28). In preclinical models of multiple myeloma, ARRY-614 inhibited tumor cell growth in murine xenografts and showed additive activity when combined with lenalidomide and dexamethasone(28). A first-in-human study conducted in healthy subjects evaluated single doses of 25 – 400 mg and repeat dosing of 50 – 400 mg once daily (QD) for 14 days (unpublished data) and found ARRY-614 to be generally well tolerated, with mean steady state half-life across all doses in the repeat dose cohort ranging from 15 to 21 hours, and showed concentration-dependent inhibition of the LPS-stimulated ex vivo production of proinflammatory cytokines in whole blood(28). As a result, a first-in-patient study with ARRY-614 was conducted to define the optimal dose and schedule, investigate pharmacokinetics (PK) and pharmacodynamics (PD) and evaluate preliminary effects on cytopenias in lower-risk MDS patients.
METHODS
Study design
This was a phase 1, multicenter, open-label, dose-escalation study with expansion cohorts of ARRY-614 administered either in a fasting or fed state to patients with IPSS low or intermediate-1 risk MDS. Sites for this study were The University of Texas MD Anderson Cancer Center (Houston, TX), the Winship Cancer Institute (Emory University, Atlanta, GA) and the H. Lee Moffitt Cancer Center (Tampa, FL). The study protocol was approved by the institutional review board at each investigational site, was conducted according to the recommendations of Good Clinical Practice and was registered with ClinicalTrials.gov (NCT00916227). Written informed consent was obtained from each patient following the guidelines of each participating institution and in accordance with the Declaration of Helsinki.
The primary objectives of the study were to determine the safety profile and maximum tolerated dose (MTD) and schedule of ARRY-614 and to characterize the PK of ARRY-614 and a metabolite. A secondary objective was to obtain a preliminary estimate of the efficacy of ARRY-614. Additional exploratory objectives included obtaining an initial assessment of target inhibition in the bone marrow and characterization of the biological activity of p38 MAPK inhibition in bone marrow and peripheral blood.
Patients
Eligible patients were ≥18 years old with a diagnosis of MDS of any World Health Organization (WHO) classification(29) with IPSS low or intermediate-1 risk and with Eastern Cooperative Oncology Group (ECOG) performance status ≤2. Patients may have received prior treatment for MDS or, if previously untreated, could be eligible for the study if they were ineligible for or refused other treatment options. Patients must have had adequate hepatic and renal function. Patients were excluded if they had a mean triplicate heart rate-corrected QT interval (QTc) ≥500 msec, a prior history of bone marrow transplant, prior malignancy unless disease-free for >2 years, or had other severe concurrent disease. For the expansion phase, patients were required to be red blood cell (RBC) transfusion dependent (TD) (defined as having ≥4 units of RBCs administered with a pretreatment hemoglobin value of ≤9 g/dL in the 8 weeks prior to first dose of treatment).
For the dose-escalation portion, prior treatment with azacitidine or decitabine had to be discontinued ≥2 weeks prior to the first dose of ARRY-614, and use of systemic corticosteroids had to be discontinued ≥4 weeks prior to the first dose. Use of hematopoietic growth factors was allowed. For the expansion portion, all treatment for MDS (including hematopoietic growth factors) had to be discontinued prior to initiation of ARRY-614, including discontinuation of growth factors ≥1 week prior, azacitidine and decitabine ≥2 weeks prior, long-acting growth factors such as darbopoetin and pegfilgrastim ≥3 weeks prior and systemic corticosteroids ≥4 weeks prior to the first dose of ARRY-614. Use of hematopoietic growth factors was allowed after Cycle 1 in the expansion phase.
Treatment
The study evaluated daily (QD) and twice daily (BID) dosing schedules of ARRY-614 provided as a neat powder-in-capsule containing 100 mg of ARRY-614. ARRY-614 was administered orally in continuous 28-day cycles under fasting conditions (no food or beverages other than water for at least 2 hours before and 1 hour after taking ARRY-614) at a starting total daily dose of 400 mg either as 400 mg QD or 200 mg BID. A single cohort of patients was evaluated under fed/fasted conditions to determine the effect of food on the PK parameters of ARRY-614. Patients in the food effect cohort received a single 400 mg oral dose of ARRY-614 in the fasted state on study day -3 followed by 400 mg QD dosing beginning on study day 1 in the fed state (patients consumed food within 45 min prior to taking ARRY-614). The starting dose in this study was selected based on the tolerability of repeat-dose administration of ARRY-614 to healthy subjects. Intra-patient dose escalation was allowed to a higher dose after that dose was determined to be tolerated. Treatment was continued until disease progression, an adverse event leading to cessation, withdrawal of consent, or investigator decision.
Dose escalation utilized a modified 3+3 design with dose increases by 50% increments until treatment-related grade 2 non-hematologic toxicity was observed and then by 30% dose increments until the MTD was reached. Patients who terminated participation in the study for any reason other than drug-related AEs prior to completing cycle 1 and patients who did not receive at least 80% of the planned doses in cycle 1 for any reason other than drug-related AEs were considered ineligible for the safety assessment required for dose escalation and were replaced. Expansion cohorts of up to 14 additional patients were planned under fasting or fed conditions at one or both of the schedule’s respective MTDs.
Safety Analysis
The safety population included all patients who received at least one dose of ARRY-614, and analysis was based on the initial dose and schedule received. Safety was assessed throughout the study and included the monitoring of adverse events (AEs), dose-limiting toxicities (DLTs), clinical laboratory evaluations (hematology, coagulation panel and clinical chemistries), vital signs, body weight and 12-lead electrocardiogram (ECG) parameters. QT intervals were corrected for heart rate using Bazett’s formula (QTcB). Adverse events were graded using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), Version 3.0 (Bethesda, MD).
The MTD was defined as the dose level below the dose resulting in DLTs in ≥33% of patients and was to be based on 6 evaluable patients. Only AEs that occurred during cycle 1 and were not clearly attributable to an extraneous cause were considered DLTs. Grade 3 or 4 nausea or vomiting was considered a DLT if either the event was >5 days in duration despite maximum supportive care if antiemetic prophylaxis was not administered or of any duration if antiemetic prophylaxis was administered. Grade 3 or 4 diarrhea was considered a DLT if the event was >5 days in duration, despite maximum supportive care if anti-diarrheal prophylaxis was not administered, or of any duration if anti-diarrheal prophylaxis was administered. Any other grade 3 or 4 non-hematologic AE was considered a DLT. For patients with grade ≥2 absolute neutrophil count (ANC) at baseline, ANC <0.1×109/L lasting >7 days and a decrease of >75% from baseline was considered a DLT; for patients with grade 0 or 1 ANC at baseline, grade 4 neutropenia lasting >7 days was considered a DLT. For patients with grade ≥2 platelets at baseline, platelet count <10×109/L lasting >7 days and a decrease of >75% from baseline was considered a DLT; for patients with grade 0 or 1 platelets at baseline, grade 4 thrombocytopenia was considered a DLT. Any AE that required an interruption of dosing beyond day 56 or a delay in starting cycle 2 beyond day 56 was considered a DLT.
Response assessment
Response data (number and timing of RBC transfusions [with triggering laboratory values], hematology counts, bone marrow pathology and cytogenetics) were evaluated using International Working Group (IWG) 2006 criteria(30). A post hoc analysis of platelet transfusion independence (TI), defined as no platelet transfusions for ≥8 weeks, was also performed. All transfusions were recorded beginning 8 weeks prior to the first day of dosing in cycle 1 and throughout the study. Hematology was assessed at screening and weekly during treatment cycles at local laboratories. Bone marrow aspirates (±biopsies) were collected at screening, at the end of cycles 2 and 4 and every 4 months thereafter and were analyzed by local pathologists. Patients were considered evaluable for response if they received at least one dose of ARRY-614 and had at least one cytopenia or transfusion requirement at baseline making them eligible for a hematologic improvement (HI) response per IWG 2006 criteria. Eligibility for IWG 2006 HI responses were based on the mean hemoglobin, platelet or neutrophil values for the 56 days prior to the first dose of ARRY-614. Baseline for all HI evaluations was based on the mean of all values during the 56 days prior to the first dose of ARRY-614 that were not influenced by a transfusion. For IWG 2006 HI evaluation, baseline RBC transfusions were counted only if triggered by a hemoglobin value ≤9 g/dL. Baseline platelet TD was defined as receiving ≥1 platelet transfusion for a platelet count below 10×103 u/L in the 56 days prior to the first dose of ARRY-614.
Pharmacokinetic analysis
For patients in the fasted dose-escalation cohorts, blood samples were collected during cycle 1 at specified time points through 12 hours postdose (for patients on the BID schedule) or 24 hours postdose (for patients on the QD schedule) on days -3 (food-effect cohort only), 1 and 15 for determination of plasma concentrations of ARRY-614. Plasma samples were analyzed for ARRY-614 using a validated quantitative liquid chromatography with tandem mass spectrometric detection (LC-MS/MS) method. The individual plasma concentration-time data for each analyte were evaluated with non-compartmental analysis using Phoenix WinNonlin, Version 6.3 (Pharsight, Sunnyvale, CA) or higher.
Pharmacodynamic analysis
Bone marrow core biopsy samples were collected at baseline and at the end of cycles 2 and 8 for immunohistochemical (IHC) analysis of target inhibition (phospho-p38 MAPK [p-p38]) and effects on apoptosis (cleaved caspase-3 [CC3]). Briefly, formalin-fixed, paraffin-embedded (FFPE), decalcified bone marrow biopsies were subjected to heat-induced epitope retrieval, blocked, and incubated with primary antibodies directed against p-p38 (Phospho-p38 MAPK D3F9 XP, Cell Signaling Technology, Inc., Danvers, MA) or CC3 (Cleaved Caspase-3 [Asp175] [5A1E], Cell Signaling Technology, Inc., Danvers, MA). 3,3′-diaminobenzidine (DAB) detection was performed following incubation with horseradish peroxidase secondary antibodies (Envision + System-HRP-DAB, Dako North America, Inc., Carpenteria, CA). Slides were scored manually (7 fields at 40X) or using automated scanning using an Aperio whole-slide scanner, 20X (Aperio, Vista, CA) and Flagship Bioscience’s (Aurora, CO) proprietary CellMap customized algorithm for p-p38 and CC3, respectively. Age-defined FFPE bone marrow biopsies from apparently healthy subjects (60–74 years old) were obtained from Folio Biosciences (Columbus, OH).
Plasma was isolated from blood samples collected at predetermined time points for evidence of p38 MAPK inhibition through changes in circulating cytokine and/or growth factor levels. Various p38-dependent analytes were measured using quantitative multiplex assays (Human Chemokine 9-Plex Ultra-Sensitive Kit and Human Hypoxia Panel, Meso Scale Discovery, Gaithersburg, MD). Baseline values represent the average of two samples taken during the screening period and/or prior to dosing on the first day on study.
Statistical considerations
Demographics and baseline disease characteristics of all treated patients were summarized descriptively. Response analyses were performed and results were presented by cohort, where appropriate; however, no formal comparisons between cohorts were performed. Responses were assessed based on the initial dose and schedule of ARRY-614 received. The time from the first dose of ARRY-614 to documentation of the first response and the maximum duration of response were calculated for each patient who achieved a response. Duration of each response was calculated from the date that response criteria were met through the date that response criteria were no longer satisfied. Patients who died or received subsequent therapy for MDS before response criteria were no longer satisfied and patients who continued to respond at study termination were censored on the date of the last response assessment. In cases with multiple responses with varying durations of response within each response category, the maximum duration was recorded for each patient within each response category.
RESULTS
Patients
Forty-five patients were enrolled between June, 2009 and Mar, 2011 at 3 sites and treated with ARRY-614 (Table 1). A majority (73%) of patients had intermediate-1 risk disease, 89% had <5% bone marrow blasts, 64% normal karyotype and 56% 2 to 3 cytopenias. By IPSS-R 3 patients were classified as having very high- or high-risk disease. At the time of enrollment, patients were a median of 3.3 (range, 0.2–16.3) years from initial diagnosis. Median number of prior therapies was 2 (range, 0–9), with 80% having received prior HMA treatment, 58% having received erythropoiesis stimulating agents (ESAs), and 42% having received treatment with lenalidomide.
Table 1.
Patient demographics and baseline disease characteristics (ITT Population)
| Demographics/Characteristics (N=45) | Value |
|---|---|
| Median age, years (range) | 72 (47 – 84) |
|
| |
| Sex, n (%) | |
| Male | 39 (87) |
| Female | 6 (13) |
|
| |
| ECOG performance status, n (%) | |
| 0 | 11 (24) |
| 1 | 28 (62) |
| 2 | 6 (13) |
|
| |
| Median time since first diagnosis, years (range) | 3.3 (0.2 – 16.3) |
|
| |
| IPSS risk score at screening, n (%) | |
| Low | 12 (27) |
| Intermediate-1 | 33 (73) |
|
| |
| WHO classification at screening, n (%) | |
| RA/RARS | 11 (24) |
| RCMD/RCMD-RS | 18 (40) |
| RAEB-1 | 7 (16) |
| RAEB-2 | 2 (4) |
| MDS-U | 6 (13) |
| MDS with isolated del(5q) | 1 (2) |
|
| |
| % Blasts (bone marrow aspirate), n (%) | |
| < 5 | 40 (89) |
| ≥ 5 | 4 (9) |
| >10 | 0 (0) |
| No data | 1 (2) |
|
| |
| Number of cytopenias, n (%) | |
| 0 | 1 (2) |
| 1 | 19 (42) |
| 2 | 12 (27) |
| 3 | 13 (29) |
|
| |
| Hemoglobin < 11 g/dL1, n (%) | 41 (91) |
|
| |
| ANC2, n (%) | |
| 0.5 – < 1.0 × 109/L | 7 (16) |
| < 0.5 × 109/L | 9 (20) |
|
| |
| Platelets3 | |
| 50 – < 100 × 109/L | 22) |
| < 50 × 109/L | 15 (33) |
|
| |
| Karyotype4, n (%) | |
| Good | |
| Normal | 29 (64.4) |
| -Y | 4 (8.9) |
| del (20q) | 2 (4.4) |
| del (5q) | 1 (2.2) |
| Intermediate | 8 (17.8) |
| Complex | 1 (2.2) |
|
| |
| Transfusions within 8 weeks prior to first day of dosing in Cycle 1, n (%) | |
| Any RBC transfusion | 38 (84) |
| RBC transfusion dependent5 | 25 (56) |
| Any platelet transfusion | 7 (16) |
|
| |
| Median number of prior therapies, n (range) | 2 (0 – 9) |
| ≥ 1 prior hypomethylating agent (HMA) 5, n (%) | 36 (80) |
| Prior lenalidomide, n (%) | 19 (42) |
| Prior erythropoiesis-stimulating agent6, n (%) | 26 (58) |
Mean hemoglobin <11 g/dL for the 56 days prior to the first dose of ARRY-614 that was not influenced by a red blood cell (RBC) transfusion in the 14 days prior to an assessment.
Mean absolute neutrophil counts for the specified ranges for the 56 days prior to the first dose of ARRY-614.
Mean platelets for the specified ranges for the 56 days prior to the first dose of ARRY-614 that was not influenced by a platelet transfusion in the 3 days prior to an assessment.
Good, Intermediate and Complex classification based on IPSS criteria.
All patients who were administered ≥ 4 units of RBCs for Hgb levels ≤9 g/dL in the 56 days prior to the first dose of ARRY-614.
Includes azacitidine and decitabine.
Includes darbepoetin alfa, erythropoietin, and epoietin alfa.
Dose Escalation
Four QD dose levels were evaluated under fasting conditions (400, 600, 900 and 1200 mg). Each of the evaluated fasted daily doses was considered tolerated. Dose-limiting toxicities (DLTs) were observed in 1 of 6 patients in the 400 mg QD cohort (grade 3 diarrhea) and in 1 of 7 patients in the 1200 mg QD dose-escalation cohort (grade 3 macular rash). Dose escalation using the QD schedule was discontinued at the 1200 mg dose level due to high capsule burden without reaching the MTD of this schedule. The 1200 mg QD dose level was expanded to include 16 patients in total. An additional 5 patients were enrolled at 400 mg QD to evaluate the effect of food on ARRY-614 exposure.
Two BID dose levels were evaluated under fasting conditions (200 and 300 mg). The 200 mg BID dose level was determined to be tolerated with no DLTs in 3 patients. Two of the first 3 patients enrolled in the 300 mg BID cohort experienced DLTs. One patient experienced grade 3 macular rash that recurred upon re-administration of ARRY-614 at a lower dose. The second patient developed grade 3 drug hypersensitivity that recurred upon re-administration at a lower dose. Due to the idiosyncratic nature of this allergic reaction, the investigator and sponsor considered the patient not evaluable for either efficacy or toxicity. A replacement patient was enrolled in the cohort and no DLT was reported for this patient. The cohort was expanded to include an additional 3 patients and each of these patients experienced DLTs; 1 patient experienced grade 3 muscular weakness and increased hepatic enzyme, 1 patient experienced grade 3 asthenia, and the third patient experienced grade 3 rash. The 300 mg BID dose level was deemed not tolerated with 4 of 6 evaluable patients experiencing a DLT. Further evaluation of the BID dosing regimen was discontinued.
Adverse Events (AEs)
All 45 patients enrolled in the study were evaluable for safety, and each patient reported at least 1 treatment-emergent AE. The most common AEs of any grade regardless of dose or relatedness were rash (defined here to include terms of rash, acne and dermatitis acneiform), diarrhea, anorexia, fatigue, anemia and prolonged ECG QT (Supplemental Table 1). Rash was generally characterized by single episodes of grade 1 or 2 that resolved with or without treatment while continuing on study drug. Atrial fibrillation was observed as a treatment-emergent AE in 6 patients (13%), and 4 of these were grade 1 or 2 in severity and none were deemed to be treatment related. The most common AEs of at least grade 3 in severity regardless of relatedness were prolonged ECG QTc, anemia, rash, diarrhea, asthenia, neutropenia and febrile neutropenia, all of which were grade 3 (Supplemental Table 1). Eight grade 4 treatment-emergent AEs occurred in 7 patients: large intestine perforation, hypokalemia, hyperuricemia, leukopenia, neutropenia, thrombocytopenia, atrial fibrillation (n=1 each) and anemia (n=2).
A majority of patients (n=37, 82%) experienced at least 1 treatment-related AE. The most common treatment-related AEs regardless of dose or severity were rash (see above), diarrhea, dry skin, fatigue, anorexia, pruritis, elevated ALT and prolonged ECG QT (Table 2). The most common treatment-related AEs of at least grade 3 in severity were rash, diarrhea, asthenia and prolonged ECG QT, all grade 3 (Supplemental Table 1). There were no treatment-related grade 4 or 5 AEs.
Table 2.
Treatment-related AEs (safety population)
| AE N (%) |
400 mg QD (n=7) |
400 mg QD FED (n=5) |
200 mg BID (n=3) |
600 mg QD (n=4) |
300 mg BID (n=7) |
900 mg QD (n=3) |
1200 mg QD (n=16) |
Total (n=45) |
|---|---|---|---|---|---|---|---|---|
| Treatment-related AEs of all grades reported in ≥5% of patients | ||||||||
| Rash | 3 (43) | 0 (0) | 0 (0) | 0 (0) | 4 (57) | 1 (33) | 6 (38) | 14 (31) |
| Diarrhea | 3 (43) | 1 (20) | 1 (33) | 0 (0) | 1 (14) | 1 (33) | 5 (31) | 12 (27) |
| Dry Skin | 1 (14) | 0 (0) | 0 (0) | 2 (50) | 0 (0) | 1 (33) | 2 (12) | 6 (13) |
| Fatigue | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 4 (25) | 5 (11) |
| Anorexia | 1 (14) | 1 (20) | 0 (0) | 0 (0) | 2 (29) | 1 (33) | 0 (0) | 5 (11) |
| Pruritus | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 1 (14) | 1 (33) | 1 (6) | 4 (9) |
| Increased ALT | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (14) | 0 (0) | 2 (12) | 3 (7) |
| Prolonged ECG QT | 0 (0) | 1 (20) | 2 (67) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (7) |
| Most common treatment-related Grade 3 AEs‡ | ||||||||
| Rash‡‡ | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (29) | 1 (33) | 2 (12) | 5 (11) |
| Diarrhea | 1 (14) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (19) | 4 (9) |
| Asthenia | 0 (0) | 1 (20) | 0 (0) | 0 (0) | 1 (14) | 0 (0) | 0 (0) | 2 (4) |
| Prolonged ECG QT | 0 (0) | 1 (20) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (4) |
No grade 4 treatment-related AEs were reported
Includes MeDRA preferred terms of rash (rash, rash maculo-papular, rash pruritic, rash macular, rash popular, rash erythematous), acne and dermatitis acneiform
SAEs occurred in 18 patients (40%), with 7 patients (16%) experiencing treatment-related SAEs. Treatment-related SAEs included diarrhea (7%), asthenia (2%), fatigue (2%), tachyarrhythmia (2%), muscular weakness (2%), dyspnea (2%) and pruritic rash (2%). The incidence of SAEs did not appear to be correlated with ARRY-614 dose. Two deaths occurred on study or within 30 days of study completion. The primary cause of death is unknown for one patient in the 400 mg QD dose group. This patient had discontinued treatment due to progressive disease 44 days prior to death. The primary cause of death for the second patient, also in the 400 mg QD dose group, was sepsis (deemed not treatment related).
Dose reductions from the initially assigned dose due to AEs occurred in 11 patients, 8 of which were in response to treatment-related AEs. Dose reductions due to treatment-related AEs were more frequently implemented in the higher QD dose groups (≥900 mg QD) and the BID dose groups, with a dose reduction required for 1 patient (33%) in the 900 mg QD cohort, 2 patients each in the 200 mg BID (66%) and 300 mg BID (29%) cohorts and 3 patients (19%) in the 1200 mg QD cohort. Treatment-related AEs leading to dose reduction were rash (n=3) and diarrhea, gastritis, fatigue, feeling jittery, and prolonged ECG QT (n=1 each).
Forty-four patients discontinued study treatment and 1 patient remains on study after >3 years of treatment at the time of submission of this report. Reasons for study treatment discontinuation include lack of efficacy (n=18), AEs (n=17), progressive disease (n=5), death (n=2), non-compliance with protocol (n=1) and withdrawal of consent (n=1). Treatment-related AEs leading to discontinuation occurred in 10 patients and included prolonged ECG QT (n=3), diarrhea and asthenia (n=2), drug hypersensitivity, muscular weakness, ataxia and macular rash (n=1 each).
Clinical activity
Of the 45 patients enrolled, 44 patients were evaluable for response. Across all doses and schedules, HI, based on IWG 2006 criteria, was observed in 14 of 44 evaluable patients (32%) (Table 3). Two additional patients achieved platelet TI for ≥56 days without meeting IWG 2006 response criteria for HI-P (overall, 5 of 7 patients who were platelet TD at baseline met platelet TI criteria on study). Responses were observed in all lineages (erythroid [HI-E]: 8 of 41 [20%], platelet [HI-P]: 6 of 25 [24%], neutrophil [HI-N]: 5 of 16 [31%]), with a bilineage response in 5 of 25 evaluable patients (20%). At the highest dose evaluated (1200 mg QD), 6 of 16 evaluable patients (38%) achieved HI, with 4 (25%) of these patients achieving bilineage responses. As of the data cutoff date, 1 patient in the 1200 mg cohort with a bilineage response (HI-E/HI-P) remained on study at 900 mg QD after >3 years of treatment. The median duration of HI was 21 weeks, with HI (RBC transfusion independence) for the ongoing patient censored at 40.9 months. The median duration was 19 weeks for erythroid response and 21 weeks for both neutrophil and platelet responses.
Table 3.
Response rates for hematologic improvement and transfusion independence
| All Patients | 1200 mg QD Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Response1 | Responders N | Evaluable Patients N | % | Median Duration of Response2 (Range) Weeks | Responders N | Evaluable Patients, N | % | Median Duration of Response2 (Range) Weeks |
| Hematologic Improvement | ||||||||
|
| ||||||||
| HI-Any | 14 | 44 | 32 | 21 (8 – 178+) | 6 | 16 | 38 | 21 (8 – 178+) |
|
| ||||||||
| HI-E | 8 | 41 | 20 | 19 (9 – 178+) | 2 | 15 | 13 | 19 (9 – 178+) |
|
| ||||||||
| HI-N | 5 | 16 | 31 | 21 (13 – 26) | 4 | 8 | 50 | 21 (15 – 26) |
|
| ||||||||
| HI-P | 6 | 25 | 24 | 21 (8 – 158+) | 4 | 11 | 36 | 12 (8 – 158+) |
| Transfusion Independence | ||||||||
|
| ||||||||
| Red blood cell | 3 | 25 | 12 | 20 (10 – 178+) | 1 | 11 | 9 | NC (178+) |
|
| ||||||||
| Platelet | 5 | 7 | 71 | 20 (12 – 66) | 1 | 2 | 50 | NC (31) |
IWG 2006 criteria were used with modifications as described in the Response Assessment section of the Methods.
One response at upper limit of duration range for HI (HI-E and HI-P) was ongoing at the time of submission of this manuscript. Data were censored as of last visit entered into the clinical database.
Abbreviations: HI, hematologic improvement; E, erythroid; N, neutrophil; NC, Not Calculated; P, platelet; TI, transfusion independence.
Of the 8 patients with an HI-E response, 2 had previously received an ESA but treatment had been discontinued prior to study entry. Treatment with an ESA was initiated while on study in 2 of the 8 patients. In one case the patient had experienced 3 periods of RBC transfusion independence ranging from 8.7 – 10.6 weeks and continued to qualify for RBC transfusion reduction at the time darbepoetin alfa was introduced (Study Day 506). The second patient received epoetin alfa and pegfilgrastim from Study Day 5 – 85, a period that partially overlapped with periods of RBC transfusion reduction.
Thirteen of the 14 HI responders had received prior treatment with HMAs. As shown in Table 4, the incidence of HI was greater in patients with prior HMA treatment than in those without (36% vs. 13%) and in patients with 2–3 cytopenias at baseline than in those with 1 baseline cytopenia (48% vs. 11%). Hematologic improvement was observed in 11 of 22 patients (50%) who previously received treatment with an HMA and had 2 to 3 cytopenias at baseline.
Table 4.
Response by baseline characteristics
| Baseline parameter | Responders/Evaluable | % |
|---|---|---|
| Total responders | 14/44 | 32 |
|
| ||
| Prior therapies | ||
| No HMA | 1/8 | 13 |
| HMA | 13/36 | 36 |
|
| ||
| Baseline cytopenias | ||
| 1 | 2/19 | 11 |
| 2–3 | 12/25 | 48 |
|
| ||
| Prior HMA and 2–3 cytopenias | 11/22 | 50 |
|
| ||
| IPSS risk | ||
| Low | 2/12 | 17 |
| Intermediate-1 | 12/32 | 38 |
|
| ||
| Phospho-p38 MAPK status at Baseline | ||
| Positive1 | 7/13 | 54 |
| Negative2 | 2/6 | 33 |
Positive defined as ≥ 5% p-p38[+] cells
One patient negative for phospho-p38 MAPK at baseline was not evaluable for HI
Pharmacokinetics
Pharmacokinetic characteristics of ARRY-614 are summarized in Supplemental Table 2. Geometric mean observed plasma drug concentration versus time profiles for all dose groups are shown in Figure 1A. In the BID cohorts, the geometric mean AUCtau values at day 15 ranged from 1,770 hr*ng/mL (geometric %CV = Not Calculated [N=2]) to 4,860 hr*ng/mL (geometric %CV = 113%) in the 200 and 300 mg BID cohorts, respectively,. Geometric mean Cmax values at day 15 ranged from 380 ng/mL (geometric %CV = Not Calculated [N=2]) in the 200 mg BID cohort to 843 ng/mL (geometric %CV = 114%) in the 300 mg BID cohort.
Figure 1. ARRY-614 Plasma Concentration-Time Profiles.
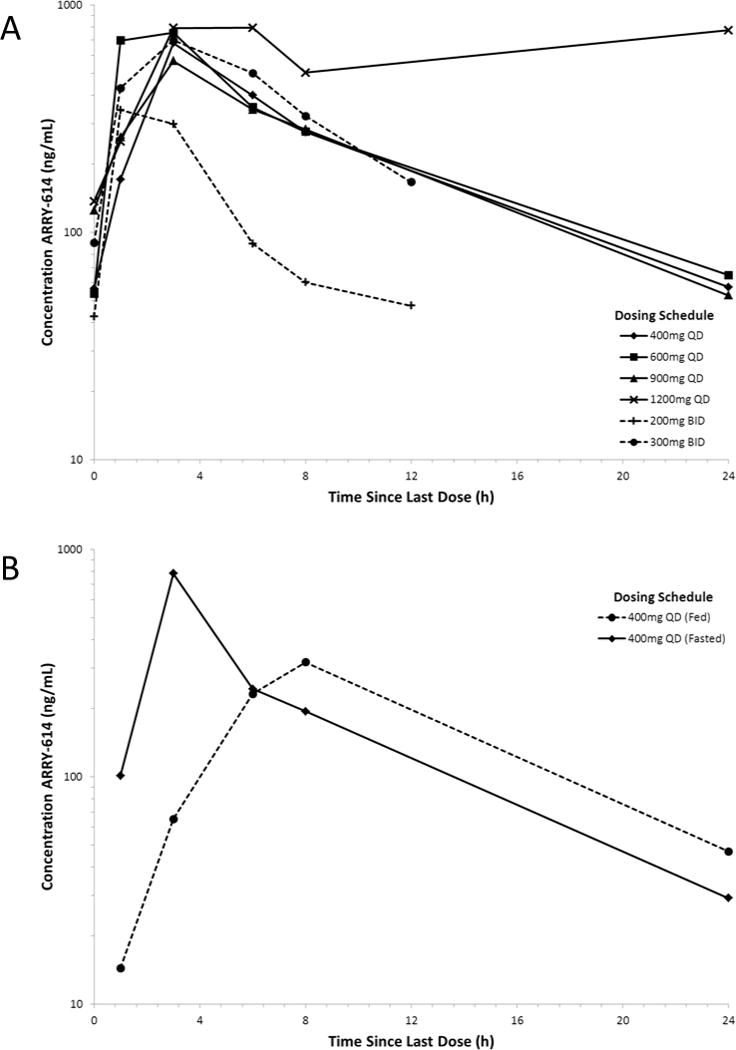
(A) Geometric mean concentration-time profile represented from each dose level in the dose escalation phase. (B) Geometric mean concentration-time profile after administration of 400 mg ARRY-614 under both fed and fasted conditions.
In the QD cohorts, the geometric mean AUCtau values at day 15 ranged from 5,840 hr*ng/mL (geometric %CV = 71.3%) in the 400 mg QD cohort to 19600 hr*ng/mL (geometric %CV = 38.4%) in the 1200 mg QD cohort, and the geometric mean Cmax values ranged from 713 ng/mL (geometric %CV = 65.2%) in the 400 mg QD cohort to 2050 ng/mL (geometric %CV = 47.1%) in the 1200 mg QD cohort. At day 15, median Tmax of ARRY-614 was 3 hours across all dose groups. Accumulation was minimal, with the geometric mean value for accumulation ratio (RAUC) across all dose groups ~1.39.
Results indicate a statistically significant (P=0.01), two-fold decrease in peak exposure (Cmax) from 783 ng/mL (geometric %CV = 125) to 387 ng/mL (geometric %CV = 159) as well as a delay in time to peak concentration (Tmax) under fed conditions. AUCtau was decreased with the administration with food, from 4520 ng*hr/mL (geometric %CV = 117) to 3780 ng*hr/mL (geometric %CV = 194). A comparison of geometric mean plasma ARRY-614 concentrations time profile following a single 400 mg dose of ARRY-614 in fed and fasted states is presented in Figure 1B.
Pharmacodynamics
Bone marrow p-p38 was evaluated to assess primary target inhibition by ARRY-614. Baseline samples were analyzed for 20 patients. The absolute percentage of cells staining positive at baseline was variable (0–44% p-p38[+] cells). Sixty-five percent (13/20) of the patients showed ≥5% p-p38[+] cells in the bone marrow at baseline. The percentage of p-p38[+] cells was reduced relative to baseline by 60% after 2 cycles of treatment (n=14), 56% after 4 cycles of treatment (n=12), and 88% after 8 cycles of treatment (n=2) (not shown) (Figure 2). Cleaved caspase 3 was analyzed by IHC as a marker for apoptosis in patient bone marrow samples. Baseline samples were available and analyzed for 19 patients. The absolute percentage of cells at baseline staining positive was variable (0.14–52% CC3[+] cells). Seventy-nine percent of patients (15/19) experienced reduction in CC3 at some point during treatment. However, the sample size is too small to make any significant correlations between response and these two biomarkers.
Figure 2. Bone marrow levels of p-p38 and CC3.
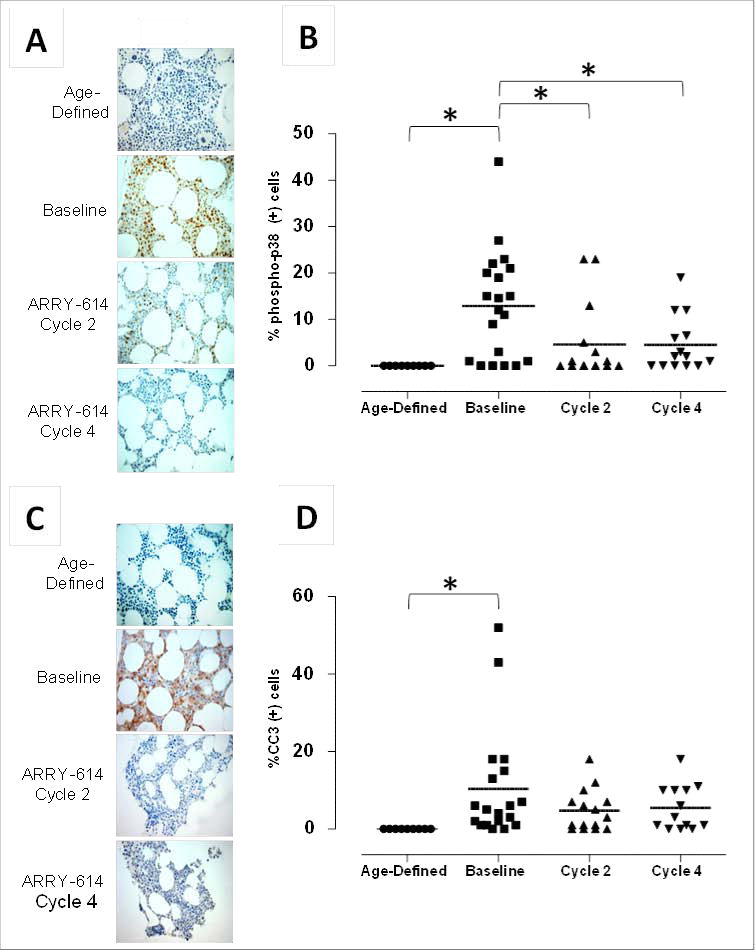
(A) Representative IHC photomicrographs for p-p38 for an individual MDS patient at baseline and after 2 or 4 cycles of treatment with ARRY-614. (B) Nine representative samples from age-comparable, normal subjects (‘Age-Defined’) were evaluated for p-p38 for comparison to MDS patient samples. (C) Representative IHC photomicrographs for CC3 for an individual MDS patient following treatment with ARRY-614. (D) CC3 expression in 9 representative samples from age-comparable, normal subjects (‘Age-Defined’) were evaluated for comparison to MDS patient samples. Data are represented as mean values analyzed by ANOVA, Tukey-Kramer post hoc (*P<0.05).
A set of plasma chemokines and growth factors were evaluated for change from baseline during ARRY-614 treatment as biomarkers of functional inhibition of p38. In the 1200 mg cohort, macrophage inflammatory protein-1β (MIP1-β), monocyte chemotactic protein-4 (MCP-4) and erythropoietin (EPO) levels decreased rapidly, although the decrease in MIP1-β was not sustained (Supplemental Figure S1).
The 44 patients evaluable for response and classified as either a responder or non-responder by IWG 2006 HI criteria (see above). Maximal reductions in EPO were observed within ~8 days of treatment for both populations (Supplemental Figure S1). Greater reductions in median EPO levels were observed in the responder population (83%) vs. the non-responder population (64%) (Supplemental Figure S2). Decreased EPO was generally sustained for >80 days in the responder population. In patients who did not demonstrate response, plasma EPO rebounded to baseline levels within ~35 days of the first dose of ARRY-614.
Discussion
In most forms of MDS, p38 MAPK is overactivated in erythroid, megakaryocyte and granulocyte progenitors(24, 31–33). Ex vivo studies in patient-derived bone marrow progenitors have shown that treatment with p38 MAPK inhibitors increases erythroid and myeloid colony formation, suggesting that the inhibition of p38 MAPK may enhance progenitor survival, numbers and/or function in patients with MDS(31, 34). It is hypothesized that p38 MAPK inhibitors, such as ARRY-614, will increase hematopoietic function in patients with MDS by blocking stress-induced apoptosis of bone marrow progenitors, and therefore, these agents are being studied in lower risk MDS.
This first-in-patient study demonstrated that continuous dosing of ARRY-614 was generally well tolerated at doses up to 1200 mg QD in patients with lower-risk MDS, and this dosing schedule is recommended for future trials. However, it is noted that this dosage schedule represents a considerable pill burden for the patients in question, and this issue may need to be addressed to increase tolerability. Adverse events were primarily mild or moderate in grade and an MTD was not definitively established in this study. Rash and diarrhea were the most commonly observed treatment-related AEs with a limited number of discontinuations resulting from these AEs.
Fourteen HI responses (based on IWG 2006 criteria) were observed with 6 of 16 evaluable patients demonstrating an HI response at the highest dose level evaluated (1200 mg QD). Responses were observed in all affected lineages with bilineage responses seen in 5 patients. Transfusion independence for both RBCs and platelets were also observed, with 5 of 7 platelet transfusion-dependent patients achieving transfusion independence for ≥56 days.
Interestingly, 13 of the 14 HI responses were noted in patients that had previously been treated with an HMA. This phenomenon may be related to HMA resistance mechanisms in MDS, which have yet to be fully characterized. Further studies of the interactions between ARRY-614 and HMA treatment may prove fruitful in not only treating MDS but also characterizing the mechanisms of HMA resistance. In addition, it will be important to understand the mechanisms of resistance to ARRAY-614.
It must be noted that ESA usage was allowed in this study. Although only 6 patients in our total cohort were treated with ESAs, their usage may still have influenced hematological outcomes. That said none of these patients experienced a response.
Inter-patient variability of pharmacokinetic parameters were relatively high in this study. As a result, assessment of dose-proportionality is difficult to ascertain due to variability in both Cmax and AUCtau. Variability associated to ARRY-614 administration can be attributed to factors such as irregularities in the absorption profile of the drug as well as limited sampling of the pharmacokinetic profile of the drug in this study. Administration of food with the drug contributed to a delay in time to peak concentration as well as a significant decrease in peak exposure.
Administration of ARRY-614 resulted in consistent inhibition of bone marrow p38 MAPK in a large fraction of patients and reduction of the apoptosis, as evidenced by the biomarker CC3, in bone marrow. Thirteen of the 20 patients evaluable for p38 MAPK evaluation (65%) showed p38 MAPK activation of ≥5% p-p38[+] cells in the bone marrow at baseline, confirming previous reports that p38 MAPK is overactivated in the bone marrow of patients with MDS. Overall, bone marrow p-p38 MAPK was dramatically reduced after 2 cycles of treatment, and inhibition was maintained for up to 8 cycles of treatment. The decrease in p-p38 MAPK in the bone marrow and reduction of p38 MAPK-dependent chemokines in the plasma suggest inhibition, both mechanistically and functionally, of the primary target, p38 MAPK.
Endogenous plasma EPO in most patients receiving ARRY-614 decreased rapidly and significantly (83% and 64% maximal reduction from baseline in HI responders and non-responders, respectively). The rapid and global effect in most patients is consistent with the presence of AU-rich sequences in the 3′ UTR of EPO, known targets for p38 MAPK-dependent mRNA stabilization(35). Persistence of the effect trended with patients experiencing HI in any lineage compared to non-responders. Although this may be due in part to improvements in erythropoietic function and RBC counts for patients achieving HI-E, sustained decreases in mean/median EPO were also observed in patients who experienced platelet and/or neutrophil improvements without erythroid improvements. These data are consistent with the known clearance of EPO, which is regulated primarily through receptor mediated endocytosis by erythroid progenitors(36) and may reflect improvements in bone marrow function in these patients. As further support, it is noteworthy that decreases in aberrant amounts of activated p38 MAPK and CC3 were observed in bone marrow from patients treated with ARRY-614, although the sample size is too small to make any clinically significant correlations.
In conclusion, ARRY-614 was generally well tolerated when dosed QD in patients with lower-risk MDS. Compound exposure was sufficient to result in target inhibition and HI responses were observed in all three lineages, with TI for both RBCs and platelets seen. Of note, responses were observed in patients who had previously received HMAs. These results support the further evaluation of ARRY-614 in lower risk MDS and in the future in combination with HMAs in higher risk disease. A dose-escalation study is currently ongoing in a patient population similar to the study presented here using an enabled formulation that provides increased bioavailability and allows dosing without regard to food.
Supplementary Material
STATEMENT.
Emerging evidence supports a role for alterations of the innate immune system and inflammatory signals in the pathogenesis of myelodysplastic syndromes (MDS). ARRY-614 is a potent p38 MAPK inhibitor. Here, we report the results of a Phase 1 trial of this agent in patients with lower-risk MDS. Results indicate that ARRY-614 is well tolerated in this patient population and inhibits its target. Furthermore, treatment with ARRY-614 was associated with clinical responses in a group of patients with heavily pretreated disease.
Acknowledgments
Aspects of these study results were presented in preliminary form at the European Hematology Association, 17th Congress, June 16, 2012, the International Congress of Targeted Anticancer Therapies, March 9, 2012 and the American Society of Hematology Annual Meeting, December 11, 2011. Sara Symons, Jen Garrus and Kari Guthrie provided medical writing support for this manuscript. The authors wish to thank the research nurses, physicians, study coordinators and other site staff, and participating patients and their families.
This clinical trial was supported by Array Biopharma, Inc. In addition, resources at MD Anderson which were used for the submission, approval and management of this study were made available in part through the NIH Cancer Center Support Grant CA016672.
Footnotes
Contributions: G.G.M., H.J.K., S.L.K., S.R., L.M., M.P., A.L., H.K. R.K. contributed to the design of the research protocol. G.G.M., H.J.K., E.J., J.L., A.L., H.K. and R.K. enrolled patients. G.G.M., H.J.K., S.L.W., L.C., S.R., L.M., G.H., M.P., A.L., H.K. and R.K. analyzed data. G.G.M., H.J.K., S.L.W., L.M., G.H., M.P., A.L., H.K., and R.K. contributed to the writing of the manuscript.
Conflict-of-interest disclosure: S.L.W., L.C., S.R., L.M., G.H. and M.P. are/were employed by Array, whose investigational drug candidate was studied in the present work. H.J.K. received honoraria from Array BioPharma Inc. for participation in advisory boards.
This study was registered at http://www.clinicaltrials.gov as NCT00916227.
References
- 1.Nimer SD. Myelodysplastic syndromes. Blood. 2011;111:4841–51. doi: 10.1182/blood-2007-08-078139. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–88. [PubMed] [Google Scholar]
- 3.PL G, H T, J S, G S, G G-M, F S, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–65. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekeres MA, Schoonen WM, Kantarjian H, List A, Fryzek J, Paquette R, et al. Characteristics of US patients with myelodysplastic syndromes: results of six cross-sectional physician surveys. J Natl Cancer Inst. 2008;100:1542–51. doi: 10.1093/jnci/djn349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malcovati L, Hellström-Lindberg E, Bowen D, Adès L, Cermak J, Del Cañizo C, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013;122:2943–64. doi: 10.1182/blood-2013-03-492884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komrokji RS, Sekeres MA, List AF. Management of lower-risk myelodysplastic syndromes: the art and evidence. Curr Hematol Malig Rep. 2011;6:145–53. doi: 10.1007/s11899-011-0086-x. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Manero G. Myelodysplastic syndromes: 2012 update on diagnosis, risk-stratification, and management. Am J Hematol. 2012;87:692–701. doi: 10.1002/ajh.23264. [DOI] [PubMed] [Google Scholar]
- 8.Adès L, Itzykson R, Fenaux P. Treatment of advanced myelodysplastic syndrome with demethylating agents: azacitidine. Seminars in Hematology. 2012;49:323–29. doi: 10.1053/j.seminhematol.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Joeckel TE, Lübbert M. Clinical results with the DNA hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in patients with myelodysplastic syndromes: an update. Seminars in Hematology. 2012;49:330–41. doi: 10.1053/j.seminhematol.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Giagounidis AA. Lenalidomide for del(5q) and non-del(5q) myelodysplastic syndromes. Seminars in Hematology. 2012;49:312–22. doi: 10.1053/j.seminhematol.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Prébet T, Gore SD, Esterni B, Gardin C, Itzykson R, Thepot S, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol. 2011;29:3322–27. doi: 10.1200/JCO.2011.35.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prébet T, Thepot S, Gore SD, Dreyfus F, Fenaux P, Vey N. Outcome of patients with low-risk myelodysplasia after azacitidine treatment failure. Haematologica. 2013;98:e18–9. doi: 10.3324/haematol.2012.071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang R, Gross CP, Halene S, Ma X. Comorbidities and survival in a large cohort of patients with newly diagnosed myelodysplastic syndromes. Leuk Res. 2009;33:1594–98. doi: 10.1016/j.leukres.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dayyani F, Conley AP, Strom SS, Stevenson W, Cortes JE, Borthakur G, et al. Cause of death in patients with lower-risk myelodysplastic syndrome. Cancer. 2010;116:2174–79. doi: 10.1002/cncr.24984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantarjian H, Giles F, List A, Lyons R, Sekeres MA, Pierce S, et al. The incidence and impact of thrombocytopenia in myelodysplastic syndromes. Cancer. 2007;109:1705–14. doi: 10.1002/cncr.22602. [DOI] [PubMed] [Google Scholar]
- 16.Huang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer. 2010;10:575–85. doi: 10.1038/nrc2894. [DOI] [PubMed] [Google Scholar]
- 17.Cowan KJ, Storey KB. Mitogen-activated protein kinases: new signaling pathways functioning in cellular responses to environmental stress. J Exp Biol. 2003;206:1107–15. doi: 10.1242/jeb.00220. [DOI] [PubMed] [Google Scholar]
- 18.Parcharidou A, Raza A, Economopoulos T, Papageorgiou E, Anagnostou D, Papadaki T, et al. Extensive apoptosis of bone marrow cells as evaluated by the in situ end-labelling (ISEL) technique may be the basis for ineffective haematopoiesis in patients with myelodysplastic syndromes. Eur J Haematol. 1999;62:19–26. doi: 10.1111/j.1600-0609.1999.tb01109.x. [DOI] [PubMed] [Google Scholar]
- 19.Kitagawa M, Saito I, Kuwata T, Yoshida S, Yamaguchi S, Takahashi M, et al. Overexpression of tumor necrosis factor (TNF)-alpha and interferon (IFN)-gamma by bone marrow cells from patients with myelodysplastic syndromes. Leukemia. 1997;11:2049–54. doi: 10.1038/sj.leu.2400844. [DOI] [PubMed] [Google Scholar]
- 20.Allampallam K, Shetty V, Mundle S, Dutt D, Kravitz H, Reddy PL, et al. Biological significance of proliferation, apoptosis, cytokines, and monocyte/macrophage cells in bone marrow biopsies of 145 patients with myelodysplastic syndrome. Int J Hematol. 2002;75:289–97. doi: 10.1007/BF02982044. [DOI] [PubMed] [Google Scholar]
- 21.Novotna B, Bagryantseva Y, Siskova M, Neuwirtova R. Oxidative DNA damage in bone marrow cells of patients with low-risk myelodysplastic syndrome. Leuk Res. 2009;33:340–43. doi: 10.1016/j.leukres.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Wei Y, Chen R, Dimicoli S, Bueso-Ramos C, Neuberg D, Pierce S, et al. Global H3K4me3 genome mapping reveals alterations of innate immunity signaling and overexpression of JMJD3 in human myelodysplastic syndrome CD34+ cells. Leukemia. 2013;27:2177–86. doi: 10.1038/leu.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei Y, Dimicoli S, Bueso-Ramos C, Chen R, Yang H, Neuberg D, et al. Toll-like receptor alterations in myelodysplastic syndrome. Leukemia. 2013;27:1832–40. doi: 10.1038/leu.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou L, Opalinska J, Verma A. p38 MAP kinase regulates stem cell apoptosis in human hematopoietic failure. Cell Cycle. 2007;6:534–37. doi: 10.4161/cc.6.5.3921. [DOI] [PubMed] [Google Scholar]
- 25.Keith T, Araki Y, Ohyagi M, Hasegawa M, Yamamoto K, Kurata M, et al. Regulation of angiogenesis in the bone marrow of myelodysplastic syndromes transforming to overt leukaemia. Br J Haematol. 2007;137:206–15. doi: 10.1111/j.1365-2141.2007.06539.x. [DOI] [PubMed] [Google Scholar]
- 26.Cheng CL, Hou HA, Jhuang JY, Lin CW, Chen CY, Tang JL, et al. High bone marrow angiopoietin-1 expression is an independent poor prognostic factor for survival in patients with myelodysplastic syndromes. Br J Cancer. 2011;105:975–82. doi: 10.1038/bjc.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koch K. The design and early development of the p38/Tie2 inhibitor, ARRY-614, in hematologic cancers. Annual Meeting of the American Association for Cancer Research. 2013:ED37. [Google Scholar]
- 28.Winski SL, Freeman BB, Remmers AE, Carter LL, Wright AD, Munson MC, et al. ARRY-614, an inhibitor of p38 MAP kinase and angiogenic targets, is active in preclinical models of hematological malignancies and significantly reduces ex vivo cytokine production in normal human subjects. Targeted Anti-Cancer Therapeutics. 2009 [Google Scholar]
- 29.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 30.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–25. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 31.Navas TA, Mohindru M, Estes M, Ma JY, Sokol L, Pahanish P, et al. Inhibition of overactivated p38 MAPK can restore hematopoiesis in myelodysplastic syndrome progenitors. Blood. 2006;108:4170–77. doi: 10.1182/blood-2006-05-023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahjahan M, Dunphy CH, Ewton A, Zu Y, Monzon FA, Rice L, et al. p38 mitogen-activated protein kinase has different degrees of activation in myeloproliferative disorders and myelodysplastic syndromes. Am J Clin Pathol. 2008;130:635–41. doi: 10.1309/2450EGK3V0XK8D9D. [DOI] [PubMed] [Google Scholar]
- 33.H P, J W, L Z, H L, CC C, Y Z, et al. A systematic modeling study on the pathogenic role of p38 MAPK activation in myelodysplastic syndromes. Mol Biosyst. 2012;8:1366–74. doi: 10.1039/c2mb05184b. [DOI] [PubMed] [Google Scholar]
- 34.Katsoulidis E, Li Y, Yoon P, Sassano A, Altman J, Kannan-Thulasiraman P, et al. Role of the p38 mitogen-activated protein kinase pathway in cytokine-mediated hematopoietic suppression in myelodysplastic syndromes. Cancer Res. 2005;65:9029–37. doi: 10.1158/0008-5472.CAN-04-4555. [DOI] [PubMed] [Google Scholar]
- 35.Lebedeva S, Jens M, Theil K, Schwanhausser B, Selbach M, Landthaler M, et al. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol Cell. 2011;43:340–52. doi: 10.1016/j.molcel.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Mufson R, Gesner TB. Binding and internalization of recombinant human erythropoietin in murine erythroid precursor cells. Blood. 1987;69:1485–90. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


