Abstract
Often seen as the paragon of higher cognition, here we suggest that cognitive control is dependent on emotion. Rather than asking whether control is influenced by emotion, we ask whether control itself can be understood as an emotional process. Reviewing converging evidence from cybernetics, animal research, cognitive neuroscience, and social and personality psychology, we suggest that cognitive control is initiated when goal conflicts evoke phasic changes to emotional primitives that both focus attention on the presence of goal conflicts and energize conflict resolution to support goal-directed behavior. Critically, we propose that emotion is not an inert byproduct of conflict but is instrumental in recruiting control. Appreciating the emotional foundations of control leads to testable predictions that can spur future research.
Keywords: cognitive control, emotion, anterior cingulate cortex, anxiety, motivation
Does cognitive control depend on emotion?
Cognitive control refers to mental processes that allow behavior to vary adaptively depending on current goals. It is multifaceted, with one of its core functions being to override, restrain, or inhibit unwanted yet dominant response tendencies [1]. Cognitive control is recruited during low-level reaction time tasks, but also during complex self-regulatory behaviors [2]. For example, cognitive control could involve inhibiting habitual reading responses on the Stroop task (see Glossary) [3], restraining one’s desire for unhealthy foods [4], or overcoming stereotypical associations about black men [5]. Cognitive control, and the related concepts of self-control and self-regulation [2], thus allows people to restrain their hearts, bodies, and minds away from the temptations of everyday life and to maintain focus on more longstanding goals. As such, cognitive control confers substantial benefits for individuals and society, including prospectively predicting better health, superior academic performance, reduced substance dependence, improved personal finances, and lower rates of criminal offending [6–8].
Cognitive control, in short, promotes the good life. But, what are the factors that prompt control and drive it forward? What are its precise mechanics? Given its myriad benefits, understanding when and how cognitive control is engaged is important. We suggest that negative affect is an integral, instantiating aspect of cognitive control. The goal of this opinion article is to suggest that cognitive control, often seen as the paragon of higher cognition, is dependent on emotion.
According to psychological construction approaches [9,10], so-called basic emotions such as joy or anger can be broken down into more primitive elements, including changes in core affect, physiology, expression, attribution, appraisal, and subjective experience. Together, these emotional primitives combine in varying degrees, with emotion emerging as a result. Here, we propose that cognitive control is initiated when goal conflicts arouse negative affect. This affect, in turn, makes goal conflicts salient and motivates goal-directed behavior that functions to resolve the conflict and minimize its recurrence.
Emotion–cognition interactions
Historically, emotion has been cast as an enemy of cognitive control, undermining any attempts to exercise restraint [11]. However, most contemporary theorists view emotion and cognition as fully integrated, only minimally decomposable, and without clear demarcation in the human brain [12,13].
To be sure, there is a rich tradition of research on the topic of emotion–cognition interactions [14], with specific research on the impact of emotion on control [15]. However, unlike past treatments, which have examined the impact of incidental moods or emotions on control [16,17] or that have examined the impact of cognitive control on the unfolding of emotional experience [18], here we examine the influence of integral emotion on cognitive control –asking not whether cognitive control is influenced by emotion (it is), but whether control itself can be understood as an emotional process.
When considering their implications for cognitive control, it is important to differentiate integral from incidental emotions [15]. As the name implies, incidental emotion is secondary, elicited by some unrelated task or mood manipulation. Integral emotion, by contrast, is elicited by features of the proximal task itself – in this case, the experience of conflict – and as such is essential for signaling the need for greater control. This differs from emotion occurring as an extrinsic factor that might moderate self-control, but operates separately from it. Such incidental emotions can have inconsistent effects on control dynamics [15,19], variously working in concert with control, competing with control, or having no impact [16,17,20].
In contrast to these ambiguous associations, here we review converging evidence from cybernetics, animal research, cognitive neuroscience, and social and personality psychology suggesting that negative affect is an integral aspect of cognitive control, alerting organisms to the need for control and energizing its execution.
What is emotion?
According to a poll of 35 distinguished emotion researchers [21], emotion can be described as consisting of ‘neural circuits (that are at least partially dedicated), response systems, and a feeling state/process that motivates and organizes cognition and action’ (p. 367). This description maps onto the common view that emotion is characterized by an organic mix of subjective experience, changes in physiological arousal, and behavioral expression [22]. It also maps onto the view that emotion prepares an organism to act or to respond to environmental demands [22,23], and that it orients organisms to cues in the environment that signal motivationally important needs and desires [24].
Although emotions have been rigorously studied for many decades, basic questions about the nature of emotion remain unanswered. In particular, there has been much recent debate about whether emotions are natural kinds [10], or whether emotions are more dynamic and fluid phenomena that are psychologically constructed anew each time [9,13]. This debate is beyond the scope of the present article; here, we simply note that there is utility in decomposing emotion to its more primitive constituent elements. In the following sections, we propose that similarly decomposing cognitive control reveals that it comprises the same types of affective, physiological, and experiential constituent elements as prototypical emotions, and thus may resemble an emotional episode [9]. As shown in Figure 1, an emotional episode is like a chain reaction: an instigating or antecedent event produces changes in a host of emotional primitives that, in turn, motivate the execution of goal-directed behavior.
Figure 1.
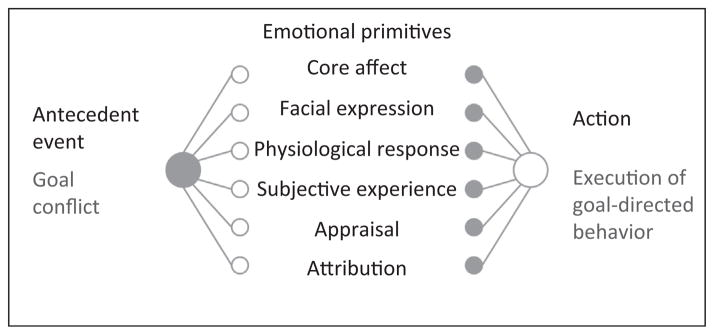
Emotional foundations of control. When decomposed, emotional episodes break down to (i) an antecedent event that (ii) produces changes in a cascade of different emotional primitives, which then (iii) motivate the execution of goal-directed behavior. When cognitive control is similarly decomposed, it becomes apparent that it is constituted by the same types of elements. Specifically, cognitive control is constituted by (i) an antecedent event (goal conflict) that (ii) triggers a host of emotional primitives (including changes in affect, facial expressions, underlying physiology, subjective experience, appraisals, and attributions) that (iii) motivate refocusing on goal-directed behavior (recruitment of control).
Cognitive control begins with conflict
Converging evidence suggests that cognitive conflict instigates control efforts. We define conflict as any disagreement or discrepancy between mental representations, response tendencies, or actual behavior. Cybernetic models, based on simple feedback loops, have been very successful in modeling control [25–27], identifying three main components: (i) goals/set points that act as desired standards with motivational value (Box 1); (ii) comparators/monitors that scan the current state of the environment to detect conflicts with desired set points; and (iii) implementers/effectors that make corrections and adjustment to the current state to reduce the size of state/set point mismatches.
Box 1. Goals, motivations, and cognitive control.
The existence of personal goals is a clear precursor to cognitive control [26]. It is only once a goal is defined that intrapersonal and contextual factors can conflict with and impede goal progress, necessitating the need for corrective action. Goals differ in their importance, however, and goals that are more highly valued are more likely to engage control processes than goals that are less valued [95]. Thus, motivation, which has an intrinsic relationship with the activation and representation of goals [96], plays a fundamental role in cognitive control [97].
Motivation can be defined as an internal state that drives behavior toward a rewarding goal or end point and away from undesirable or punishing outcomes [16]. According to some theorists, emotion and motivation cannot be considered separately from one another [16,75,98], with emotion as the readout or output of some motivated potential [98]. Accordingly, when motivationally relevant goals are at risk of not being met (e.g., during goal conflict), negative affect is produced [58]. More highly valued goals in turn produce more intense motivation, and thus generate more negative affect at the prospect of goal failure.
The motivation and willingness to engage control efforts depends on a trade-off between the expected payoff from a controlled process (e.g., acquiring some desirable reward or avoiding some undesirable punishment) and the perceived cost of engaging control in terms of cognitive effort [56]. When these factors lead individuals to feel motivated, cognitive conflict is particularly salient, resulting in considerable task-related negative affect when control fails or is at risk of failing [58]. This negative affect is not merely output, however, and can result in goal reprioritization and the mobilization of further goal-directed actions [27,41]. Emotion, in our view, is consequently both an output of motivation and an input to the execution of goal-directed behavior.
For example, people who are intrinsically motivated to control prejudice exhibit better control of their biased impulses than those who are not similarly motivated, and do so because of heightened neuroaffective response to their own errors [99]. Similar results have been found with monetary incentives, the advent of choice, and the threat of punishment [84,95,100]. Motivation, in short, directs cognitive control [16,97], with emotion as output of and input to processes that engender control.
Cybernetic principles, especially the principle of a conflict monitor, are found in nearly every model of control. For example, revised reinforcement sensitivity theory, based on animal models and behavioral pharmacology, suggests that behavioral inhibition is instigated by conflict within and between appetitive and aversive motivational systems [28]. Thus, goal conflicts lead to the overriding of ongoing behavior – a form of control – as organisms determine the optimal course of action [28,29]. For example, behavioral inhibition might occur following conflicts between a dieter’s longstanding goal of not gaining weight and the situational goal of consuming sweets [30]; it might also occur when deciding between small immediate rewards and larger delayed rewards [31,32].
Cognitive neuroscience models of control place similar importance on systems responsible for monitoring response conflicts or prediction errors [33,34]. Conflict monitoring theory focuses on how the monitoring system plays an important role in scrutinizing the moment-to-moment representations of action tendencies for potential conflicts so that inhibitory mechanisms may be engaged to override the unwanted tendency and promote effective goal pursuit [33,35]. For example, because reading is an overlearned response for literate adults, the word ‘red’ presented in green font will activate both the urge to read the word (i.e., say ‘red’) and the Stroop goal of naming the color (i.e., say ‘green’), which conflict with one another. When conflict is detected by these systems, a second regulatory system is engaged, biasing behavior toward the goal-relevant response while suppressing incompatible responses. These functions are thought to be implemented by the dorsal anterior cingulate cortex (dACC) and the dorsolateral prefrontal cortex, respectively [36].
Social and personality psychology theories similarly stress the importance of conflict in instigating control, with theorists suggesting that the presence of conflict is the ‘defining feature of self-control phenomena’ ([37], p. 77; [38,39]). Several theories explicitly use feedback loop dynamics to model control. The control of one’s thoughts, for example, requires an operating process that promotes the intended state and a monitoring process that scans for thoughts that are inconsistent or in conflict with the intended state ([40]; see also [27]).
Conflict is aversive
Conflict is thus at the heart of control. Conflict, however, is not affectively neutral. According to some cybernetic theorists, the detection of conflict is accompanied by various affective states, including negative affect when the rate of conflict reduction is too slow [27,41]. According to revised reinforcement sensitivity theory [28], the system that detects conflict not only inhibits ongoing behavior and enhances risk assessment but also produces feelings of worry, caution, and uncertainty [42]. Finally, the classic social psychological concept of cognitive dissonance –referring to the coactivation of conflicting mental representations – has for decades been characterized by its aversive, emotional essence [43,44].
These early perspectives on conflict have been confirmed more recently by cognitive and affective neuroscience, which has made it clear that conflict has an emotional cost [45]. What is more, these emotional costs have not been limited to the types of conflicts that threaten cherished goals or values [46], but have also been found in low-level cognitive tasks containing simple response conflict. For example, when participants are presented with conflict-instigating, incongruent Stroop words as primes, they are quicker to identify negatively valenced targets [47] and to evaluate neutral targets as negative [48] in comparison to when presented with congruent Stroop words. These results imply that conflict produces negative affect. This affect, however, is very short lived [49], implying that the emotional aspect of cognitive control is best characterized as transiently tied to the conflict itself; for minor conflicts, it is more like a quick twinge of affect that arises and dissipates rapidly [50] rather than a full-blown emotion that is slow to rise and slow to fall [51].
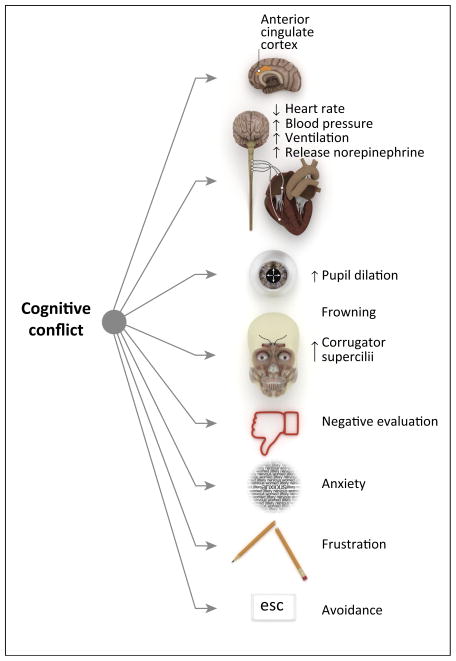
Further evidence for the emotionally aversive nature of conflict comes from work relating response conflict to a number of other emotional primitives (Figure 2). For example, performing laboratory control tasks, such as the Stroop task, increases activity in the sympathetic nervous system [52,53], including heart rate deceleration, as well as increases in blood pressure, ventilation, and plasma norepinephrine. These same types of tasks also produce feelings of anxiety and frustration [54,55]. Such laboratory tasks are high in conflict because they contain both a proportion of high-conflict trials (e.g., ‘red’ presented in green) and also because they lead participants to make errors, which some consider an extreme form of (unresolved) conflict [35,56]. Focusing specifically on the presentation of high-conflict stimuli (but not necessarily errors), other research indicates that conflict increases pupil dilation [57], strengthens contraction of the corrugator supercilii (frowning) muscle in the face [58], and encourages the behavioral tendency to avoid conflict-laden tasks [59].
Figure 2.
Cognitive conflict is aversive. Cognitive conflict is associated with a number of emotional primitives. Performing a laboratory control task, such as the Stroop task, increases activity in the sympathetic nervous system, including heart rate deceleration, as well as increases in blood pressure, ventilation (defined as the product of breathing rate and tidal volume), and plasma norepinephrine. Exposure to high-conflict stimulus (such as the word ‘red’ printed in green) activates the dorsal anterior cingulate cortex (dACC), evokes pupil dilation, leads to contraction of the corrugator supercilii muscle of the face, primes negative evaluation of subsequent stimuli, and produces conscious self-reports of anxiety and frustration. The aversive nature of conflict is also evident because it fosters a behavioral tendency to avoid conflict-laden tasks.
Cognitive conflict is also registered in the human brain, with much work over the past two decades pointing to the dACC as playing a prominent role [33,34,36,56,60]. Much of this work centers around errors and the discovery of an evoked brain potential that accompanies errors, called error-related negativity (ERN; [60]). That the ERN is generated by the dACC is not surprising given the aversive nature of errors and the long-held view that the ACC plays a key role in the evaluation of pain and distress [61,62]. The involvement of the dACC in both conflict and negative affect [63,64] further suggests functional overlap between the two domains.
The reactivity of the dACC to errors is thus likely to represent more than just the cold registration of conflict but may also register as an aversive signal [65], representing a distress- or threat-related response to potential or actual goal failure [19,66]. In support of this, errors in a cognitive task are associated with a host of physiological changes consistent with the mobilization of defensive motives, such as increased skin conductance, greater cardiac reactivity [67], corrugator muscle contraction [58], and increased startle reflexes [68]. Increased ERN amplitudes are likewise observed among people who are most sensitive to negative affect, such as those suffering from anxiety disorders [69,70].
Although the conflict produced by the commission of errors and the presentation of high-conflict stimuli are related, they are not identical. Recent neuroimaging work suggests that although errors and conflict recruit overlapping regions of the presupplementary motor area, they are also distinguishable, with errors especially activating the rostral cingulate zone [71]. This might suggest that although both errors and conflict involve the inhibition of competing motor plans [72], errors might be particularly affective, enlisting stronger emotional responses and, as a result, greater adjustments in subsequent control.
Variation in conflict-related emotion predicts variation in control
Considerable evidence indicates that the experience of conflict is aversive [54,58,68], and that conflict instigates control [36,73]. Does this mean that the affective quality of conflict impels control? Or, is negative affect merely epiphenomenal – a mechanistically inert byproduct of the neural activities that control behavior [74]?
There are many reasons why emotion would play an important role in the engagement of cognitive and behavioral resources to resolve conflicts. Most notably, emotion is especially good at recruiting attention and mobilizing an organism for action [75], even when the attentional system is otherwise occupied [76]. Emotional stimuli preferentially capture attention because they signify the presence of motivationally relevant information [77], and in so doing help promote adaptive behavioral responses [78]. Thus, an incongruent Stroop trial will register as an aversive signal [47], which not only helps make response conflict salient but also helps in preparing corrective actions to manage the conflict.
The idea that negative affect might be instrumental to cognitive control first emerged in research examining the effects of anxiolytic drugs on post-error adjustments in control. Specifically, it is now well known that a moderate dose of alcohol attenuates ERN amplitudes and impairs post-error behavioral adjustment [79]. Similar effects have been reported with other anxiolytic agents, including lorazepam and triazolam, but not with stimulant medications [80,81]. Although these effects were first attributed to drug-induced changes in attention and awareness [79], they are now thought to be a product of the anxiolytic properties of the drugs, with evidence that the effects of alcohol on both the ERN and post-error adjustment are mediated by alcohol-induced reductions in subjective negative affect [82].
Other evidence indicates that when the aversive quality of conflict is muted, control suffers. Conflict adaptation, for example, is eliminated when conflict trials are followed by monetary gain (compared with both neutral and loss conditions) [83]. This suggests that conflict per se is insufficient for instigating control adjustment, with the affective consequences of conflict being crucial.
Additional research indicates that the magnitude of both the ERN and post-error behavioral adjustment are larger when errors are punished than when they are not [84], consistent with the argument that more aversive errors elicit larger dACC activation and instigate greater post-error adjustments in control. Similarly, high-conflict trials during an inhibition task elicit greater activity in the corrugator supercilii muscle, an unambiguous reflection of negative affect [85], than do low-conflict trials [58]. Critically, error-related corrugator activity predicts post-error control adjustments, consistent with the idea that variation in negative affect predicts variation in control. Finally, psychological manipulations that reduce negative affect –by changing cognitive appraisals and attributions – not only directly reduce ERN but also indirectly reduce cognitive control [55,86].
Together, findings from these studies point to a causal role for conflict-related negative affect in instigating control. A functional role for negative affect makes sense when considering that across virtually all mammalian species emotion systems have evolved to motivate adaptive behavior [87].
Emotion is necessary but not sufficient for recruiting control
It is important to note that although conflict-related emotion may be a necessary precursor for control, it is likely to be not sufficient. People high in trait anxiety (Box 2), for example, respond to conflict with heightened emotion but may ruminate and worry about the significance of that emotion instead of using it to motivate corrective behavior [70,88]. There are a variety of ways to regulate affective states, only some of which focus on the source of the emotion, with other solutions focusing on the emotions themselves [89]. Dealing directly with a conflict-laden event through the mobilization of cognitive control is an effective strategy for reducing negative affect. Nonetheless, people may focus on trying to make themselves feel better when they lack the ability to address the conflict itself, or if the emotional intensity of the conflict is too threatening.
Box 2. Trait negative affect and cognitive control.
Accumulating evidence points to an association between trait negative affect (i.e., trait anxiety) and enhanced neural responses to conflict and errors [69,70]. Given that increased neural responses to conflict are associated with the enhancement of cognitive control [36,69], one might be tempted to conclude that highly trait-anxious individuals would be better at recruiting control than less anxious people. Research, however, suggests the opposite: highly anxious people tend to have difficulty controlling their attention and inhibiting task-irrelevant information [88,101]. How can these apparently discrepant sets of findings be reconciled? The answer might lie in the ability to use conflict-related negative affect adaptively.
The heightened sensitivity to punishment and uncertainty that is characteristic of trait-anxious individuals makes them highly susceptible to attentional capture by potential threats in the environment [102]. These potential threats summon attentional resources through bottom-up orienting processes, disrupting pre-existing goal frames. As a result of this persistent capturing of attention by potential threats, highly anxious people have fewer attentional resources available to support active goal maintenance [70] and cognitive control [16]. In effect, highly anxious individuals are less able to efficiently deploy their control resources for two reasons: (i) they have fewer cognitive resources available to support proactive forms of control, leading to greater reliance on less efficient, reactive processes [70,103]; and (ii) the aversive signals arising from cognitive conflict cannot effectively compete with the chorus of task-irrelevant threat signals that they experience. As a result, highly anxious individuals must exert greater cognitive effort simply to perform at the same level as less anxious people [88,104].
People with high levels of trait anxiety also tend to feel overwhelmed by the intensity of their emotions, making it harder to understand and identify the most adaptive behavioral response to their affective signals [105]. Indeed, such people can have difficulty evaluating their own performance, such that negative and positive performance outcomes both produce similar affective responses and dACC activity [106,107]. Consequently, although trait anxiety is associated with greater conflict-related activity in the dACC [69,70], this activity does not necessarily translate to improved cognitive control.
Concluding remarks
Interest in cognitive control has blossomed in the past decade, perhaps unsurprisingly given that it is predictive of so many important life outcomes [6]. Despite this interest, however, answers to basic questions of what control is and how it is initiated remain elusive. The main contribution of this opinion article is to suggest that cognitive control can be understood as an emotional process; emotion, in our view, is an essential component of control, alerting organisms to its need and energizing its execution.
Such a view is not merely descriptive but also generative of several interesting hypotheses. For example, it offers a mechanism by which increasing emotional sensitivity and acceptance – such as through mindfulness meditation training – can increase control [90]. It also provides a mechanism by which pathological deficits in emotional sensitivity, such as in psychopathy [91], may disrupt the engagement of cognitive control. Similarly, given suggestions that BOTOX® (which paralyzes muscles of facial expression) can shape emotional experience [92], BOTOX® injections to the corrugator may interfere with the experience of conflict, and thus control. Likewise, given the provocative work relating the administration of acetaminophen (i.e., Tylenol) to reductions in social pain [93] and perhaps to negative affect more broadly [94], acetaminophen may reduce conflict-related negative affect and impair control as a consequence. More generally, a novel prediction of the present framework is that any manipulation that disrupts the emotional primitives that comprise conflict-related negative affect should also disrupt the effective engagement of cognitive control in response to that conflict.
Although it has traditionally been cast as the enemy of control, affect appears crucial to its instigation. We suspect that a greater appreciation of the integral role of affect in higher mental operations will help the field gain a richer understanding of cognitive control and stimulate future research.
Acknowledgments
This research was supported by grants from the Natural Sciences and Engineering Research Council of Canada and the Ontario Ministry of Research and Innovation to M.I., and by grant R21 AA017282 from the National Institute on Alcohol Abuse and Alcoholism to B.D.B. We thank Blair Saunders, Elliot Berkman, Greg Hajcak Proudfit, Nathaniel Elkins-Brown, Elizabeth Page-Gould, William Cunningham, Rimma Teper, and Naomi Sarah Ball for valuable insights and help along the way.
Glossary
- Affect
is an umbrella term for psychological states that involve valuation, or a ‘good-for-me’ versus ‘bad-for-me’ judgment. Affective states include transient states, full-blown emotional states with clear instigators, and more diffuse mood states where the instigator in unclear
- Anterior cingulate cortex (ACC)
is a thick belt of cortex that surrounds the corpus callosum. Although early research segregated functions of the ACC into a more emotional rostral part and a more cognitive dorsal part, more recent integrationist accounts indicate that negative affect, pain, and cognitive control activate overlapping regions of the dorsal ACC (dACC)
- Conflict adaptation
is the phenomenon whereby response times on high-conflict trials in speeded reaction time tasks tend to be faster and less error-prone when they follow a high-conflict trial in comparison to when they follow a low-conflict trial. Conflict adaptation is thought to occur because the experience of conflict prompts an increase in regulatory control processes that result in improved performance on the next trial
- Core affect
is an emotional primitive that is thought to be at the core of all emotion-laden events and refers to a nonreflective feeling state characterized by a blend of valence (pleasure–displeasure) and arousal (sleepy–activated)
- Corrugator supercilii
is a small narrow muscle close to the eye, located at the medial end of the eyebrow. It draws the eyebrow downward and medially, resulting in the appearance of frowning. The corrugator is regarded as the principal muscle in the expression of negative affect
- Emotional primitives
are the most basic and simple building blocks of emotion, including changes in core affect, physiology, and subjective conscious experience. The concept of emotional primitives acknowledges that emotions can be broken down into more basic elements or dimensions and runs counter to the idea of emotions as irreducible natural types
- Error-related negativity (ERN)
is a sharp, negative-going evoked electrical brain potential measured through electroencephalography (EEG) that is time-locked to the commission of an error. Peaking around 50–100 ms after an erroneous response, the ERN is thought to be generated by the ACC and to reflect an evaluative aspect of conflict or error detection
- Post-error slowing (or post-error adjustment)
refers to the phenomenon whereby response times and accuracy tend to increase following errors, thought to reflect the strategic engagement of controlled responding when desired outcomes are not occurring
- Stroop task
consists of color word stimuli that are presented in font colors that are either congruent (e.g., the word ‘red’ presented in red) or incongruent (e.g., the word ‘red’ presented in green) with the semantic meaning of the word. During the task, participants are asked to name the color of the word, but not to read it. Because incongruent trials involve conflict between the prepotent reading response and the task-appropriate color-naming response, such trials typically generate longer reaction times and more errors
References
- 1.Miyake A, et al. The unity and diversity of executive functions and their contributions to complex ‘Frontal Lobe’ tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann W, et al. Executive functions and self-regulation. Trends Cogn Sci. 2012;16:174–180. doi: 10.1016/j.tics.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 3.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 4.Nederkoorn C, et al. Control yourself or just eat what you like? Weight gain over a year is predicted by an interactive effect of response inhibition and implicit preference for snack foods. Heal Psychol. 2010;29:389–393. doi: 10.1037/a0019921. [DOI] [PubMed] [Google Scholar]
- 5.Payne BK. Conceptualizing control in social cognition: how executive functioning modulates the expression of automatic stereotyping. J Pers Soc Psychol. 2005;89:488–503. doi: 10.1037/0022-3514.89.4.488. [DOI] [PubMed] [Google Scholar]
- 6.Moffitt TE, et al. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci USA. 2011;108:2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duckworth AL, Seligman MEP. Self-discipline outdoes IQ in predicting academic performance of adolescents. Psychol Sci. 2005;16:939–944. doi: 10.1111/j.1467-9280.2005.01641.x. [DOI] [PubMed] [Google Scholar]
- 8.Hall PA, Fong GT. Conscientiousness versus executive function as predictors of health behaviors and health trajectories. Ann Behav Med. 2013;45:398–399. doi: 10.1007/s12160-012-9466-2. [DOI] [PubMed] [Google Scholar]
- 9.Russell J. Core affect and the psychological construction of emotion. Psychol Rev. 2003;110:145–172. doi: 10.1037/0033-295x.110.1.145. [DOI] [PubMed] [Google Scholar]
- 10.Barrett LF. Are emotions natural kinds? Perspect Psychol Sci. 2006;1:28–58. doi: 10.1111/j.1745-6916.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- 11.Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychol Rev. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- 12.Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- 13.Lindquist K, Barrett LF. A functional architecture of the human brain: emerging insights from the science of emotion. Trends Cogn Sci. 2012;16:533–540. doi: 10.1016/j.tics.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson MD, et al. Handbook of Cognition and Emotion. Guilford Press; 2013. [Google Scholar]
- 15.Schmeichel B, Inzlicht M. Incidental and integral effects of emotions on self-control. In: Robinson MD, et al., editors. Handbook of Cognition and Emotion. Guilford Press; 2013. pp. 272–290. [Google Scholar]
- 16.Pessoa L. How do emotion and motivation direct executive control? Trends Cogn Sci. 2009;13:160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray JR. Integration of emotion and cognitive control. Curr Dir Psychol Sci. 2004;13:46–48. [Google Scholar]
- 18.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Proudfit GH, et al. Anxiety and error monitoring: the importance of motivation and emotion. Front Hum Neurosci. 2013;7:636. doi: 10.3389/fnhum.2013.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larson MJ, et al. What are the influences of orthogonally-manipulated valence and arousal on performance monitoring processes? The effects of affective state. Int J Psychophysiol. 2013;87:327–339. doi: 10.1016/j.ijpsycho.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Izard CE. The many meanings/aspects of emotion: definitions, functions, activation, and regulation. Emot Rev. 2010;2:363–370. [Google Scholar]
- 22.Levenson RW. Human emotion: a functional view. In: Ekman P, Davidson RJ, editors. The Nature of Emotion: Fundamental Questions. Oxford University Press; 1994. pp. 123–126. [Google Scholar]
- 23.Frijda NH. The laws of emotion. Am Psychol. 1988;43:349–358. doi: 10.1037//0003-066x.43.5.349. [DOI] [PubMed] [Google Scholar]
- 24.Bradley MM. Natural selective attention: orienting and emotion. Psychophysiology. 2009;46:1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiener N. Cybernetics or Control and Communication in the Animal and the Machine. John Wiley; 1948. [Google Scholar]
- 26.Inzlicht M, et al. Exploring the mechanisms of self-control improvement. Curr Dir Psychol Sci. 2014;23:302–307. [Google Scholar]
- 27.Carver CS, Scheier MF. On the Self-Regulation of Behavior. Cambridge University Press; 1998. [Google Scholar]
- 28.Gray JA, McNaughton N. The Neuropsychology of Anxiety: An Enquiry Into the Functions of the Septo-hippocampal System. Oxford University Press; 2000. [Google Scholar]
- 29.Corr PJ. The Reinforcement Sensitivity Theory of Personality. Cambridge University Press; 2008. [Google Scholar]
- 30.Stroebe W, et al. Why dieters fail: testing the goal conflict model of eating. J Exp Soc Psychol. 2008;44:26–36. [Google Scholar]
- 31.Hoch SJ, Loewenstein GF. Time-inconsistent preferences and consumer self-control. J Consum Res. 1991;17:492. [Google Scholar]
- 32.Mischel W, et al. Delay of gratification in children. Science. 1989;244:933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- 33.Botvinick MM, et al. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 34.Holroyd CB, Coles MGH. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- 35.Yeung N, et al. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- 36.Kerns JG, et al. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 37.Hofmann W, Kotabe H. A general model of preventive and interventive self-control. Soc Personal Psychol Compass. 2012;6:707–722. [Google Scholar]
- 38.Myrseth KOR, Fishbach A. Self-control: a function of knowing when and how to exercise restraint. Curr Dir Psychol Sci. 2009;18:247–252. [Google Scholar]
- 39.Baumeister RF. Yielding to temptation: reflections and reviews impulsive purchasing, and consumer behavior. J Consum Res. 2002;28:670–677. [Google Scholar]
- 40.Wegner DM. Ironic processes of mental control. Psychol Rev. 1994;101:34–52. doi: 10.1037/0033-295x.101.1.34. [DOI] [PubMed] [Google Scholar]
- 41.Carver CS, Scheier MF. Self-regulation of action and affect. In: Vohs KD, Baumeister RF, editors. Handbook of Self-regulation: Research, Theory, and Applications. 2. Guilford Press; 2011. pp. 3–21. [Google Scholar]
- 42.Hirsh JB, et al. Psychological entropy: a framework for understanding uncertainty-related anxiety. Psychol Rev. 2012;119:304–320. doi: 10.1037/a0026767. [DOI] [PubMed] [Google Scholar]
- 43.Zanna MP, Cooper J. Dissonance and the pill: an attribution approach to studying the arousal properties of dissonance. J Pers Soc Psychol. 1974;29:703–709. doi: 10.1037/h0036651. [DOI] [PubMed] [Google Scholar]
- 44.Elliot AJ, Devine PG. On the motivational nature of cognitive dissonance: dissonance as psychological discomfort. J Pers Soc Psychol. 1994;67:382–394. [Google Scholar]
- 45.Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- 46.Proulx T, et al. Understanding all inconsistency compensation as a palliative response to violated expectations. Trends Cogn Sci. 2012;16:285–291. doi: 10.1016/j.tics.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Dreisbach G, Fischer R. Conflicts as aversive signals. Brain Cogn. 2012;78:94–98. doi: 10.1016/j.bandc.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Fritz J, Dreisbach G. Conflicts as aversive signals: conflict priming increases negative judgments for neutral stimuli. Cogn Affect Behav Neurosci. 2013;13:311–317. doi: 10.3758/s13415-012-0147-1. [DOI] [PubMed] [Google Scholar]
- 49.Fritz J, Dreisbach G. The time course of the aversive conflict signal. Exp Psychol. 2014 doi: 10.1027/1618-3169/a000271. Published online September 30, 2014 http://dx.doi.org/10.1027/1618-3169/a000271. [DOI] [PubMed]
- 50.Winkielman P, Berridge KC. Unconscious emotion. Curr Dir Psychol Sci. 2004;13:120–123. [Google Scholar]
- 51.Baumeister RF, et al. How emotion shapes behavior: feedback, anticipation, and reflection, rather than direct causation. Pers Soc Psychol Rev. 2007;11:167–203. doi: 10.1177/1088868307301033. [DOI] [PubMed] [Google Scholar]
- 52.Hoshikawa Y, Yamamoto Y. Effects of Stroop on the autonomic color-word conflict test nervous system responses. Am J Physiol. 1997;272:H1113–H1121. doi: 10.1152/ajpheart.1997.272.3.H1113. [DOI] [PubMed] [Google Scholar]
- 53.Critchley HD, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 54.Spunt RP, et al. The phenomenology of error processing: the dorsal ACC response to stop-signal errors tracks reports of negative affect. J Cogn Neurosci. 2012;24:1753–1765. doi: 10.1162/jocn_a_00242. [DOI] [PubMed] [Google Scholar]
- 55.Inzlicht M, Al-Khindi T. ERN and the placebo: a misattribution approach to studying the arousal properties of the error-related negativity. J Exp Psychol Gen. 2012;141:799–807. doi: 10.1037/a0027586. [DOI] [PubMed] [Google Scholar]
- 56.Shenhav A, et al. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Steenbergen H, Band GPH. Pupil dilation in the Simon task as a marker of conflict processing. Front Hum Neurosci. 2013;7:215. doi: 10.3389/fnhum.2013.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindström BR, et al. In your face: risk of punishment enhances cognitive control and error-related activity in the corrugator supercilii muscle. PLoS ONE. 2013;8:e65692. doi: 10.1371/journal.pone.0065692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kool W, et al. Decision making and the avoidance of cognitive demand. J Exp Psychol Gen. 2010;139:665–682. doi: 10.1037/a0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gehring WJ, et al. The error-related negativity (ERN/Ne) In: Luck SJ, Kappenman ES, editors. The Oxford Handbook of Event-related Potential Components. Oxford University Press; 2011. pp. 231–291. [Google Scholar]
- 61.Rainville P, et al. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 62.Mobbs D, et al. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc Natl Acad Sci USA. 2010;107:20582–20586. doi: 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shackman AJ, et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koban L, Pourtois G. Brain systems underlying the affective and social monitoring of actions: an integrative review. Neurosci Biobehav Rev. 2014;46P1:71–84. doi: 10.1016/j.neubiorev.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 65.Aarts K, et al. Erroneous and correct actions have a different affective valence: evidence from ERPs. Emotion. 2013;13:960–973. doi: 10.1037/a0032808. [DOI] [PubMed] [Google Scholar]
- 66.Bartholow BD, et al. Strategic control and medial frontal negativity: beyond errors and response conflict. Psychophysiology. 2005;42:33–42. doi: 10.1111/j.1469-8986.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- 67.Hajcak G, et al. To err is autonomic: error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology. 2003;40:895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- 68.Hajcak G, Foti D. Errors are aversive: defensive motivation and the error-related negativity. Psychol Sci. 2008;19:103–108. doi: 10.1111/j.1467-9280.2008.02053.x. [DOI] [PubMed] [Google Scholar]
- 69.Cavanagh JF, Shackman AJ. Frontal midline theta reflects anxiety and cognitive control: meta-analytic evidence. J Physiol Paris. 2014 doi: 10.1016/j.jphysparis.2014.04.003. Published online April 29, 2014 http://dx.doi.org/10.1016/j.jphysparis.2014.04.003. [DOI] [PMC free article] [PubMed]
- 70.Moser J, et al. Anxiety and error monitoring: a meta-analysis and conceptual framework. Front Hum Neurosci. 2013;7:466. doi: 10.3389/fnhum.2013.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iannaccone R, et al. Conflict monitoring and error processing: new insights from simultaneous EEG–fMRI. Neuroimage. 2014;105:395–407. doi: 10.1016/j.neuroimage.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 72.Nachev P, et al. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 73.Gratton G, et al. Optimizing the use of information: strategic control of activation of responses. J Exp Psychol Gen. 1992;121:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- 74.Yeung N. Relating cognitive and affective theories of the error-related negativity. In: Ullsperger M, Falkenstein M, editors. Errors, Conflicts, and the Brain: Current Opinions on Performance Monitoring. Max Planck Institute of Cognitive Neuroscience; 2004. pp. 63–70. [Google Scholar]
- 75.Bradley MM, et al. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- 76.Anderson AK. Affective influences on the attentional dynamics supporting awareness. J Exp Psychol Gen. 2005;134:258–281. doi: 10.1037/0096-3445.134.2.258. [DOI] [PubMed] [Google Scholar]
- 77.Weinberg A, Hajcak G. Beyond good and evil: the time-course of neural activity elicited by specific picture content. Emotion. 2010;10:767–782. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- 78.Damasio A. Descartes’ Error: Emotion, Reason and the Human Brain. Random House; 1994. [Google Scholar]
- 79.Ridderinkhof KR, et al. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298:2209–2211. doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- 80.DeBruijn ERA, et al. Drug-induced stimulation and suppression of action monitoring in healthy volunteers. Psychopharmacology (Berl) 2004;177:151–160. doi: 10.1007/s00213-004-1915-6. [DOI] [PubMed] [Google Scholar]
- 81.McNaughton N, et al. Anti-anxiety drugs reduce conflict-specific ‘theta’ – a possible human anxiety-specific biomarker. J Affect Disord. 2013;15:104–111. doi: 10.1016/j.jad.2012.11.057. [DOI] [PubMed] [Google Scholar]
- 82.Bartholow BD, et al. Alcohol effects on performance monitoring and adjustment: affect modulation and impairment of evaluative cognitive control. J Abnorm Psychol. 2012;121:173–186. doi: 10.1037/a0023664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Steenbergen H, et al. Reward counteracts conflict adaptation. Evidence for a role of affect in executive control. Psychol Sci. 2009;20:1473–1477. doi: 10.1111/j.1467-9280.2009.02470.x. [DOI] [PubMed] [Google Scholar]
- 84.Riesel A, et al. Punishment has a lasting impact on error-related brain activity. Psychophysiology. 2012;49:239–247. doi: 10.1111/j.1469-8986.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- 85.Larsen JT, et al. Effects of positive and negative affect on electromyographic activity over zygomaticus major and corrugator supercilii. Psychophysiology. 2003;40:776–785. doi: 10.1111/1469-8986.00078. [DOI] [PubMed] [Google Scholar]
- 86.Hobson NM, et al. Emotion down-regulation diminishes cognitive control: a neurophysiological investigation. Emotion. 2014;14:1014–1026. doi: 10.1037/a0038028. [DOI] [PubMed] [Google Scholar]
- 87.Panksepp J, Biven L. The Archaeology of Mind: Neuroevolutionary Origins of Human Emotions. W.W. Norton; 2012. [Google Scholar]
- 88.Eysenck MW, Derakshan N. New perspectives in attentional control theory. Pers Individ Dif. 2011;50:955–960. [Google Scholar]
- 89.Lazarus RS, Folkman S. Stress, Appraisal, and Coping. Springer; 1984. [Google Scholar]
- 90.Teper R, et al. Inside the mindful mind: how mindfulness enhances emotion regulation through improvements in executive control. Curr Dir Psychol Sci. 2013;22:449–454. [Google Scholar]
- 91.Blair J, et al. The Psychopath: Emotion and the Brain. Blackwell; 2005. [Google Scholar]
- 92.Davis JI, et al. The effects of BOTOX injections on emotional experience. Emotion. 2010;10:433–440. doi: 10.1037/a0018690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dewall CN, et al. Acetaminophen reduces social pain: behavioral and neural evidence. Psychol Sci. 2010;21:931–937. doi: 10.1177/0956797610374741. [DOI] [PubMed] [Google Scholar]
- 94.Randles D, et al. The common pain of surrealism and death: acetaminophen reduces compensatory affirmation following meaning threats. Psychol Sci. 2013;24:966–973. doi: 10.1177/0956797612464786. [DOI] [PubMed] [Google Scholar]
- 95.Legault L, Inzlicht M. Self-determination, self-regulation, and the brain: autonomy improves performance by enhancing neuroaffective responsiveness to self-regulation failure. J Pers Soc Psychol. 2013;105:123–138. doi: 10.1037/a0030426. [DOI] [PubMed] [Google Scholar]
- 96.Kruglanski AW, et al. A theory of goal systems. Adv Exp Soc Psychol. 2002;34:331–378. [Google Scholar]
- 97.Chiew KS, Braver TS. Positive affect versus reward: emotional and motivational influences on cognitive control. Front Psychol. 2011;2:279. doi: 10.3389/fpsyg.2011.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Buck R. The biological affects: a typology. Psychol Rev. 1999;106:301–336. doi: 10.1037/0033-295x.106.2.301. [DOI] [PubMed] [Google Scholar]
- 99.Amodio DM, et al. Individual differences in the regulation of intergroup bias: the role of conflict monitoring and neural signals for control. J Pers Soc Psychol. 2008;94:60–74. doi: 10.1037/0022-3514.94.1.60. [DOI] [PubMed] [Google Scholar]
- 100.Boksem MAS, et al. Mental fatigue, motivation and action monitoring. Biol Psychol. 2006;72:123–132. doi: 10.1016/j.biopsycho.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 101.Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci. 2008;12:92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- 102.Bar-Haim Y, et al. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 103.Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn Sci. 2012;16:106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lyons IM, Beilock SL. Mathematics anxiety: separating the math from the anxiety. Cereb Cortex. 2012;22:2102–2110. doi: 10.1093/cercor/bhr289. [DOI] [PubMed] [Google Scholar]
- 105.Mennin DS, et al. Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder. Behav Res Ther. 2005;43:1281–1310. doi: 10.1016/j.brat.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 106.Aarts K, et al. Evidence for the automatic evaluation of self-generated actions. Cognition. 2012;124:117–127. doi: 10.1016/j.cognition.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 107.Aarts K, Pourtois G. Anxiety disrupts the evaluative component of performance monitoring: an ERP study. Neuropsychologia. 2012;50:1286–1296. doi: 10.1016/j.neuropsychologia.2012.02.012. [DOI] [PubMed] [Google Scholar]