Summary
Myoclonic twitches are jerky movements that occur exclusively and abundantly during active (or REM) sleep in mammals, especially in early development [1–4]. In rat pups, limb twitches exhibit a complex spatiotemporal structure that changes across early development [5]. However, it is not known whether this developmental change is influenced by sensory experience, which is a prerequisite to the notion that sensory feedback from twitches not only activates sensorimotor circuits, but modifies them [4]. Here we investigated the contributions of proprioception to twitching in newborn ErbB2 conditional knockout mice that lack muscle spindles and grow up to exhibit dysfunctional proprioception [6–8]. High-speed videography of forelimb twitches unexpectedly revealed a category of reflex-like twitching—comprising an agonist twitch followed immediately by an antagonist twitch—that developed postnatally in wild types/heterozygotes but not in knockouts. Contrary to evidence from adults that spinal reflexes are inhibited during twitching [9–11], this finding suggests that twitches trigger the monosynaptic stretch reflex and, by doing so, contribute to its activity-dependent development [12–14]. Next, we assessed developmental changes in the frequency and organization (i.e., entropy) of more complex, multi-joint patterns of twitching; again, wild types/heterozygotes exhibited developmental changes in twitch patterning that were not seen in knockouts. Thus, targeted deletion of a peripheral sensor alters the normal development of local and global features of twitching, demonstrating that twitching is shaped by sensory experience. These results also highlight the potential use of twitching as a uniquely informative diagnostic tool for assessing the functional status of spinal and supraspinal circuits.
Keywords: sleep, myoclonic twitching, muscle spindle, proprioception, spinal circuitry, sensorimotor, monosynaptic stretch reflex, knockout, ErbB2, neurotrophin, activity-dependent development
Results and Discussion
Contrary to the longstanding view of twitching as an aimless, disjointed, and uncoordinated by-product of a dreaming brain [15], it is in fact a complex behavior with its own developmental dynamics [5]. This new perspective raises the question of whether the development of twitching is shaped by sensory experience, particularly in early infancy when active sleep and twitching are prominently expressed [1, 2, 16]. Muscle-specific ErbB2 knockouts provide a unique opportunity to address this question: Few if any muscle spindles are present at birth and they are completely lacking by postnatal day (P) 9 [6]. This phenotype arises because normal spindle development requires activation of ErbB2 receptors, located on spindles, by neuregulin 1 (Nrg1) released from Ia afferents [6, 7, 17]. Muscle-specific ErbB2 knockouts survive to adulthood [6], at which time they exhibit abnormal limb extension reflexes and ataxia [6–8]. However, motor outflow is spared in these knockouts, so twitching continues to be expressed. Thus, by comparing twitching in wild types (WTs) and heterozygotes (Hets) with that of knockouts (KOs) across the early postnatal period, the developmental contributions of muscle spindles to twitching can be revealed.
To confirm the phenotypes of WTs, Hets, and KOs, we analyzed skeletal muscles for the presence of spindles (see Supplemental Information). As expected [6], whereas spindles were readily observed in the WTs and Hets, none was observed in the KOs. Next, we used high-speed videography and 3D motion tracking to assess twitching in P4 and P10 mice. At P4, 9 WTs, 11 Hets, and 7 KOs yielded 37–54 video segments containing 4020-6985 twitches per genotype. At P10, 7 WTs, 8 Hets, and 8 KOs yielded 40–47 video segments containing 1824–2176 twitches per genotype.
The analyses below focus exclusively on shoulder (adduction, abduction) and elbow (flexion, extension) twitches in the left and right forelimbs. We first perform pairwise analyses of twitch movements, followed by an analysis of more complex patterns of multi-joint twitching.
Absence of muscle spindles differentially affects agonist-antagonist twitches
We first assessed genotype- and age-related differences in the organization of twitching by computing inter-twitch intervals (ITIs) and plotting their frequency distributions. After initial review of the data, we focused our attention on ITIs for four categories of twitch pairs. Each pair was composed of an initial “agonist” twitch and (i) a succeeding twitch that occurred within the same joint but in the opposite direction (“agonist-antagonist twitches”; e.g., right elbow flexion → right elbow extension; Movie S1), (ii) a succeeding twitch that was a repeat of the first (“agonist-agonist twitches”; e.g., right elbow flexion → right elbow flexion), (iii) a succeeding twitch that occurred within the same limb but at the other joint (e.g., right elbow flexion → right shoulder abduction), and (iv) a succeeding twitch that occurred in the other limb in a homologous fashion (e.g., right elbow flexion → left elbow flexion). The ITI distributions for the last two twitch pairs exhibited negligible genotypic differences at P4 and P10 and so are not discussed further (Figure S1).
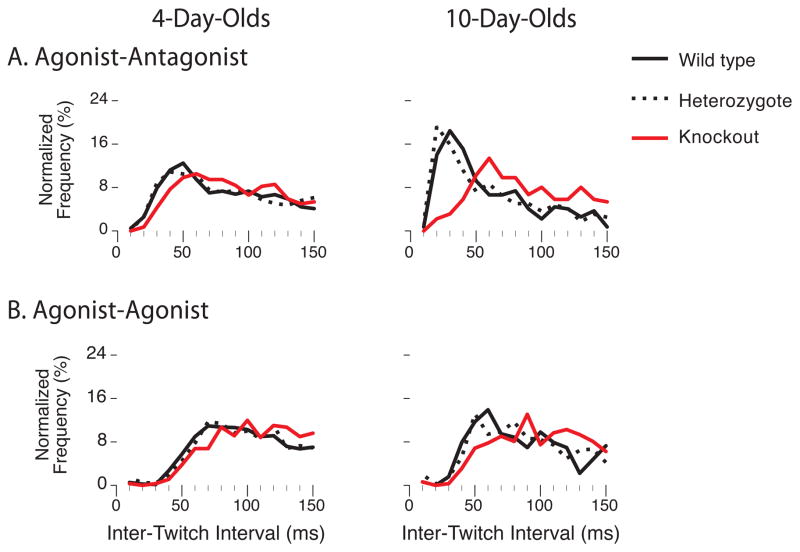
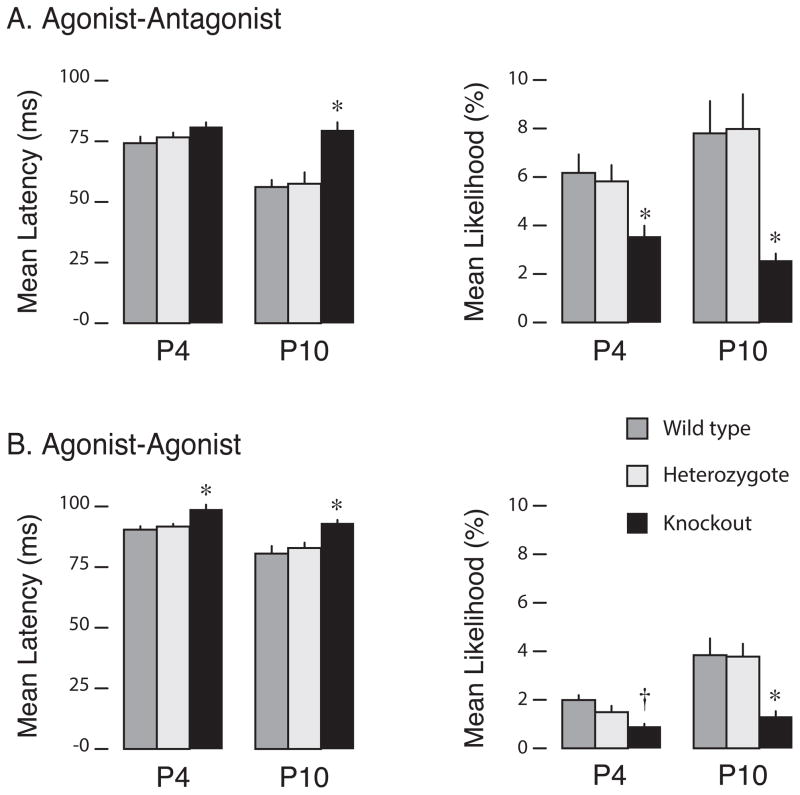
For agonist-antagonist twitches, the three genotypes segregate substantially between P4 and P10, with the P10 WTs and Hets—but not the KOs—exhibiting a pronounced developmental increase in frequency at short ITIs (Figure 1A). These differences were assessed statistically by performing two-factor (age, genotype) between subject analysis of variance (ANOVA) on mean twitch latencies computed over a 150-ms window. There was a marked decrease in latency at P10, but only in the WTs and Hets (Figure 2A, left). The ANOVA revealed significant main effects of age (F[1,44] = 27.2, p < 0.001) and genotype (F[2,44] = 14.2, p < 0.001) and a significant age × genotype interaction (F[2,44] = 5.2, p < 0.01). Next, we tested the mean likelihood that an antagonist twitch occurred within 50 ms of the agonist (Figure 2A, right). We found genotypic differences at both ages; by P10, agonist-antagonist twitches were 4x more likely in the WTs and Hets than in the KOs. ANOVA revealed a significant main effect of genotype (F[2,44] = 16.6, p < 0.001).
Figure 1. Frequency distributions of inter-twitch intervals for wild type, heterozygous, and knockout mice at 4 and 10 days of age.
Inter-twitch intervals were restricted to one of two movement categories. For each category, the first twitch of a pair could occur at any joint, whereas the second immediately succeeding twitch was (A) an antagonist twitch (e.g., right elbow flexion → right elbow extension) or (B) a repeat of the first twitch (e.g., right elbow flexion → right elbow flexion). Frequencies were normalized over the 150-ms window. P4: Ns = 3995–6931 ITIs; P10: Ns = 1784–2131 ITIs.
Figure 2. Mean latencies and likelihoods for (A) agonist-antagonist and (B) agonist-agonist twitch pairs in wild type, heterozygous, and knockout mice at 4 (P4) and 10 (P10) days of age.
These movement categories correspond to those in Figure 1. Mean latencies between twitch pairs were calculated over 150-ms windows (and thus are longer than the peak latencies observed in Figure 1) and mean likelihoods of each pair were calculated over a 0- to 50-ms window. Means were calculated across individual pups (N = 7–11 pups per group). * significant difference from both WT and Het; † significant difference from WT only. Mean + SEM.
For the second category of twitch pairs—the agonist-agonist twitches—the genotypic differences were small, especially at ITIs less than 50 ms (Figure 1B). KOs exhibited slightly longer mean latencies than the WTs and Hets at both ages, with latencies in all three genotypes decreasing slightly with age (Figure 2B, left); ANOVA revealed significant main effects of age (F[1,44] = 29.9, p < 0.001) and genotype (F[2,44] = 17.9, p < 0.001). Conversely, the mean likelihood that an agonist-agonist twitch occurred within 50 ms was lower for the KOs at both ages, with likelihoods in all three genotypes increasing with age (Figure 2B, right); again, ANOVA revealed significant main effects of age (F[1,44] = 21.2, p < 0.001) and genotype (F[2,44] = 14.6, p < 0.001).
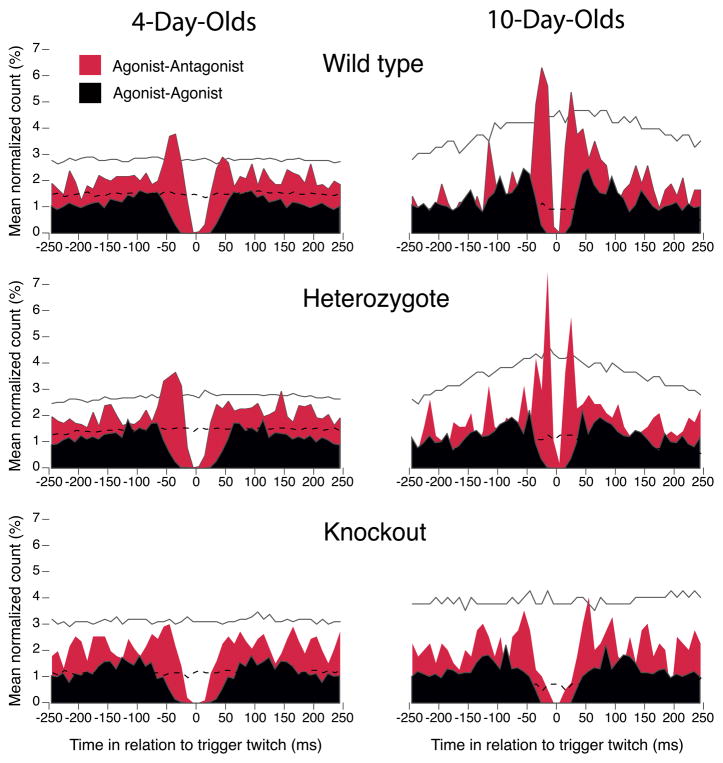
To better visualize differences in agonist-antagonist and agonist-agonist twitching across age and genotype, we constructed perievent histograms from data pooled across subjects (Figure 3). As shown previously [5], strongly coupled twitch pairs exhibit pronounced peaks on either side of the “trigger” twitch at 0 ms (preceding and following peaks occur depending on whether the trigger twitch is the second or first twitch in the pair, respectively). For the agonist-antagonist pairs in the WTs and Hets, such peaks were weak at P4 and pronounced by P10; note the clustering of the peaks within 50 ms of the trigger and the narrow peri-trigger gaps. However, this developmental progression was not seen in the KOs—peaks were weak or absent at both ages in this genotype. In contrast, perievent histograms of agonist-agonist twitches exhibited weak or absent peaks and wide peri-trigger gaps in all three genotypes at both ages. The contrast between the two types of twitch pairs is striking because, at the completion of each initial agonist twitch, it is theoretically equiprobable that the second twitch will be an antagonist or an agonist. That they are not, in fact, equiprobable leads us to conclude that the early postnatal development of agonist-antagonist twitches uniquely depends upon the presence of muscle spindles.
Figure 3. Perievent histograms comparing temporal pairwise relationships for agonist-antagonist (red) and agonist-agonist (black) twitches for wild type, heterozygous, and knockout mice at 4 and 10 days of age.
Perievent histograms were computed from data pooled across subjects. Significant bins for the agonist-antagonist plots are indicated by upper and lower confidence bands (solid and dashed black lines; p < 0.01). The agonist-agonist perievent histograms exhibit mirror imaging because they are auto-correlations. See Supplemental Experimental Procedures for details.
In trying to account for the spatiotemporal structure of twitching in newborn rats in a previous report [5], we discounted the possibility that sensory feedback triggers reflex-mediated activation of twitches. This view derived, in part, from evidence in adult cats that monosynaptic and polysynaptic spinal reflexes are powerfully inhibited during twitching ([9–11]; see also [18]). The present results led us to reconsider a role for reflexes during twitching.
When a skeletal muscle is stretched and muscle spindles are activated, spinally projecting Ia afferents activate motoneurons projecting back to that same muscle (or a related one). In this way, the monosynaptic stretch reflex counteracts the stretch on the muscle and controls muscle length. But, do limb twitches, by contracting one muscle and stretching an opposing one, trigger spinally mediated reflexive reactions to result in opposite-going twitches? There are several reasons to believe that they might. First, agonist-antagonist twitches developed substantially in the WTs and Hets between P4 and P10, consistent with evidence that the monosynaptic reflex circuit strengthens over the early postnatal period [13, 14, 19]. Second, adult ErbB2 KOs exhibit deficient hindlimb extension reflexes as well as a near-complete absence of Ia afferent connections with spinal motoneurons [6, 8]; accordingly, if the stretch reflex were indeed triggered during twitching, one would expect that, of all four twitch pairs, the agonist-antagonist twitches would be most affected by the absence of muscle spindles, which was clearly the case. Third, the peak latency from the first (agonist) to the second (antagonist) twitch was approximately 30 ms (Figure 1A), which is both shorter than the latency for agonist-agonist twitches (Figure 1B) and longer than the latencies for the two other twitch pairs (Figure S1); in other words, the timing of agonist-antagonist twitches is unique in relation to the other twitch pairs.
Given this evidence of reflexive twitches in the WTs and Hets, we returned to an earlier dataset comprising typically developing rats [1] to determine whether they exhibit similar patterns of twitching across the first postnatal week (although it should be noted that P2 and P8 rats may be more functionally similar to P0 and P7 mice, respectively; see http://www.translatingtime.net/translate). Figure S2 compares the ITIs of agonist-antagonist and agonist-agonist twitches at P2 and P8. At both ages, the rats exhibit agonist-antagonist twitches that peak at shorter ITIs than do the agonist-agonist twitches. Thus, these data in rats parallel those reported here in mice (see Figures 1 and 3). It also appears that agonist-antagonist twitching is more developed at P2 in rats than at P4 in mice. In fact, the peak latency of approximately 50 ms for agonist-antagonist twitches in the P2 rats is close to the reported latency of 69.7+ 9.2 ms for the monosynaptic stretch reflex in P0-1 rats [20], thus providing additional evidence that agonist-antagonist twitches are produced by a reflex mechanism.
Latent Class Analysis of Multi-Joint Twitch Patterns
Whereas the analyses thus far have focused on pairwise movements, twitching also comprises complex multi-joint combinations of actions [1]. Moreover, these complex combinations exhibit large-scale developmental changes that are consistent with a selectionist scheme: Complex twitches that are frequently expressed early in development become better organized later in development, and twitches that are better organized early in development become more frequent later in development. Our question here is whether these developmental changes are altered in ErbB2 KOs due to diminished proprioceptive feedback.
Using latent class analysis (LCA), we first identified and characterized the complex twitch patterns across age and genotype. To do this, we conducted six separate LCA analyses on the WT, Het, and KO mice at each age. These analyses were conducted on windowed datasets in which, for each 50-ms increment, we designated whether each of the 8 possible twitch movements (2 movements x 2 joints x 2 limbs) did or did not occur. The resulting vectors were pooled across all subjects within a given age and genotype (litter was used as a random effect). From this, LCA yielded a set of clusters, each representing a distinct pattern of twitch movements. Moreover, each cluster was associated with a frequency of occurrence and a vector of the likelihoods that each joint movement belonged to the cluster (see Figure S3). Table S1 provides a summary description of the clusters. The clusters identified by LCA generally represented motor patterns comprising more than two twitches. Averaged across all 6 groups, the clusters included 2.28 joint movements that were strongly associated (probability > 0.5) with a given cluster, and an additional 1.41 joint movements that were moderately associated (probability = 0.1–0.5).
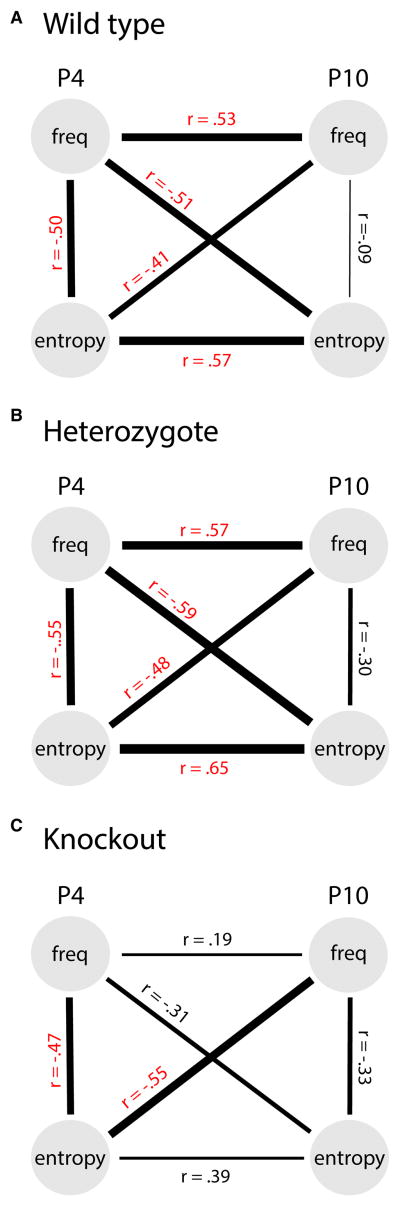
We examined how these clusters changed over development. To do this, we matched clusters across P4 and P10 within each genotype using a procedure similar to that described previously [5]. Table S1 shows that across the three genotypes, 14 to 18 clusters were identified as matching, thus leaving 11 to 15 clusters at P4 that did not have a match at P10 (they were “lost”) and 2 clusters at P10 that did not have a match at P4 (they “appeared”). Next, for each genotype we conducted regression analyses on these clusters to examine the patterns of change across age and genotype. These analyses considered two factors within and across age: The frequency of occurrence of a cluster and its entropy (computed using Shannon’s information over the profile probabilities of each cluster). Here entropy is a measure of the organization of joints within a cluster: Higher entropy clusters can be seen as having more random activity across all joints, and lower entropy clusters as having a few strongly associated joints and little “noise” in the weakly associated ones. As shown in Figure 4, all three genotypes at P4 showed strong and significant negative correlations between frequency and entropy (WT: r = −0.50; Het: r = −0.55; KO: r = −0.47): Clusters that were less structured (high entropy) were also expressed less frequently. By P10, however, these correlations were no longer significant for any genotype (WT: r = −0.09; Het: r = −0.30; KO: r = −0.33).
Figure 4. Regression analyses of LCA cluster frequency (freq) and entropy for (A) wild type, (B) heterozygous, and (C) knockout mice at 4 (P4) and 10 (P10) days of age.
Correlation coefficients (r) in red are statistically significant (p < 0.05) and the width of each connecting line is scaled to the magnitude of r.
Both the WTs and Hets showed strong developmental continuity in that clusters that were highly frequent at P4 tended to remain highly frequent at P10 (Figure 4A,B, upper horizontal connections: WT: r = 0.53; Het: r = 0.57), and the clusters that were highly structured at P4 tended to remain highly structured at P10 (lower horizontal paths: WT: r = 0.57; Het: r = 0.65). This continuity was markedly different in the KOs: neither frequency (r = 0.19) nor entropy (r = 0.39) was significantly correlated across age in these mice (Figure 4C).
Analyses of the cross-correlations across genotypes present a mixed picture. Specifically, frequency at P4 was significantly and negatively correlated with entropy at P10 in the WTs and Hets (WT: r = −0.51; Het: r = −0.59). This suggests that the higher-frequency twitch patterns at P4 tended to become more organized (lower entropy) at P10 as minor components of the twitch patterns were pruned. However, this was not observed in the KOs (KO: r = −0.31), suggesting muscle spindles may be required for this within-cluster pruning. In contrast, entropy at P4 was significantly and negatively correlated with frequency at P10 in all three genotypes (WT: r = −0.41; Het: r = −0.48; KO: r = −0.55). Here, the better-organized (lower entropy) twitch patterns tended to become more frequent later in development.
These results reaffirm in newborn mice a major finding from our earlier report in newborn rats [5]: Clusters of twitching develop such that more frequent clusters become more organized (in the WTs and Hets, but not the KOs), and more organized clusters become more frequent (in all three genotypes). These relationships are illustrated by the diagonal pathways in Figures 4A and 4B. What is novel here is the use of ErbB2 KOs to assess the role that proprioception plays in this developmental process. If we focus on the downward-going diagonal path, muscle spindles appear necessary for highly frequent (“well-practiced”) twitches to become more organized across development; we might think of this developmental process as “pruning” of irrelevant features of clusters such that they become more refined. In contrast, if we focus on the upward-going diagonal path, muscle spindles appear unnecessary for highly organized twitches to become more frequent across development; we might think of this process as “amplification.” Whereas “pruning” entails changes within a cluster, “amplification” entails changes to clusters as a whole. That only the latter process was intact in the KOs suggests that “pruning” and “amplification” are governed by distinct mechanisms acting over developmental time.
There was a strong propensity for highly frequent and organized clusters at P4 to remain highly frequent and organized by P10; however, this was true only for the WTs and Hets. That is, focusing on the horizontal paths in Figure 4, muscle spindles appear necessary to maintain the frequency and structure of clusters across development. In other words, this continuity across age may not come for free, but rather must be actively maintained through proprioception.
Conclusions
Here we have presented evidence for the first time that the development of twitching is shaped by sensory experience. We obtained this evidence by comparing the spatiotemporal structure of twitching at two postnatal ages in mice with and without normal proprioception. Critically, had we not obtained such evidence, it would be difficult to sustain the theory that animals use twitches to build, refine, and maintain sensorimotor maps [4].
Sensory feedback from a twitching limb may modulate subsequent twitching in several non-mutually exclusive ways [5]. First, feedback may activate local spinal circuits—including but not limited to reflex-mediated activation of twitches—thereby triggering cascades of subsequent twitches in one or both limbs; this process could contribute to the moment-to-moment spatiotemporal structure of twitching. Second, sensory feedback may exert long-term influences on sensorimotor integration, thereby modifying the future production of twitches (as well as wake movements). The present findings provide support for both mechanisms.
The specific engagement by twitching of the monosynaptic reflex circuit raises the question of whether twitches contribute to its activity-dependent development. In fact, it has been suggested that the activity-dependent release of neurotrophin 3 by muscle spindles strengthens Ia afferent connections on motoneurons during the second postnatal week [13, 14]. Thus, in light of the early postnatal functional development of muscle spindles [21] and the monosynaptic stretch reflex [12–14], we propose twitching as a source of discrete activity for developing spinal circuits.
It is possible that other proprioceptors (i.e., Golgi tendon organs) and tactile receptors contribute to aspects of twitching that were not affected in the ErbB2 KOs. In fact, twitching has been implicated in the self-organization of the withdrawal reflex, which involves the integration of tactile and proprioceptive information [22]. Moreover, a computational model of twitching suggests that different types of peripheral sensors contribute differentially to the development of spinal circuits [4, 23]. It is not known if and how these various sensors interact through development to shape the patterning of twitching.
It is possible that twitching plays no causal role in sensorimotor development and that the effects observed here on twitching are mediated entirely by wake movements; in that case, developmental changes in twitching would merely come along for the ride. However, evidence is accumulating in favor of a causal role for twitching. This claim is based, in part, on overwhelming evidence that the nervous system pays close attention to sensory feedback arising from twitches [3, 24–29], and the recent demonstration that sensory feedback from twitching limbs is processed very differently from feedback arising from wake movements [29]. Moreover, and of particular relevance to the present report, twitch-dependent neural activity in week-old ErbB2 knockouts is substantially disrupted in the cerebellum [30] and sensorimotor cortex [31]. Nonetheless, selective manipulations of sleep and wake movements may be needed to establish the precise functional contributions of twitching across early development.
There are tangible benefits to taking more seriously the notion that the motor system during wake functions very differently than it does during sleep [29, 32, 33]. For example, just as REM behavior disorder—a movement disorder expressed exclusively during REM sleep—is predictive of the later onset of Parkinson disease [34, 35], twitching in early infancy may prove useful for diagnosing neurodevelopmental disorders before they can be detected using standard neurological assessments, which are routinely performed only during wakefulness. The present results underscore this unrecognized value of twitching as a sensitive indicator of the functional status of spinal and supraspinal circuits. Ultimately, these findings should be extended to other peripheral sensory receptors, at later ages, and to humans—in healthy and disordered populations—if we wish to grasp the full potential of twitching as both a diagnostic and explanatory tool.
Experimental Procedures
All experiments were approved by the Institutional Animal Care and Use Committee of The University of Iowa. Subjects were male and female ErbB2+/+ (wild type), ErbB2+/− (heterozygous), and ErbB2−/− (knockout) mice. As described previously [5], small dots of ultraviolet (UV) fluorescent paint were applied on the two forelimbs and chest at strategic locations for identifying individual joint movements. Pups were secured in a supine position in custom-made silicone molds appropriate to their age and size. Two high-speed (250 frames/s) digital video cameras (Integrated Design Tools, Tallahassee, FL) with 105 mm micro-Nikkor lenses (Nikon, Melville, NY) were used to record twitches. Recordings began when the pup was acclimated in an incubator (35°C) and cycling between sleep and wakefulness. Under UV illumination, multiple 20-s recordings were acquired. For each subject, a minimum of three and a maximum of eight videos were acquired. As described previously [5], automatic motion tracking of the joints was performed using ProAnalyst (Xcitex, Boston, MA). Three-dimensional reconstruction of the pups’ limb movements in space was done with a calibration fixture with an accuracy of approximately 0.1 mm. Data were imported into Spike2 (Cambridge Electronic Design, Cambridge, UK) as eight continuous waveforms representing the four joints across the two forelimbs. For pup-specific parametric analyses, analysis of variance (ANOVA) was performed using SPSS (IBM, Armonk, NY). When appropriate, follow-up ANOVAs and post hoc tests (LSD) were used. For all inferential statistics, alpha was set at 0.05. Perievent histograms were used to assess pairwise within-joint relationships using the “event correlation” function in Spike2. As described previously [29], statistical significance was assessed using a jitter protocol implemented in MATLAB (MathWorks, Natick, MA) [36]. Latent class analysis (LCA; Latent GOLD software, Statistical Innovations, Belmont, MA) and follow-up analyses were performed as described previously [5], with some modifications (see Supplemental Experimental procedures). All means are presented with their standard error.
Supplementary Material
Acknowledgments
We thank Hugo Gravato Marques, Alexandre Tiriac, and Carlos Del Rio-Bermudez for helpful comments on an earlier draft of the manuscript. This work was supported by a grant from the NIH (NS73869) to M.S.B.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, one table, and one movie and can be found with this article online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gramsbergen A, Schwartze P, Prechtl HFR. The postnatal development of behavioral states in the rat. Dev Psychobiol. 1970;3:267–280. doi: 10.1002/dev.420030407. [DOI] [PubMed] [Google Scholar]
- 2.Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- 3.Tiriac A, Uitermarkt BD, Fanning AS, Sokoloff G, Blumberg MS. Rapid whisker movements in sleeping newborn rats. Curr Biol. 2012;22:2075–2080. doi: 10.1016/j.cub.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blumberg MS, Marques HG, Iida F. Twitching in sensorimotor development from sleeping rats to robots. Curr Biol. 2013;23:R532–R537. doi: 10.1016/j.cub.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumberg MS, Coleman CM, Gerth AI, McMurray B. Spatiotemporal structure of REM sleep twitching reveals developmental origins of motor synergies. Curr Biol. 2013;23:2100–2109. doi: 10.1016/j.cub.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leu M, Bellmunt E, Schwander M, Farinas I, Brenner H, Muller U. Erbb2 regulates neuromuscular synapse formation and is essential for muscle spindle development. Development. 2003;130:2291–2301. doi: 10.1242/dev.00447. [DOI] [PubMed] [Google Scholar]
- 7.Andrechek E, Hardy W, Girgis-Gabardo A, Perry R, Butler R, Graham F, Kahn R, Rudnicki M, Muller W. ErbB2 is required for muscle spindle and myoblast cell survival. Molecular and Cellular Biology. 2002;22:4714. doi: 10.1128/MCB.22.13.4714-4722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Li L, Frank E. The role of muscle spindles in the development of the monosynaptic stretch reflex. J Neurophysiol. 2012;108:83–90. doi: 10.1152/jn.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldissera F, Broggi G, Mancia M. Monosynaptic and polysynaptic spinal reflexes during physiological sleep and wakefulness. Arch Ital Biol. 1966;104:112. [PubMed] [Google Scholar]
- 10.Kubota K, Tanaka R. Fusimotor unit activities and natural sleep in cat. Jpn J Physiol. 1968;18:43–58. doi: 10.2170/jjphysiol.18.43. [DOI] [PubMed] [Google Scholar]
- 11.Gassel MM, Marchiafava PL, Pompeiano O. Tonic and phasic inhibition of spinal reflexes during deep, desynchronized sleep in unrestrained cats. Arch Ital Biol. 1964;102:471. [PubMed] [Google Scholar]
- 12.Mendell L, Munson J, Arvanian V. Neurotrophins and synaptic plasticity in the mammalian spinal cord. J Physiol. 2001;533:91–97. doi: 10.1111/j.1469-7793.2001.0091b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen HH, Hippenmeyer S, Arber S, Frank E. Development of the monosynaptic stretch reflex circuit. Curr Opin Neurobiol. 2003;13:96–102. doi: 10.1016/s0959-4388(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 14.Mentis GZ, Alvarez FJ, Shneider NA, Siembab VC, O’Donovan MJ. Mechanisms regulating the specificity and strength of muscle afferent inputs in the spinal cord. Ann N Y Acad Sci. 2010;1198:220–230. doi: 10.1111/j.1749-6632.2010.05538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blumberg MS. Beyond dreams: Do sleep-related movements contribute to brain development? Front Neurol. 2010;1:140. doi: 10.3389/fneur.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 17.Hippenmeyer S, Shneider NA, Birchmeier C, Burden S, Jessell TM. A role for neuregulin I signaling in muscle spindle differentiation. 2002;36:1035–1049. doi: 10.1016/s0896-6273(02)01101-7. [DOI] [PubMed] [Google Scholar]
- 18.Waldenström A, Christensson M, Schouenborg J. Spontaneous movements: Effect of denervation and relation to the adaptation of nociceptive withdrawal reflexes in the rat. Physiol Behav. 2009;98:532–536. doi: 10.1016/j.physbeh.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Seebach B, Mendell L. Maturation in properties of motoneurons and their segmental input in the neonatal rat. J Neurophysiol. 1996;76:3875–3885. doi: 10.1152/jn.1996.76.6.3875. [DOI] [PubMed] [Google Scholar]
- 20.Kudo N, Yamada T. Development of the monosynaptic stretch reflex in the rat: an in vitro study. J Physiol (Lond) 1985;369:127–144. doi: 10.1113/jphysiol.1985.sp015892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vejsada R, Hník P, Payne R, Ujec E, Paleĉek J. The postnatal functional development of muscle stretch receptors in the rat. Somatosens Mot Res. 1985;2:205–222. doi: 10.3109/07367228509144564. [DOI] [PubMed] [Google Scholar]
- 22.Petersson P, Waldenström A, Fåhraeus C, Schouenborg J. Spontaneous muscle twitches during sleep guide spinal self-organization. Nature. 2003;424:72–75. doi: 10.1038/nature01719. [DOI] [PubMed] [Google Scholar]
- 23.Marques HG, Bharadwaj A, Iida F. From spontaneous motor activity to coordinated behaviour: A developmental model. PLoS Comput Biol. 2014;10:e1003653. doi: 10.1371/journal.pcbi.1003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- 25.Sokoloff G, Uitermarkt BD, Blumberg MS. REM sleep twitches rouse nascent cerebellar circuits: Implications for sensorimotor development. Dev Neurobiol. 2014 doi: 10.1002/dneu.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohns EJ, Blumberg MS. Neocortical activation of the hippocampus during sleep in newborn rats. J Neurosci. 2010;30:3438–3449. doi: 10.1523/JNEUROSCI.4832-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohns EJ, Blumberg MS. Synchronous bursts of neuronal activity in the developing hippocampus: Modulation by active sleep and association with emerging gamma and theta rhythms. J Neurosci. 2008;28:10134–10144. doi: 10.1523/JNEUROSCI.1967-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McVea DA, Mohajerani MH, Murphy TH. Voltage-sensitive dye imaging reveals dynamic spatiotemporal properties of cortical activity after spontaneous muscle twitches in the newborn rat. J Neurosci. 2012;32:10982–10994. doi: 10.1523/JNEUROSCI.1322-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiriac A, Del Rio-Bermudez C, Blumberg MS. Self-generated movements with “unexpected” sensory consequences. Curr Biol. 2014;24:2136–2141. doi: 10.1016/j.cub.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uitermarkt BD, Sokoloff G, Weiner JA, Fritzsch B, Blumberg MS. Newborn mice lacking muscle spindles exhibit reduced twitch-related Purkinje cell activity during active sleep. Neuroscience Meeting Planner; San Diego, CA: Society for Neuroscience; 2013. Program No. 647.19.2013. Online. [Google Scholar]
- 31.Tadjalli A, Weiner JA, Fritzsch B, Blumberg MS. Proprioceptive feedback is necessary for the generation of twitch-related spindle bursts during active sleep in newborn mice. Neuroscience Meeting Planner; San Diego, CA: Society for Neuroscience; 2013. Program No. 610.17.2013. Online. [Google Scholar]
- 32.De Cock V, Vidailhet M, Leu S, Texeira A, Apartis E, Elbaz A, Roze E, Willer J, Derenne J, Agid Y, et al. Restoration of normal motor control in Parkinson’s disease during REM sleep. Brain. 2007;130:450–456. doi: 10.1093/brain/awl363. [DOI] [PubMed] [Google Scholar]
- 33.Bliwise D, Trotti L, Greer S, Juncos J, Rye D. Phasic muscle activity in sleep and clinical features of Parkinson disease. Annals of Neurology. 2010 doi: 10.1002/ana.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schenck C, Bundlie S, Mahowald M. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996;46:388–393. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 35.Claassen DO, Josephs KA, Ahlskog JE, Silber MH, Tippmann-Peikert M, Boeve BF. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology. 2010;75:494–499. doi: 10.1212/WNL.0b013e3181ec7fac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amarasingham A, Harrison MT, Hatsopoulos NG, Geman S. Conditional modeling and the jitter method of spike resampling. J Neurophysiol. 2012;107:517–531. doi: 10.1152/jn.00633.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.