Abstract
OBJECTIVE
To determine how inter-hemispheric balance in stroke, measured using transcranial magnetic stimulation (TMS), relates to balance defined using neuroimaging (functional magnetic resonance (fMRI) and diffusion tensor imaging (DTI)), and how these metrics of balance are associated with clinical measures of upper limb function and disability.
DESIGN
Cross-Sectional
SETTING
Clinical Research Laboratory
PARTICIPANTS
Ten chronic stroke patients (63±9 years) in a population based sample with unilateral upper-limb paresis.
INTERVENTION
Not applicable
MAIN OUTCOME MEASURES
Inter-hemispheric balance was measured with TMS, fMRI and DTI. TMS defined inter-hemispheric differences in recruitment of corticospinal output, the size of the corticomotor output maps and the degree of mutual transcallosal inhibition they exerted upon one another. fMRI studied whether cortical activation during the movement of the paretic hand was lateralized to the ipsilesional or to the contralesional primary motor (M1), premotor (PMC) and supplementary motor cortices (SMA). DTI was used to define inter-hemispheric differences in the integrity of the corticospinal tracts projecting from M1. Clinical outcomes tested function (upper-extremity Fugl-Meyer (UEFM) and the perceived disability in the use of the paretic hand [Motor Activity Log (MAL)].
RESULTS
Inter-hemispheric balance assessed with TMS relates differently to fMRI and DTI. Patients with high fMRI lateralization to the ipsilesional hemisphere possessed stronger ipsilesional corticomotor output maps [M1 (r=.831, p=.006), PMC (r=.797, p=.01)], and better balance of mutual transcallosal inhibition (r=.810, p=.015). Conversely, we have found that patients with less integrity of the corticospinal tracts in the ipsilesional hemisphere show greater corticospinal output of homologous tracts in the contralesional hemisphere (r=.850, p=.004). However, neither an imbalance in their integrity nor an imbalance of their output relates to transcallosal inhibition. Clinically, while patients with less integrity of corticospinal tracts from the ipsilesional hemisphere showed worse impairments (UEFM) (r = −.768, p=.016), those with low fMRI lateralization to the ipsilesional hemisphere had greater perception of disability (MAL) [M1 (r=.883, p=.006), PMC (r=.817, p=.007) and SMA (r=.633, p=.062).
CONCLUSIONS
In patients with chronic motor deficits of the upper limb, fMRI may serve to mark perceived disability as well as transcallosal influence between hemispheres. DTI-based integrity of corticospinal tracts, however, may be useful in categorizing the range of functional impairments of the upper-limb. Further, in patients with extensive corticospinal damage, DTI may help infer the role of the contralesional hemisphere in recovery.
Keywords: Diffusion tensor imaging (DTI), Functional magnetic resonance imaging (fMRI), Transcranial magnetic stimulation (TMS), Inter-hemispheric imbalance, transcallosal inhibition, Stroke, Motor cortex
In chronic stroke it is believed that hand deficits persist because of an imbalance between the ipsilesional and the contralesional hemisphere activity.1–3 Neurophysiologically, this inter-hemispheric imbalance is thought to arise from altered transcallosal inhibition (TCI), where inhibition exerted from the ipsilesional hemisphere (lesioned) upon the contralesional hemisphere (intact) is weaker than inhibition exerted from the contralesional hemisphere upon the ipsilesional hemisphere.4–6 The inter-hemispheric imbalance in chronic stroke has been examined using many different modalities; however, it has yet to be determined whether these modalities truly reflect TCI.
Transcranial magnetic stimulation (TMS) is one the most popular noninvasive methods used to define inter-hemispheric imbalance. It can study activity of motor cortices via electromagnetic induction. The action of passing a brief and strong current through an insulated coiled wire placed on the scalp induces a perpendicular magnetic field that can pass unimpeded through the skull and induce weak current flow in an area of the brain. This causes depolarization and triggers action potentials or post-synaptic potentials in neurons of the targeted cortex.7 TMS has been used to describe inter-hemispheric imbalance in a couple different ways. First, TMS can denote inter-hemispheric differences in corticospinal output.3 When single pulses of TMS are delivered at incrementally greater intensities, the responses evoked in the contralateral muscle (hemisphere opposite of the target limb) can be plotted as a recruitment curve. Second, with single pulses of TMS applied over multiple scalp sites, one can study the entire representation of the corticomotor output for the contralateral muscle- also known as a corticomotor output map.3,8
Functional magnetic resonance imaging (fMRI) captures inter-hemispheric imbalance during movement of the paretic hand. It records regions showing higher changes in blood flow during voluntary movement than during rest. Comparison of activation in the ipsilesional versus the contralesional motor cortex yields a metric called laterality index signifying inter-hemispheric imbalance with regard to cortical control in the movement of the paretic hand.2
Lastly, diffusion tensor imaging (DTI) is a relatively new MRI modality that is being used to characterize inter-hemispheric imbalance.9 DTI assesses integrity of the white matter tracts based on the principle of water diffusion, where white matter normally has anisotropic diffusion.10 The magnitude and directionality of anisotropic diffusion yields DTI metrics of tract integrity such as fractional anisotropy (FA).11 In stroke, DTI measures inter-hemispheric imbalance by comparing the mean FA of the corticospinal tracts within the ipsilesional and the contralesional hemisphere, termed FAasymmetry.9,12,13
The varying metrics that are used to define inter-hemispheric imbalance are often used interchangeably. Also, these metrics are presumed to reflect altered TCI in patients, but this has never been confirmed.12,14 Further, it is unclear which metric of imbalance represents upper-limb functional impairments and disability. Therefore, the purpose of the present study was three-fold: 1) examine if the various metrics of imbalance can be used interchangeably by finding their mutual relationships; 2) confirm if they are an accurate representation of TCI by examining relationships between fMRI laterality, and DTI asymmetry to a commonly used TCI metric, ipsilateral silent period i.e the suppression of ongoing voluntary motor output due to a supra-threshold TMS pulse delivered to the ipsilateral hemisphere; 15–17 and 3) examine the relation of metrics to two widely used clinical measures- upper-extremity Fugl-Meyer (UEFM)18 and Motor Activity Log (MAL). Our study aimed to better understand if multiple metrics of inter-hemispheric imbalance are able to relate to the degree of impairment and disability of the paretic hand in chronic stroke.19
Our report is significant because TMS, fMRI and DTI remain investigational and none of the techniques are currently FDA approved as a clinical diagnostic tool for stroke. They are time-, resource- and cost-intensive. Knowing their similarities (objective 1 and 2), as well as their unique clinical strengths (objective 3) would help future studies eliminate redundancies or harness their potentially complementary features. In addition, our preliminary study allows us to understand which modality/modalities relate to impairments as well as perceived use of the extremity, so that future studies may be able to identify which one(s) could define functional recovery along the course of treatment. These steps are critical to justify their use as diagnostic tools in future clinical practice.
1. METHODS
Participants
The local research ethics board reviewed and approved this study. In a cross-sectional design, data from 10 patients with stroke (63 ± 9 years) have been included. Location and side of stroke are presented in Table 1. Our inclusion criteria were: age ≥ 21 years, chronic phase of recovery (> 6 months) after first-ever (ischemic or hemorrhagic) stroke, trace ability to extend fingers or thumb or wrist ≥10° and chief complaint of inadequate ability to use the paretic hand in daily life. The exclusionary criteria related to contraindications of TMS20, 21 and imaging.22,23 Briefly, these include cardiac pacemaker, metallic implant in the head, seizure disorder and use of any neuro- or psycho-active medications as published in recommendations.20
Table 1.
Age (years). Gender (M = Male; F= Female). Time since stroke (months). MMI = Mini Mental Exam (Maximum 30). Hand Dominance (R = Right; L = Left). UEFM = Upper Extremity Fugl-Meyer (Maximum 30). MAL = Motor Activity Log Amount Score (Maximum 5)
| Patient | Age | Gender | Time Since Stroke (mo) | Lesioned Hemisphere | Stroke Etiology | Stroke Location | MMI | Hand Dominance | UEFM | MAL |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 68 | F | 32 | R | Ischemic | Basal Ganglia | 30 | R | 21 | 1.12 |
| 2 | 66 | M | 19 | R | Ischemic | Mesial Frontal Cortical | 24 | R | 26 | 1.29 |
| 3 | 58 | F | 24 | R | Ischemic | Basal Ganglia | 30 | L | 24 | 0.66 |
| 4 | 69 | M | 23 | L | Hemorrhagic | Thalamus & Putamen | 28 | R | 24 | 2.45 |
| 5 | 54 | M | 29 | L | Ischemic | Centrum Semi-Ovale & Basal Ganglia | 29 | R | 22 | 1.16 |
| 6 | 72 | M | 84 | R | Ischemic | Pontine | 26 | R | 25 | 2.31 |
| 7 | 55 | F | 48 | L | Hemorrhagic | Dorsal Thalamus | 30 | R | 20 | 3.32 |
| 8 | 59 | F | 23 | L | Ischemic | Caudate head, globus pallidus, insula, punctate cortical foci | 29 | R | 20 | 1.6 |
| 9 | 76 | M | 24 | R | Ischemic | Basal Ganglia and Corona Radiata | 28 | R | 8 | 0.35 |
| 10 | 50 | M | 54 | L | Hemorrhagic | Thalamus | 30 | R | 22 | 1.03 |
Assessments and Procedures
TMS
We used TMS to examine how TCI was exerted mutually between both hemispheres, and compared the corticomotor output devoted to the paretic vs. non-paretic muscles using recruitment curves and corticomotor output maps for both hemispheres. Patients were seated comfortably in a chair with both arms and forearms supported and hands resting on a flat surface. Single pulse TMSa was delivered using a figure-of-eight coil (diameter 70mm). Patients’ fMRI activation (see below) was used to stereotactically guide TMS [BRAINSIGHT software (Rogue Research, Montreal, Canada)] to M1. TMS-evoked responses in the contralateral muscle, called motor evoked potentials, were recorded in the first dorsal interosseous (FDI) muscle using surface electromyographyb via bipolar electrodes (silver-silver chloride, 8 mm diameter) positioned over the muscle belly. A reference electrode was placed over the lateral epicondyle.
TMS was performed on both hemispheres. First, we found the scalp site that was able to elicit a motor evoked potential with the lowest stimulator output i.e resting motor threshold (≥50μV in at least 3/5 trials), termed motor hot spot. At the motor hotspot, we then created a recruitment curve by increasing the stimulator intensity by increments of 10% of the resting motor threshold (from 90%–150%) (10 trials each). Next, we defined the corticomotor map for the FDI muscle. This was achieved by delivering single pulses of TMS (5 pulses each, @ 110% of resting motor threshold, randomized) to sites on a 7×5 grid (10 mm spatial resolution) centered on the motor hotspot. Finally, to signify TCI, we examined the ipsilateral silent period (iSP).15,17 Patients were asked to volitionally contract their FDI muscle at 50% of their maximum voluntary contraction. They were provided visual feedbackc so they could accurately maintain the designated level of force. We delivered supra-threshold TMS (150% motor threshold, 15 stimulations) ipsilateral to the contracting muscle.
fMRI
We recorded movement related changes in blood flow using the blood oxygen level-dependent (BOLD) fMRI signal, using a Siemens Trio 3Td scanner.24–26 For anatomically based images, 176 axial slices with a thickness of 1mm and field of view (FOV) = 256×256 mm were collected. An inversion time/echo time (TE)/repetition time (TR) and flip angle of 1900 msec/1.71 msec/900 msec and 8 degrees was used. BOLD Echo-planar imaging (EPI) was acquired with 160 repetitions of 31-4-mm thick axial slices. Imaging parameters consisted of TE= 29ms, TR= 2.8s, flip angle= 80°, matrix = 128×128 and field of view = 256×256 mm2 providing an in-plane resolution of 2×2 mm2.
For the movement related paradigm, patients were asked to perform self-paced flexion-extension of fingers of the paretic hand in a block design (each block lasting 45 seconds). fMRI preprocessing included head motion correction and EPI alignment to the high resolution T1 image. Head motion correction was performed using a voxel-specific motion regression following the method of Bullmore et al. 199927 to reduce artifactual effects of motion at each voxel. EPI data was smoothed with a 3-mm full width at half maximum Gaussian kernel. The difference in magnetic resonance signals between rest and movement period was analyzed using 3dDeconvolve with Analysis of Functional Neuroimages (AFNI) softwaree. Voxels (volume) that showed significantly greater BOLD signal during finger movements versus rest were considered active at a corrected threshold of P < 5×10−6 (determined through AlphaSim with a single voxel threshold of P < .01 and a cluster size > 13 voxels).28 All images were transformed to Talairach space and activation was examined in M1, PMC, and SMA. These regions were defined for each subject based on guidelines published in our previous work and of others.22,29
DTI
With DTI, we examined the inter-hemispheric imbalance in integrity of corticospinal tracts. We acquired a High Angular Resolution Diffusion Imaging dataset with 71 diffusion-weighting gradients (b = 1000 s/mm2) and 7 b=0 s/mm2 image volumes, whole- brain coverage and 2mm isotropic voxels (field of view 256×256mm, image matrix 128×128, 52 2-mm thick slices). All data were corrected for eddy currents and head motion. On FA maps, regions-of-interest were drawn at the level of the posterior limb of internal capsule, M1, PMC and SMA. For the posterior limb of internal capsule, an axial image was chosen at an approximate level where the foramen of monroe was visible. M1, PMC and SMA were drawn on single axial images using similar method to that of fMRI. Corticospinal fiber tracts were reconstructed (virtually) using probabilistic tractography from the internal capsule to each of the regions separately.12, 30
Clinical Assessment of function
UEFM is an impairment-based test rated on an ordinal scale (0 to 2) by an investigator.18 We focused on the distal portion of the UEFM, which evaluates wrist, hand and coordination for a maximum score of 30.12 We have chosen UEFM because it is a commonly-used clinical test with acceptable inter-rater reliability (0.97)31 and concurrent validity.32
MAL is a semi-structured interview assessing the patient’s perceived use of the paretic hand in 14 different activities of daily living. Here, we employed the portion of MAL that records the Amount of Use.19 Ratings vary from 0 (absence of use or inability to use) to 5 (as frequent as in pre-morbid state). The test has strong validity,33 internal consistency and stability in chronic stroke.34
Data Analysis: TMS
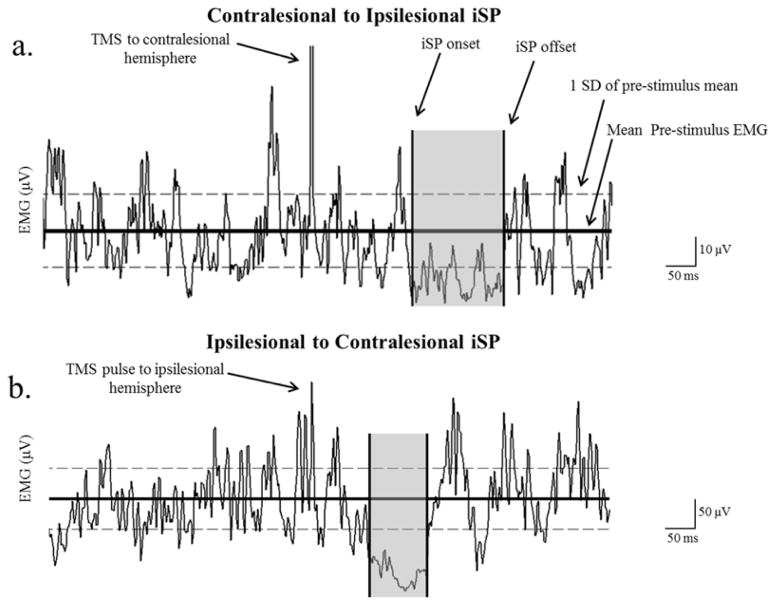
iSP for measurement of TCI
Onset of iSP was defined when EMG of the contracting muscle was suppressed below 1 standard deviation of the pre-stimulus EMG for 5ms. Offset was defined when EMG of the contracting muscle returned to within 1SD of the pre-stimulus value for 5 ms16 (Fig. 1). We computed the level of TCI as the percentage decrease in mean EMG during iSP as a proportion of pre-stimulus EMG (equation 1).
Figure 1.
Example of an ipsilateral silent period (iSP) from (a) contralesional to ipsilesional and (b) ipsilesional to contralesional hemisphere. The ISP ratio was the % EMG decrease of contralesional to ipsilesional/% EMG decrease of ipsilesional to contralesional hemisphere. ISP onset and offset were determined as the point where the EMG went below 1SD of the pre-stimulus mean and returned back within 1 SD of the pre-stimulus mean. Even though the patients maintained a contraction at 50% of the maximum voluntary contraction, it is important to notice the difference between the EMG scale of the paretic hand (a) and the non-paretic hand (b), where the paretic hand EMG reached a maximum of ~ 50 μV, and the non-paretic hand reached a maximum of ~ 400 μV. Despite the difference in EMG activity it has recently been shown that level of EMG does not influence the degree of inhibition.51 Results are reported as ISP ratio, which is defined as the Contralesional to Ipsilesional iSP/Ipsilesional to Contralesional iSP. The subject shown had an iSP ratio of .75.
| Equation 1 |
Ultimately, we computed a ratio of iSP exerted from contralesional hemisphere upon the paretic muscle and from ipsilesional hemisphere upon the non-paretic muscle. A balanced ratio (~1) would indicate a balanced level of TCI exerted between both hemispheres, whereas an iSP ratio <1 indicates greater inhibition exerted from the ipsilesional upon contralesional hemisphere, and >1 indicates greater inhibition from the contralesional upon ipsilesional hemisphere.35
Recruitment Curve
At the hotspot, the stimulator intensity was increased from 90% to 150% of the resting motor threshold in a randomized order, at 10% increments while amplitude of corresponding MEPs was plotted as a recruitment curve.36 Similar to Talelli et al 200737, recruitment curves were variable among patients. This made it difficult to fit the curves to a single model. Instead, we measured area under the curve (AUC) to define the corticospinal output of the M1 from the ipsilesional and the contralesional hemisphere.
Corticomotor Output Maps
Scalp sites consistently eliciting motor evoked potentials > 30μV were analyzed (3 of 5 pulses/trials). The size of the map was defined as the number of sites that met the motor evoked potential criteria.
Data Analysis: fMR
Laterality Index
To examine inter-hemispheric imbalance during movement of the paretic hand, we calculated a laterality index,2,22 which expresses whether activation during movement of the paretic hand is mainly ipsilesional or contralesional. Laterality index varies from −1 to 1, with 1 indicating purely ipsilesional and −1 indicating purely contralesional activation (equation 2).
| Equation 2 |
Data Analysis: DTI
Asymmetry Index
Structural integrity of the corticospinal tracts was compared between the two hemispheres using asymmetry index. Mean FA along the tracks were compared between the ipsilesional and contralesional sides.12 These asymmetry indices for M1, PMC and SMA were given by (equation 3):
| Equation 3 |
0 indicates perfect symmetry between both sides, while positive values signify reduced tract integrity in the ipsilesional hemisphere.
Statistics
Statistical analysis was performed using Statistical Package for the Social Sciences (v18, SPSS Inc., Chicago, IL). We defined the bivariate relation between TCI and Laterality index, TCI and FAasymmetry, map count and Laterality index, AUC and FAasymmetry, and map count and FAasymmetry. To identify metrics that are associated with function, we computed correlations between UEFM and each of the physiologic and imaging metrics (TCI, map count, AUC, Laterality index and FAasymmetry) and between MAL and each of the parameters. All relationships were assessed using non-parametric Spearman’s correlation test at alpha value of significance set at 0.05.
2. RESULTS
All patients were able to maintain volitional contraction of the paretic muscle for the iSP measurement; however one patient was excluded due to technical issues. All but one patient could elicit motor evoked potentials in the paretic muscle with TMS pulses applied to the ipsilesional hemisphere and all patients were able to perform the self-paced finger flexion-extension task with fMRI. Due to excessive head motion within the scanner, one subject had to be excluded from fMRI calculations (laterality index) and one patient had to be excluded from the DTI calculations (FAasymmetry). The same patient was excluded for the DTI and iSP metric and an additional patient was excluded for the fMRI metric.
fMRI and TMS: Laterality versus TCI, recruitment curve and corticomotor output map
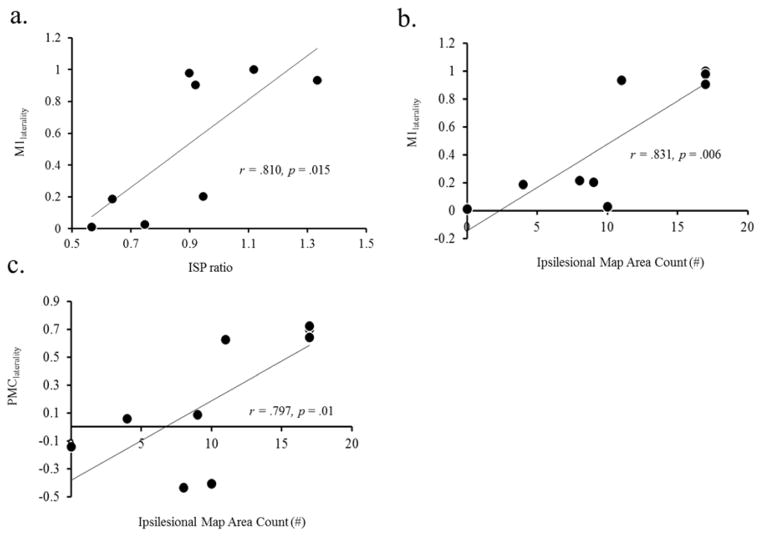
We first examined the relationship between TCI (iSP) ratio and laterality index. We found that patients with greater fMRI laterality to the ipsilesional M1 also had a more balanced TCI (ratio closer to 1) (Fig. 2a; r = .810, p = .015). Next, we examined relationships between fMRI laterality index and corticomotor output maps devoted to the paretic muscle. We found that patients with greater ipsilesional fMRI laterality in the M1 and PMC also had larger ipsilesional corticomotor output maps measured with TMS (Fig. 2b & 2c; r = .831 p =.006 & r =.797 p=.01).
Figure 2.
Correlation between fMRI laterality index and TMS. (a) There was a positive correlation between the ratio of ISP and fMRI laterality index within the M1. (b & c) There was a significant positive correlation between fMRI laterality index within the M1 and PMC to the total ipsilesional map area count with TMS.
DTI and TMS: FAasymmetry versus TCI, corticomotor output map, recruitment curve
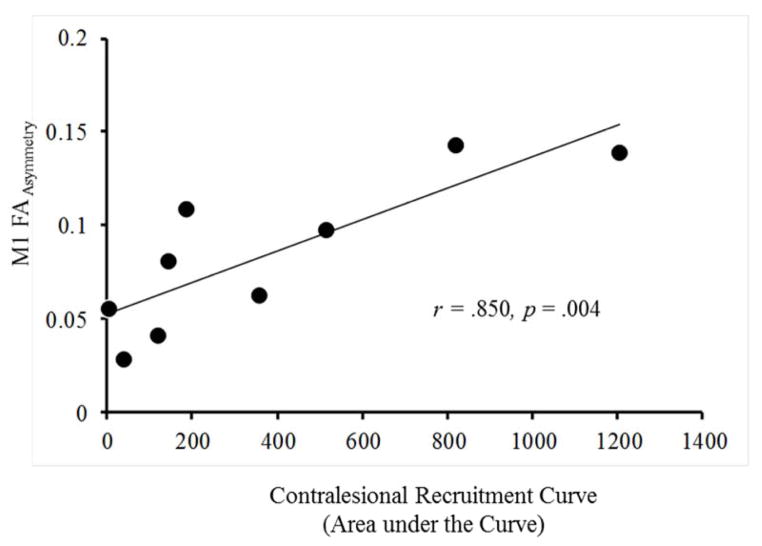
FAasymmetry of corticospinal tracts was not related to TCI or corticomotor output maps (not shown), but it shared a positive relation with the recruitment curve of the contralesional hemisphere. Patients with less corticospinal tract integrity from the ipsilesional M1 (greater FAasymmetry) also showed greater corticospinal output from the contralesional hemisphere measured as the recruitment curve (Fig. 3; r = .850 p = .004).
Figure 3.
Correlation between DTI and TMS. There was a significant positive correlation between FAasymmetry of corticospinal tracts originating from M1 and the recruitment curve of the contralesional hemisphere discerned with TMS.
Metrics of Inter-hemispheric imbalance and Clinical Assessment of Function
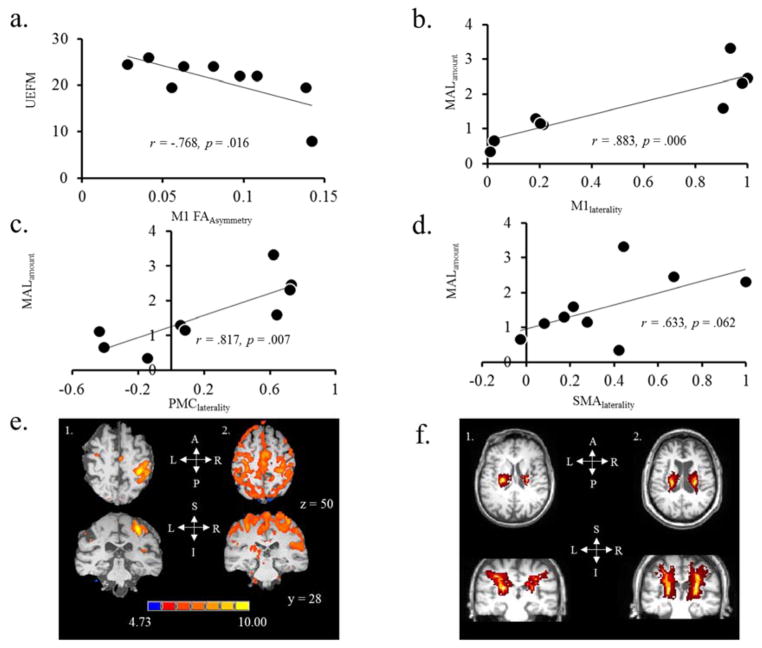
When relating fMRI, DTI and TMS measures of inter-hemispheric balance to outcomes of function, we have found different types of association with clinical measures. Patients with greater UEFM scores (better function) showed favorable value of FAasymmetry of the corticospinal tracts originating from M1 (Fig. 4a; r = −.768 p = .016). UEFM was not correlated with TMS (data not shown). MAL on the other hand was not correlated with measures of TMS or DTI, but patients with greater MAL scores (perceived use of the paretic hand) showed greater fMRI lateralization, favoring ipsilesional M1 and PMC (Fig 4b & 4c; r = .883 & r = .817; p < .05) and showed a similar trend with the laterality of SMA (Fig. 4d; r = .633 p = .062). Figure 4e and 4f show fMRI activation maps and DTI tractography, respectively.
Figure 4.
Relationship between clinical assessment scores (UEFM & MAL) and neurophysiologic and neuroimaging outcomes. (a) There was a negative correlation between UEFM and M1 FAasymmetry. (b–d) there was a positive correlation between MAL and fMRI laterality index within the M1, PMC and SMA. (e) FMRI based images in two different patients. Subject (1) shows high fMRI laterality signifying good ipsilesional hemisphere dominance during contraction of the paretic hand. Subject (2) shows low fMRI laterality signifying weaker ipsilesional hemispheric dominance during contraction of the paretic hand. Their MAL scores were 2.3 and .66, respectively, signifying that patient with higher laterality showed higher MAL. (f) DTI-based images in two different patients. Subject (1) shows poor M1 FAasymmetry signifying weaker integrity of corticospinal tracts originating from the ipsilesional side. Subject (2) shows good M1 FAasymmetry signifying similar integrity of tracts in both hemispheres. Their UEFM scores were 35 and 50, respectively, signifying that patient with higher function showed favorable integrity on ipsilesional side
S = superior, I = inferior, A = anterior, P = posterior, L = left & R = right.
Discussion
We examined the associations between neurophysiologic and imaging-based metrics that characterize inter-hemispheric imbalance, and explored how they related to two widely used clinical measures of hand function- UEFM and MAL. We have found that even though metrics noted with imaging (fMRI and DTI) relate to those measured neurophysiologically (TMS), the nature of such relationship varies. Patients with higher laterality of fMRI activation in the ipsilesional motor cortices also show larger ipsilesional corticomotor output maps and favorable balance of TCI between hemispheres. However, patients with poor ipsilesional tract integrity recorded with DTI, showed greater contralesional corticospinal output. Metrics of both fMRI and DTI were related to clinical outcomes, albeit differently. fMRI was associated with MAL- patient’s perceived report of their disability in using the paretic hand, while DTI was associated with the objective assessment of the patients’ impairments (UEFM). Although preliminary and exploratory, our findings carry implications for clinical practice. Because fMRI laterality during voluntary movement relates to overall global perceived use of the paretic hand as well neurophysiologic outcomes, we propose that fMRI may serve to mark perceived disability and could be used as a surrogate measure of neurophysiologic imbalance in chronic stroke deficits. However, DTI-based integrity of the corticospinal tracts may be useful in categorizing range in the severity of the motor impairments, an outcome expressed by a number of other studies. 11, 12, 40–42 Further, DTI could be used as a tool to explore the role of the contralesional corticomotor output during recovery in patients with extensive damage.
Neurophysiologic metrics of inter-hemispheric imbalance versus fMRI laterality
fMRI laterality has been used since 19972 in stroke to signify changes in inter-hemispheric imbalance with volitional movements of the paretic hand. Previous studies have reported relationships between fMRI and TMS in healthy subjects. It has been shown that fMRI activation is positively related to corticomotor output maps,38 while BOLD fMRI signal intensity of activation is related to TCI.39, 40 With chronic stroke patients, it has been shown that reduced fMRI laterality to the ipsilesional hemisphere relates to smaller motor evoked potentials.41 This suggests that patients with weaker corticospinal output of the ipsilesional hemisphere favor the contralesional hemisphere to a greater extent. Similarly, previous fMRI studies have reported in chronic stroke that prior to treatment12 as well as post treatment,2 the fMRI laterality tends to favor the contralesional hemisphere. Our study supports this work by showing evidence that greater fMRI laterality towards the ipsilesional hemisphere is associated with increased ipsilesional corticomotor maps measured with TMS (Fig 2b–c). Further, to our knowledge, this is the first study to show an association between fMRI laterality and TCI balance in chronic stroke. Because of these findings we propose that fMRI laterality may be a surrogate marker of the altered TCI in chronic stroke deficit (Fig 2a).
Neurophysiologic metrics versus FAasymmetry
fMRI laterality has been the primary method to show that there is an increase in activation of the contralesional hemisphere following stroke.2,12 We further expand on these findings by showing a similar phenomenon using FAasymmetry and TMS. We show that patients with greater ipsilesional corticospinal tract damage, indeed, had a greater corticospinal output of the contralesional hemisphere measured with TMS (Fig 3). The importance of the contralesional hemisphere is still unknown as it relates to motor recovery. One hypothesis is that the increase in contralesional excitability is a result of learned nonuse of the paretic limb- the maladaptive phenomenon where patients learn to restrict use of the paretic hand in daily activities for fear of failing.42 Learned nonuse will result in greater use of the unaffected limb thus increasing excitability of the unaffected hemisphere. However, a more optimistic hypothesis is that the contralesional hemisphere may have a role in the recovery of the paretic limb. Previous studies have found that contralesional cortices reorganize (show greater fMRI activation) in recovery of motor skill following stroke.43 In fact, when the contralesional hemisphere is inactivated, animals with large infarcts lose the ability to perform a reaching task,44 and patients show poorer reaction times.45 Here, we suggest the contralesional hemisphere as a potential substrate for recovery; future studies could explore whether ipsilateral corticospinal output or if alleviated callosal influence is critical for the role of the contralesional hemisphere.
We were unable to show any relationship between FAasymmetry and TCI. These results differ from that of Lindberg et al. 2007. They not only demonstrate a relationship with the slope of the recruitment curve, but they also show a relationship with the degree of TCI balance in their chronic stroke patients.46 The differences reflect the variance in ways used to analyze outcomes. For example, DTI is a relatively new modality and studies differ from each other as to where they place the seed (e.g. anterior cerebral peduncle vs. posterior limb of the internal capsule). Further, there are differences as to whether we should analyze at a single slice or across the entire length of the corticospinal tract. The differences in analysis provide different, yet important results. However, because of these differences, one should be aware when interpreting results.
Neuroimaging and Neurophysiological correlates of Clinical Outcomes of function
Previous studies have shown that corticospinal tract integrity measured with DTI has high predictive value for motor function in the moderate to severely impaired patients. 11, 12, 40–42 We add to this by showing such a relation in a sample of mild to severely impaired. Stinear et al. 2007 indicated predictive value of DTI’s FAasymmetry only in those patients who were not able to evoke a motor potential with TMS. Our study is able to demonstrate an association between UEFM and DTI even in patients that elicit motor evoked potentials. The variance may emerge from the choice of DTI analysis as previously mentioned. Here we measure integrity along the entire length of tract using a method less affected by confounds from the lesion (details 47, 48,49). Hence, we may have been able to witness the ‘detailed’ degeneration of the tracts even in less impaired patients.
While DTI is critical in marking overall function of the paretic hand, we propose fMRI is important in capturing overall use of the paretic hand. It has previously been suggested that MAL scores are reflective of frontal system changes, which are involved in learned nonuse behavior following stroke.42 Our results support this interpretation by showing that global activity of fMRI in frontal areas (M1, PMC and SMA) is positively correlated to MAL scores. Our result is supported by a previous study that showed that decreasing the use of the paretic limb related to greater fMRI activation in the contralesional PMC and M1.50 Our results offer an important extension of the previous study by characterizing the inter-hemispheric imbalance as it relates to MAL rather than absolute activation. By showing a relationship between fMRI laterality and MAL we have found an important neuronal marker that may provide insight into the patients’ perceived outlook of their disability. Future studies will determine if this translates to longitudinal treatment protocols.
Limitations and Conclusions
We acknowledge caution in interpreting our results because of its limited sample size. In addition to a small sample size, another limitation of our study is the cross-sectional design. Therefore, we can only provide preliminary evidence of correlations across metrics currently serving investigational purpose in clinical studies/trials, without suggesting their causative interactions. In order to fully understand the relationship across different modalities and their ability to prognosticate stroke recovery, larger longitudinal studies must be performed.
In summary, we have shown in a small cross-sectional study that metrics of inter-hemispheric imbalance noted with fMRI and DTI are associated with those of TMS, albeit differently. Further, we show that fMRI and DTI are related to clinical outcomes; however, correlations differ based on the nature of the clinical assessment. In demonstrating that fMRI has a real-time perspective of inter-hemispheric imbalance in movement as it relates to favorable TCI and output from the ipsilesional hemisphere, we conjecture that fMRI may be critical in capturing chronic deficits of the patient’s perceived use of the paretic hand and neurophysiologic outcomes. DTI’s examination of corticospinal tracts, however, may or may not directly infer TCI, but it may be an important metric to classify patients across levels of motor impairment severity. In a clinical setting each modality offers similar yet unique perspective, which can ultimately aid in determining what interventions may best enhance rehabilitation outcomes.
Acknowledgments
This work was supported by the National Institutes of Health (1K01HD069504) and American Heart Association (13BGIA17120055) to EP.
Dr. Sakaie reports grants from Novartis, outside the submitted work.
Abbreviations
- BOLD
Blood Oxygen Level-Dependent
- DTI
Diffusion tensor imaging
- EMG
Electromyography
- EPI
Echo-planar imaging
- FA
Fractional anisotropy
- FDI
First Dorsal Interosseous
- fMRI
Functional magnetic resonance imaging
- iSP
Ipsilateral silent period
- M1
Primary motor cortex
- PMC
Premotor cortex
- SMA
Supplementary Motor area
- TMS
Transcranial magnetic stimulation
- TCI
Transcallosal Inhibition
Footnotes
Magstim 2002, The Magstim Company Limited, Sprinf Gardens, Whitland Carmarthenshire, SA 0HR, UK
BRAINSIGHT software, Rogue Research Inc., 206-4398 boul. St – Laurent, Montreal Quebec, H2W 1Z5
Powerlab 4/25T and LabChart, ADInstruments Inc., 2205 Executive Circle, Colorado Springs CO 80906, USA
Siemens Trio 3T scanner, Wittelsbacherplatz 2, 80333 Munich, Germany
Analysis of Functional Neuroimages (AFNI) software., http://afni.nimh.nih.gov/afni/
Conflicts of Interest: AM has the following conflicts of interest to disclose: Intelect medical (advisory board, consultant, shareholder), ATI, Enspire and Cardionomics (distribution rights from intellectual property), Functional Neurostimulation (consultant), Deep Brain Innovations (consultant).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. NeuroImage. 2005 Dec;28(4):940–946. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 2.Cramer SC, Nelles G, Benson RR, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke; a journal of cerebral circulation. 1997 Dec;28(12):2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- 3.Liepert J, Miltner WH, Bauder H, et al. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neuroscience letters. 1998 Jun 26;250(1):5–8. doi: 10.1016/s0304-3940(98)00386-3. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu T, Hosaki A, Hino T, et al. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain : a journal of neurology. 2002 Aug;125(Pt 8):1896–1907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- 5.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Annals of neurology. 2004 Mar;55(3):400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 6.Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. Journal of the neurological sciences. 1996 Dec;144(1–2):160–170. doi: 10.1016/s0022-510x(96)00222-5. [DOI] [PubMed] [Google Scholar]
- 7.Terao Y, Ugawa Y. Basic mechanisms of TMS. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2002 Aug;19(4):322–343. doi: 10.1097/00004691-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Sawaki L, Butler AJ, Leng X, et al. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabilitation and neural repair. 2008 Sep-Oct;22(5):505–513. doi: 10.1177/1545968308317531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werring DJ, Toosy AT, Clark CA, et al. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. Journal of neurology, neurosurgery, and psychiatry. 2000 Aug;69(2):269–272. doi: 10.1136/jnnp.69.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996 Jun;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 11.Moller M, Frandsen J, Andersen G, Gjedde A, Vestergaard-Poulsen P, Ostergaard L. Dynamic changes in corticospinal tracts after stroke detected by fibretracking. Journal of neurology, neurosurgery, and psychiatry. 2007 Jun;78(6):587–592. doi: 10.1136/jnnp.2006.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain : a journal of neurology. 2007 Jan;130(Pt 1):170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 13.Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010 Jan 26;74(4):280–287. doi: 10.1212/WNL.0b013e3181ccc6d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke; a journal of cerebral circulation. 2000 Jun;31(6):1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 15.Harris-Love ML, Morton SM, Perez MA, Cohen LG. Mechanisms of short-term training-induced reaching improvement in severely hemiparetic stroke patients: a TMS study. Neurorehabilitation and neural repair. 2011 Jun;25(5):398–411. doi: 10.1177/1545968310395600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003 Mar;89(3):1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- 17.Avanzino L, Teo JT, Rothwell JC. Intracortical circuits modulate transcallosal inhibition in humans. The Journal of physiology. 2007 Aug 15;583(Pt 1):99–114. doi: 10.1113/jphysiol.2007.134510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. [PubMed] [Google Scholar]
- 19.Taub E, Uswatte G, King DK, Morris D, Crago JE, Chatterjee A. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke; a journal of cerebral circulation. 2006 Apr;37(4):1045–1049. doi: 10.1161/01.STR.0000206463.66461.97. [DOI] [PubMed] [Google Scholar]
- 20.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2009 Dec;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: State of the art 2008. Brain stimulation. 2008 Jul;1(3):206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Plow (Bhatt) E, Nagpal A, Greer KH, et al. Effect of finger tracking combined with electrical stimulation on brain reorganization and hand function in subjects with stroke. Experimental brain research. Experimentelle Hirnforschung Experimentation cerebrale. 2007 Oct;182(4):435–447. doi: 10.1007/s00221-007-1001-5. [DOI] [PubMed] [Google Scholar]
- 23.Ward NS, Newton JM, Swayne OB, et al. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. The European journal of neuroscience. 2007 Mar;25(6):1865–1873. doi: 10.1111/j.1460-9568.2007.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Logothetis NK, Pfeuffer J. On the nature of the bold fmri contrast mechanism. Magnetic resonance imaging. 2004;22(10):1517–1531. doi: 10.1016/j.mri.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Ekstrom A. How and when the fMRI BOLD signal relates to underlying neural activity: the danger in dissociation. Brain Res Rev. 2010 Mar;62(2):233–244. doi: 10.1016/j.brainresrev.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews PM, Jezzard P. Functional magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2004 Jan;75(1):6–12. [PMC free article] [PubMed] [Google Scholar]
- 27.Bullmore ET, Brammer MJ, Rabe-Hesketh S, et al. Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Human brain mapping. 1999;7(1):38–48. doi: 10.1002/(SICI)1097-0193(1999)7:1<38::AID-HBM4>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward BD. Simultaneous Inference for FMRI Data. 2000 http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf.
- 29.Dassonville P, Lewis SM, Zhu XH, Ugurbil K, Kim SG, Ashe J. The effect of stimulus-response compatibility on cortical motor activation. NeuroImage. 2001 Jan;13(1):1–14. doi: 10.1006/nimg.2000.0671. [DOI] [PubMed] [Google Scholar]
- 30.Lowe MJ, Beall EB, Sakaie KE, et al. Resting state sensorimotor functional connectivity in multiple sclerosis inversely correlates with transcallosal motor pathway transverse diffusivity. Human brain mapping. 2008 Jul;29(7):818–827. doi: 10.1002/hbm.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deakin A, Hill H, Pomeroy VM. Rough Guide to the Fugl-Meyer Assessment-Upper Limb Section. Physiotherapy. 2003;89(12):751–763. [Google Scholar]
- 32.Berglund K, Fugl-Meyer AR. Upper extremity function in hemiplegia. A cross-validation study of two assessment methods. Scand J Rehabil Med. 1986;18(4):155–157. [PubMed] [Google Scholar]
- 33.Uswatte G, Taub E, Morris D, Vignolo M, McCulloch K. Reliability and validity of the upper-extremity Motor Activity Log-14 for measuring real-world arm use. Stroke; a journal of cerebral circulation. 2005 Nov;36(11):2493–2496. doi: 10.1161/01.STR.0000185928.90848.2e. [DOI] [PubMed] [Google Scholar]
- 34.van der Lee JH, Beckerman H, Knol DL, de Vet HC, Bouter LM. Clinimetric properties of the motor activity log for the assessment of arm use in hemiparetic patients. Stroke; a journal of cerebral circulation. 2004 Jun;35(6):1410–1414. doi: 10.1161/01.STR.0000126900.24964.7e. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi N, Tada T, Matsuo Y, Ikoma K. Low-frequency repetitive TMS plus anodal transcranial DCS prevents transient decline in bimanual movement induced by contralesional inhibitory rTMS after stroke. Neurorehabilitation and neural repair. 2012 Oct;26(8):988–998. doi: 10.1177/1545968311433295. [DOI] [PubMed] [Google Scholar]
- 36.Liepert J, Kucinski T, Tuscher O, Pawlas F, Baumer T, Weiller C. Motor cortex excitability after cerebellar infarction. Stroke. 2004 Nov;35(11):2484–2488. doi: 10.1161/01.STR.0000143152.45801.ca. [DOI] [PubMed] [Google Scholar]
- 37.Talelli P, Greenwood RJ, Rothwell JC. Exploring Theta Burst Stimulation as an intervention to improve motor recovery in chronic stroke. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2007 Feb;118(2):333–342. doi: 10.1016/j.clinph.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 38.McGregor KM, Carpenter H, Kleim E, et al. Motor map reliability and aging: a TMS/fMRI study. Experimental brain research. Experimentelle Hirnforschung Experimentation cerebrale. 2012 May;219(1):97–106. doi: 10.1007/s00221-012-3070-3. [DOI] [PubMed] [Google Scholar]
- 39.Sarfeld AS, Diekhoff S, Wang LE, et al. Convergence of human brain mapping tools: neuronavigated TMS parameters and fMRI activity in the hand motor area. Human brain mapping. 2012 May;33(5):1107–1123. doi: 10.1002/hbm.21272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brocke J, Schmidt S, Irlbacher K, Cichy RM, Brandt SA. Transcranial cortex stimulation and fMRI: electrophysiological correlates of dual-pulse BOLD signal modulation. NeuroImage. 2008 Apr 1;40(2):631–643. doi: 10.1016/j.neuroimage.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 41.Milot MH, Spencer SJ, Chan V, et al. Corticospinal Excitability as a Predictor of Functional Gains at the Affected Upper Limb Following Robotic Training in Chronic Stroke Survivors. Neurorehabilitation and neural repair. 2014 Mar 18; doi: 10.1177/1545968314527351. [DOI] [PMC free article] [PubMed]
- 42.Malcolm MP, Triggs WJ, Light KE, et al. Repetitive transcranial magnetic stimulation as an adjunct to constraint-induced therapy: an exploratory randomized controlled trial. American journal of physical medicine & rehabilitation/Association of Academic Physiatrists. 2007 Sep;86(9):707–715. doi: 10.1097/PHM.0b013e31813e0de0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaechter JD, Perdue KL. Enhanced cortical activation in the contralesional hemisphere of chronic stroke patients in response to motor skill challenge. Cereb Cortex. 2008 Mar;18(3):638–647. doi: 10.1093/cercor/bhm096. [DOI] [PubMed] [Google Scholar]
- 44.Biernaskie J, Szymanska A, Windle V, Corbett D. Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. The European journal of neuroscience. 2005 Feb;21(4):989–999. doi: 10.1111/j.1460-9568.2005.03899.x. [DOI] [PubMed] [Google Scholar]
- 45.Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002 Oct 29;99(22):14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindberg PG, Skejo PH, Rounis E, et al. Wallerian degeneration of the corticofugal tracts in chronic stroke: a pilot study relating diffusion tensor imaging, transcranial magnetic stimulation, and hand function. Neurorehabilitation and neural repair. 2007 Nov-Dec;21(6):551–560. doi: 10.1177/1545968307301886. [DOI] [PubMed] [Google Scholar]
- 47.Lindenberg R, Zhu LL, Ruber T, Schlaug G. Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Human brain mapping. 2012 May;33(5):1040–1051. doi: 10.1002/hbm.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plow EB, Cunningham DA, Beall E, et al. Effectiveness and neural mechanisms associated with tDCS delivered to premotor cortex in stroke rehabilitation: study protocol for a randomized controlled trial. Trials. 2013;14(1):331. doi: 10.1186/1745-6215-14-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cunningham DAMA, Rajagopalan V, Lowe M, Jones S, Beall E, Sakaie K, Plow E. DTI versus fMRI: Accuracy and reliability in predicting response to TMS in stroke. Annals of neurology. 2013;74:S1–S120. [Google Scholar]
- 50.Kokotilo KJ, Eng JJ, McKeown MJ, Boyd LA. Greater activation of secondary motor areas is related to less arm use after stroke. Neurorehabilitation and neural repair. 2010 Jan;24(1):78–87. doi: 10.1177/1545968309345269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tazoe T, Perez MA. Speed-dependent contribution of callosal pathways to ipsilateral movements. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013 Oct 9;33(41):16178–16188. doi: 10.1523/JNEUROSCI.2638-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]