Abstract
BACKGROUND
Insecticide resistance in the malaria mosquito, Anopheles gambiae, is well documented and widespread agricultural use of pyrethroids may exacerbate development of resistance when pyrethroids are used in vector control. We have developed carbamate anticholinesterases that possess a high degree of An. gambiae: human selectivity for enzyme inhibition. The purpose of this study was to assess the spectrum of activity of these carbamates against other mosquitoes and agricultural pests.
RESULTS
Experimental carbamates were potent inhibitors of mosquito acetylcholinesterases, with IC50 values in the nanomolar range. Similar potencies were observed for Musca domestica and Drosophila melanogaster enzymes. Although meta-substituted carbamates were potent inhibitors, two ortho-substituted carbamates displayed poor enzyme inhibition (IC50 ≥ 10−6 M) in honey bee (Apis mellifera), Asian citrus psyllid (Diaphorina citri), and lepidopteran agricultural pests (Plutella xylostella and Ostrinia nubilalis). Enzyme inhibition results were confirmed by toxicity studies in caterpillars, where the new carbamates were 2- to 3-fold less toxic than propoxur and up to 10-fold less active than bendiocarb, indicating little utility of these compounds for crop protection.
CONCLUSION
The experimental carbamates were broadly active against mosquito species but not agricultural pests, which should mitigate selection for mosquito insecticide resistance by reducing agricultural uses of these compounds.
Keywords: acetylcholinesterase, Anopheles gambiae, selectivity, mosquito
1 INTRODUCTION
Insecticides remain the principal component of the integrated management approach for the control of vector borne diseases.1 If available, residents of malaria endemic countries sleep under insecticide treated nets (ITNs) to reduce malarial transmission.2, 3 Currently, pyrethroids are the only class of insecticides approved for use in ITNs due to their high efficacy, excito-repellent properties, and low toxicity to mammals.3, 4 Pyrethroids have been effective in controlling the malaria vector, Anopheles gambiae (Giles) for a number of years, but the increased prevalence of pyrethroid resistant mosquitoes, primarily through a sodium channel mutation (kdr), has reduced the efficacy of ITNs and is forcing researchers to develop new mosquitocides with a novel modes of action.5 Besides malaria, diseases such as dengue fever/dengue hemorrhagic fever (vector: Aedes aegypti), lymphatic filariasis (vector: Culex quinquefasciatus), and West Nile virus (vector: Aedes albopictus) are also highly prevalent and often deadly. Of large concern is the viral infection dengue fever, that is estimated to hospitalize 500,000 people annually and approximately 2.5 billion people are deemed at risk for the disease.6 It is apparent that the design of novel mosquitocides is necessary for the reduction of vector-borne disease transmission and minimizing mosquito-borne deaths.
Acetylcholinesterase (AChE) is a well-validated insecticide target site that has been exploited for many years through the use of organophosphates and carbamates.7 AChE is a serine hydrolase necessary for regulation of the neurotransmitter acetylcholine in human and insect central nervous systems, and anticholinesterases react with a serine residue located at the catalytic site to inactivate the enzyme.7 The inactivated enzyme is no longer capable of hydrolyzing acetylcholine, resulting in the buildup of acetylcholine (Ach) in the nerve synapse, leading to convulsions and death.7 Although highly toxic to insects, toxicity to humans through concurrent human AChE inhibition8 has limited the uses of anticholinesterases in malaria control programs.
Insecticide resistance in mosquitoes due to agricultural uses has been documented and specifically affects insecticide design for disease control. For example, widespread agricultural use of pyrethroids has been implicated in exacerbating development of resistance to insecticides with the same mode of action, thus reducing the effectiveness of ITNs.9 It has been suggested that irrigated agriculture and crop spraying has subjected mosquito vectors to selection in the larval stages, especially with pyrethroids.9, 10 Development of more selective insecticides with reduced toxicity to agricultural pests could mitigate resistance selection by reducing or eliminating use on crops.
We have synthesized a collection of phenyl substituted carbamates that possess novel structures and increased An. gambiae:human selectivity at the enzyme level.11, 12, 13 The objective of the present investigation was to determine the activity of these experimental carbamates to other mosquito disease vectors and agricultural pests, in an effort to further explore species selectivity and to evaluate any advantageous properties for resistance management. The activity of the experimental carbamates was compared to three commercial materials. The commercial carbamates propoxur and bendiocarb were selected for this study because they are WHOPES approved for mosquito control (http://www.who.int/whopes/Insecticides_IRS_Malaria_25_Oct_2013.pdf?ua=1), and carbofuran was used because of its potent and broad spectrum of insecticidal activity.12
2 EXPERIMENTAL METHODS
2.1 Inhibitors, solvents, and assay reagents
Propoxur (99% purity), bendiocarb (99% purity), and carbofuran (99% purity) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Experimental carbamates were prepared as described in Hartsel et al., 2012.12 All experimental compounds were purified by column chromatography and/or re-crystallization and are >95% pure by 1H NMR analysis. Structures of the experimental carbamates referred to in this study are shown in Figure 1.
Figure 1.
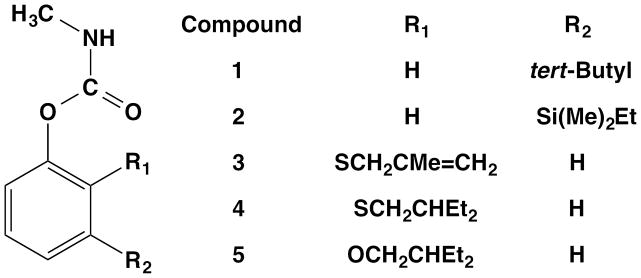
Structures of experimental methylcarbamates used in this study
Ellman assay14 reagents are composed of acetylthiocholine iodide (ATCh; ≥ 99% purity), 5,5′-dithiobis-(2-nitro)benzoic acid (DTNB; 99% purity), and sodium phosphate buffer, all of which were purchased from Sigma-Aldrich (St. Louis, MO, USA). Molecular sieve UOP type 3Å were purchased from Sigma (St. Louis, MO, USA) and were used to prevent water absorption within the DMSO stock. Fifty beads were added into a 100 mL stock solution. These sieves have a diameter of ~2 mm, a pore size of 3Å, and a water absorbing capacity of ≥ 15%. The solvents, dimethyl sulfoxide and absolute ethanol were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2 Insects and enzyme sources
Wild type Anopheles gambiae AChE (An.gAChE; Diptera: Culicidae) was prepared from mosquitoes provided by the Center for Disease Control (Atlanta, GA) that were reared from the egg stage at the University of Florida (Department of Entomology and Nematology, Emerging Pathogens Institute, Gainesville, FL, USA). Anopheles albimanus AChE (An.aAChE; Diptera: Culicidae), Anopheles quadrimaculatus AChE (An.qAChE; Diptera: Culicidae), and Aedes aegypti AChE (Ae.aAChE; Diptera: Culicidae) were cultured at the United States Department of Agriculture, Agricultural Research Service (Gainesville, FL, USA). Aedes albopictus AChE (Ae.bAChE; Diptera: Culicidae) was extracted from insects provided by Dr. Phil Kaufman at the University of Florida (Department of Entomology, Medical and Veterinary Laboratory, Gainesville, FL, USA). Culex quinquefasciatus AChE (CqAChE) was extracted from susceptible mosquitoes supplied by Dr. Bill Walton at the University of California, Riverside, cultured for over 40 years. The housefly, Musca domestica (MdAChE; FS strain; Diptera: Muscidae), was also provided by Dr. Phil Kaufman at the University of Florida and has been in culture for over 40 years. Drosophila melanogaster (DmAChE; Orgeon-R wild type strain; Diptera: Drosophilidae) was cultured at the University of Florida (Department of Entomology and Nematology, Emerging Pathogens Institute, Gainesville, FL, USA). Asian Citrus Psyllids, Diaphorina citri (DcAChE; Hemiptera: Psyllidae) were provided by the Department of Entomology and Nematology, Lake Alfred CREC station, University of Florida (Gainesville, FL, USA). Apis mellifera (Ap.mAChE; Hymenoptera: Apidae), were provided by Dr. James Ellis of the University of Florida (Department of Entomology, Bee Unit, Gainesville, FL, USA). Ostrinia nubilalis (OnAChE; Lepidoptera: Crambidae) were purchased from French Agricultural Research (Lamberton, MN). Plutella xylostella (PxAChE; Lepidoptera: Plutellidae) was provided by Dr. Anthony Shelton of Cornell University (Ithaca, NY, USA). Neither lepidopteran species as provided are known to possess resistance to any insecticides. Acetylcholinesterase enzymes were obtained from groups of ten whole non-blood fed adult female mosquitoes, three housefly or bee heads, six whole bodied fruit flies, twenty whole bodied psyllids, or twenty lepidopteran heads. Each enzyme preparation was from tissue homogenized in 1 mL of ice-cold sodium phosphate buffer (0.1 M, pH 7.8) containing 0.3% Triton x-100, with an electric motor driven glass tissue homogenizer. The homogenate was centrifuged at 5000 × g at 4 °C for 5 minutes. The supernatant was used as the crude enzyme source for the Ellman assay without further purification.
2.3 Enzyme inhibition assays
IC50 values (concentration needed to inhibit 50% of control enzyme activity) were determined using slight modifications of the Ellman et al. protocol14 outlined in Jiang et al. (2013).13 Briefly, 10 μL of enzyme solution was added to each well of a 96-well micro assay plate, along with 20 μL of dissolved compound and 150 μL of ice-cold phosphate buffer. The assay plate was incubated at 25°C for ten minutes. Ellman assay reagents, ATCh (0.4 mM, final conc.) and DTNB (0.3 mM, final conc.), were prepared fresh for each experiment and 20 μL was added to the enzyme to initiate the reaction. Changes in absorbance were recorded by a DYNEX Triad spectrophotometer (DYNEX Technologies, Chantilly, VA, USA) at 405 nm. Six inhibitor concentrations were used in triplicate to construct concentration-response curves using Graphpad Prism 4 (GraphPad Software, San Diego, CA, USA). Inhibitors were prepared using DMSO and contained a final concentration of 0.1% DMSO (v/v) for each inhibitor concentration. Enzyme concentrations used were within the linear range of measured catalytic activity, therefore eliminating the need for protein quantification. IC50 values for each species were calculated by nonlinear regression using Prism™ (GraphPad Software, San Diego, CA, USA). All data were fit to a sigmoid curve with r2 ≥ 0.98 in all experiments and Hill slope values ≥ 0.8. The nonlinear regression equation used was as follows:
where x = the logarithm of the concentration and Y = the response.
2.4 Topical toxicity assays
Topical toxicity bioassays were performed based on the method of Pridgeon et al (2008).15 Briefly, insects were chilled on ice for three minutes (one minute for Anopheline mosquitoes), during which the appropriate volume (200 nL for mosquitoes, 1 μL for lepidopteran larvae) of chemical (dissolved in 95% ethanol) was applied onto the abdomen of the insect using a handheld Hamilton® microapplicator. For each compound, five doses were applied to ten insects each, and repeated three times. An ethanol-only treatment was included in each experiment as a negative control. Insects were transferred into holding containers covered with netting. Mosquitoes had free access to sugar water and the caterpillars were provided food substrate for the duration of the experiment. Mortality was recorded at the 24-hour time point. Mortality data was pooled and analyzed by log-probit using Poloplus® to determine 24 hour LD50 values. Three LD50 values were obtained and the mean LD50 value was used for statistical analysis.
2.5 Statistical Analyses
IC50 values were averaged (n = 3 replicates, minimum) and compared by a one-way ANOVA followed by Tukey’s multiple comparison test using GraphPad InStat™ (GraphPad Software, San Diego, CA, USA). IC50 values were compared for each inhibitor among mosquito species and for each species among all inhibitors (Table 1 and Table 2). Mortality was recorded 24 hours post treatment and an LD50 was calculated using Poloplus®. Three LD50 values were obtained and the mean LD50 value was used for statistical analysis. For all toxicity assays, control mortality was corrected for using Abbot’s formula.16
Table 1.
Inhibition potencies and selectivity ratios of commercial and experimental carbamates against AChE of five mosquito species. Values for each inhibitor were tested for statistical differences across species, and those not labeled by the same lower case letter in that row represent significance at P < 0.05. Values for each species were also tested for statistical differences across compounds, and those not labeled by the same upper case letter in that column represent significance at P < 0.05.
| Mean IC50, nM (95% CI) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| An.gAChE | An.aAChE | An.qAChE | Ae.aAChE | Ae.bAChE | CqAChE | ||||||
| IC50 | a IC50 | b SR | IC50 | SR | IC50 | SR | IC50 | SR | IC50 | SR | |
| Propoxur | 445 aA (267–623) | 481 aA (319–643) | 1.1 | 428 aA (335–521) | 1.0 | 369 aA (304–433) | 0.8 | 352 aA (257–447) | 0.8 | 505 aA (318–691) | 1.1 |
| Carbofuran | 47 aB (25–68) | 55 aB (43–68) | 1.2 | 43 aB (29–57) | 0.9 | 41 aB (27–54) | 0.9 | 46 aB (10–81) | 1.0 | 111 aB (45–176) | 2.3 |
| Bendiocarb | 172 aC (80–264) | 182 aC (96–268) | 1.0 | 137 aC (83–190) | 0.8 | 127 aC (81–173) | 0.7 | 137 aC (96–177) | 0.8 | 215 aC (158–271) | 1.2 |
| 1 | 104 aC (80–128) | 100 aB (80–119) | 1.0 | 93 aC (73–111) | 0.9 | 79 aC (39–118) | 0.8 | 87 aB (73–100) | 0.8 | 123 aB (60–186) | 1.2 |
| 2 | 221 aC (116–325) | 217 aC (153–282) | 1.0 | 281 aD (223–338) | 1.3 | 341 bA (253–429) | 1.5 | 288 bA (223–353) | 1.3 | 67 cB (39–94) | 0.3 |
| 3 | 476 aA (356–596) | 379 bA (283–474) | 0.8 | 390 aA (342–439) | 0.8 | 275 cA (213–337) | 0.6 | 332 cA (280–384) | 0.7 | 375 bD (287–463) | 0.8 |
| 4 | 106 aC (85–128) | 93 aB (75–110) | 0.9 | 92 aC (66–117) | 0.9 | 208 bD (146–270) | 2.0 | 177 bC (110–245) | 1.7 | 245 bE (126–363) | 2.3 |
| 5 | 431 aA (254–607) | 546 aA (392–700) | 1.3 | 565 aA (383–746) | 1.3 | 756 bE (708–803) | 1.8 | 574 aD (452–695) | 1.3 | 571 aA (436–705) | 1.3 |
IC50 values are shown as mean (n > 3) values and are expressed in nM (95% CI)
SR = selectivity index = IC50 of mosquito AChE/IC50 of An. gambiae (rounded to the nearest decimal point).
Table 2.
IC50 and selectivity ratio data obtained from six agriculturally relevant insect species AChE. Values for each inhibitor were tested for statistical differences across species, and those not labeled by the same lower case letter in that row represent significance at P < 0.05. Values for each species were tested for statistical differences across compounds, and those not labeled by the same upper case letter in that column represent significance at P < 0.05.
| Inhibitor | PxAChE | OnAChE | DmAChE | MdAChE | DcAChE | Ap.mAChE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| a IC50 | b SR | IC50 | SR | IC50 | SR | IC50 | SR | IC50 | SR | IC50 | SR | |
| Propoxur | 3322 aA (2520–4120) | 7.5 | 6710 bA (4770–8650) | 15 | 104 dA (48–159) | 0.2 | 152 dA (106–199) | 0.3 | 942 cA (689–1194) | 2 | 941 cA (853–1030) | 2 |
| Bendiocarb | 217 aB (143–291) | 1.3 | 336 aB (229–442) | 2 | 27 cB (9–44) | 0.2 | 58 bB (36–79) | 0.3 | 92 bB (62–121) | 0.5 | 49 bB (34–64) | 0.3 |
| 1 | 675 aC (370–980) | 6.5 | 2933 bC (2540–3330) | 28 | 97 cA (66–129) | 0.6 | 181 dA (142–220) | 1.0 | 128 cB (100–156) | 1.2 | 32 dB (17–47) | 0.3 |
| 2 | 3411 aA (3160–3660) | 15 | 7794 bA (7070–8520) | 35 | 279 cC (215–343) | 1.3 | 488 eC (434–541) | 2.2 | 210 cC (147–273) | 0.9 | 77 dB (29–125) | 0.3 |
| 3 | 1681 aD (1400–2000) | 3.5 | 10847 bD (8150–13500) | 23 | 131 dA (78–183) | 0.3 | 156 dA (129–182) | 0.3 | 1067 aD (812–1345) | 2.2 | 379 cC (307–451) | 0.8 |
| 4 | 52166 aE (42800–62000) | 492 | 20640 aE (14800–26400) | 194 | 23 dB (8–38) | 0.2 | 51 dB (14–87) | 0.5 | 3193 bE (2830–3550) | 30 | 1242 cA (883–1601) | 11 |
| 5 | > 100 uM aF | >232 | 81940 bF (71800–92000) | 190 | 482 dD (334–630) | 1.1 | 654 dC (493–815) | 1.5 | 6189 cF (5790–6600) | 14 | 5440 cD (3990–6890) | 12 |
IC50 values are shown as mean (n > 3) values and are expressed in nM (95% CI)
SR = selectivity index = IC50 of agricultural insect AChE/IC50 of An.gAChE (rounded to the nearest decimal point).
3 RESULTS
3.1. Mosquito enzyme sensitivity
The IC50 results of mosquito enzymes, presented in Table 1, show that carbofuran displayed more potent inhibition across all mosquito species than all other methylcarbamates. Among commercial carbamates, the rank order of potency was always carbofuran > bendiocarb > propoxur (Table 1). Within each mosquito species, carbofuran was found to be approximately 10-fold more potent than the least potent commercial carbamate, propoxur, and about 3-fold more active than bendiocarb. Each difference in potency was statistically significant within a given species. In contrast, no statistical significance of inhibition potencies was observed across mosquito species for any commercial carbamate (Table 1).
The inhibitory potencies of experimental carbamates for the six mosquito species were usually in the range of either bendiocarb or propoxur, but in some cases approached that of carbofuran (Table 1). For An.gAChE, compounds matched the activity of bendiocarb (1, 2, 4) or propoxur (3, 5). Compounds 1 and 4 were similar in activity to carbofuran against An.aAChE, as they were significantly more potent than propoxur and bendiocarb, whereas 2 was similar to bendiocarb and 3 and 5 were similar to propoxur (Table 1). For An.qAChE, 2 was intermediate in potency between propoxur and bendiocarb, 1 and 4 were similar to bendiocarb, and 3 and 5 were similar to propoxur. Compound 4 on Ae.aAChE was intermediate in potency between propoxur and bendiocarb, while 5 was less active than propoxur. The other compounds matched either bendiocarb (1) or propoxur (2, 3) in potency. Compound 1 on Ae.bAChE approached the level of activity of carbofuran, 4 was equal to bendiocarb, 2 and 3 equaled propoxur, and 5 was less active than propoxur. For CqAChE, 5 matched the potency of propoxur, while the others were equal to carbofuran (1 and 2) or fell between bendiocarb and propoxur (3 and 4), with 4 being the more potent of the two.
Among the Anopheles spp. studied, experimental compounds 1 and 4 were the most active and essentially equipotent (IC50 ca. 100 nM), but 4 had less activity against the other mosquito species (Table 1). Compounds 1 and 2 were experimental inhibitors containing a meta-substituted side chain (Fig. 1). Of these two, compound 1 was the more potent carbamate, typically by about 2–3 fold, or up to 4-fold for Ae.aAChE, against all mosquito species, except CqAChE. Compound 2 was only more potent than 1 against CqAChE (about 2-fold). Compound 4 was found to be the most potent experimental inhibitor containing an ortho-substituted side chain (Fig. 1) in all mosquito species studied; however, 4 was approximately two-fold less potent when compared to 1 in both Aedes and Culex mosquitoes (Table 1). Potency differences for ortho-substituted experimental carbamates (least potent IC50/most potent IC50), when compared within a mosquito species, ranged from 3.2-fold (Ae.bAChE; compounds 3 and 4) to 6.1-fold (An.qAChE; compound 4 and 5). Across all compounds and all mosquito species, SR ratios were ≤ 2, indicating little difference (Table 1).
3.2 Agricultural pest enzyme sensitivity
Five pest species and one economically important pollinator were studied to determine the activity of carbamate AChE inhibitors to agriculturally relevant insects (Table 2). For the commercial insecticides bendiocarb and propoxur, the former was uniformly more potent against all species tested (Table 2). Bendiocarb was found to be 3- and 461-fold more potent against PxAChE and 8- to 243-fold more potent against OnAChE when compared to the most (1) and least potent (5, using a 100 μM value) experimental inhibitors. Within a given lepidopteran species, all experimental inhibitors were significantly less active (P < 0.05) when compared to bendiocarb, whereas 1 and 2 were found to be greater than, and equally potent to propoxur, respectively. Of the commercial carbamates, propoxur was significantly more potent against PxAChE when compared to OnAChE, while no significant differences in inhibition potencies was observed between the two lepidopteran species with bendiocarb. The inhibition potency of 1, the most potent experimental inhibitor, was significantly less than bendiocarb and significantly more active than propoxur, for both lepidopteran insects. When assessing the range of activity of experimental carbamates against lepidoptera, inhibitor 1 was shown to be > 150-fold and 27-fold more potent than 5, the least potent inhibitor, against PxAChE and OnAChE, respectively (Table 2).
The acetylcholinesterases of the pestiferous flies, Drosophila melanogaster and Musca domestica, were significantly more sensitive to inhibition by all standard and experimental carbamates when compared to PxAChE, OnAChE, or DcAChE (Table 2). The most potent commercial inhibitor, bendiocarb, was significantly more active against DmAChE but not against MdAChE when compared to Ap.mAChE or DcAChE. Against the fly species, bendiocarb was shown to be 2.6-fold (MdAChE) to 3.9-fold (DmAChE) more potent than propoxur. Experimental inhibitor 4 was not significantly different from the potencies of bendiocarb for both fly species, and was the most potent experimental inhibitor with IC50 values 21- to 13-fold more active than 5 (the least active compound) against DmAChE and MdAChE, respectively (Table 2).
Inhibition potencies with the serious citrus pest DcAChE showed IC50 values for commercial carbamates that were intermediate between those of lepidopteran and dipteran species (Table 2). For the experimental insecticides, 1 was the most potent inhibitor (matching bendiocarb) and was 1.6-fold more potent than 2, the other meta-substituted experimental methylcarbamate (Fig. 1). The experimental carbamates possessing an ortho-substituted side chain were 8- to 48-fold less active against D. citri when compared to 1 (Table 2). Within DcAChE, all inhibition potencies of experimental carbamates were statistically significantly different from that of bendiocarb, except for 1, which was more potent than all the other experimental inhibitors tested.
The economically important pollinator, Apis mellifera, displayed a wide range of enzyme inhibition potencies that appeared to be based upon the position of the substituted side chain. Similar to D. citri, all ortho-substituted inhibitors were substantially less potent when compared to meta-substituted compounds (Table 2). A 19-fold difference in IC50 value was observed between propoxur and bendiocarb, whereas a 170-fold difference in inhibition potency was observed between 1 and 5, the most and least potent of the experimental carbamates. For the experimental carbamates, a 2.4-fold difference was observed among meta-substituted compounds, and up to a 14-fold difference was observed among the ortho substituted compounds. The experimental inhibitors 1 and 2 were not significantly different in potency when compared to bendiocarb, but were significantly more potent than all other inhibitors on Ap.mAChE. The IC50 value of 4 was not significantly different from the IC50 value of propoxur against Ap.mAChE, but was significantly different when compared to the potency values of 4 against all other species (Table 2).
3.3 Selectivity of carbamates on enzymes from mosquitoes and agricultural pests
Selectivity ratios (SR) were calculated from An. gambiae IC50 values, since the experimental carbamates were designed to control this particular species of mosquito (Table 2). When compared to An.gAChE, O. nubilalis displayed up to a 194-fold increase (compound 4) in IC50 value, whereas the commercial carbamate bendiocarb had IC50 values for these species that differed by only 2-fold. This pattern of decreased inhibition potencies was also observed with Plutella xylostella AChE, as experimental inhibitors displayed a statistically significant decrease (e.g., 492-fold; compound 4) in inhibition, whereas bendiocarb showed no significant difference in SR between An.gAChE and PxAChE. Interestingly, the commercial carbamate propoxur displayed a 7-fold decrease in potency between PxAChE and An.gAChE, a substantially smaller decrease when compared to bendiocarb. Propoxur was 15-fold selective for An.gAChE compared to O. nubilalis, but was 13-fold less selective than 4 (194-fold overall) when considering this same species comparison (Table 2). Similarly, compound 4 was found to be 97-fold more selective than bendiocarb for An.gAChE compared to OnAChE. The overall range of the selectivity ratios of An. gambiae and P. xylostella were generally similar to those observed with O. nubilalis. Propoxur and bendiocarb showed little selectivity (≤7-fold) between An.gAChE and that of P. xylostella, whereas the experimental carbamates ranged from 5- to 490-fold selective.
Commercial carbamates were found to be poorly selective (0.3- to 2-fold) over the An.gAChE enzyme for DcAChE and Ap.mAChE (Table 2). Experimental carbamates 1-3 had values similar to propoxur and bendiocarb, but 4 and 5 were more selective. Selectivity of experimental carbamates ranged from 0.9-fold (compound 2) to 30-fold (compound 4), the latter of which is 15-fold more selective than either commercial carbamate studied (Table 2). Similarly, propoxur was only 2-fold selective for An.gAChE over Ap.mAChE, while bendiocarb was about 3-fold more active on Ap.mAChE enzyme compared to An.gAChE. Experimental carbamates 1, 2, and 3 were negatively selective for Ap.mAChE compared to An.gAChE (SR < 1); however, 4 and 5 were 11- and 12-fold more active, respectively on An.gAChE than Ap. mAChE (Table 2).
The flies, DmAChE and MdAChE, were found to be highly sensitive to all carbamates, with IC50 values greater than or equal to those found for An.gAChE, and which often yielded SR ratios ≤ 1 (Table 2). Greatest negative selectivity for the flies (5-fold) was observed with propoxur and bendiocarb against DmAChE, and was similar to that observed with MdAChE (3-fold). Similar levels of selectivity for fly enzymes was observed for compounds 3 and 4. Compound 2 was the most selective inhibitor studied for both DmAChE and MdAChE with SR values of 1.3 and 2-fold, respectively (Table 2).
3.4 Toxicity of methylcarbamates to mosquitoes and European corn borer
Toxicity of carbamates was assessed through topical bioassays to determine LD50 values (Table 3). For mosquitoes, sensitivity to all carbamates was An. gambiae = Ae. aegypti > Cu. quinquefasciatus (all significantly greater LD50 values in Culex except 5). Within a species, experimental carbamates were found to differ in toxicity by approximately 20-fold to An. gambiae and Ae. aegypti and 6-fold to Cx. quinquefasciatus. Compounds 1 and 5 were the most and least toxic to An. gambiae and Ae. aegypti, respectively, whereas 1 and 2 were most and least toxic to Cx. quinquefasciatus. These data also support the in vitro data, as 1 and 5 were the most and least potent enzyme inhibitors against their respective enzyme sources (Table 1). It is interesting to note, 1 and 4 are equipotent inhibitors of An.gAChE and Ae.aAChE yet 4 is 2.5-fold less toxic to the adult mosquitoes. The two commercial carbamates had toxicities to An. gambiae and Ae. aegypti adults that were essentially matched by compound 1 (Table 3). The other experimental carbamates were less toxic, with 2 having significant activity, but less for compound 5. Toxicity of all carbamates but 5 to Cx. quinquefasciatus was 2- to 6-fold less when compared to An. gambiae (Table 3).
Table 3.
Topical toxicity of methylcarbamates (LD50, ng/mg) to three mosquito species and Ostrinia nubilalis. LD50 values for each inhibitor were tested for statistical differences across species, and those not labeled by the same lower case letter in that row represent significance at P < 0.05. LD50 values for each species were also tested for differences across compounds, and those not labeled by the same upper case letter in that column represent statistical significance at P < 0.05. Average (n=25) insect weights were as follows: An gambiae: 1.2 mg, Ae. aegypti: 1.8 mg, Cx. quinquefasciatus: 2.4 mg, O. nubilalis L2: 25 mg.
| An. gambiae | Ae. aegypti | Cu. quinquefasciatus | O. nubilalis | ||
|---|---|---|---|---|---|
| Insecticide | LD50 (95% CI) | LD50 (95% CI) | LD50 (95% CI) | LD50 (95% CI) | SRa |
| Propoxur | 3 (2–4) aA | 3 (1–4) aA | 8 (5–11) bA | 42 (34–49) cA | 14 |
| Bendiocarb | 2 (1–4) aA | 1 (0.5–3) aA | 2 (1–4) aB | 4 (2–6) aB | 2 |
| 1 | 3 (2–6) aA | 4 (1–8) aA | 6 (3–10) aA | 95 (79–111) cC | 32 |
| 2 | 6 (3–9) aB | 7 (3–11) aB | 38 (28–47) bC | 128 (109–148) cC | 21 |
| 3 | 10 (5–15) aB | 11 (6–17) aB | 35 (25–46) bC | 132 (117–148) cC | 13 |
| 4 | 8 (3–15) aB | 13 (8–18) aB | 21 (17–25) bD | 114 (99–129) cC | 14 |
| 5 | 67 (45–88) aC | 51 (39–64) aC | 34 (28–41) bC | 153 (129–176) cC | 2 |
SR = Selectivity Ratio = O. nubilalis LD50/An. gambiae LD50 (to the nearest whole number).
Interestingly, both commercial and experimental carbamates possessed significantly reduced toxicity to Ostrinia nubilalis of between 2- to 30-fold (Table 3). When compared within this species, bendiocarb was found to have an LD50 of 4 ng/mg, 24-fold more toxic than the most toxic experimental carbamate (1). Otherwise, experimental carbamates and propoxur were all toxic at low microgram doses to Ostrinia. Propoxur was shown to possess relatively high selective toxicity for An. gambiae over O. nubilalis. However, the most selective experimental carbamate (1) was shown to be 1.7-fold less toxic than propoxur. The least selective experimental carbamate (5) was shown to be as selective as bendiocarb in the toxicity studies (Table 3). The relatively low toxicity of the experimental carbamates to O. nubilalis are consistent with the poor potency against lepidopteran AChE that was observed in the in vitro enzyme experiments.
4 DISCUSSION
All the mosquito species studied were sensitive to the experimental carbamates (Table 1), and experimental carbamates 1 – 4 were found to be toxic at low nanogram doses (Table 3), which suggests they could be useful for controlling a variety of mosquito borne diseases. Past literature reports that alkyl substituents at the meta position of the phenyl ring are generally more potent inhibitors than substitutions at the ortho position.17, 18 Although this structure-activity relationship was true for the compounds and insects tested at that time, this trend was not observed for potency against mosquitoes, as 1 and 4 were nearly equipotent in all five mosquito species studied. Among the ortho-substituted carbamates, propoxur and 5 both possess an alkoxy linkage to the phenyl ring while 3 and 4 are thioethers (Figure 1). The structural similarities between 5 and propoxur likely explain the similar inhibition potencies observed across all mosquito species; however, propoxur was more toxic than 5 (Table 3). It is interesting to note that although both 3 and 4 are thioethers substituted in the ortho- position, 4 is approximately four-fold more potent than 3 across all mosquito species. This loss of potency could be due to reduced structural flexibility or hydrophobicity of 3 compared to 4. The high activity observed with 4 was reduced four- to five-fold by replacing the sulfur with an oxygen (5), confirming a previous observation that oxygen in this position reduces activity.12 Similarly, meta substitution of a silicon group in the side chain of 2 caused a reduction in AChE inhibition potency when compared to the tert-butyl group of 1, suggesting the longer silicon atom bond length reduces binding within the catalytic site in all mosquito enzymes except CqAChE, where 2 had the highest observed potency of any experimental carbamate (Table 1). Unfortunately, the high enzyme inhibition potency of 2 did not translate into a low LD50 for Cu. quinquefasciatus, suggesting that pharmacokinetics factors are responsible. Although we did not perform toxicity studies on D. melanogaster or M. domestica, the high enzyme inhibition potency of the experimental carbamates suggests that they would be potent lethal agents on these species.
Although the experimental carbamates were found to possess high activity against mosquito species and low activity against human (0.23 μM–113 μM) and avian enzymes (1 μM–250 μM)13, 19, it is also critical to understand the activity of the chemicals to agricultural pests. Broad-spectrum insecticides were once favored for commercialization due to the ability to target numerous pests with the same chemical. However, insecticide resistance of mosquitoes due to agricultural uses has been documented, and specifically affects insecticide design for disease control. Widespread agricultural use of pyrethroids has been implicated in exacerbating development of resistance to insecticides with the same mode of action when used in ITNs.9 Currently, lepidopteran insect pests are considered to be the most important insect pest of maize in Africa, and are the cause of substantial food loss throughout the continent.20 Specifically, Chilo partellus (Swinhoe; Lepidoptera: Crambidae) is a lepidopteran stem borer known to cause large amounts of economic damage in Eastern Africa.20 The European corn borer, Ostrinia nubilalis (Lepidoptera: Crambidae), was studied due to its relatedness to Chilo partellus in an effort to determine the activity of novel carbamates to lepidopteran pests. The present results show high selectivity for An.gAChE over lepidopteran AChE enzyme with experimental carbamates (Table 2). In toxicity studies (Table 3), lethal activity against Ostrinia caterpillars was up to 4-fold less than propoxur and 38-fold less than bendiocarb, suggesting advantageous properties for mitigation of resistance selection of An. gambiae populations through reducing the ancillary uses of the chemicals in crop pest control.
Low potency of mosquitocides against the honey bee, Apis mellifera, is equally as important as the low activity against agricultural pests as honey bees represent a economically critical insect pollinator. Additionally, pesticides are postulated to serve a principal role in the phenomenon termed Colony Collapse Disorder21, 22, 23, suggesting insecticides with minimal activity against A. mellifera are of obvious interest. The novel carbamates with meta-positioned side chains showed at best no increase in selectivity for A. mellifera and D. citri, whereas the ortho-substituted carbamates were up to 12- and 30-fold selective for mosquitoes, respectively (Table 2). These data suggest ortho-substituted, branched carbamates comprise a promising approach to more selective carbamates against An. gambiae and Ae. aegypti based upon the high mosquito activity, poor agricultural insect activity, and poor activity against Ap.mAChE.
CONCLUSION
The potent inhibition of mosquito AChE, previously documented levels of low human and mouse enzyme activity13 and low mouse oral toxicity19, when coupled with the low activity to agricultural insects, suggest these carbamates could be useful for mosquito control programs. The general lack of activity against agricultural pests suggests avoidance of cross resistance development in non-target species from incidental insecticide exposure from agricultural uses.
Acknowledgments
The authors would like to thank Phil Kaufman (University of Florida), Dan Kline (USDA-ARS, CMAVE), and Paul Howell (CDC, Atlanta) for providing insects used in the course of this study. Financial support was provided by the National Institute of Allergy and Infectious Diseases (AI082581) and the Foundation for the National Institutes of Health (GCGH-1497) through the Grand Challenges in Global Health initiative.
References
- 1.Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annual Review of Entomology. 2000;45:369–389. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- 2.Lines J. Mosquito nets and insecticides for net treatment: a discussion of existing and potential distribution systems in Africa. Tropical Medicine and International Health. 1996;1:616–632. doi: 10.1111/j.1365-3156.1996.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 3.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database of Systematic Reviews. 2004;2 doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Zaim M, Aitio A, Nakashima N. Safety of pyrethroid-treated mosquito nets. Medical and Veterinary Entomology. 2000;14:1–5. doi: 10.1046/j.1365-2915.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- 5.N’Guessan R, Corbel V, Akogbeto M, Rowland M. Reduced efficacy of insecticide treated nets and indoor residual spraying formalaria control in pyrethroid resistance area, Benin. Emerging Infectious Diseases. 2007;13:199–206. doi: 10.3201/eid1302.060631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gubler D. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends in Microbiology. 2002;10:100–103. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien R. Insecticides action and metabolism. Academic Press; New York: 1967. [Google Scholar]
- 8.Thompson C, Richardson R. Anticholinesterase insecticides. In: Marrs TC, Ballantye B, editors. Pesticide Toxicology and International Regulation. John Wiley & Sons Ltd; Chichester: 2004. pp. 89–127. [Google Scholar]
- 9.Yadouleton A, Asidi A, Diouaka R, Braima J, Agossou C, Akogbeto M. Development of vegetable farming: a cause of the emergence of insecticide resistance in populations of Anopheles gambiae in urban areas of Benin. Malaria Journal. 2009;8:103. doi: 10.1186/1475-2875-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ljumba J, Lindsay S. Impact of irrigation on malaria in Africa: paddies paradox. Medical and Veterinary Entomology. 2008;15:1–11. doi: 10.1046/j.1365-2915.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- 11.Wong D, Li J, Lam P, Hartsel J, Mutunga J, Totrov M, Bloomquist J, Carlier P. Aryl methylcarbamates: potency and selectivity towards wild-type and carbamate-insensitive (G119S) Anopheles gambiae acetylcholinesterase, and toxicity to G3 strainAn. gambiae. Chemico Biological Interactions. 2013;203:314–318. doi: 10.1016/j.cbi.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartsel J, Wong D, Mutunga J, Ma M, Anderson T, Wysinski A, Islam R, Wong E, Paulson S, Li J, Lam P, Totrov M, Bloomquist J. Re-engineering aryl methylcarbamates to confer high selectivity for inhibition of Anopheles gambiae versus human acetylcholinesterase. Bioorganic and Medicinal Chemistry Letters. 2012;22:4593–4598. doi: 10.1016/j.bmcl.2012.05.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang Y, Swale D, Carlier P, Hartsel J, Ma M, Ekstrom F, Bloomquist J. Evaluation of novel carbamate insecticides for neurotoxicity to non-target species. Pesticide Biochemistry and Physiology. 2013;106:156–161. [Google Scholar]
- 14.Ellman G, Courtney K, Andres V, Featherstone R. A new rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 15.Pridgeon J, Pereira R, Becnel J, Allan S, Clark G, Linthicum K. Susceptibility of Aedes aegypti, Culex quinquefasciatus, and Anopheles quadrimaculatus to 19 pesticides with different modes of action. Journal of Medical Entomology. 2008;45:82–87. doi: 10.1603/0022-2585(2008)45[82:soaacq]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Abbot WS. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology. 1925;18:265–267. [Google Scholar]
- 17.Metcalf R. Structure-activity relationships for carbamates. Bulletin of World Health Organization. 1971;4:43–78. [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhr R, Dorough H. Carbamate Insecticide: Chemistry, Biochemistry, and Toxicology. CRC Press; Cleveland, Ohio: 1976. pp. 189–220. [Google Scholar]
- 19.Swale D, Tong F, Temeyer K, Li A, Lam P, Totrov M, Carlier P, Perez de Leon A, Bloomquist J. Inhibitor profile of bis(n)-tacrines and N-methylcarbamates on acetylcholinesterase from Ripicephalus (Boophilus) microplus and Phlebotomus papatasi. Pesticide Biochemistry and Physiology. 2013;106:85–92. doi: 10.1016/j.pestbp.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong’Amo G, Ru B, Dupas S, Moyal P, Calatayud P, Silvain J. Distribution, pest status and agro-climatic preferences of lepidopteran stem borers of maize in Kenya. Annual Entomological Society of France. 2006;42:171–177. [Google Scholar]
- 21.Johnson R, Ellis M, Mullin C, Frazier M. Pesticides and honey bee toxicity – USA. Apidologie. 2010;41:312–331. [Google Scholar]
- 22.Chauzat M, Faucon J, Martel A, Lachaize J, Cougoule N, Auber M. A survey of pesticide residues in pollen loads collected by honey bees in France. J Econ Entomol. 2006;99:253–262. doi: 10.1603/0022-0493-99.2.253. [DOI] [PubMed] [Google Scholar]
- 23.Henry M, Beguin M, Requier F, Rollin O, Odoux J, Aupinel P, Aptel J, Tchamitchian S, Decourtye A. A common pesticide decreases foraging success and survival in honey bees. Science. 2012;336:348–350. doi: 10.1126/science.1215039. [DOI] [PubMed] [Google Scholar]


