Abstract
Self-reactivity was once seen as a potential characteristic of T cells that was eliminated by clonal selection to protect the host from autoimmune pathology. We now appreciate that the T cell repertoire is in fact, broadly self-reactive, one could even say self-centered. The strength with which a T cell reacts to self ligands, and the environmental context that this reaction occurs in, influences almost every aspect of T cell biology: from development to differentiation to effector function. In this review we highlight recent advances and discoveries that relate to T cell self-reactivity, with a particular emphasis on TCR signaling thresholds.
Introduction
The physical elimination of self-reactive lymphocyte clones (clonal deletion) is a central tenet of the clonal selection theory, and has been a major focus of immunologists since introduction of the concept by F. Macfarlane Burnet and Peter Medawar in the 1950s. Indeed, many self-reactive clones are eliminated each day in the thymus, and from numerous studies of TCR transgenic mice crossed to self-antigen expressing mice, we consider clonal deletion to be a particularly efficient process1. Given this, it would be natural to assume that clonal deletion plays a central role in immunological tolerance, and that the remainder of T cells that exist in healthy animals are not self-reactive. The former assumption is not readily apparent from the experimental literature, however, and the later is clearly not the case. In the first part of this review, we discuss how pervasive clonal deletion is, how important it is for overall immunological tolerance, and how T cells interpret low and high affinity interactions to result in life or death fates respectively. Next, we discuss how strong TCR signals can also induce the differentiation of unique lineages, including regulatory T cell (Treg) cells, invariant natural killer T cells (iNKT cells), and intraepithelial lymphocytes (IEL), in the thymus. Finally, we discuss how the “weak” interactions of T cells with self, which are selected for by positive selection, yield a repertoire of naïve T cells with substantial heterogeneity in their ability to respond to foreign antigens.
Clonal deletion in the thymus
For T cells, clonal deletion occurs in the thymus, and is most efficient for clones that have high affinity for self-antigens presented by professional APC, such as dendritic cells1. That deleted clones have a higher affinity for self-p/MHC than positively selected clones has been extensively validated in both monoclonal and polyclonal experimental models, although it has been unclear precisely how many clones achieve this high signaling threshold and become deleted, relative to the number that are positively selected. It had been widely assumed that the number of clones that interact with any given peptide/MHC (p/MHC) complex with high affinity (and are deleted) would be smaller than the number of clones that could interact with low affinity (and are positively selected), because the CDR3 region of the TCR is produced by random assortment and non-templated nucleotide addition. However, several groups recently addressed this question with new approaches, and their data suggest that many more clones undergo clonal deletion in the thymus than positive selection. Two groups used an approach that focused on Bim deficient mice, which have impaired clonal deletion. These groups used novel transgenic (Nur77GFP)2 or endogenous (Helios)3 markers to enumerate the strongly signaled cells that are generated in mice lacking the pro-apoptotic molecule Bim. They reported that 55%3 – 57%2 of all signaled thymocytes at the double positive (DP) stage in the cortex are deleted, and that another roughly 50% of the positively selected single positive (SP) cells were subsequently deleted in the medulla. Thus more than three quarters of the cells that respond to self-p/MHC in the thymus are deleted. These studies were remarkably concordant with those generated by a completely different approach, where a synchronous cohort of thymocytes developing in normal mice was analyzed, and mathematical modeling of death and differentiation was used to explain the numbers of thymocytes at each stage. That data suggested 75% of cells that start selection fail to complete it4. All of these data favor the notion that the TCR repertoire has a germline encoded bias toward recognition of MHC molecules5, rather than a bias that is strictly rendered by thymic selection processes6. Although it is worth emphasizing that while the T cell repertoire is overtly MHC reactive on the whole, thymic selection processes further skew it toward recognition of the specific MHC alleles present in the individual.
Since many self-reactive clones are eliminated each day in the thymus, and we consider clonal deletion to be a particularly efficient process from the study of TCR transgenics, we might assume that clonal deletion plays an essential role in immunological tolerance. However, studies have attempted to evaluate clonal deletion from the perspective of a given self-antigen, and these reports, which used p/MHC tetramers to ask how many self-antigen reactive clones are present in animals that do or do not express the self-antigen, suggested that deletion may not be particularly efficient. Bounead et al. found only a 3 fold reduction in the number of male specific cells in male versus female mice using male-antigen/Db tetramers in TCRβ chain transgenic mice7, and similar results were observed for a tissue-specific antigen in a different TCRβ chain transgenic model8. Although it might be argued that TCRβ transgenic mice are not a physiologic model because of their elevated frequency of reactive T cells, this type of decrease was also seen in studies that used enrichment techniques to enumerate Class II restricted self-reactive T cells mice with a normal polyclonal T cell repertoire9, 10. One consideration is that all of these studies proposed a lower TCR avidity of the remaining T cells, consistent with a lower tetramer staining intensity. Two of the studies included TCR repertoire analysis and found that certain receptor specificities were indeed eliminated from the tetramer binding pool7, 10. Thus from the perspective of a given high affinity receptor, clonal deletion may be as highly efficient in the polyclonal pool as it is in monoclonal TCR transgenic models. On the other hand, from the perspective of numbers of T cells that can react to a particular self-p/MHC complex, clonal deletion is incomplete. While tetramers are useful for comparative studies of defined self-reactive T cell populations in mice with specific mutations11, or in autoimmune prone strains12, and transgenic models are useful for exploring deletion mechanisms in animal models, the true measure of clonal deletion efficiency cannot be addressed using either approach alone. In the future, technology will likely allow a comprehensive evaluation of clonal deletion where self-specific T cells are defined at the level of the sequenced TCR repertoire.
Irrespective of the precise degree of deletion efficiency, it is somewhat surprising that there are no autoimmune diseases where impaired clonal deletion has been shown to be a pathogenic mechanism. It had been assumed that Aire deficiency in APECED patients reflects autoimmunity due to a failure to clonally delete self-reactive T cells13. However, new evidence suggests that Aire is also crucial for the development of tissue specific regulatory T cells14, so this inherited autoimmune syndrome may represent a more complex loss of tolerance. It is remarkable that Bim deficient mice, which have a profound increase in the number of self-reactive clones2,3, do not have widespread autoimmunity, even in a sensitized screen using hematopoietic reconstitution of RAG1−/− mice15. In part this is because clonal deletion is not required to purge the repertoire of clones reactive to ubiquitous antigens16. The reason for this has to do with the very nature of differential TCR signaling in the thymus: In DP thymocytes, high affinity ligands cause a strong signal of short duration that results in rapid induction of apoptosis17, 18. In contrast, low affinity ligands have a preferential ability to result in sustained signals, which are required for positive selection18, 19. Thus strong stimuli at the DP stage, even when they fail to induce apoptosis, do not support positive selection and inclusion of those clones in the repertoire16. Once cells undergo positive selection and encounter different self-antigens in the medulla, they are again susceptible to deletion. However, in this case, if apoptosis is prevented, autoreactive clones will accumulate20. Therefore any autoimmune pathology that would result from a failure of clonal deletion, such as in Bim deficient mice, is predicted to be focused on tissue specific antigens. That such mice do not display autoimmunity suggests that high frequencies of self-reactive T cells can be controlled by other tolerance mechanisms. Interestingly, double deficiency in Bim and another pro-apoptotic protein, Puma, was recently shown to result in spontaneous multi-organ autoimmunity15. Thus, a very extensive (perhaps complete) impairment of clonal deletion is presumably required to breakdown the protective effects of tolerance mechanisms such as clonal anergy and extrinsic regulation by regulatory (Treg) cells.
Specialized mechanisms are at play in the cortex, to select T cells with low self-reactivity
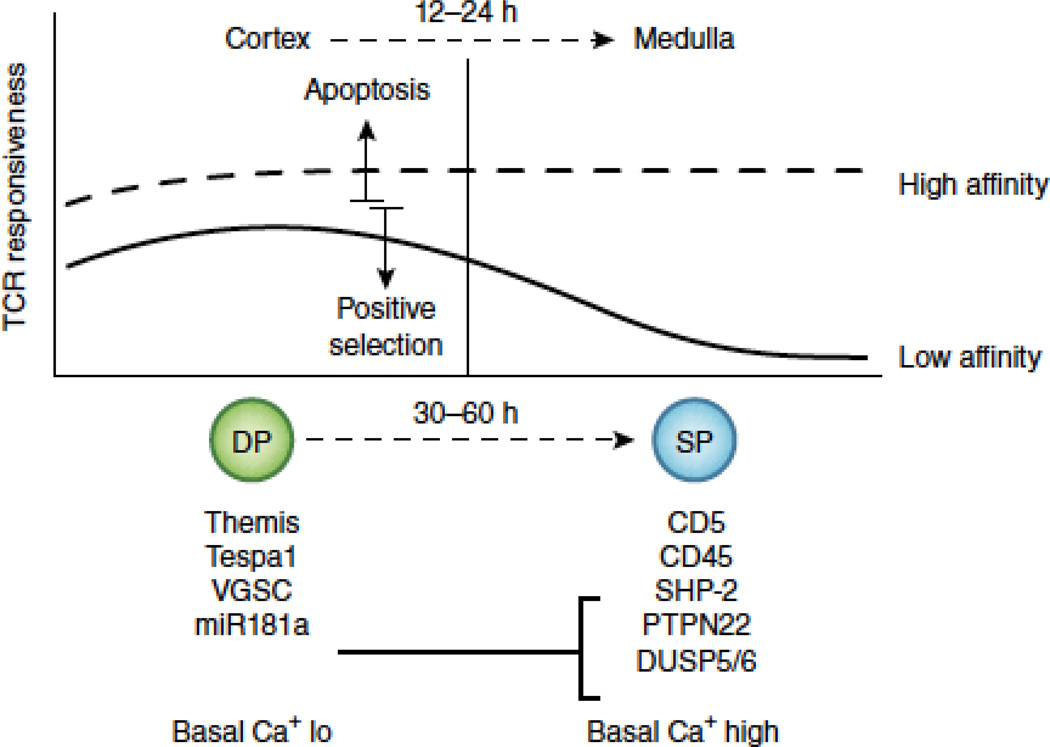
T cell progenitors undergo TCR gene rearrangement in the thymus. As soon as an intact TCRαβ heterodimer is expressed on the cell surface, the clone is exposed to potential ligands in its environment. The specific environment where this first recognition event occurs in the thymus is the thymic cortex, which is a highly specialized environment established by the activities of the transcription factor Foxn1. This factor is conserved throughout evolution in species that have a thymus21, and speaks to the importance of the “first-exposure” environment in establishing a repertoire of T cells that can function properly in the animal. It has long been known that cortical, or CD4/CD8 double positive (DP) thymocytes, are very sensitive to TCR ligands and can respond to low affinity ligands more readily that single positive (SP) thymocytes22 (Figure 1). DP cells have a dramatically different gene expression profile compared to SP cells, and at least some of these genes are presumed to account for this enhanced sensitivity. Several genes have been identified that are highly expressed in DP but reduced or absent in mature T cells, and that function to support positive selection and/or tuning. These include Themis23, Tespa124, and Scn4b25, which encode proteins involved in TCR proximal signaling events. Scn4b encodes the regulatory subunit of a voltage gated sodium channel (VGSC). Inhibition of the VGSC inhibited Ca++ influx and positive selection in thymocytes25. Furthermore, ectopic VGSC expression in mature T cells allowed them to respond to low affinity ligands, thus VGSCs appear to be crucial for the enhanced sensitivity of thymocytes (Figure 1). Both positive and negative selection invoke Ca++ signaling, although the patterns are distinct, which was demonstrating directly in thymic tissue using two-photon microscopy recently26. Interestingly, store-operated Ca++ entry, which is crucial for mature T cell responses, is dispensable for positive selection of conventional T cells27. Rather, store-operated Ca++ entry seems to function in DP thymocytes to promote “agonist-selection” of T cells (inKT cells, Treg cells, and IEL)27—discussed further below. Thus mechanistically distinct Ca++ responses are at play when thymocytes perceive weak versus strong TCR signals. It was recently shown that positive selection causes a gradual increase in the basal level of intracellular Ca++ 28. This may also contribute to the tuning that thymocytes experience, as there are data from other biological contexts that elevated basal Ca++ correlates with reduced receptor-evoked Ca++ responses29.
Figure 1. TCR sensitivity changes as cells mature from cortical (DP) to medullary (SP) thymocytes.
DP thymocytes reside in the cortex and respond efficiently to both low and high affinity TCR ligands. High affinity ligands can trigger apoptosis (clonal deletion), whereas low affinity ligands are more likely to induce survival and differentiation (positive selection). 12–24 hours after positive selection, DP thymocytes migrate to the medulla. At the same time they begin differentiating to the SP stage—a process that is not complete for another 1–2 days. In the medulla, SP thymocytes remain responsive to high affinity ligands, but their response to low affinity ligands decreases, a process sometimes referred to as “tuning”. DP thymocytes have a unique gene expression profile that serves two purposes. First, it allows them to interpret graded TCR signals in a digital fashion, to induce life or death in the cortex. The protein Themis is hypothesized to be crucial for this feature. Second, it allows them to respond more efficiently than SP thymocytes to low affinity ligands. Evidence suggests that the protein Tespa1, the voltage gated sodium channel (VGSC) and the microRNA mir181a all contribute to this property. Mir181a represses expression of several phosphatases (SHP-2, PTPN22, and DUSP5/6) that are known to negatively regulate TCR signaling. Another phosphatase (CD45) and a cell surface protein known to interact with phosphatases (CD5) are upregulated in SP thymocyte independently of mir181a. These gene expression changes, together with an increase in the basal level of intracelluar Ca++, are all thought to contribute to the developmental tuning of the TCR response.
The precise signaling functions of Tespa1 and Themis remain to be fully elucidated, although Tespa1 deficiency also influences Ca++ signaling24. An important recent study showed that Themis-deficiency allows low affinity ligands to elicit negative selection-like signaling characteristics30. Thus Themis acts to attenuate or modify weak signals through the TCR to facilitate positive selection. Likewise, Schnurri-2, which encode a zinc finger protein that possibly acts downstream of Themis, was shown to inhibit apoptosis in response to positive selection signals in DP thymocytes31. These unique signals transduced by the TCR during positive selection must be sustained for a surprisingly long time. Blocking the TCR signal 24 – 48 hours after initiation with anti-MHC antibodies19, ERK inhibitors19, Zap-70 inhibitors18, or by induced loss of Zap-7032, all were shown to impact positive selection. Intriguingly, cortex to medulla migration was recently shown to occur about 12–24 hours after positive selection initiation28, (Figure 1) suggesting that at least some of this sustained signaling may be occurring in the medulla. Nonetheless, there is little evidence that medullary epithelial cells33 or any MHC ligands in the medulla34 are needed for positive selection, so this issue needs further investigation.
It is important to consider how multiple genes that act at different levels of the signaling pathway are regulated in concert to tune TCR responsiveness. The microRNA miR-181a is highly expressed in DP thymocytes but not SP, and was shown to repress expression of several phosphatases, including SHP-2, PTPN22, and DUSP5/6, to alter TCR responsiveness35, 36. Its ectopic expression in mature T cells enhanced their sensitivity35, and its loss in DP thymocytes impaired clonal deletion37 and agonist selection of iNKT cells36. In contrast, the expression of a negative regulatory proteins, such as CD45 and CD5, increase during T cell development38 (Figure 1). CD5 is an interesting membrane protein that can negatively regulate signaling, yet its expression level seems to be directly proportional to the signal strength initially perceived39. Although the precise mechanisms of how these changes over time act together to influence the signal network remain to be fully elucidated, it is clear that specific genetic mechanisms exist to allow cortical thymocytes to preferentially respond to low affinity TCR ligands, and to do so in such a way as to not undergo apoptosis.
Not only are cortical thymocytes specialized for selection, the cortical antigen presenting cells (APCs) are as well (Figure 2). Cortical thymic epithelial cells (cTEC) display a distinct repertoire of peptides compared to other APC. The cEC unique peptides, which have been called “private peptides”, are apparently crucial for selection of the repertoire, since deletion of cTEC unique genes such as Psmb11 (encoding the β5t subunit of the proteasome), CtsL (encoding cathepsin L), or Prss16 (encoding thymus-specific serine protease or TSSP) resulted in reduced CD8 or CD4 T cell numbers [reviewed in40]. In the case of β5t, this unique private peptide diversity was suggested to be important for positive selection, rather than reducing the impact of negative selection (either of which could result in reduced T cell numbers)41. Interestingly, naïve CD8 T cells that were CD5lo and had lower reactivity for self were selectively affected by β5t deficiency, suggesting that private ligands on cTEC enhance selection of T cells with low affinity for self41. How having a distinct peptide repertoire expressed by cTEC favors positive selection was discussed recently40, and will not be reviewed in detail here. Suffice it to say that the naïve T cell repertoire in normal animals contains clones with a range of reactivity to self (CD5lo to CD5hi). In the steady state, these clones do not respond to their selecting self-ligands, either because the specific peptides are not presented by peripheral APC, and/or because the TCR signaling threshold was adjusted by tuning. It is important to stress that positive selection does not absolutely require expression of private peptides – indeed, cells selected on “public peptides” (those shared between cTEC and other cell types) have the opportunity to reencounter those ligands in the periphery, leading to enhanced basal TCR signaling (Figure 3A). Recent studies showed that a relatively abundant self-pMHC was capable of positively selecting CD4 T cells of defined foreign antigen specificity42, in support of this concept. What affect having a range of self-reactivity within the naïve repertoire has on peripheral T cell function is discussed in greater detail in the section on self-reactivity in the periphery, below.
Figure 2. The anatomic context of TCR signaling is crucial for thymocyte fate.
Cortex: TCR signal strength is central in DP thymocyte fate outcome, but extrinsic factors provided by antigen presenting cells in the cortex are also key. Weak interactions between the nascent TCR and self-pMHC complexes on cortical thymic epithelial cells (cTEC) are crucial for positive selection. The pMHC repertoire expressed by cTEC is unique, due to the function of several genes involved in endogenous or endosomal proteolysis, such as β5T, TSSP, and cathepsin L. Although this unique repertoire is essential for efficient positive selection, we do not fully understand why. Strong interactions between the newly expressed TCR and self-pMHC complexes in the cortex classically induces apoptosis (clonal deletion) and co-stimulation by B7 family members enhances this outcome. Strong TCR signals can also induce cells to become IEL precursors (IELp), although it remains unclear how frequently this occurs (relative to deletion), if it requires unique extrinsic factors, and whether IELp express a transcription factor that acts as a “master-regulator” of this fate. In the case of iNKT cells, other DP thymocytes expressing CD1d are a crucial APC. The semi-invariant iNKT TCR must recognize a high affinity CD1d/lipid ligand, but SAP-dependent signals from homotypic interactions between SLAM family members on DP thymocytes is also required. These integrated signals result in the upregulation of PLZF.
Medulla: The cells positively selected by weak TCR signals in the cortex will migrate to the medulla and, by downregulating the inappropriate co-receptor, will form the medullary SP thymocyte pool. SP thymocytes interact with distinct APC, including medullary TEC (mTEC), which express the nuclear factor AIRE. As AIRE promotes the expression of tissue-specific antigens, mTEC also display a unique pMHC repertoire. Strong interactions between the TCR and new pMHC complexes displayed by mTEC or DC classically induce apoptosis (clonal deletion), and both express B7 family members as a source of co-stimulation. Strong TCR signals can also induce CD4+ SP cells to become Treg cells, through the induction of the transcription factor FoxP3. Treg cell induction requires signals from TNFR family members, including OX40 and GITR, the ligands of which are expressed on mTEC. Treg cell induction also requires TGFβ, which is abundantly produced by mTEC. Like for IELp cells in the cortex, it remains unclear how frequently Treg cell induction occurs (relative to deletion). SP thymocytes that continue to weakly recognize self-pMHC ligands ultimately emigrate from the thymus and form the naïve T cell pool in peripheral lymphoid organs.
Figure 3. Self-reactivity establishes the activation potential of naïve T cells.
A. Naïve T cells, as a population, display a heterogeneous level of CD5, and this heterogeneity reflects the strength with which the TCR of a particular clone recognizes self-pMHC. This was confirmed using Nur77GFP mice, where CD5 expression levels correlate with GFP expression. An additional means by which CD5 heterogeneity may arise is by recognition of public versus private self-pMHC. We refer to public self-pMHC as those that are displayed by both positive-selecting APC in the thymic cortex (cTEC) and dendritic cells in the periphery. Private self-pMHC are those displayed only by cTEC, due to their unique expression of proteolysis factors. A clone selected on private pMHC will fail to continue to recognize the same pMHC in the periphery, resulting in lower CD5 levels. CD5hi clones have a higher level of basal TCRζ phosphorylation and altered gene expression patterns compared to CD5lo clones, factors that contribute to their activation potential.
B. CD5 expression levels on naïve T cells correlate with their ability to respond to foreign antigens during an immune response. CD5lo cells are less efficiently recruited into immune responses, produce IL-2 more slowly, and undergo less clonal expansion. Interestingly, naïve CD4 T cells have an overall higher level of CD5 (and GFP in Nur77GFP mice) than naïve CD8 T cells and are more effective at rapidly producing IL-2. Because CD4 T cells can undergo activation induced cell death (AICD) when over-stimulated, the CD4 clones with the very highest CD5 levels will not be as well represented in memory response.
Strong self-reactivity drives multiple T cell fates in the thymus: Treg cells, IEL, and iNKT cells
While is it abundantly evident that strong TCR signals can drive clonal deletion, increasing evidence suggests that strong TCR signals can also drive the survival and differentiation of other T cell populations, including foxP3+ Treg cells, invariant natural killer T cells (iNKT) and intraepithelial lymphocytes (IEL). Regarding Treg cells, support for the concept of agonist selection has been extensively reviewed elsewhere43,44, but the seminal observations are: 1) co-expression of an agonist ligand in TCR transgenic mice promotes Treg cell development45, at least in some models, 2) FoxP3+ Treg cells have an overlapping repertoire with autoreactive T cells derived from FoxP3-deficient mice46, and 3) Treg cells show signs of stronger and/or more recent activation in both the thymus and periphery of TCR signal reporter mice47. These data have led to the frequent discussion of a model where Treg cell selection is supported optimally by TCR affinities for self-p/MHCII that are intermediate between positive selection and clonal deletion. Recently Hsieh and colleagues examined a panel of six TCRs with varying degrees of reactivity for a model antigen and then studied the thymic development of cells with transgenic and/or retrogenic expression of those TCRs in mice that expressed the model antigen as a self-antigen. They observed a direct correlation between the degree of antigen reactivity and Treg cell development; and negative selection was apparent only with the most self-reactive TCRs. This would support the model stated above. However, the range of reactivities over which they observed Treg cell development was surprisingly broad, providing an explanation for the observed overlap between the TCR repertoires of Treg and non-Treg cells43, and suggesting that either the TCR signaling thresholds between positive selection, clonal deletion, and Treg cell development are not rigid, or that natural variation in signaling capacity between cells with the identical TCR is large. Theoretical modeling posits an integration of different signal thresholds together with loss of TCR sensitivity over time that allows the divergent fate outcomes of positive selection and Treg cell induction, which is an interesting idea that will need further experimental testing48. In the future, it will also be important to have a comprehensive understanding of the TCR repertoire overlap between conventional CD4 T cells, Treg cells, and MHCII-restricted deleted TCRs to fully understand the role of the antigen receptor.
The medulla is a specialized environment for Treg cell induction
Transgenic and retrogenic models using TCRs cloned from Treg cells have confirmed the crucial role of antigen receptor signaling49, yet also made clear that a particular TCR specificity alone is not sufficient to induce Treg cell differentiation, since Treg cell generation was only detectable at low precursor frequencies, and never observed to be complete (where 100% of the cells were foxP3+). Thus, there is a limiting “niche” for Treg cell development, whether it be at the level of competition for TCR ligands or due to competition for other factors. In addition to TCR signaling, Treg cell differentiation is known to require multiple other factors, including CD28, IL-2, TGFβ, PI3K signaling (reviewed in44), and as recently described, TNFR superfamily co-stimulation50. The availability of one or more of these factors may account for the recent observation that Treg cell development is dependent on medullary epithelial cells33 (Figure 2). An intriguing idea recently proposed is that thymocyte apoptosis may enhance the generation of regulatory T cells through production of TGFβ by phagocytic cells, particularly in the medulla51. Likewise, certain TNFR ligands, like OX40L and GITRL are also expressed on medullary APC50. Furthermore, it may be an intrinsic property of medullary thymocytes to respond to these cues, since semi-mature SP thymocytes that reside in the medulla were shown to have enhanced susceptibility to foxP3 induction52. This may reflect the requirement for TNFR signaling, as TNFR families member, such as GITR are upregulated only in medullary thymocytes, through a TAK1 dependent process during positive selection50. In this context, it is interesting to consider that tumor infiltrating self-antigen specific Treg cells were shown to be dependent on AIRE, expressed in medullary epithelial cells14. Although AIRE deficient animals are not profoundly lacking Treg cells, the extent to which the Treg cell repertoire is affected by AIRE deficiency, and whether this contributes to the autoimmune pathogenesis in mice and humans, is now of great interest.
New data on iNKT cells is consistent with a threshold model
iNKT cells have a unique semi-invariant Vα14-Jα18 TCR that recognizes lipid molecules presented by CD1d. They develop in the thymus, like other αβ T cells, but have many distinctions from conventional T cells, including their dependence on SLAM family member signals, and the use of other DP thymocytes as APC which makes the cortex a unique environment for their development (Figure 2). iNKT cells have a previously activated phenotype, like Treg cells, which suggested they may perceive strong TCR signals during development. Indeed, analysis of Egr253 and Nur7747 reporter mice was consistent with this idea. A crucial role for strong signaling comes from observations that iNKT cell development required a full complement and ITAMs54, and was abrogated in mice lacking miR-181a/b36. Recently, Bedel and colleagues more fully explored the extent to which TCR affinity for agonist self-ligands controlled iNKT cell differentiation55. They created a TCRβ chain transgenic animal using a TCRβ chain that increased the affinity for self-lipid/CD1d complexes when paired with canonical Vα14-Jα18 rearrangements. iNKT cells that expressed the high affinity canonical Vα14-Jα18 rearrangements did not mature in these TCRβ chain transgenic mice, proving that iNKT cells are subject to clonal deletion. A small proportion of cells escaped deletion in these mice, and were shown to utilize a non-canonical Vα14-Jα18 rearrangement with a 40 fold lower affinity for self-ligands. However those TCRs did not did not lead to the efficient induction of the iNKT-lineage specific transcription factor PLZF, and thus displayed aberrant proliferation, trafficking, and effector function55. This study thus suggests that proper iNKT cell differentiation requires a specific window of TCR signal perception, together with other anatomically restricted lineage induction factors, much like Treg cells (Figure 2).
Some highly self-reactive clones divert to IEL
The intestinal epithelium is interspersed with a heterogenous lymphocyte population. IEL expressing an αβ TCR and CD8αα are referred to as natural IELs and are thought to be important for mucosal tolerance and homeostasis56. Such natural IELs are generally thought to be derived from thymic precursors and to require TGFβ57 and IL-1558 for survival. This natural IEL pool has an activated phenotype, is known to contain ‘forbidden clones’, and TCR transgenics models suggested that IEL were dependent on high affinity TCR interactions in the thymus59. More recently, it was shown that rescuing cells from thymic clonal deletion through deficiency in CD28 or transgenic overexpression of bcl-2, led to increased IEL development60, suggesting that the precursors of gut IEL are cells destined for clonal deletion in the thymus. It is challenging to understand why cells destined for deletion in the thymus would sometimes traffic to the gut and adopt this fate. Nor do we understand how frequently it occurs and whether it is stochastic or regulated by specific mechanisms. Nonetheless there are a large number of such cells in the gut, and their potential to play an important role in mucosal tolerance and homeostasis merits further examination.
Self-reactivity in the periphery and naïve T cell homeostasis
The majority of thymocytes that emerge after thymic selection are “conventional” naïve TCRαβ T cells rather than the agonist selected populations discussed above. Considerable evidence suggests that these naïve T cells maintain a low-level response to self-p/MHC ligands, and that those interactions are important for sustaining a basal TCR signal and naïve T cell homeostasis. At the same time, accumulating evidence indicates that the strength of the T cell interaction with self-p/MHC is not uniform among naïve T cell clones, and that even within this “low affinity” range, the extent of self-recognition has a substantial impact on the T cell’s ability to respond to homeostatic cues and to react against foreign p/MHC ligands in an immune response. Hence, like developing thymocytes, a continuum in intensity of self-awareness has significance for function and maintenance of the peripheral naïve T cell pool. Furthermore, as will be discussed below, accumulating data suggest the impact of self-pMHC interactions in the periphery is not identical for CD4 and CD8 T cells, suggesting an unexpected layer of subset specialization.
It has been known for many years that naïve CD8 and CD4 T cells exhibit a basal TCR signal (manifest as partial phosphorylation of the TCRζ chain), and that this is lost with deprivation of self-MHCI and MHCII molecules respectively61, 62, 63, 64. Likewise, naïve T cells express a basal level of GFP in Nur77GFP transgenic mice, but this expression is rapidly lost when naïve CD4 T cells are deprived of self-MHCII molecules47. However, the significance of these weak TCR signals has been more difficult to discern. Initial studies on T cell homeostasis suggested an essential role for self-p/MHC ligands in naïve T cell survival65, 66 but interpreting these findings became more complicated when it was discovered that self-p/MHC recognition is one of the cues that induces naïve T cells to proliferate and acquire memory-phenotype in situations of lymphopenia (which were often employed in studies on T cell homeostasis)61, 67. There is general consensus that self-p/MHC recognition provokes lymphopenia-induced proliferation (LIP) of both CD4 and CD8 T cells, and that similar TCR encounters are needed for naïve CD8 T cell survival in a lymphoreplete environment. Determining whether CD4 T cell survival requires TCR binding to self-pMHC Class II has proven more controversial61, 67, however comprehensive studies by Martin and colleagues suggest a more complex picture in which competition with CD8 T cells (but not other CD4 T cells) enforces dependence on self-pMHC Class II for naïve CD4 T cell survival, although the molecular mechanisms for this regulation are currently unclear68. Also, while memory CD8 T cell maintenance appears completely independent of self-p/MHC recognition (or even of TCR expression)61, 67, studies using a TCR loss model show that a subset of memory-phenotype CD4 T cells continue to depend on TCR signals for maintenance69.
An uneven playing field: heterogeneity in self-sensitivity leads to heterogeneity in naïve T activation potential
Part of the difficulty in getting a clear impression of how self-p/MHC affects T cell homeostasis is that not all naïve T cells respond to homeostatic cues in the same way: Analysis of TCR transgenic models showed radically divergent capacity of naïve CD8 and CD4 T cells to proliferate and acquire memory-like properties in LIP models67. Likewise, there was notable variability in the capacity of different TCR transgenic naïve CD8 T cells to respond to the cytokines IL-7 and IL-270, 71. Relating this heterogeneity to self-p/MHC recognition is inherently difficult. For conventional T cells, the affinity of the TCR for self-p/MHC ligands is predicted to be very low. The p/MHC complexes that drive thymic positive selection are known to bind the TCR with low affinity (and similar ligands can promote naïve T cell homeostasis), and it is likely that multiple self-peptides can engage a given TCR in such interactions, making it impractical to identify and characterize all relevant self-p/MHC complexes in a physiological setting72, 73.
Fortunately, studies in mice have revealed valuable phenotypic markers that correlate with the intensity of TCR recognition of self. Of these, the molecule CD5, has proven the most reliable (Figure 3A). Analysis of TCR transgenic and polyclonal naïve T cells (of either CD4 or CD8 subsets) revealed that increasing levels of CD5 expression correlate positively with: a) degree of basal TCRζ phosphorylation63, 64, 74; b) capacity to rapidly produce IL-2 and induce ERK phosphorylation following T cell stimulation63 and c) capacity of T cells to undergo LIP71, 75, 76. We have observed that CD5hi naïve CD4 and CD8 T cells also show increased expression of GFP expression in Nur77GFP mice, and that CD5hi and CD5lo naïve CD8 T cells differ in gene expression profiles77, suggesting that the CD5hi pool has improved readiness for activation and functional differentiation (see below). CD5 itself is a cell surface molecule that can, through associated SHP-1, negatively regulate TCR signals, and has been proposed to function as a rheostat in dampening TCR signaling during thymic development39. Indeed, CD5 expression levels are set during thymic development and are typically maintained in the periphery63, 78 although deprivation of T cell interaction with self-p/MHC in the periphery can cause a decline in CD5 expression79. Since it is T cells with the highest levels of CD5 that appear to show the strongest reactivity to self-p/MHC, however, its proposed rheostat function appears insufficient to normalize TCR reactivity for all clones, making this marker valuable for isolating cells with different levels of active self-sensitivity. Studies using CD5-deficient TCR transgenic T cells support the concept that this molecule restrains TCR signaling, yet CD5 loss did not effectively normalize the differential responses of clones that are normally selected into CD5hi and CD5lo pools63, 64. Hence, though CD5 expression levels have proven to be an effective and useful maker for naïve T cell subsetting, the functional relevance of CD5 per se is controversial.
Intriguingly, differences emerge in comparison between naïve CD4 and CD8 T cell populations (Figure 3B). Expression levels of GFP in Nur77GFP reporter mice are greater in naïve CD4 T cells than in naïve CD8 T cells47 and there is a notably greater range in resting p-TCRζ levels when comparing CD5lo and CD5hi populations in the CD4 pool, rather than the CD8 subset64. Further, while pharmacologically induced IL-2 production increases with CD5 expression levels on both populations, the overall expression is notably greater in the CD4 subset63. On the other hand, CD5hi naïve CD8 T cells are provoked into proliferation when exposed to sustained high dose IL-2 stimulation (in the absence of foreign pMHC ligands), while this response was not observed for naïve CD4 T cells (regardless of their CD5 expression levels). That cytokine-induced proliferation was found to correlate with preferential localization of CD122 (the IL-2Rbeta chain) to lipid rafts in CD5hi CD8 T cells71, and hence the failure of naïve CD4 T cells to engage in this response may reflect the lower expression levels of CD122 on CD4 subsets compared to their CD8 counterparts. We observed that CD5hi naïve CD8 T cells show elevated expression of factors associated with early T cell activation (including T-bet, Eomes, CXCR3, Xcl-1), and patterns of gene expression that indicate improved preparation for responsiveness and cell division77, yet such changes have not been reported for naïve CD4 T cell subsets. Thus there may be additional distinct features of the CD5hi and CD5lo populations from naïve CD4 versus CD8 T cell populations.
Although basal TCR signaling, and associated CD5 expression levels, are considered to reflect the intensity of self-recognition by naïve T cells, this does not necessarily mean they reflect the affinity/avidity of the TCR interaction with self-p/MHC. Instead, these signals might reflect the tissue distribution of suitable self-p/MHC ligands (Figure 3A). Whether a given T cell would encounter the relevant self-p/MHC frequently or rarely might dictate its steady state basal TCR signal. A potential reflection of this is the finding that selection of some CD8 TCR transgenic clones strongly depends on β5t expression, while others are independent of β5t. Intriguingly, this generally correlates with the CD5 express status of the T cells – with CD5hi cells being less dependent on β5t80. Consistent with this, the CD8+ T cells selected in β5t−/− animals are enriched for the CD5hi population. It is tempting to conclude that cells selected on self-MHC molecules containing β5t -independent “public peptides” may encounter the very same pMHC complex on other cells in the periphery (imparting a basal TCR signal), while this would be less likely to occur for cells selected on ligands containing private β5t-dependent peptides. It is currently unclear whether the same pattern applies to CD4 T cells selected on private versus public peptides presented by Class II MHC ligands. This concept was discussed in a recent review40, and hence will not be further explored here.
Self-sensitivity regulates foreign antigen reactivity
Although the degree of self-p/MHC sensitivity impacts TCR basal signaling intensity, gene expression patterns and the proliferative response to lymphopenia, those differences, perhaps surprisingly, do not seem to affect steady state survival of naïve T cells – hence, studies on CD5hi and CD5lo polyclonal CD4 and CD8 T cells reported similar maintenance (and preservation of their CD5hi/lo status) after adoptive transfer into a lymphoreplete environment63, 64, 77. This suggests that a minimum TCR signal suffices for T cell survival, while greater self-awareness provides naïve CD8 T cells with increased sensitivity to homeostatic opportunities.
However, multiple studies suggest the response to foreign antigen is influenced by naïve T cell self-p/MHC sensitivity – though the exact nature and basis of that effect remains controversial. Studies from Mandl et al. reported that CD5 expression levels on naïve CD4 T cells not only corresponded with the level of basal TCR signaling but also predicted the capacity of these cells to bind specific foreign p/MHC ligands (as revealed by p/MHC tetramer staining)64. This led to the intriguing hypothesis that the interaction with self-pMHC during thymic positive selection not only provides a signal for T cell maturation, but also produces T cells better able to bind foreign pMHC. However, other studies reached divergent conclusions. Allen and colleagues generated two TCR transgenic strains, which bound the same foreign pMHC ligand with very similar TCR affinity, and yet were selected into CD5hi or CD5lo populations (with corresponding differences in basal TCR signaling)63, 81. Likewise, studies on TCR transgenic and normal polyclonal CD8 T cells did not indicate a consistent difference in foreign p/MHC tetramer binding to CD5hi versus CD5lo populations77.
There is better consensus with the concept that the intensity of the basal encounter with self-pMHC impacts reactivity to foreign antigen. There are two aspects of this that have been explored –the contribution of self-pMHC recognition during foreign antigen encounter itself, and the effects of self-pMHC on intrinsic properties of naïve T cells prior to encounter with foreign antigen. Davis and colleagues first introduced the concept that non-stimulatory self-pMHC complexes may serve as “co-agonists” to assist the T cell response to foreign pMHC ligands82, 83. However, it remains unclear whether co-agonists contribute to all T cell responses, and even in situations where a co-agonist role could be observed there was considerable controversy over whether co-agonist encounters were peptide specific or not, with divergent conclusions being drawn for CD4 and CD8 T cells82, 83. A recent report from Gascoigne’s group nicely resolves this discrepancy by showing that there are two modes of co-agonist contribution, in which the significance of the TCR affinity for the co-agonist pMHC depends critically on the strength of co-receptor binding to the foreign pMHC target84 – since the CD8 co-receptor is thought to have a higher affinity for Class I MHC molecules (albeit not all alleles) compared to the CD4-Class II MHC interaction, TCR specificity for co-agonist ligands may be a much more stringent limitation for CD4 compared to CD8 T cells84.
Three recent studies investigated the foreign antigen-specific response of CD5hi versus CD5lo naïve CD4 T cells63, 64 and naïve CD8 T cells77, using a combination of polyclonal and TCR transgenic model systems. These reports included studies on the properties of CD5hi and CD5lo naïve T cells prior to antigen encounter, and in situations where a co-agonist role could be excluded, in order to determine whether and how self-pMHC sensitivity (reflected as CD5 expression levels) alters the intrinsic capacity of naïve T cells to respond to foreign ligands. As discussed above, CD5hi populations of both CD4 and CD8 naïve T cell subsets show evidence of the increased basal TCR signaling and improved functional characteristics, and this was found to correlate with greater expansion in response to foreign antigen in two of those studies64, 77. While Mandl et al. correlated this response pattern with changes in TCR binding to foreign pMHC by CD5hi naïve CD4 T cells64, 77, Fulton and colleagues found that the CD5hi naïve CD8 T cells exhibited more efficient clonal recruitment into the immune response, and superior augmentation of their response by inflammatory cues77. In apparent contrast to both of these reports, studies using two TCR transgenic strains that recognize the same foreign pMHC ligand but were positively selected into CD5hi and CD5lo populations, exhibited a different pattern, in which the CD5lo clones displayed greater expansion than the CD5hi group during the primary immune response63, 81. A possible resolution of these discrepancies reflects the concept that the CD5hi population in naïve CD4 T cells may maintain a higher basal activation state than CD5hi naïve CD8 T cells (Figure 3B) – and hence may be more susceptible to activation induced cell death pathways after strong stimulation. Indeed, Allen and colleagues suggested that the poor expansion of their CD5hi TCR transgenic clone was not due to ineffective early activation but rather to IL-2 driven apoptosis63. In this context, is interesting to note that, compared to polyclonal cells, the CD5 levels on this “CD5hi” TCR transgenic clone were greater than those on TCR transgenic clones analyzed by Mandl et al.63, 64 potentially suggesting an even greater degree of self-pMHC reactivity. Hence, superior intrinsic sensitivity in the CD5hi naïve T cell pool may (at least in the CD4 subset) come at the cost of increased vulnerability to elimination mechanisms. Furthermore, it is worth noting that in secondary immune responses, Persaud et al. reported that the CD5hi clones expanded to a greater extent than the CD5lo population63. Whether this arises from avoidance of IL-2 induced cell death pathways during the recall response or some other mechanism is unclear.
While these reports are consistent in that the CD5hi populations of naïve CD4 and CD8 T cells possess enhanced intrinsic reactivity toward foreign pMHC stimulation, the underlying mechanism and immediate consequences for clonal recruitment and expansion are harder to predict and may depend on where cells lie on the scale of self-reactivity.
In conclusion, T cells experience a broad range of self-reactivity continually; from the time they first express a surface receptor in the thymus, and throughout their lifespan and residency in peripheral lymphoid organs or tissues. The developmental stage of the T cell, as well as the affinity and co-stimulatory/cytokine context of these interactions, all impact what kind of response is ultimately made and maintained. Thus self-interactions are crucial to the sophisticated and highly controlled adaptive immune response.
Acknowledgements
This work was supported by grants from the National Institutes of Health to K.A.H. (PO1 AI35296, RO1 AI088209, and R37 AI39560) and S.C.J. (R01 AI75168 and R37 AI38903).
References
- 1.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nature reviews Immunology. 2005;5(10):772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 2. Stritesky GL, Xing Y, Erickson JR, Kalekar LA, Wang X, Mueller DL, et al. Murine thymic selection quantified using a unique method to capture deleted T cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(12):4679–4684. doi: 10.1073/pnas.1217532110. Using Bim deficient Nur77GFP reporter mice, this study reported that the extent of negative selection is far greater than previously appreciated.
- 3. Daley SR, Hu DY, Goodnow CC. Helios marks strongly autoreactive CD4+ T cells in two major waves of thymic deletion distinguished by induction of PD-1 or NF-kappaB. The Journal of experimental medicine. 2013;210(2):269–285. doi: 10.1084/jem.20121458. This study reports that helios expression distinguishes cells undergoing positive and negative selection in the thymus. Analogous to the previous study, they analyzed helios expresion in Bim deficient mice to define the extent of clonal deletion.
- 4. Sinclair C, Bains I, Yates AJ, Seddon B. Asymmetric thymocyte death underlies the CD4:CD8 T-cell ratio in the adaptive immune system. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(31):E2905–E2914. doi: 10.1073/pnas.1304859110. Sinclair and colleagues estimated rates of death and differentiation using mathematical analysis of synchronized cohorts of thymocytes developing in an inducible ZAP70 model. Their results suggested an asymmetry in the death rates of Class I and Class II restricted thymocytes, and concurred remarkably well with the previous two studies that the majority of cells that start selection fail to complete it.
- 5.Garcia KC, Gapin L, Adams JJ, Birnbaum ME, Scott-Browne JP, Kappler JW, et al. A closer look at TCR germline recognition. Immunity. 2012;36(6):887–888. doi: 10.1016/j.immuni.2012.05.018. author reply 889–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tikhonova AN, Van Laethem F, Hanada K, Lu J, Pobezinsky LA, Hong C, et al. alphabeta T cell receptors that do not undergo major histocompatibility complex-specific thymic selection possess antibody-like recognition specificities. Immunity. 2012;36(1):79–91. doi: 10.1016/j.immuni.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13(6):829–840. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 8.Zehn D, Bevan MJ. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 2006;25(2):261–270. doi: 10.1016/j.immuni.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu HH, Moon JJ, Kruse AC, Pepper M, Jenkins MK. Negative selection and peptide chemistry determine the size of naive foreign peptide-MHC class II-specific CD4+ T cell populations. Journal of immunology. 2010;185(8):4705–4713. doi: 10.4049/jimmunol.1002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon JJ, Dash P, Oguin TH, 3rd, McClaren JL, Chu HH, Thomas PG, et al. Quantitative impact of thymic selection on Foxp3+ and Foxp3− subsets of self-peptide/MHC class II-specific CD4+ T cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(35):14602–14607. doi: 10.1073/pnas.1109806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taniguchi RT, DeVoss JJ, Moon JJ, Sidney J, Sette A, Jenkins MK, et al. Detection of an autoreactive T-cell population within the polyclonal repertoire that undergoes distinct autoimmune regulator (Aire)-mediated selection. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(20):7847–7852. doi: 10.1073/pnas.1120607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pauken KE, Linehan JL, Spanier JA, Sahli NL, Kalekar LA, Binstadt BA, et al. Cutting edge: type 1 diabetes occurs despite robust anergy among endogenous insulin-specific CD4 T cells in NOD mice. Journal of immunology. 2013;191(10):4913–4917. doi: 10.4049/jimmunol.1301927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathis D, Benoist C. Aire. Annual review of immunology. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 14.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339(6124):1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray DH, Kupresanin F, Berzins SP, Herold MJ, O'Reilly LA, Bouillet P, et al. The BH3-only proteins Bim and Puma cooperate to impose deletional tolerance of organ-specific antigens. Immunity. 2012;37(3):451–462. doi: 10.1016/j.immuni.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Q, Sader A, Parkman JC, Baldwin TA. Bim-mediated apoptosis is not necessary for thymic negative selection to ubiquitous self-antigens. Journal of immunology. 2009;183(12):7761–7767. doi: 10.4049/jimmunol.0902181. [DOI] [PubMed] [Google Scholar]
- 17.Dzhagalov IL, Chen KG, Herzmark P, Robey EA. Elimination of self-reactive T cells in the thymus: a timeline for negative selection. PLoS biology. 2013;11(5):e1001566. doi: 10.1371/journal.pbio.1001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Au-Yeung B, Melichar HJ, Ross JO, Cheng DA, Zikherman J, Shokat KM, et al. Quantitative and Temporal Requirements revealed for Zap-70 catalytic activity during T cell development. Nature immunology. 2014 doi: 10.1038/ni.2918. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNeil LK, Starr TK, Hogquist KA. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(38):13574–13579. doi: 10.1073/pnas.0505110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suen AY, Baldwin TA. Proapoptotic protein Bim is differentially required during thymic clonal deletion to ubiquitous versus tissue-restricted antigens. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(3):893–898. doi: 10.1073/pnas.1114834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajoghli B, Aghaallaei N, Hess I, Rode I, Netuschil N, Tay BH, et al. Evolution of genetic networks underlying the emergence of thymopoiesis in vertebrates. Cell. 2009;138(1):186–197. doi: 10.1016/j.cell.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Davey GM, Schober SL, Endrizzi BT, Dutcher AK, Jameson SC, Hogquist KA. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. The Journal of experimental medicine. 1998;188(10):1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gascoigne NR, Palmer E. Signaling in thymic selection. Current opinion in immunology. 2011;23(2):207–212. doi: 10.1016/j.coi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Zheng M, Lei L, Ji J, Yao Y, Qiu Y, et al. Tespa1 is involved in late thymocyte development through the regulation of TCR-mediated signaling. Nature immunology. 2012;13(6):560–568. doi: 10.1038/ni.2301. [DOI] [PubMed] [Google Scholar]
- 25.Lo WL, Donermeyer DL, Allen PM. A voltage-gated sodium channel is essential for the positive selection of CD4(+) T cells. Nature immunology. 2012;13(9):880–887. doi: 10.1038/ni.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melichar HJ, Ross JO, Herzmark P, Hogquist KA, Robey EA. Distinct temporal patterns of T cell receptor signaling during positive versus negative selection in situ. Science signaling. 2013;6(297):ra92. doi: 10.1126/scisignal.2004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh-Hora M, Komatsu N, Pishyareh M, Feske S, Hori S, Taniguchi M, et al. Agonist-selected T cell development requires strong T cell receptor signaling and store-operated calcium entry. Immunity. 2013;38(5):881–895. doi: 10.1016/j.immuni.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross JO, Melichar HJ, Au-Yeung B, Herzmark P, Weiss A, Robey EA. Distinct phases in the positive selection of CD8+ T cells distinguished by intrathymic migration and TCR signaling patterns. Proceedings of the National Academy of Sciences of the United States of America. 2014 doi: 10.1073/pnas.1408482111. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann A, Kann O, Ohlemeyer C, Hanisch UK, Kettenmann H. Elevation of basal intracellular calcium as a central element in the activation of brain macrophages (microglia): suppression of receptor-evoked calcium signaling and control of release function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(11):4410–4419. doi: 10.1523/JNEUROSCI.23-11-04410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fu G, Casas J, Rigaud S, Rybakin V, Lambolez F, Brzostek J, et al. Themis sets the signal threshold for positive and negative selection in T-cell development. Nature. 2013;504(7480):441–445. doi: 10.1038/nature12718. This study showed that Themis deficiency results in activation-induced death of DP thymocytes that are normally positively selected; supporting the idea that Themis selectively dampens low-affinity TCR signals via recruiting the phosphatase SHP1. Themis deficiency had no effect on responses to high-affinity ligands or on the development of agonist-selected T cell populations.
- 31.Staton TL, Lazarevic V, Jones DC, Lanser AJ, Takagi T, Ishii S, et al. Dampening of death pathways by schnurri-2 is essential for T-cell development. Nature. 2011;472(7341):105–109. doi: 10.1038/nature09848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinclair C, Seddon B. Overlapping and Asymmetric Functions of TCR Signaling during Thymic Selection of CD4 and CD8 Lineages. Journal of immunology. 2014 doi: 10.4049/jimmunol.1303085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cowan JE, Parnell SM, Nakamura K, Caamano JH, Lane PJ, Jenkinson EJ, et al. The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development. The Journal of experimental medicine. 2013;210(4):675–681. doi: 10.1084/jem.20122070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dyall R, Nikolic-Zugic J. The final maturation of at least some single-positive CD4(hi) thymocytes does not require T cell receptor-major histocompatibility complex contact. The Journal of experimental medicine. 1999;190(6):757–764. doi: 10.1084/jem.190.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129(1):147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Zietara N, Lyszkiewicz M, Witzlau K, Naumann R, Hurwitz R, Langemeier J, et al. Critical role for miR-181a/b-1 in agonist selection of invariant natural killer T cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(18):7407–7412. doi: 10.1073/pnas.1221984110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ebert PJ, Jiang S, Xie J, Li QJ, Davis MM. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nature immunology. 2009;10(11):1162–1169. doi: 10.1038/ni.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. The Journal of experimental medicine. 1998;188(12):2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azzam HS, DeJarnette JB, Huang K, Emmons R, Park CS, Sommers CL, et al. Fine tuning of TCR signaling by CD5. Journal of immunology. 2001;166(9):5464–5472. doi: 10.4049/jimmunol.166.9.5464. [DOI] [PubMed] [Google Scholar]
- 40.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see and don’t see. Nature reviews Immunology. 2014 doi: 10.1038/nri3667. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xing Y, Jameson SC, Hogquist KA. Thymoproteasome subunit-beta5T generates peptide-MHC complexes specialized for positive selection. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(17):6979–6984. doi: 10.1073/pnas.1222244110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lo WL, Solomon BD, Donermeyer DL, Hsieh CS, Allen PM. T cell immunodominance is dictated by the positively selecting self-peptide. eLife. 2014;3:e01457. doi: 10.7554/eLife.01457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nature reviews Immunology. 2012;12(3):157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 44.Huynh A, Zhang R, Turka LA. Signals and pathways controlling regulatory T cells. Immunological reviews. 2014;258(1):117–131. doi: 10.1111/imr.12148. [DOI] [PubMed] [Google Scholar]
- 45.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nature immunology. 2001;2(4):301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 46.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nature immunology. 2006;7(4):401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 47.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. The Journal of experimental medicine. 2011;208(6):1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bains I, van Santen HM, Seddon B, Yates AJ. Models of self-peptide sampling by developing T cells identify candidate mechanisms of thymic selection. PLoS computational biology. 2013;9(7):e1003102. doi: 10.1371/journal.pcbi.1003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, Luo J, et al. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nature immunology. 2009;10(6):610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mahmud SA, Manlove LS, Schmitz HM, Xing Y, Wang Y, Owen DL, et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nature immunology. 2014 doi: 10.1038/ni.2849. This study showed that developing thymocytes having a stronger interaction with self-pMHC have higher expression of TNF receptor family members, allowing them to preferentially undergo Treg cell induction by allowing more effective competition for IL-2.
- 51.Konkel JE, Jin W, Abbatiello B, Grainger JR, Chen W. Thymocyte apoptosis drives the intrathymic generation of regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(4):E465–E473. doi: 10.1073/pnas.1320319111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wirnsberger G, Mair F, Klein L. Regulatory T cell differentiation of thymocytes does not require a dedicated antigen-presenting cell but is under T cell-intrinsic developmental control. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(25):10278–10283. doi: 10.1073/pnas.0901877106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seiler MP, Mathew R, Liszewski MK, Spooner CJ, Barr K, Meng F, et al. Elevated and sustained expression of the transcription factors Egr1 and Egr2 controls NKT lineage differentiation in response to TCR signaling. Nature immunology. 2012;13(3):264–271. doi: 10.1038/ni.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Becker AM, Blevins JS, Tomson FL, Eitson JL, Medeiros JJ, Yarovinsky F, et al. Invariant NKT cell development requires a full complement of functional CD3 zeta immunoreceptor tyrosine-based activation motifs. Journal of immunology. 2010;184(12):6822–6832. doi: 10.4049/jimmunol.0902058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bedel R, Berry R, Mallevaey T, Matsuda JL, Zhang J, Godfrey DI, et al. Effective functional maturation of invariant natural killer T cells is constrained by negative selection and T-cell antigen receptor affinity. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(1):E119–E128. doi: 10.1073/pnas.1320777110. Using a TCR engineered to have supra-physiologically high-affinity for CD1d self-lipid ligands, this study showed that iNKT cells can be susceptible to clonal deletion. They also showed that lowering the affinity for CD1d led to poor induction of PLZF and the iNKT lineage, suggesting that iNKT development is constrained by a limited range of affinity.
- 56.Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nature reviews Immunology. 2011;11(7):445–456. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konkel JE, Maruyama T, Carpenter AC, Xiong Y, Zamarron BF, Hall BE, et al. Control of the development of CD8alphaalpha+ intestinal intraepithelial lymphocytes by TGF-beta. Nature immunology. 2011;12(4):312–319. doi: 10.1038/ni.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lai YG, Gelfanov V, Gelfanova V, Kulik L, Chu CL, Jeng SW, et al. IL-15 promotes survival but not effector function differentiation of CD8+ TCRalphabeta+ intestinal intraepithelial lymphocytes. Journal of immunology. 1999;163(11):5843–5850. [PubMed] [Google Scholar]
- 59.Stritesky GL, Jameson SC, Hogquist KA. Selection of self-reactive T cells in the thymus. Annual review of immunology. 2012;30:95–114. doi: 10.1146/annurev-immunol-020711-075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pobezinsky LA, Angelov GS, Tai X, Jeurling S, Van Laethem F, Feigenbaum L, et al. Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nature immunology. 2012;13(6):569–578. doi: 10.1038/ni.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nature reviews Immunology. 2009;9(12):823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 62.Dorfman JR, Stefanova I, Yasutomo K, Germain RN. CD4+ T cell survival is not directly linked to self-MHC -induced TCR signaling. Nature immunology. 2000;1:329–335. doi: 10.1038/79783. [DOI] [PubMed] [Google Scholar]
- 63. Persaud SP, Parker CR, Lo WL, Weber KS, Allen PM. Intrinsic CD4(+) T cell sensitivity and response to a pathogen are set and sustained by avidity for thymic and peripheral complexes of self peptide and MHC. Nature immunology. 2014;15(3):266–274. doi: 10.1038/ni.2822. This report found that CD5hi naïve CD4 T cells show superior intrinsic responsiveness (compared to CD5lo cells), which could be uncoupled from the specificity of TCR engagement. However, this stronger reactivity of the CD5hi population made them more susceptible to IL-2 driven cell death, limiting the expansion of this population during the primary immune response.
- 64. Mandl JN, Monteiro JP, Vrisekoop N, Germain RN. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 2013;38(2):263–274. doi: 10.1016/j.immuni.2012.09.011. In this report, the authors show that CD5hi naïve T cell exhibit enhanced reactivity during a primary immune response, and introduces the novel concept that TCR affinity for foreign pMHC is directly related to the strength of the interaction with self pMHC.
- 65.Takeda S, Rodewald HR, Arakawa H, Bluethmann H, Shimizu T. MHC class II molecules are not required for survival of newly generated CD4+ T cells, but affect their long-term life span. Immunity. 1996;5(3):217–228. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- 66.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276(5321):2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 67.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29(6):848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Martin B, Becourt C, Bienvenu B, Lucas B. Self-recognition is crucial for maintaining the peripheral CD4+ T-cell pool in a nonlymphopenic environment. Blood. 2006;108(1):270–277. doi: 10.1182/blood-2006-01-0017. [DOI] [PubMed] [Google Scholar]
- 69.Leignadier J, Hardy MP, Cloutier M, Rooney J, Labrecque N. Memory T-lymphocyte survival does not require T-cell receptor expression. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(51):20440–20445. doi: 10.1073/pnas.0806289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palmer MJ, Mahajan VS, Chen J, Irvine DJ, Lauffenburger DA. Signaling thresholds govern heterogeneity in IL-7-receptor-mediated responses of naive CD8+ T cells. Immunol Cell Biol. 2011 doi: 10.1038/icb.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho JH, Kim HO, Surh CD, Sprent J. T cell receptor-dependent regulation of lipid rafts controls naive CD8+ T cell homeostasis. Immunity. 2010;32(2):214–226. doi: 10.1016/j.immuni.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annual review of immunology. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 73.Lo WL, Allen PM. Self-awareness: how self-peptide/MHC complexes are essential in the development of T cells. Molecular immunology. 2013;55(2):186–189. doi: 10.1016/j.molimm.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith K, Seddon B, Purbhoo MA, Zamoyska R, Fisher AG, Merkenschlager M. Sensory adaptation in naive peripheral CD4 T cells. The Journal of experimental medicine. 2001;194(9):1253–1261. doi: 10.1084/jem.194.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kieper WC, Burghardt JT, Surh CD. A role for TCR affinity in regulating naive T cell homeostasis. Journal of immunology. 2004;172(1):40–44. doi: 10.4049/jimmunol.172.1.40. [DOI] [PubMed] [Google Scholar]
- 76.Johnson LD, Jameson SC. Self-specific CD8+ T cells maintain a semi-naive state following lymphopenia-induced proliferation. Journal of immunology. 2010;184(10):5604–5611. doi: 10.4049/jimmunol.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fulton RB, Xing Y, Best JA, Goldrath AW, Hogquist KA, Jameson SC. TCR sensitivity to self peptide-MHC dictates naive CD8 T cells' capacity to respond to foreign antigens. Submitted. 2014 doi: 10.1038/ni.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saini M, Sinclair C, Marshall D, Tolaini M, Sakaguchi S, Seddon B. Regulation of Zap70 expression during thymocyte development enables temporal separation of CD4 and CD8 repertoire selection at different signaling thresholds. Science signaling. 2010;3(114):ra23. doi: 10.1126/scisignal.2000702. [DOI] [PubMed] [Google Scholar]
- 79.Takada K, Jameson SC. Self-class I MHC molecules support survival of naive CD8 T cells, but depress their functional sensitivity through regulation of CD8 expression levels. The Journal of experimental medicine. 2009;206(10):2253–2269. doi: 10.1084/jem.20082553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nitta T, Murata S, Sasaki K, Fujii H, Ripen AM, Ishimaru N, et al. Thymoproteasome shapes immunocompetent repertoire of CD8+ T cells. Immunity. 2010;32(1):29–40. doi: 10.1016/j.immuni.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 81.Weber KS, Li QJ, Persaud SP, Campbell JD, Davis MM, Allen PM. Distinct CD4+ helper T cells involved in primary and secondary responses to infection. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(24):9511–9516. doi: 10.1073/pnas.1202408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krogsgaard M, Juang J, Davis MM. A role for "self" in T-cell activation. Seminars in immunology. 2007;19(4):236–244. doi: 10.1016/j.smim.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gascoigne NR, Zal T, Yachi PP, Hoerter JA. Co-receptors and recognition of self at the immunological synapse. Current topics in microbiology and immunology. 2010;340:171–189. doi: 10.1007/978-3-642-03858-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hoerter JA, Brzostek J, Artyomov MN, Abel SM, Casas J, Rybakin V, et al. Coreceptor affinity for MHC defines peptide specificity requirements for TCR interaction with coagonist peptide-MHC. The Journal of experimental medicine. 2013;210(9):1807–1821. doi: 10.1084/jem.20122528. These studies shed new light on the way in which pMHC ligands can act as co-agonists in the response to foreign pMHC complexes, though showing that the TCR specificity requirement in recognition of a co-agonist depends on both TCR and CD8 coreceptor affinity for the agonist ligand.