Abstract
Methylmercury (MeHg) exposure through fish consumption is a worldwide health concern. Saltwater fish account for most dietary MeHg exposure in the general U.S. population, but less is known about seasonal variations in MeHg exposure and fish consumption among millions of freshwater anglers. This longitudinal study examined associations between MeHg exposure and fish consumption in a rural, low-income population relying on a freshwater reservoir (Oklahoma, USA) for recreational and subsistence fishing. We interviewed 151 participants, primarily anglers and their families, seasonally for one year using 90-day recall food frequency questionnaires to assess general and species-specific fish consumption, and tested hair biomarker samples for total mercury (THghair). Mean THghair was 0.27 μg/g (n=595, range: 0.0044–3.1 μg/g), with 4% of participants above U.S. EPA's guideline for women of childbearing age and children. Mean fish consumption was 58 g/d (95% CI: 49–67 g/d), within the range previously reported for recreational freshwater anglers and above the national average. Unlike the general U.S. population, freshwater species contributed the majority of fish consumption (69%) and dietary Hg exposure (60%) among participants, despite relatively low THg in local fish. THghair increased with fish consumption, age, and education, and was higher among male participants and lowest in winter. Our results suggest that future studies of anglers should consider seasonality in fish consumption and MeHg exposure and include household members who share their catch. Efforts to evaluate benefits of reducing Hg emissions should consider dietary patterns among consumers of fish from local freshwater bodies.
Keywords: exposure, fish consumption, fishing, mercury, season
1. Introduction
Mercury (Hg), especially its methylated form, methylmercury (MeHg), is associated with neurotoxicity in humans (Harada 1995), with the most severe effects in fetuses and children (Grandjean et al. 1999; Karagas et al. 2012). In adults, low levels of MeHg exposure have been associated with cardiovascular disease in some studies (Choi et al. 2009; Virtanen et al. 2005) but not others (Mozaffarian et al. 2011), and with neurological effects, such as deficits in motor or cognitive functions (Lebel et al. 1998).
The primary non-occupational route of human MeHg exposure is fish/shellfish consumption (Mahaffey et al. 2004), and thousands of mercury-related fish consumption advisories have been issued in the U.S. (U.S. EPA 2004). Although commercial saltwater species account for the majority of fish consumption and dietary MeHg exposure in the general U.S. population (Carrington et al. 2004) and other populations worldwide (Davidson et al. 2008), locally-caught freshwater fish may contribute substantially to dietary MeHg intake in inland, rural communities (Turyk et al. 2012). Of 27 million U.S. anglers, 83% fished in freshwater lakes, reservoirs, or ponds, and freshwater fishing accounted for 81% of all fishing trips in 2011 (U.S. FWS 2013). Despite the popularity of freshwater fishing, few studies have quantified Hg exposure in freshwater anglers, and the extent of seasonal variability in the quantities and types of fish consumed is poorly characterized. Furthermore, studies of anglers often focus on men and do not include women and children in their families sharing their catch, despite greater susceptibility of children and fetuses to detrimental effects of MeHg and other pollutants. Low-income and minority populations are often at elevated risk of contaminant exposure through consumption of self-caught fish because of their greater reliance on local resources and closer proximity to pollution sources (Burger and Gochfeld 2011).
We assessed the influence of fishing behaviors, local fish consumption, and season on MeHg exposure in a rural, low-income population through a longitudinal study of primarily freshwater anglers and their families. This community-based participatory research (CBPR) project, developed collaboratively by university researchers and community partners, was designed to address community concerns about potential mercury exposure among recreational and subsistence anglers in Grand Lake (Oklahoma, USA; Figure S1), due to the presence of six coal-fired power plants within 100 km of the lake. Our goals were: (1) to assess overall MeHg exposure and the contribution of locally-caught freshwater fish consumption among anglers and their families who consume fish from the watershed; (2) to evaluate seasonal variability in fish consumption rates, diet composition, and MeHg exposure; and (3) to examine associations in MeHg exposure and fish consumption among family members. Understanding patterns of fish consumption and Hg exposure is important for identifying populations at risk of elevated Hg exposure and assessing benefits of Hg emission reductions.
2. Materials and methods
2.1. Recruitment of participants
We recruited 151 self-identified consumers (≥14 years old) of fish from the Grand Lake watershed (Oklahoma, USA), trying to include more individuals with high rates of fish consumption. Participants were recruited in person at events (e.g., fishing tournaments, health fairs, fish fries, and meetings of fishing-related organizations) and through personal contact with study team members.
To explore seasonal patterns in MeHg exposure and fish consumption, we aimed to interview each participant five times, at approximately three-month intervals over the course of one year. Target dates for follow-up visits were generated for each participant based on the date of their first interview, with an acceptable window of ±1.5 months around each target date. During each interview, participants completed a 90-day recall food frequency questionnaire (FFQ) and provided a hair sample for Hg analysis. Overall, 611 FFQs (80% based on the initial sample size) and 599 hair samples were collected between July 2010 and March 2013, with only eight participants officially withdrawing. We recorded relationships among spouses and other household members to compare their consumption patterns and Hg exposure. Thirty-three pairs of spouses and domestic partners participated in our study.
Informed consent was obtained from every participant at the time of enrollment. All study materials and research protocols relating to human subjects were approved by the Office of Human Research Administration at Harvard School of Public Health.
2.2. Biomarker sampling
Hair was selected as a biomarker because it is a commonly-used biomarker (Grandjean et al. 2002; McDowell et al. 2004) for capturing MeHg exposure over weeks or months (NRC 2000) and is less invasive and easier to sample than blood. Since hair grows at a rate of approximately 1 cm/month (WHO 1990), and it takes about one month for hair to emerge at the skin surface (NRC 2000), the first 2 cm of hair from the scalp roughly corresponds to a recall period 1-3 months prior to sample collection.
A small bundle of hair, approximately 0.5 cm in diameter, was collected at each interview by a study team member, cut close to the scalp with scissors that were wiped with alcohol wipes after each use. The hair sample was tied with dental floss to indicate the end closest to the scalp. Within three months of collection, participants received a report-back letter with the Hg concentration in each hair sample they provided that graphically depicted their hair Hg level relative to three benchmarks: median hair Hg level in adult women in the US (0.19 μg/g; McDowell et al. 2004), U.S. EPA's guideline for women of childbearing age (1.1 μg/g), and hair Hg level associated with cardiovascular effects in middle-aged men in some studies (2 μg/g, Choi et al. 2009; Virtanen et al. 2005). The letter encouraged participants concerned about their result to talk with their doctor or a study team member. Additional information was also provided about sources of Hg into the environment, Hg levels in fish, and health effects associated with Hg.
2.3. Food frequency questionnaire
Our FFQ (available at: http://grandlakemercurystudy.org/images/GLWMS_FFQ.pdf) was based on an FFQ used by Lincoln et al. (2011), which was modified from a semiquantitative FFQ for the Nurses’ Health Study (Hu et al. 2002). Our FFQ asked about general and species-specific fish/shellfish consumption frequencies over the previous three months in 8 categories: never, once in last three months, once a month, two or three times a month, once a week, two or three times a week, four to six times a week, and once a day or more. We included fish species commonly caught from the Grand Lake watershed and commonly-consumed non-local, primarily saltwater, fish. In addition, we asked about both typical fish portion size and number of portions at a typical fish meal, after participants viewed a plaster model depicting filets of four different portion sizes (2, 4, 6, and 8 oz). The FFQ also included demographic questions and questions on fishing and sharing and storage of fish. The FFQ was reviewed and tested by members of a community advisory board and multiple focus groups that encompassed a range of ethnicities before administration to participants.
To improve recall accuracy, participants received a fish consumption log at their first four visits. The log included a blank calendar for recording each fish meal, pictures of fish species, a full scale photograph of the portion model, and a map of the watershed. 73% of participants reported using their log between visits.
2.4. Hair Hg analysis
Total Hg concentration in hair (THghair) is a reasonable surrogate for MeHg since 80-98% of THg in hair is present as MeHg (Mergler et al. 2007). THghair is used hereafter in this paper to indicate THg or MeHg. The first 2 cm from the scalp-end of each hair sample was trimmed using titanium scissors and analyzed for THg by thermal decomposition, amalgamation and atomic absorption spectrophotometry (U.S. EPA 2007), using a DMA-80 Direct Mercury Analyzer (Milestone Inc., Shelton, CT). At least one method blank and one certified reference material (CRM), GBW-07601 (human hair powder), were tested every 10 samples. Average recovery for the CRM was 108.2% with an RSD of 10.7%.
2.5. Fish Hg data
To calculate Hg intake from local fish, we used average fish THg concentrations (THgfish) measured in a companion study of commonly-consumed fish from the Grand Lake watershed (Table S1). MeHg was generally >90% of THg in these fish (unpublished data). About 1000 filet samples from Grand Lake watershed fish, including more than 30 species, were collected from the watershed by either local volunteers or Oklahoma Department of Wildlife Conservation and analyzed for THg on the DMA-80. For non-local fish, we used mean THgfish from a U.S. market basket survey (U.S. FDA 2006).
2.6. Data analyses
FFQ results were entered into a Microsoft Access database through a Microsoft Infopath form, which replicated the FFQ layout. Body mass index (BMI, kg/m2) was calculated for each participant by dividing body mass by the square of height. Fish consumption frequency was converted into a fish consumption rate (FCR; g/d) using the typical portion size and number of portions reported by each participant. A general FCR was calculated based on overall reported fish consumption, and a species-specific FCR was calculated as the sum of FCRs across all species. Since over-reporting has been observed for species-specific consumption rates (Björnberg et al. 2005), a scaling factor (SF) was calculated to scale Hg dose according to general FCRs (Lincoln et al. 2011):
| (1) |
where SFi is the scaling factor for participant i, and FCRij (g/d) is the fish consumption rate of species j among a total of n species for participant i.
Hg intake was quantified using unscaled and scaled Hg doses (μg/kg/d), calculated as (Lincoln et al. 2011):
| (2) |
| (3) |
in which bwi is body weight (kg) for participant i, and THgj (μg/g) is mean THg in fish species j.
Since participants enrolled over a period of 18 months (July 2010-January 2012), we used season instead of visit date as the index for repeated measurements. Each FFQ and hair sample was classified according to the season to which a majority of the 91 prior days belonged. Data from two visits of two married participants were excluded from all analyses since they reported frequent consumption (once per day) of non-local, high Hg fish during a two-month trip outside of the study area.
We used a mixed effects regression model to analyze the association between THghair and fish consumption, and other potential predictors of Hg exposure (i.e., gender, age, ethnicity, BMI, education, percent local fish consumed, season) were included as covariates. THghair was log-transformed because its distribution was positively skewed. Random intercepts were used to model random effects within each participant. To control for temporal autocorrelation over repeated visits, an AR(1) covariance structure was applied, which was chosen over compound symmetry and unstructured covariance structure based on likelihood ratio tests. We also modeled random effects within each family using a nested mixed model. This model generated similar results to those from the model only accounting for within-participant correlations, but had much fewer degrees of freedom (27 vs. 139 for demographic covariates). Therefore, results were reported for the model with participant random effects only.
THghair was modeled using one of five metrics as the main exposure variable: general fish consumption frequency (categorical, Model 1), general (Model 2) and species-specific (Model 3) FCR, and unscaled (Model 4) and scaled (Model 5) Hg dose. Due to small sample sizes in the lowest and highest consumption categories, fish consumption frequency was regrouped into four categories: once a month or less, 2-3 times a month, once a week, and more than once a week.
R 3.0.1 was used to perform all statistical analyses.
3. Results
3.1. Study population
The demographics of our study cohort generally reflect the population in the four counties surrounding Grand Lake, although our cohort had a slightly higher proportion of men (55% vs. 50%) and American Indians (29% vs 16%) and a higher median age (54 vs. 41) (Table S2). On average, more people in this area (19%) live below the poverty level than in Oklahoma (16%) or the whole U.S. (14%). We intended to recruit additional Asian/Pacific Islanders, given the sizable local Micronesian community, but only one of more than 100 Micronesians we approached met our recruitment criterion of eating local fish.
3.2. Fishing and fish consumption patterns
Many of our participants were active anglers. 79% of participants reported fishing at least once during their five visits, and these anglers fished an average of 3.8 times per month, about three times the national average of 16 fishing trips per year (1.3 trips per month) among freshwater anglers (U.S. FWS 2013). Another 14% of participants lived with a household member who had gone fishing. For each season, over two-thirds of participants reported fishing or living with someone who fished during the prior three months, ranging from 69% (fall) to 83% (spring) (Figure S2). 77% of anglers reported sharing their catch with others, including adults (62%) and children (38%) in their household and people outside their household (75%). A majority (72%) of participants froze some fish to eat later. Among 33 couples or domestic partners, 74% of men went fishing, compared to only 34% of women.
Fish consumption rates among participants were above those of the general U.S. population and similar to those in other studies of recreational anglers. Most participants ate fish 2-3 times a month (35%) or once a week (29%), and 20% ate fish at least 2-3 times a week. The mean and median general FCR among participants were 58 g/d (95% CI: 49–67 g/d) and 28 g/d, respectively. These are within the range of mean FCRs for freshwater fish compiled by U.S. EPA (2011) for recreational freshwater anglers, which ranged from 5 to 70 g/d, after accounting for the portion of local fish consumed by our participants. Our FCRs were above the estimated FCR for the general U.S. population (mean: 22 g/d, median: 0 g/d; includes non-consumers of fish), and lower than for U.S. fish consumers (mean: 102 g/d, median: 70 g/d) (U.S. EPA 2011).
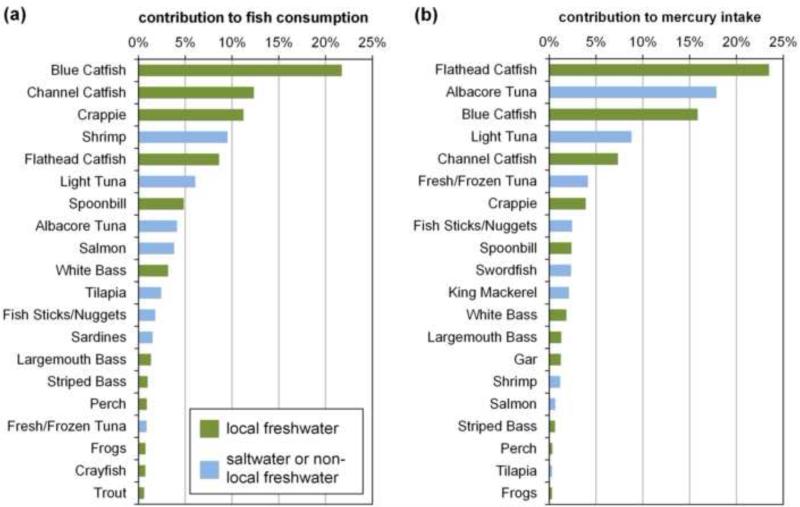
Local fish contributes a majority of fish consumption and dietary Hg among our participants. Across all visits, on average 69% of FCR consisted of local species, primarily species of catfish (43% of total), crappie (11%) and bass (6%) (Figure 1a). These are consistent with species most frequently caught by Oklahoma anglers (U.S. FWS 2014). Based on FCRs and species-specific THgfish (Table S1), an estimated 60% of dietary Hg was contributed by local fish, primarily catfish species (47%). Most non-local Hg intake came from tuna (31% of total Hg) (Figure 1b).
Figure 1.
Species-specific contributions to (a) overall fish consumption and (b) dietary Hg intake.
3.3. Hair Hg
Mean THg in hair (THghair) in participants was 0.27 μg/g (range: 0.0044–3.1 μg/g; Table S3). Eleven participants (7% of study population), 10 of whom were male, had at least one THghair value of 1.1 μg/g or above, which corresponds to U.S. EPA's reference dose (RfD) of 0.1 μg/kg/d MeHg for children and women of child-bearing age (Rice et al. 2003). Three participants (<2%), all of whom were male, had a THghair value above 2 μg/g (Figure S3), a level associated with cardiovascular effects in middle-aged men in some studies (Choi et al. 2009; Virtanen et al. 2005). THghair levels for female participants (mean: 0.18 μg/g, median: 0.12 μg/g) are lower than those of adult women in the U.S. (mean: 0.47 μg/g , median: 0.19 μg/g; McDowell et al. 2004) and in the inland southern U.S. (mean: 0.32 μg/g, median: 0.20 μg/g, using a blood-hair Hg conversion factor of 250 from Mahaffey et al. 2009).
3.4. Hg Dose
Estimated unscaled Hg dose (mean: 0.13 μg/kg/d, 95% CI: 0.10–0.15 μg/kg/d, median: 0.040 μg/kg/d) in our female participants was much higher than the average Hg intake of adult women in the inland southern U.S. (mean: 0.023, 95% CI: 0.020–0.026 μg/kg/d, Mahaffey et al. 2009), while the scaled Hg dose (mean: 0.052 μg/kg/d, 95% CI: 0.043–0.062 μg/kg/d, median: 0.018 μg/kg/d) was closer to, though still above, the regional average.
Using the unscaled Hg dose, 27% of all participants (across all visits) were above the EPA RfD for children and women of child-bearing age (Rice et al. 2003); using the scaled Hg dose, this percentage went down to 11%, still above the observed 4% of participants exceeding the U.S. EPA guideline based on THghair. Given that THghair levels in our cohort were generally lower than those among NHANES participants, this suggests that our calculations based on FFQ results and THgfish were overestimating actual Hg doses.
3.5. Association of hair Hg with fish consumption
Five regression models using different exposure metrics generated comparable results (Table 1): age, gender, education, percent local fish consumed, and season were all significantly associated with THghair, after controlling for the main effect of FCR or Hg dose. BMI and ethnicity were not significant predictors of THghair in any model. Age was positively associated with THghair, with a one decade increase in age associated with ~12% higher THghair. Women had ~57% lower THghair than men. Participants who had college or post-graduate degrees had ~45% higher in THghair than those with high school or less education. A 1% increase in local fish consumption was associated with a ~0.15% increase in THghair.
Table 1.
Mixed-effects regressions of natural log-transformed hair Hg (μg/g) against fish consumption frequency, general fish consumption rate, species-specific fish consumption rate, unsealed Hg dose, or scaled Hg dose.
| Predictors | Model 1 Fish Consumption Frequency (AICa = 1071.6) | Model 2 General Fish Consumption (AIC = 1075.1) | Model 3 Species-Specific Consumption (AIC=1077.5) | Model 4 Unscaled Hg Dose (AIC=1062.8) | Model 5 Scaled Hg Dose (AIC = 1059.1) |
|---|---|---|---|---|---|
| β-Estimate (95% CI)b | β-Estimate (95% CI) | β-Estimate (95% CI) | β-Estimate (95% CI) | β-Estimate (95% CI) | |
| Age | 0.012 (0.0040, 0.020)** |
0.013 (0.0043, 0.021)** |
0.013 (0.0044, 0.021)** |
0.013 (0.0044, 0.021)** |
0.013 (0.0043, 0.021)** |
|
Gender (referent = Male) |
|||||
| Female | −0.57 (−0.86, −0.28)*** |
−0.58 (−0.87, −0.28)*** |
−0.58 (−0.87, −0.28)*** |
−0.58 (−0.88, −0.29)*** |
−0.58 (−0.87, −0.28)*** |
| BMI (kg/m2) | −0.0078 (−0.031, 0.015) |
−0.0077 (−0.031, 0.016) |
−0.0083 (−0.032, 0.015) |
−0.0077 (−0.031, 0.016) |
−0.0072 (−0.031, 0.016) |
| Ethnicity (referent = White/Caucasian)c | |||||
| American Indian | 0.0042 (−0.33, 0.33) | −0.0028 (−0.34, 0.33) | −0.0066 (−0.34, 0.33) | −0.0073 (−0.34, 0.33) |
−0.004 (−0.34, 0.33) |
| African American | −0.77 (−2.5, 0.96) | −0.83 (−2.6, 0.93) | −0.82 (−2.6, 0.94) | −0.82 (−2.6, 0.93) | −0.82 (−2.6, 0.94) |
| Hispanic | 0.11 (−0.57, 0.8) | 0.13 (−0.57, 0.82) | 0.13 (−0.57, 0.82) | 0.13 (−0.57, 0.82) | 0.11 (−0.58, 0.81) |
| Education (referent = High school or less) | |||||
| Some college | −0.17 (−0.52, 0.19) | −0.16 (−0.52, 0.20) | −0.16 (−0.52, 0.20) | −0.15 (−0.51, 0.21) |
−0.16 (−0.52, 0.20) |
| Graduated college or more |
0.43 (0.066, 0.79)* | 0.45 (0.081, 0.82)* | 0.45 (0.083, 0.82)* | 0.46 (0.088, 0.83)* |
0.45 (0.085, 0.83)* |
|
Season (referent = Fall) |
|||||
| Spring | −0.014 (−0.1, 0.074) | −0.0084 (−0.095, 0.079) |
−0.0047 (−0.091, 0.082) |
0.00027 (−0.086, 0.086) |
−0.012 (−0.099, 0.074) |
| Summer | −0.028 (−0.12, 0.059) | −0.033 (−0.12, 0.054) | −0.027 (−0.11, 0.059) | −0.021 (−0.11, 0.065) |
−0.032 (−0.12, 0.055) |
| Winter | −0.18 (−0.27, −0.092)*** |
−0.17 (−0.25, −0.080)*** |
−0.16 (−0.25, −0.079)*** |
−0.16 (−0.25, −0.079)*** |
−0.17 (−0.26, −0.082)*** |
|
Percent local fish consumed |
0.0016 (0.00024, 0.003)* |
0.0016 (0.00028, 0.003)* |
0.0015 (2e-04, 0.0029)* |
0.0015 (2e-04, 0.0029)* |
0.0015 (0.00019, 0.0029)* |
|
Fish Consumption Frequency (referent = ≤ Once a month) |
General FCR (g/d) |
Species-Specific FCR (g/d) |
Hg Dose (μg/kg/d) |
Scaled Hg Dose (μg/kg/d) |
|
| 2 or 3 times a month |
0.067 (−0.041, 0.17)^ | 0.00047 (0.00011, 0.00083)* |
0.00018 (0.000037, 0.00032)* |
0.17 (0.046, 0.29)** |
0.38 (0.056, 0.71)* |
| Once a week | 0.17 (0.041, 0.29)** | ||||
| More than once a week |
0.21 (0.073, 0.34)** | ||||
AIC: Akaike Information Criterion; a smaller AIC indicates a better goodness-of-fit.
p<0.1
p<0.1
p<0.05
p<0.01
p<0.001.
Asian American not included due to extremely small sample size (n=1)
As the main exposure variable in the models, fish consumption frequency, FCR, and Hg dose were all significant predictors of THghair. Compared to participants eating fish once a month or less, those who ate fish once a week or more had ~19% higher THghair. A one ounce (28 g) per day increase in general or species-specific fish consumption was associated with a 1.3% or 0.51%, respectively, increase in THghair. A 0.1 μg/kg/d increase in unscaled or scaled Hg dose was associated with a 1.7% or 3.8% increase in THghair, respectively. Overall, the most parsimonious model used scaled Hg dose, based on the AIC (Akaike Information Criterion) score.
3.6. Seasonal variations in hair Hg and fish consumption
Many participants showed substantial variability in THghair over time, with differences between minimum and maximum THghair for individual participants ranging from 0 to 2.2 μg/g (mean = 0.19 μg/g). The relative standard deviation (RSD) for each participant across all five visits varied from 3.4% to 93%, while 42% of participants had an RSD >50%, regardless of THghair.
Overall, THghair was significantly (on average 13–19%) lower in winter than in the other seasons based on regression models (Table 1). THghair was the highest in summer, followed by fall and spring (Figure S4), although differences among these seasons were not significant. While FCRs were similar in spring and summer, FCR was significantly lower in fall than other seasons, despite similar THghair. This discrepancy may be related to diet composition; the contribution to total fish consumption and Hg intake from locally-caught catfish was highest in the fall and lowest in winter (Figure S5).
3.7. Correlation among family members
Simple linear regressions between husbands and wives yielded significant correlations using both weight-normalized general FCR (adjusted R2 = 0.70, p<0.0001) and THghair (adjusted R2=0.44, p<0.0001) (Table S4). Overall, THghair in husbands was 1.4 times higher than in their wives, while they consumed 32% less fish per unit body weight. Seasonal differences may exist for these associations, with the strongest correlations (largest adjusted R2) observed in spring and summer and lowest in fall.
4. Discussion
In this longitudinal exposure study, we investigated fish consumption and MeHg exposure among consumers of local fish in a rural, low-income community, in which nearly all participants were either freshwater anglers or lived with an angler. We found that local fish constituted the majority of fish consumption and dietary Hg intake in our cohort, and that a higher proportion of local fish consumption was associated with higher THghair despite relatively low Hg in fish from this watershed. The prevalence of local freshwater fish consumption has been observed in other inland regions (e.g., Gerstenberger and Eccleston 2002), notably in American Indian communities (Dellinger 2004) although these are often based on one-time assessments. Fish consumption rates were higher than for the general population and similar to other studies of freshwater anglers (summarized in U.S. EPA 2011), but lower than fish consumers in the U.S., despite a high frequency of fishing trips among many participants. This discrepancy may be due to a combination of factors, including catch-and-release behaviors, concerns about pollution (expressed by ~30% of participants), and a preference for other foods.
Many other lakes in Oklahoma have higher THgfish than Grand Lake (Table S1), particularly in southeastern Oklahoma downwind of large coal-fired power plants (CFPPs). To assess MeHg exposure among anglers in more impacted watersheds, we combined fish consumption patterns of our participants with THg concentrations in fish from Lake Eufaula (Oklahoma Department of Environmental Quality, unpublished data), a commonly-fished reservoir 170 miles south of Grand Lake with 2-6 times higher THgfish. Our calculations indicate that consumption of local fish from Lake Eufaula would contribute about 84% of dietary Hg and that predicted Hg dose would be 2-3 times higher on average. Actual fish consumption patterns may be different for anglers who fish in Lake Eufaula, where fish consumption advisories have been issued for six fish species, although compliance with and awareness of fish consumption advisories may be low among freshwater anglers (Ney and Ney 2008). In our study, about half of participants said they were aware of fish consumption advisories in Oklahoma, while less than a third said they followed them. Similarly, using average THgfish across lakes throughout Oklahoma (Oklahoma Department of Environmental Quality, unpublished data), local fish were estimated to contribute 86% of Hg intake for freshwater anglers and their families. These calculations indicate that anglers in other watersheds more impacted by Hg may have much higher Hg exposures and higher Hg contributions from local fish.
One strength of our study was the use of watershed-specific THg concentrations from locally-caught fish, of which ~ 25% were donated by community members and study participants. Thus, our assessment is highly relevant to the local population. However, Hg exposure estimates are very sensitive to THgfish, and using single-point estimates for calculating Hg intake does not account for variability in THgfish. For instance, THgfish may fluctuate according to changes in Hg biogeochemical cycling within the watershed, fish growth rates, and the types and Hg content of the fish prey. Furthermore, some of our THgfish values may have been higher than fish typically consumed by study participants because volunteers may have chosen to donate relatively large fish, which tend to have higher THg, from a limited number of locations. This potential bias may explain our overestimation of Hg intake, although our average concentrations for largemouth bass, spotted bass, and white bass were below concentrations reported by Oklahoma Department of Environmental Quality for Grand Lake (unpublished data).
Another strength of our study was the inclusion of nearly equal numbers of male and female participants, including 33 husband-wife pairs. In general, male participants had significantly higher THghair than female participants, after controlling for BMI and FCR. Among husband-wife pairs, both FCR and THghair were highly correlated between husbands and wives within the same household. Husband's THghair was on average 40% higher than in their wives, while their weight-normalized FCR was 32% lower. This suggests that the gender difference in THghair is more closely related to the dietary composition rather than overall FCR. Overall, female participants tended to eat a smaller proportion of high Hg fish, such as flathead catfish and largemouth bass (9.6% vs. 6.8%, p=0.04). This is consistent with a recent U.S. EPA study that showed while overall fish consumption in women has been constant over the past decade, Hg exposure has decreased 34% (U.S. EPA 2013), suggesting that women are heeding recommendations to eat lower Hg fish. Moreover, physiological factors such as protective metabolism in females may have also played a role, as suggested in studies in the Amazon (Ashe 2012; Barbosa et al. 2001).
Nevertheless, the high correlation between men and women and frequent sharing of local fish suggest that in communities where male anglers have high Hg exposures, their spouses or children, who are often not included in exposure studies, may also be at risk. Compared to other female participants, women whose husband or domestic partner was an angler had higher average THghair (0.21 μg/g vs. 0.14 μg/g, p<0.001) and overall FCR (63 g/d vs. 38 g/d, p=0.06), while there was no significant difference between men whose wives were anglers and other male participants. This indicates that women living with an angler tend to consume more fish and may be exposed to more Hg than women in general. Similar trends have been found in mothers and children who lived with a licensed sport-fish angler in a multi-state survey (Imm et al. 2007).
In addition to gender, we also found that THghair was associated with age, an effect found in some studies of anglers (e.g., Lincoln et al. 2011), but not others (Knobeloch et al. 2007). THghair was positively associated with age among our participants, and this increase may be due to differences in lifestyle or fish consumption patterns as a function of age (Dumont et al. 1998). Compared to younger participants (≤51 years old, the median age), our older participants (>51 years) fished more often (11 times vs. 7 times in previous three months, p=0.01), and ate more local fish (72% vs. 64%, p=0.04). In addition, the association between THghair and age could be due to long-term Hg accumulation in human body through chronic exposure (Laks 2009).
Education was also a significant predictor of THghair, with higher THghair in people with college or post-graduate degrees. Other studies have observed a similar increase in Hg exposure with education level (e.g., Lincoln et al. 2011, Cole et al. 2004), while Burger et al. (1999) saw higher FCRs in anglers with less than a high school education or at least some college than in anglers with only a high school degree. Among our participants, higher education or socioeconomic status may be associated with a shift in preference or ability to afford non-local, high Hg fish species such as albacore or fresh tuna. Compared to high school graduates or those who did not finish college, the college graduates in our study ate a greater proportion of albacore and fresh tuna (5.7% vs. 2.0%, p=0.002). Participants with more education may also be more aware of the health benefits of fish (Burger et al. 1999).
While American Indians often have high rates of fish consumption through practice of traditional fishing activities (Burger and Gochfeld 2011), American Indian participants in our study did not have higher FCR or THghair than Caucasian participants. Many reported anecdotally that they do not actively practice tribal traditions. The local American Indian population is well-integrated into the broader community and thus less likely to have unique exposure patterns.
The longitudinal component of our study provides a unique and more integrated assessment of year-round fish consumption and Hg exposure. The observed seasonal variability in THghair and FCR highlights the importance of a repeated approach in capturing MeHg exposure and fish consumption patterns. For most participants, THghair was highest in summer and lowest in winter, while reported fish consumption was lowest in the fall. The reason for this lag is unknown. One possibility is that participants were more likely to report their most recent fish consumption when asked to recall over three months, since recent events are more accurately recalled and more likely to shape retrospection (Reis and Gable 2000). Thus, FFQs completed in the fall may match more closely hair samples collected in the winter, since the hair samples represent exposure 1-3 months prior to collection. Furthermore, while the anglers in this study reported the most fishing trips in spring, many also reported freezing their catch for later, so FCRs may be less variable than expected based on fishing behavior alone. Meanwhile, seasonality in Hg exposure is influenced by multiple factors, including seasonal shifts in fishing activities and dietary composition, recall and reporting inaccuracies over time, and temporal variations in THgfish. The potential for seasonal fluctuations also limits direct comparisons of our results, which are integrated over a year, to results from other studies of anglers that often survey anglers during active fishing months.
Our results suggest that participation in the study and report-back of hair testing results may have changed the fish consumption patterns of some participants. Participants with high THghair at their first visit, especially those above 1.1 μg/g, generally showed lower levels at subsequent visits (Figure S6a), regardless of which season they enrolled. A quantile regression of THghair (Figure S6b) showed a significant decreasing trend as a function of visit number in participants with the top 10% and 4% of THghair values (corresponding to THghair above 0.58 μg/g and 1.1 μg/g, respectively, at the first visit). Within these participants, THghair dropped on average 0.06 μg/g (p = 0.03, 95% CI: 0.007–0.11 μg/g) and 0.18 μg/g (p = 0.003, 95%CI: 0.06– 0.30 μg/g) in each subsequent visit, respectively. Among 10 participants who had hair Hg > 1.1 g/g at their first two visits, only 4 were still above 1.1 μg/g at their last visit.
These changes are consistent with interactions between study team members and participants and with qualitative responses to questions on the FFQ. Anecdotally, six of 11 participants with a hair Hg result above 1.1 μg/g directly contacted local study team members (or for one participant, indirectly through a spouse who was also above the guideline) to discuss their results and seek additional information. Study team members made suggestions to eat smaller fish and/or select lower Hg fish. All participants, regardless of their hair mercury levels, were asked a question on their fifth (final) FFQ to assess whether they had changed their fish consumption patterns based on their involvement in the study. Among 120 participants who answered this question (80% of study population), 19 reported a change in fish consumption frequency, of whom seven ate fish more frequently, five ate fish less frequently, and another three switched to smaller fish. These responses suggest that report-back of initial results in our study led to reductions in Hg exposure in participants with high THghair as well as increases in fish consumption in those with low THghair through report-back of the hair testing results. Previous studies have also found that reporting the results of hair mercury testing, along with suggestions for reducing MeHg exposure, can reduce the proportion of fish consumers with hair Hg above the EPA guideline (Knobeloch et al. 2011).
Despite the dominance of saltwater species in the U.S. domestic market (Carrington et al. 2004) and Hg intake of the U.S. general population (Sunderland 2007), local freshwater fish contribute the majority of fish consumption and Hg exposure in our study population despite relatively low Hg concentrations in local fish. Since water bodies closer to CFPPs generally receive greater atmospheric Hg deposition (Carpi 1997), reductions in Hg emissions from CFPPs throughout the U.S. could reduce Hg exposure in many communities that live in close proximity to the plants, in addition to reducing the global mercury pool. Fish consumption advisories, especially those targeting specific water bodies or communities, need to take into account these unique dietary patterns of the local population. As more research on region-specific sources of Hg exposure emerges, environmental health authorities need to consider regional and population specific strategies to protect public health.
5. Conclusions
We found that consumption of locally-caught freshwater fish was the primary source of methylmercury exposure in a rural, low-income population of primarily anglers and their families. Hg exposure, as assessed by hair Hg, was significantly associated with consumption of local fish, and increased as functions of age and reliance on local fish. Women had lower dietary Hg exposure relative to men, although women living with an angler ate more fish and had higher hair Hg than women who did not. Since our study population may be representative of anglers in other rural, inland communities where consumption of local freshwater fish is common, our results suggest that efforts to evaluate benefits of Hg pollution control measures should consider dietary patterns among anglers who fish in local freshwater bodies. While our participants ate more fish on average than the general U.S. population, hair Hg levels were not elevated, demonstrating that eating low mercury fish can provide the health benefits of fish without excessive Hg exposure. Our results also highlight the complexity of associations between fish consumption and Hg exposure and suggest that future exposure studies should address potential seasonal variability.
Supplementary Material
Highlighhts.
We assessed fish consumption and hair Hg in freshwater anglers and their families.
Hair Hg increased with higher fish consumption rate, age, and education.
Majority of fish and Hg intake came from local freshwater fish deptite low fish Hg.
Fish consumption rates and hair Hg showed some seasonal variation.
Women ate more fish per unit body weight but had lower Hg intake relative to men.
Acknowledgments
This study was funded by National Institute of Environmental Health Sciences (NIEHS) grant number 1R21ES017941 and NIEHS Center Grant 2 P30-ES00002. We thank: study participants, fish donors, and members of our community advisory board and focus groups for their time and generosity; Kindel Maymi and Gina Manders for interviewing study participants and collecting hair samples; Robert Lynch for thoughtful feedback and suggestions; and Oklahoma Department of Environmental Quality for providing fish mercury data.
Informed consent was obtained from every participant at the time of enrollment. All study materials and research protocols relating to human subjects were approved by the Office of Human Research Administration at Harvard School of Public Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashe K. Elevated mercury concentrations in humans of Madre De Dios, Peru. PLOS One. 2012;7 doi: 10.1371/journal.pone.0033305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa AC, Jardim W, Dorea JG, Fosberg B, Souza J. Hair mercury speciation as a function of gender, age, and body mass index in inhabitants of the Negro River Basin, Amazon, Brazil. Arch Environ Contam Toxicol. 2001;40:439–444. doi: 10.1007/s002440010195. [DOI] [PubMed] [Google Scholar]
- Björnberg KA, Vahtera M, Grawe KP, Berglund M. Methyl mercury exposure in Swedish women with high fish consumption. Sci Total Environ. 2005;341:45–52. doi: 10.1016/j.scitotenv.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Conceptual environmental justice model for evaluating chemical pathways of exposure in low-income, minority, native american, and other unique exposure populations. Am J Pub Health. 2011;101:S64–S73. doi: 10.2105/AJPH.2010.300077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Stephens WL, Boring CS, Kuklinski M, Gibbons JW, Gochfeld M. Factors in exposure assessment: Ethnic and socioeconomic differences in fishing and consumption of fish caught along the Savannah River. Risk Anal. 1999;19:427–438. doi: 10.1023/a:1007048628467. [DOI] [PubMed] [Google Scholar]
- Carpi A. Mercury from combustion sources: A review of the chemical species emitted and their transport in the atmosphere. Water Air Soil Pollut. 1997;98:241–254. [Google Scholar]
- Carrington CD, Montwill B, Bolger PM. An intervention analysis for the reduction of exposure to methylmercury from the consumption of seafood by women of child-bearing age. Regul Toxicol Pharmacol. 2004;40(3):272–280. doi: 10.1016/j.yrtph.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Choi AL, Pal WH, Budtz-Jorgensen E, Jorgensen PJ, Salonen JT, Tuomainen TP, et al. Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environ Health Perspect. 2009;117:367–372. doi: 10.1289/ehp.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DC, Kearney J, Sanin LH, Leblanc A, Weber JP. Blood mercury levels among Ontario Anglers and sport-fish eaters. Environ Res. 2004;95:305–314. doi: 10.1016/j.envres.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Strain JJ, Myers GJ, Thurston SW, Bonham MP, Shamlaye CF, et al. Neurodevelopmental effects of maternal nutritional status and exposure to methylmercury from eating fish during pregnancy. Neurotoxicology. 2008;29:767–775. doi: 10.1016/j.neuro.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger JA. Exposure assessment and initial intervention regarding sh consumption of tribal members of the Upper Great Lakes Region in the United States. Environ Res. 2004;95:325–340. doi: 10.1016/j.envres.2003.07.012. [DOI] [PubMed] [Google Scholar]
- Dumont C, Girard M, Bellavance F, Noel F. Mercury levels in the Cree Population of James Bay, Quebec, from 1988 to 1993/94. Can Med Assoc J. 1998;158:1439–1445. [PMC free article] [PubMed] [Google Scholar]
- Gerstenberger SL, Eccleston B. Development of a fish contaminant monitoring protocol for Lake Mead, Nevada. Lake Reserv Manage. 2002;18:118–128. [Google Scholar]
- Grandjean P, Budtz-Jorgensen E, White RF, Jorgensen PJ, Weihe P, Debes F, et al. Methylmercury exposure biomarkers as indicators of neurotoxicity in children aged 7 years. Am J Epidemiol. 1999;150:301–305. doi: 10.1093/oxfordjournals.aje.a010002. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Jorgensen PJ, Weihe P. Validity of mercury exposure biomarkers. In: Wilson S, Suk W, editors. Biomarkers of Environmentally Associated Disease. CRC Press/Lewis Publishers; Boca Raton, FL: 2002. pp. 235–247. [Google Scholar]
- Harada M. Minamata disease - Methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- Hu FB, Bronner L, Willett WC, Stampfer MJ, Rexrode KM, Albert CM, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–1821. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- Imm P, Knobeloch L, Anderson HA. Maternal recall of children's consumption of commercial and sport-caught fish: Findings from a multi-state study. Environ Res. 2007;103:198–204. doi: 10.1016/j.envres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, et al. Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect. 2012;120:799–806. doi: 10.1289/ehp.1104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobeloch L, Gliori G, Anderson H. Assessment of methylmercury exposure in Wisconsin. Environ Res. 2007;103:205–210. doi: 10.1016/j.envres.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Knobeloch L, Tomasallo C, Anderson H. Biomonitoring as an intervention against methylmercury exposure. Pub Health Rep. 2011;126:568–574. doi: 10.1177/003335491112600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laks DR. Assessment of chronic mercury exposure within the US population, National Health and Nutrition Examination Survey, 1999-2006. Biometals. 2009;22:1103–1114. doi: 10.1007/s10534-009-9261-0. [DOI] [PubMed] [Google Scholar]
- Lebel J, Mergler D, Branches F, Lucotte M, Amorim M, Larribe F, et al. Neurotoxic effects of low-level methylmercury contamination in the Amazonian Basin. Environ Res. 1998;79:20–32. doi: 10.1006/enrs.1998.3846. [DOI] [PubMed] [Google Scholar]
- Lincoln RA, Shine JP, Chesney EJ, Vorhees DJ, Grandjean P, Senn DB. Fish consumption and mercury exposure among Louisiana Recreational anglers. Environ Health Perspect. 2011;119:245–251. doi: 10.1289/ehp.1002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey KR, Clickner RP, Bodurow CC. Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ Health Perspect. 2004;112:562–570. doi: 10.1289/ehp.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey KR, Clickner RP, Jeffries RA. Adult women's blood mercury concentrations vary regionally in the United States: Association with patterns of fish consumption (NHANES 1999-2004). Environ Health Perspect. 2009;117:47–53. doi: 10.1289/ehp.11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell MA, Dillon CF, Osterloh J, Bolger PM, Pellizzari E, Fernando R, et al. Hair mercury levels in US Children and women of childbearing age: Reference range data from NHANES 1999-2000. Environ Health Perspect. 2004;112:1165–1171. doi: 10.1289/ehp.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergler D, Anderson HA, Chan LHM, Mahaffey KR, Murray M, Sakamoto M, et al. Methylmercury exposure and health effects in humans: a worldwide concern. Ambio. 2007;36:3–11. doi: 10.1579/0044-7447(2007)36[3:meahei]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Shi PL, Morris JS, Spiegelman D, Grandjean P, Siscovick DS, et al. Mercury exposure and risk of cardiovascular disease in two U.S. cohorts. N Engl J Med. 2011;W364:1116–1125. doi: 10.1056/NEJMoa1006876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney JJ, Ney JP. Risky Business: Evaluation of US consumption advisories for freshwater sport fish. In: Allen MS, Sammons S, Maceina MJ, editors. Balancing Fisheries Management and Water Uses for Impounded River Systems. Vol. 62. Amer Fisheries Soc; Bethesda, MD: 2008. pp. 559–569. [Google Scholar]
- NRC (National Research Council) Toxicological Effects of Methylmercury. National Academy Press; Washington, DC: 2000. [Google Scholar]
- Reis HT, Gable SL. Event-sampling and other methods for studying everyday experience. In: Reis HT, Judd CM, editors. Handbook of Research Methods in Social and Personality Psychology. Cambridge University Press; New York, NY: 2000. pp. 190–222. [Google Scholar]
- Rice DC, Schoeny R, Mahaffey K. Methods and rationale for derivation of a reference dose for methylmercury by the US EPA. Risk Anal. 2003;23:107–115. doi: 10.1111/1539-6924.00294. [DOI] [PubMed] [Google Scholar]
- Sunderland EM. Mercury exposure from domestic and imported estuarine and marine fish in the US seafood market. Environ Health Perspect. 2007;115:235–242. doi: 10.1289/ehp.9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk ME, Bhavsar SP, Bowerman W, Boysen E, Clark M, Diamond M, et al. Risks and benefits of consumption of Great Lakes Fish. Environ Health Perspect. 2012;120:11–18. doi: 10.1289/ehp.1003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency) [15 May 2014];Fish Consumption Advisories: What You Need to Know About Mercury in Fish and Shellfish. 2004 Available: http://www.epa.gov/mercury/advisories.htm.
- U.S. EPA . Method 7473: Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry. U.S. Environmental Protection Agency; Washington DC: 2007. [15 May 2014]. Available: http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/7473.pdf. [Google Scholar]
- U.S. EPA [15 May 2014];Exposure Factors Handbook. 2011 Report no. EPA/600/R-090/052F. Available: http://www.epa.gov/ncea/efh/pdfs/efh-complete.pdf.
- U.S. EPA [15 May 2014];Trends in blood mercury concentrations and fish consumption among U.S. women of childbearing age NHANES, 1999-2010. 2013 doi: 10.1016/j.envres.2014.02.001. Report no. EPA-823-R-13-002. Available: http://water.epa.gov/scitech/swguidance/fishshellfish/fishadvisories/upload/Trends-in-Blood-Mercury-Concentrations-and-Fish-Consumption-Among-U-S-Women-of-Childbearing-Age-NHANES-1999-2010.pdf. [DOI] [PubMed]
- U.S. FDA (U.S. Food and Drug Administration) [15 May 2014];Mercury Levels in Commercial Fish and Shellfish (1990-2010) 2006 Available: http://www.fda.gov/Food/FoodborneIllnessContaminants/Metals/ucm115644.htm.
- U.S. FWS (U.S. Fish and Wildlife Service) [15 May 2014];2011 National Survey of Fishing, Hunting and Wildlife-Associated Recreation. Report no. FHW/11-NAT (RV) 2013 Available: http://www.census.gov/prod/2012pubs/fhw11-nat.pdf.
- U.S. FWS. [15 May 2014];2011 National Survey of Fishing, Hunting and Wildlife-Associated Recreation: Oklahoma. 2014 Report no. FHW/11-OK (RV). Available: http://www.census.gov/prod/2013pubs/fhw11-ok.pdf.
- Virtanen JK, Voutilainen S, Rissanen TH, Mursu J, Tuomainen TP, Korhonen MJ, et al. Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in Eastern Finland. Arterioscler Thromb Vasc Biol. 2005;25:228–233. doi: 10.1161/01.ATV.0000150040.20950.61. [DOI] [PubMed] [Google Scholar]
- WHO . Methylmercury, Environmental Health Criteria 101. World Health Organization; Geneva, Switzerland: 1990. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.