Abstract
Background and aim of the study
Detailed analyses of risk-adjusted outcomes after mitral valve surgery have documented significant survival decrements with tissue valves at any age. Several recent studies of prosthetic aortic valve replacement (AVR) also have suggested a poorer performance of tissue valves, although analyses have been limited to small matched series. The study aim was to test the hypothesis that AVR with tissue valves is associated with a lower risk-adjusted survival, as compared to mechanical valves.
Methods
Between 1986 and 2009, primary isolated AVR, with or without coronary artery bypass grafting (CABG), was performed with currently available valve types in 2,148 patients (1,108 tissue valves, 1,040 mechanical). Patients were selected for tissue valves to be used primarily in the elderly. Baseline and operative characteristics were documented prospectively with a consistent variable set over the entire 23-year period. Follow up was obtained with mailed questionnaires, supplemented by National Death Index searches. The average time to death or follow up was seven years, and follow up for survival was 96.2% complete. Risk-adjusted survival characteristics for the two groups were evaluated using a Cox proportional hazards model with stepwise selection of candidate variables.
Results
Differences in baseline characteristics between groups were (tissue versus mechanical): median age 73 versus 61 years; non-elective surgery 32% versus 28%; CABG 45% versus 35%; median ejection fraction 55% versus 55%; renal failure 6% versus 1%; diabetes 18% versus 7% (p<0.01). Unadjusted Kaplan-Meier survival was significantly lower with tissue than mechanical valves; however, after risk adjustment for the adverse profiles of tissue valve patients, no significant difference was observed in survival after tissue or mechanical AVR. Thus, the hypothesis did not hold, and risk-adjusted survival was equivalent, of course qualified by the fact that selection bias was evident.
Conclusion
With selection criteria that employed tissue AVR more frequently in elderly patients, tissue and mechanical valves achieved similar survival characteristics across the spectrum of patient risk. Further studies of the relative outcomes of mechanical versus tissue valves across the spectrum of patient age seem indicated.
Selection of the ideal prosthesis for aortic valve replacement (AVR) remains controversial (1). The most recent American Heart Association guidelines recommend mechanical prostheses for patients aged <65 years and biological prostheses for those aged >65 years (1). Despite a recent trend of increased bioprosthetic valve use in both the aortic and mitral positions, patients with biological mitral prostheses have shown inferior survival to those with mechanical prostheses (2–4). Similarly, the two large randomized trials comparing mechanical and bioprosthetic AVR -the Edinburgh Valve Trial and the Department of Veterans Affairs (VA) trial - both demonstrated a trend towards better survival in patients who received mechanical valves (5,6). These trials ended, however, prior to the availability of the newer generations of bioprosthetic and mechanical valves. With the improved durability of newer bioprostheses, many groups believe that bioprosthetic valves should be used in younger patients (7). Evidence for employing bioprosthetic aortic valves remains contradictory, however, with some recent studies having confirmed that even the newer generation of bioprosthetic aortic valves performed poorly compared to mechanical valves (4).
Hence, the study aim was to test the hypothesis that AVR with a bioprosthesis is associated with a lower risk-adjusted survival compared to mechanical valve replacement.
Clinical material and methods
Patients
The records of 2,148 patients with isolated aortic valve disease who underwent AVR between January 1986 and December 2009 were retrieved from the Duke Databank for Cardiovascular Disease, and reviewed. Patients having concomitant coronary artery bypass grafting (CABG) or electrophysiologic procedures were included, but those having other major cardiac procedures were excluded (e.g. tricuspid valve procedures, mitral valve operations, repair of postinfarct ventricular septal defect, ventricular aneurysm repair or restoration). Patients receiving prostheses that have subsequently been withdrawn from the market (e.g. Ionescu-Shiley or Björk valves) also were excluded. This selection process resulted in 2,148 consecutive patients who underwent primary isolated AVR with currently available valves.
The preoperative baseline characteristics and intraoperative observations for all patients were recorded prospectively during the entire 23 years, with consistent variable collection throughout the period. Late outcome data were collected prospectively for patients with significant concomitant coronary disease, as per the Duke Databank protocols. A National Death Index (NDI) search was conducted through 2009 to acquire mortality results for patients without coronary disease. Patients were allocated to two groups: group 1 (n = 1,108) consisted of patients receiving a bioprosthetic valve, while group 2 (n = 1,040) included patients receiving a mechanical valve. Survival outcomes and causes of mortality were obtained from mailed self-administered questionnaires or telephone follow up (in patients with coronary disease), as well as review of hospital records. Mortality data were adjudicated by a multidisciplinary committee. Survival data were supplemented with information from the NDI and Social Security Death Index. Only all-cause mortality data were available consistently for analysis.
This study was performed with approval from the Duke Institutional Review Board and under a waiver of informed consent.
Data acquisition
Baseline characteristics and clinical event rates were described using medians with 25th and 75th percentiles for continuous variables, and frequencies and proportions for categorical variables. Descriptive data were compared using the Wilcoxon rank-sum test for continuous and ordinal variables, and a Pearson χ2 or Fisher’s exact test for categorical variables, as appropriate. The analysis strategy was to adjust for the impact of baseline characteristics on survival using multivariable Cox proportional hazards regression modeling techniques. In order to develop the risk-adjustment model, a pool of all covariates that have been shown to be important in previous analyses was chosen. The candidate variable list for baseline adjustment included the following factors: age, gender, race, diabetes mellitus, hypertension, hyperlipidemia, peripheral vascular disease, cerebrovascular disease, renal failure, body mass index, smoking history, chronic lung disease, prior myocardial infarction, prior CABG, prior percutaneous coronary intervention, glomerular filtration rate, congestive heart failure, NYHA functional class, ejection fraction, number of diseased vessels, concomitant CABG, preoperative arrhythmia, and year of surgery.
Statistical analysis
Continuous and ordinal variables were tested for linearity with the log hazard and transformed as necessary to satisfy this modeling assumption. Cox regression analysis was used to identify the significant independent predictors of mortality in the multivariable setting. The adjusted survival estimates for each group were calculated by applying its estimated baseline hazard function, along with covariate Cox model parameter estimates, to all patients in the entire cohort and then averaged for all patients at each time point. All statistical analyses were performed using SAS version 8.2 (SAS Institute, Cary, NC, USA). A p-value <0.05 was considered to be statistically significant.
Results
Baseline characteristics for the entire group of patients undergoing AVR with currently available valves (n = 2,148) are listed in Table I. Bioprosthetic valves were employed in 1,108 patients and mechanical valves in 1,040. Patients receiving bioprosthetic valves were older (median age 73 years) than those receiving mechanical valves (median age 61 years). There were no significant differences between the two groups with regard to gender or body mass index. Patients receiving bioprosthetic aortic valves were more likely to undergo concomitant CABG (44.8%) than those receiving mechanical valves (34.8%). Diabetes mellitus was also more prevalent in the bioprosthetic group (17.5%) than in mechanical valve patients (7.2%). Along with older age, bioprosthetic patients also had higher rates of hypercholesterolemia, renal failure, preoperative dialysis, hypertension, chronic lung disease, endocarditis, peripheral vascular disease, and cerebrovascular disease. The median ejection fraction was the same in both groups (55%). Aortic stenosis was the predominant lesion in 74.5% of the bioprosthetic group and in 66.2% of the mechanical valve group. The median period to death after AVR was 4.8 years in the bioprosthetic group and 7.8 years in the mechanical valve group.
Table I.
Distribution of baseline characteristics.
| Variable | Level | Tissue valve (n = 1,108) | Mechanical valve (n = 1,040) |
|---|---|---|---|
| Age (years) | Median | 73 | 61 |
| 25th | 67 | 52 | |
| 75th | 78 | 69 | |
| Gender | Male | 665 (60) | 645 (62) |
| Female | 443 (40) | 345 (38) | |
| Body mass index | Median | 27 | 27 |
| 25th | 24 | 24 | |
| CABG surgery | 495 (44.8) | 362 (34.8) | |
| Prior tobacco use | 313 (28.2) | 217 (21.9) | |
| Diabetes | 194 (17.5) | 75 (7.2) | |
| Hypercholesterolemia | 360 (32.5) | 206 (19.8) | |
| Renal failure | 66 (6.0) | 9 (0.9) | |
| Preoperative dialysis | 33 (2.9) | 5 (0.48) | |
| GFR | Median | 75 | 81 |
| 25th | 57 | 88 | |
| 75th | 88 | 98 | |
| Hypertension | 598 (54.0) | 303 (29.1) | |
| Chronic lung disease/COPD | 139 (12.5) | 65 (6.3) | |
| Infective endocarditis | 25 (2.3) | 15 (1.4) | |
| PVD | 107 (9.7) | 34 (3.3) | |
| History of CVD | 97 (8.8) | 41 (3.9) | |
| Prior PCI | 50 (4.5) | 30 (2.9) | |
| Prior CABG | 40 (3.6) | 18 (1.7) | |
| Diseased coronary vessels | 0 | 544 (49) | 634 (61) |
| 1 | 177 (16) | 147 (14) | |
| 2 | 177 (16) | 123 (12) | |
| 3 | 210 (19) | 136 (13) | |
| Left main disease | 93 (8.4) | 60 (5.8) | |
| Ejection fraction (%) | Median | 55 | 55 |
| 25th | 43 | 44 | |
| 75th | 65 | 64 | |
| History of MI | 217 (19.6) | 148 (14.2) | |
| CHF within 2 weeks | 611 (55.1) | 335 (32.2) | |
| NYHA class | I | 550 (49.6) | 728 (70.0) |
| II | 129 (11.6) | 68 (6.5) | |
| III | 266 (24.0) | 168 (16.2) | |
| IV | 163 (14.7) | 76 (7.3) | |
| Arrhythmia | 69 (6.2) | 28 (2.7) | |
| Cardiogenic shock | 9 (0.8) | 7 (0.7) | |
| CPR within 1 h of procedure | 4 (0.4) | 0 (0) | |
| Angina | 212 (19.1) | 117 (11.3) | |
| Acute presentation | 312 (32.1) | 173 (27.7) | |
| Mitral stenosis | 2 (0.2) | 2 (0.2) | |
| Aortic stenosis | 825 (74.5) | 688 (66.2) | |
| Aortic insufficiency grade | 0–1 | 739 (66.7) | 593 (57.0) |
| 2 | 155 (14.0) | 151 (14.5) | |
| 3 | 120 (10.8) | 139 (13.4) | |
| 4 | 94 (8.5) | 157 (15.1) | |
| No. of coronary grafts placed | 1 | 161 (33) | 147 (41) |
| 2 | 142 (29) | 116 (32) | |
| 3 | 153 (31) | 73 (20) | |
| 4 | 34 (7) | 23 (6) | |
| 5 | 5 (1) | 3 (1) | |
| IMA graft | 325 (29.3) | 221 (21.3) | |
| Years to death | Median | 4.8 | 7.8 |
| 25th | 2.5 | 3.7 | |
| 75th | 8.8 | 12.3 |
Values in parentheses are percentages.
CABG: Coronary artery bypass grafting; CHF: Congestive heart failure; COPD: Chronic obstructive pulmonary disease; CPR: Cardiopulmonary resuscitation; CVD: Cerebrovascular disease; GFR: Glomerular filtration rate; IMA: Internal mammary artery; MI: Myocardial infarction; PCI: Percutaneous coronary intervention; PVD: Peripheral vascular disease.
The types of aortic valve inserted are listed in Table II. The majority of the 1,108 bioprosthetic valves were Carpentier-Edwards porcine (72%), followed by Carpentier-Edwards pericardial (21%). Aortic valve homografts accounted for only 6% of the total bioprosthetic aortic valves. St. Jude Medical (SJM) mechanical valves accounted for the majority (81%) of the 1,040 mechanical valves; the remainder of the mechanical valves included Starr-Edwards (13%), SJM Hemodynamic Plus (5%), or CarboMedics (2%).
Table II.
Valve types.
| Valve type | No. implanted |
|---|---|
| Bioprosthetic (n = 1,108) | |
| Carpentier-Edwards Porcine | 802 (72) |
| Cryolife Homograft | 70 (6) |
| Carpentier-Edwards Pericardial | 236 (21) |
| Mechanical (n = 1,040) | |
| St. Jude Medical - Masters | 839 (81) |
| Starr-Edwards | 130 (13) |
| St. Jude Medical - Hemodynamic Plus | 55 (5) |
| CarboMedics | 16 (2) |
Values in parentheses are percentages.
The results of the Cox proportional hazards model are shown in Table III. Risk predictors with the greatest hazard of mortality after AVR were renal failure (hazard ratio (HR) 1.9, p <0.0001), age (HR 1.7, p <0.0001), and the presence of coronary artery disease (HR 1.8, p <0.0001). Chronic lung disease, diabetes, prior myocardial infarction and endocarditis also were significant predictors of decreased survival after AVR. Caucasian race was associated with a better survival over time (HR 0.7, p <0.0001). Bioprosthetic valves were primarily placed in older patients; details of the age range of bioprosthetic and mechanical valve patients are shown in Figure 1. The median age of bioprosthetic valve recipients was 73 years (95% CI: 67,78). The youngest patient to receive a bioprosthesis was aged 16 years, and the oldest 92 years. The median age of mechanical valve recipients was 61 years (95% CI: 52,69); the youngest recipient was aged 16 years, and the oldest 86 years.
Table III.
Cox proportional hazards model.
| Parameter | Wald χ2 | Hazard ratio (HR) | CI | p-value |
|---|---|---|---|---|
| Age (HR per 10 years) | 196 | 1.7 | (1.6,1.8) | <0.0001 |
| Coronary artery disease | <0.0001 | |||
| Three-vessel | 32 | 1.8 | (1.5,2.2) | |
| Two-vessel | 20 | 1.5 | (1.3,1.9) | |
| One-vessel | 7 | 1.3 | (1.1,1.5) | |
| White race | 29 | 0.7 | (0.6,0.8) | <0.0001 |
| Renal failure | 18 | 1.9 | (1.4,2.5) | <0.0001 |
| COPD | 15 | 1.5 | (1.2,1.8) | <0.0001 |
| Diabetes | 14 | 1.4 | (1.2,1.7) | 0.0002 |
| History of MI | 13 | 1.3 | (1.1,1.5) | 0.0003 |
| Endocarditis | 10 | 1.7 | (1.2,2.4) | 0.0003 |
| Acute presentation | 8 | 1.2 | (1.1,1.4) | 0.0040 |
| Congenital etiology | 8 | 0.8 | (0.6,0.9) | 0.0050 |
| Hyperlipidemia | 5 | 0.8 | (0.7,0.9) | 0.0200 |
| Utilization of left IMA | 4 | NA | NA | 0.0600 |
| Depressed ejection fraction | 3 | NA | NA | 0.0700 |
| Mechanical vs. tissue replacement | 1 | NA | NA | 0.4400 |
CI: Confidence interval; COPD: Chronic obstructive pulmonary disease; IMA: Internal mammary artery; MI: Myocardial infarction; NA: Not available.
Figure 1.
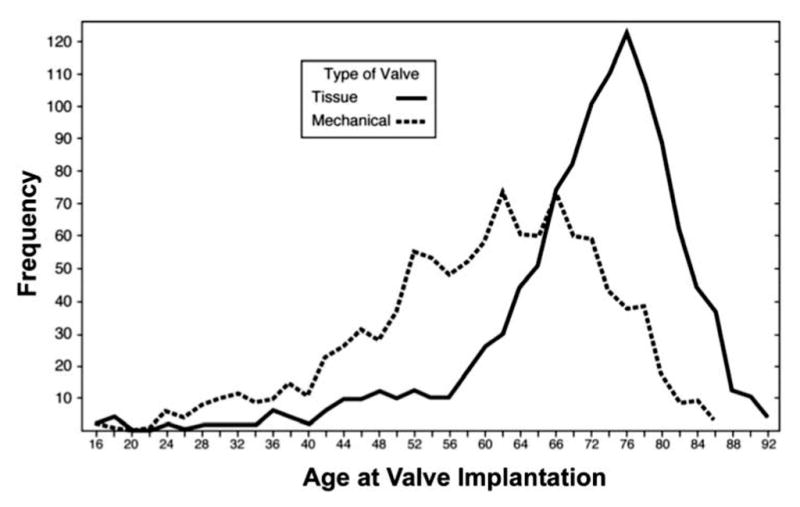
Valve distribution by patient age.
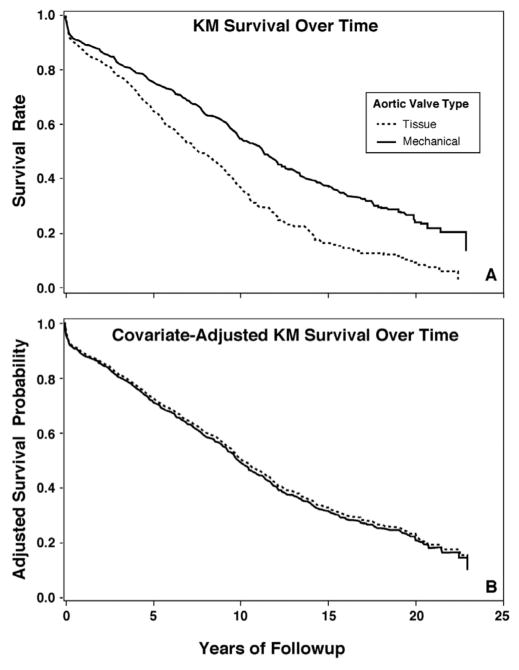
The results of the unadjusted Kaplan-Meier survival analysis are shown in Figure 2. Mechanical valve patients displayed a significantly improved unadjusted survival compared to bioprosthetic valve recipients (log rank <0.01). The 10-year unadjusted survival for bioprosthetic valve recipients was approximately 40%, compared to mechanical valve patients who experienced a 10-year unadjusted survival of 60%. Bioprosthetic valve recipients, however, were older with more comorbidities (as detailed in Table I). Consequently, when taking into account any differences in baseline characteristics between groups, the risk-adjusted Kaplan-Meier survival estimates (Fig. 2) demonstrated no survival advantage with either a tissue or mechanical valve (log rank, p <0.44). Both groups of patients exhibited 10-year survivals of approximately 50%.
Figure 2.
Unadjusted and risk-adjusted survival.
Discussion
Over a 23-year experience with AVR at the authors’ institution, no significant difference in risk-adjusted survival was observed among 2,148 patients receiving either a mechanical or bioprosthetic aortic valve. This finding agrees with an earlier report from their center demonstrating identical 10-year survival in 841 patients receiving either mechanical or bioprosthetic valves (8). In the present study, patients who received bioprosthetic aortic valves were older with significantly higher rates of coronary artery disease, renal failure and diabetes mellitus. Despite these differences in preoperative characteristics the risk-adjusted survival was similar, suggesting that the current strategy of placing bioprosthetic valves in older patients with more comorbidities is approximately sound.
Prior randomized data comparing mechanical and bioprosthetic valves are limited to two major studies. The Veterans Administration Cooperative Study on Valvular Heart Disease was a prospective, randomized trial that concluded in 1982 and compared survival in 394 patients who had received either a mechanical or bioprosthetic valve (5). In that trial, the mechanical valve patients demonstrated a superior 15-year survival compared to bioprosthetic valve recipients (79 ± 3% versus 66 ± 3%; p = 0.02) (5). Similarly, the Edinburgh Valve Trial demonstrated a survival advantage for mechanical valves at 12 years, but the difference had disappeared at 20 years, as the survival curves converged (9).
Larger retrospective data sets comparing bioprosthetic and mechanical aortic valves are available, but do not conclusively answer the question of appropriate prosthesis selection. Khan et al. (10) reported no differences in the 20-year survival of 1,389 patients receiving either a mechanical or bioprosthetic aortic valve. In this study, the risk of valve reoperation increased significantly between years 6 to 8 and rose progressively with time (10). Therefore, older patients with more comorbidities, and who are not expected to live beyond this period, would seem the best candidates for bioprostheses. Several other non-randomized retrospective studies also failed to demonstrate survival differences between mechanical and bioprostheses (8,11,12). In contrast to these studies, Brown et al. (13) analyzed case-matched data from 440 patients aged 50 to 70 years who underwent either mechanical or bioprosthetic AVR and demonstrated significant five- and ten-year survival advantages for mechanical valve recipients. In this study, patients receiving mechanical valves had lower reoperation rates at 10 years (2% versus 9%; p = 0.06) but higher rates of hemorrhagic complications (15% versus 7%; p = 0.01) (13).
Study limitations
Although the present study was one of the largest comparisons of survival after mechanical and bioprosthetic AVR to have been reported, its non-randomized and retrospective nature limited the findings. As shown by baseline differences between groups with respect to age and comorbidities, a significant selection bias was evident, as occurs with most studies of this type. Despite efforts to account comprehensively for baseline characteristics in the risk-adjustment process, the likelihood exists that factors not available in this model influenced outcomes, and that these factors were distributed differently in the two groups. A second weakness of the study was the lack of information on non-fatal events, including hemorrhagic or thrombotic complications after AVR. Rates of thromboembolism and major hemorrhage for mechanical AVR were approximately 1.9% per patient-year (pt-yr) and 3.0% per pt-yr, respectively (14). These data compared well to the bioprosthetic AVR rates of thromboembolism of 2.3% per pt-yr and major hemorrhage of 1.1% per pt-yr (10). The rate of hemorrhage for mechanical valve patients was significantly higher, and thus, mechanical valves are not ideal for patients with long life expectancies. Newer generations of bioprosthetic valves have demonstrated promising durability and may lower the acceptable age of implantation (7,15), and emerging techniques of aortic valve repair may be preferable in selected younger patients (16). Due to the long duration of the study, multiple valve types were utilized, the majority of bioprosthetic valves being second-generation porcine bioprostheses that have subsequently been largely replaced by third-generation pericardial valves. Nevertheless, both porcine and pericardial valves demonstrated similar durability and hemodynamic characteristics (17). Since the majority of mechanical valves used in the present study (either SJM Masters or SJM Hemodynamic Plus) are still widely used today (14), these results remain applicable to modern cardiac surgical practice.
In conclusion, the results of the study, showing outcome equivalence of mechanical and tissue valves, support current management algorithms that favor mechanical prostheses in younger patients and tissue valves in the elderly. Ultimately, the choice of aortic prosthesis should be individualized on the basis of the patient’s age, life expectancy, and ability to take warfarin. However, many patients who are ideal for mechanical prostheses are younger, active and have a strong desire to avoid lifelong anticoagulation. Aortic valve repair may represent an attractive option in selected younger patients, and has demonstrated promising durability and hemodynamic performance without the attendant risks of hemorrhage, thromboembolism or endocarditis (18). In the future, the choice of aortic prosthesis may also be influenced by the development of non-warfarin-based anticoagulation regimens for mechanical valves, and the expanding field of trans-catheter aortic valve technology (19,20).
Footnotes
Presented at the Sixth Biennial Meeting of the Society for Heart Valve Disease, 25th–28th June 2011, Palau de Congressos de Catalunya, Barcelona, Spain
References
- 1.Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e1–e142. doi: 10.1016/j.jacc.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Gammie JS, Sheng S, Griffith BP, et al. Trends in mitral valve surgery in the United States: Results from the Society of Thoracic Surgeons Adult Cardiac Database. Ann Thorac Surg. 2009;87:1431–1439. doi: 10.1016/j.athoracsur.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 3.Daneshmand MA, Milano CA, Rankin JS, et al. Mitral valve repair for degenerative disease: A 20-year experience. Ann Thorac Surg. 2009;88:1828–1837. doi: 10.1016/j.athoracsur.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Brown JM, O’Brien SM, Wu C, Sikora JAH, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: Changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009;137:82–90. doi: 10.1016/j.jtcvs.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: Final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36:1152–1158. doi: 10.1016/s0735-1097(00)00834-2. [DOI] [PubMed] [Google Scholar]
- 6.Bloomfield P, Wheatley DJ, Prescott RJ, Miller HC. Twelve-year comparison of a Björk-Shiley mechanical heart valve with porcine bioprostheses. N Engl J Med. 1991;324:573–579. doi: 10.1056/NEJM199102283240901. [DOI] [PubMed] [Google Scholar]
- 7.Rizzoli G, Mirone S, Ius P, et al. Fifteen-year results with the Hancock II valve: A multicenter experience. J Thorac Cardiovasc Surg. 2006;132:602–609. doi: 10.1016/j.jtcvs.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 8.Peterseim DS, Cen YY, Cheruvu S, et al. Long-term outcome after biologic versus mechanical aortic valve replacement in 841 patients. J Thorac Cardiovasc Surg. 1999;117:890–897. doi: 10.1016/S0022-5223(99)70368-5. [DOI] [PubMed] [Google Scholar]
- 9.Oxenham H, Bloomfield P, Wheatley DJ, et al. Twenty year comparison of a Björk-Shiley mechanical heart valve with porcine bioprostheses. Heart. 2003;89:715–721. doi: 10.1136/heart.89.7.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan SS, Trento A, DeRobertis M, et al. Twenty-year comparison of tissue and mechanical valve replacement. J Thorac Cardiovasc Surg. 2001;122:257–269. doi: 10.1067/mtc.2001.115238. [DOI] [PubMed] [Google Scholar]
- 11.Chan V, Jamieson WRE, Germann E, et al. Performance of bioprostheses and mechanical prostheses assessed by composites of valve-related complications to 15 years after aortic valve replacement. J Thorac Cardiovasc Surg. 2006;131:1267–1273. doi: 10.1016/j.jtcvs.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 12.Prasongsukarn K, Jamieson WRE, Lichtenstein SV. Performance of bioprostheses and mechanical prostheses in age group 61–70 years. J Heart Valve Dis. 2005;14:501–508. discussion 509. [PubMed] [Google Scholar]
- 13.Brown ML, Schaff HV, Lahr BD, et al. Aortic valve replacement in patients aged 50 to 70 years: Improved outcome with mechanical versus biologic prostheses. J Thorac Cardiovasc Surg. 2008;135:878–884. doi: 10.1016/j.jtcvs.2007.10.065. discussion 884. [DOI] [PubMed] [Google Scholar]
- 14.Toole JM, Stroud MR, Kratz JM, et al. Twenty-five year experience with the St. Jude Medical mechanical valve prosthesis. Ann Thorac Surg. 2010;89:1402–1409. doi: 10.1016/j.athoracsur.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 15.Cohen G, Zagorski B, Christakis GT, et al. Are stentless valves hemodynamically superior to stented valves? Long-term follow-up of a randomized trial comparing Carpentier-Edwards pericardial valve with the Toronto Stentless Porcine Valve. J Thorac Cardiovasc Surg. 2010;139:848–859. doi: 10.1016/j.jtcvs.2009.04.067. [DOI] [PubMed] [Google Scholar]
- 16.Rankin JS, Gaca JG. Techniques of aortic valve repair. Innovations. 2011;6:348–354. doi: 10.1097/IMI.0b013e31824641d7. [DOI] [PubMed] [Google Scholar]
- 17.Jamieson WRE, Germann E, Aupart MR, Neville PH, Marchand MA, Fradet GJ. 15-year comparison of supra-annular porcine and PERIMOUNT aortic bioprostheses. Asian Cardiovasc Thorac Ann. 2006;14:200–205. doi: 10.1177/021849230601400306. [DOI] [PubMed] [Google Scholar]
- 18.Aicher D, Fries R, Rodionycheva S, Schmidt K, Langer F, Schafers HJ. Aortic valve repair leads to a low incidence of valve-related complications. Eur J Cardiothorac Surg. 2010;37:127–132. doi: 10.1016/j.ejcts.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Garnock-Jones KP. Dabigatran etexilate: A review of its use in the prevention of stroke and systemic embolism in patients with atrial fibrillation. Am J Cardiovasc Drugs. 2011;11:57–72. doi: 10.2165/11206400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]