Abstract
Traumatic brain injury (TBI) is an increasingly frequent and poorly understood condition lacking effective therapeutic strategies. Inflammation and oxidative stress (OS) are critical components of injury, and targeted interventions to reduce their contribution to injury should improve neurobehavioral recovery and outcomes. Recent evidence reveals potential protective, yet short-lived, effects of the endocannabinoids (ECs), 2-arachidonoyl glycerol (2-AG) and N-arachidonoyl-ethanolamine (AEA), on neuroinflammatory and OS processes after TBI. The aim of this study was to determine whether EC degradation inhibition after TBI would improve neurobehavioral recovery by reducing inflammatory and oxidative damage. Adult male Sprague-Dawley rats underwent a 5-mm left lateral craniotomy, and TBI was induced by lateral fluid percussion. TBI produced apnea (17±5 sec) and a delayed righting reflex (479±21 sec). Thirty minutes post-TBI, rats were randomized to receive intraperitoneal injections of vehicle (alcohol, emulphor, and saline; 1:1:18) or a selective inhibitor of 2-AG (JZL184, 16 mg/kg) or AEA (URB597, 0.3 mg/kg) degradation. At 24 h post-TBI, animals showed significant neurological and -behavioral impairment as well as disruption of blood–brain barrier (BBB) integrity. Improved neurological and -behavioral function was observed in JZL184-treated animals. BBB integrity was protected in both JZL184- and URB597-treated animals. No significant differences in ipsilateral cortex messenger RNA expression of interleukin (IL)-1β, IL-6, chemokine (C-C motif) ligand 2, tumor necrosis factor alpha, cyclooxygenase 2 (COX2), or nicotinamide adenine dinucleotide phosphate oxidase (NOX2) and protein expression of COX2 or NOX2 were observed across experimental groups. Astrocyte and microglia activation was significantly increased post-TBI, and treatment with JZL184 or URB597 blocked activation of both cell types. These findings suggest that EC degradation inhibition post-TBI exerts neuroprotective effects. Whether repeated dosing would achieve greater protection remains to be examined.
Key words: : 2-AG, AEA, endocannabinoids, neuroinflammation, TBI
Introduction
Traumatic brain injury (TBI) is an increasingly frequent occurrence in the military population, resulting from explosive or blast attacks.1,2 Reports indicate that the number of closed brain injuries has increased with TBI, accounting for 66% of all army war-zone evacuations.3 TBI is not limited to the military population and has become a well-recognized medical problem in contact sports, such as football and boxing.4 The majority of TBI victims are young, otherwise healthy adults—in fact, TBI is now recognized as a leading cause of death in young adults (<45 years of age).5 The early-period post-TBI is characterized by neuroinflammation and oxidative stress (OS), then followed by neurological and -behavioral changes that include, but are not limited to, increased incidence of anxiety and depression, stress sensitivity, anhedonia, impulse control deficits, sleep disturbances, and pain sensitivity.6–10
The initial mechanical insult induces increased and sustained inflammation, which is characterized by acute up-regulation of proinflammatory cytokines (interleukin [IL]-1α, IL-6, and tumor necrosis factor [TNF]-α), activation of astrocytes and microglia, and disruption of blood–brain barrier (BBB).11 The acute response to TBI is followed by a long-term injury, which involves neuronal damage, cytotoxicity, and cognitive impairment.12,13 Thus, timely modulation of neuroinflammation early on becomes critical in preventing prolonged neuroinflammation that can be damaging when in excess, while not interfering with the reparative contribution of endogenous neuromodulators, and activated astrocytes and glia.14,15 Current available treatment and management of TBI is palliative, owing to incomplete understanding of its pathophysiology, with no effective therapies identified to improve outcomes after injury.16 Long-term effects of TBI, including neurobehavioral dysfunction, may be ameliorated by interventions aimed at reducing short-term neuroinflammation, OS, and excessive astrocyte and microglial activation.10,14,15,17
Over the past decade, the neuroprotective effects of the endocannabinoid (EC) system have received increased attention.18–21 The EC system primarily consists of two G-protein-coupled transmembrane receptors, cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2), and several lipid-derived endogenous ligands. The CB1 receptor is expressed throughout the brain, and CB2 receptor distribution is predominantly in cells and tissues of the immune system.22 Two principal ECs have been identified, 2-arachidonoyl glycerol (2-AG) and N-arachidonoyl-ethanolamine (AEA), of which 2-AG is the most bioactive and abundant EC in the brain.18,23 In contrast to preformed neurotransmitters, which are stored in secretory vesicles, ECs are synthesized “on demand” in response to specific stimuli. Similar to neurotransmitters, degradation of these ECs is rapid. Once released, ECs act locally and their effects are quickly terminated by cellular uptake and enzymatic degradation by fatty acid amide hydrolase (FAAH), monoacylglycerol lipase (MAGL), and cyclooxygenase 2 (COX2).24 Degradation is predominantly mediated by FAAH for AEA and MAGL for 2-AG; however, COX2 has also been shown to metabolize both AEA and 2-AG.25–27 Several investigators have reported neuroprotective effects of cannabinoid receptor agonists (CRAs).18,19,21,28–31 CRAs have been demonstrated to decrease glutamatergic toxicity, OS, and inflammation as well as improve motor function recovery, reduce BBB breakdown, and attenuate cerebral edema after head injury in rodents.28,30 However, use of CRAs has resulted in conflicting outcomes in clinical trials in severe head injury.32 In addition, therapeutic cannabinoid agonist administration may produce psychotropic effects through CB1 receptor activation and this has limited their widespread use.
An alternative approach to achieving cannabinoid-mediated neuroprotection is that of modulating EC degradation. ECs are synthesized in response to specific stimuli, including traumatic injury or inflammatory challenges, and their (2-AG and AEA) rapid degradation is mediated primarily by two enzymes, FAAH and MAGL.20,33–35 Thus, we hypothesized that decreased EC degradation would improve outcomes from TBI, without producing overt neuropsychological dysfunction, thereby presenting a unique pharmacological intervention for TBI.21 The aim of this study was to test the prediction that inhibition of enzymatic degradation of EC after TBI provides neuroprotection and, in turn, improves neurobehavioral outcomes as reflected in motor and cognitive function.
Methods
Animals
All animal procedures were approved by the institutional animal care and use committee of the Louisiana State University Health Sciences Center (LSUHSC; New Orleans, LA) and were in accord with the National Institute of Health (NIH) guidelines. Adult male Sprague-Dawley rats (Charles River, Raleigh, NC) weighing 250–275 g at the time of arrival were housed in the Division of Animal Care at LSUHSC and were exposed to a 12-h light/dark cycle and fed a standard rat diet (Purina Rat Chow; Ralston Purina, St. Louis, MO) ad libitum for 1 week before surgical procedures.
Surgical procedures
Animals were anesthetized (intramuscular injection of ketamine 90 mg/kg and xylazine 9 mg/kg) and positioned in a stereotaxic apparatus (model 900; Kopf Instruments, Tujunga, CA), and craniotomy (5.0 mm) was performed (2.0 mm posterior to bregma, 3.0 mm lateral from mid-line, over the left sensory motor cortex). Extreme care was taken to ensure that the dura matter was not penetrated. A female Luer Loc connector was positioned directly over the craniotomy and secured in place with cyanoacrylate glue. Once the glue was dry, dental cement (Lang Dental Manufacturing, Wheeling, IL) was applied around the female Luer Loc connector and surrounding exposed skull approximately 2 mm in thickness. The female Luer Loc was filled with sterile normal saline and capped. A subset of animals was also surgically implanted with a carotid catheter for BBB measurements. Briefly, using aseptic surgical procedures, catheters (PE50; BD Diagnostic Systems, Sparks, MD) were inserted into the left carotid artery, then advanced approximately 3 cm in length. Catheters were flushed with sterile saline, then sealed, and subcutaneously routed to the nape of the neck, where they were exteriorized through a small incision and secured with tape. After surgery, animals were placed in individual cages, allowed to recover from anesthesia, and given food and water ad libitum for 3 days before randomization to either sham or TBI groups.
Traumatic brain injury model
After recovery from surgery, animals were either subjected to TBI by lateral fluid percussion (LFP) or given no injury [shams]). The LFP model is the most extensively used and well-characterized model of nonpenetrating and nonischemic TBI and provides consistent, reproducible injury.36–39 Animals were anesthetized with isoflurane (4% induction, 3% maintenance) and positioned into a stereotaxic frame; the cranial female Luer Loc was connected to an LFP system by pressure tubing. LFP (pressure wave of ∼2 atm and 25-ms duration) was delivered to the dura. Animals were immediately monitored for signs of apnea after TBI, removed from the stereotaxic frame, and placed on their right side for observation of respiratory rate. Righting reflex was recorded as the time it took for the animal to regain complete consciousness and standing on all four limbs. Time-matched sham controls were anesthetized and connected to the LFP system, but not subjected to LFP. All animals were placed back into their individual home cage and continuously monitored for 2 h postinjury with free access to food and water.
Endocannabinoid modulation
Selective inhibitors of MAGL and FAAH, the enzymes responsible for 2-AG and AEA hydrolysis (JZL184 [16 mg/kg] and URB597 [0.3 mg/kg]) were dissolved in alcohol, emulphor, and saline (1:1:18) as vehicle. Either JZL184 (TBI/JZL) or URB597 (TBI/URB) was injected intraperitoneally at 30 min post-TBI. Time-matched controls received equal volumes (10 μL/kg body weight) of vehicle (TBI/VEH). Animals were studied during the acute postinjury period (2–24 h).
Neurological and neurobehavioral assessments
As previously described, neurological (neurological severity scores; NSS) and neurobehavioral (neurobehavioral scores; NBS) function was assessed at baseline (1 h before TBI) and at 2 and 24 h post-TBI, as previously described.40 All animals were exposed to all tasks, trained, and evaluated before TBI using the testing parameters that were adapted from previously published methods of assessing cognition and behavior.39,41 To reduce any impact on behavior assessments potentially caused by the process of relocation, they were transported to the test room 1 h in advance of the start of testing. NSS scores ranged from 0 to 25 and NBS scores ranged from 0 to12 and were based on the animal's ability to carry out each task. A score of 0 represented normal or pass, whereas higher scores correlated to the animal's severity of injury and NSS or NBS impairment. NSS evaluates motor function, sensory, reflexes, beam walking, and beam balancing. Pinna, corneal, startle, and righting reflexes were assessed, where one is no reflex and 0 indicates the reflex is intact. Beam walking assessed motor coordination, animals were placed on beams of decreasing width (10, 8, 5, and 2.5 cm) and allowed 60 min to traverse each beam. In addition, beam balance was assessed where animals were placed on a 1.5-cm-wide beam and given 60 sec to balance. Failure to walk all beams and/or balance for 60 sec resulted in increased NSS total. NBS tests sensorimotor, proprioception, exploratory behavior, and novel object exploration. Proprioception was assessed by pushing each animal laterally (lateral pulsion) on each side of its body. Each side was assessed and failure to resist lateral pulsion on one or both sides increased the NBS total. Exploratory behavior was assessed immediately after the animal's cage top was removed. Uninjured animals actively explore the top of the cage and surroundings.
Blood–brain barrier permeability
Integrity of the BBB was examined by dye tracer extravasation, as previously described.42,43 Animals received a 1-mL injection of a Ringer's lactate solution containing 2% Evans Blue (EB; Sigma-Aldrich, St. Louis, MO) into the carotid catheter. After 10–15 min, animals were deeply anesthetized with isoflurane and then transcardially perfused for 15 min with normal saline to remove dye from the vasculature. The brain was removed and flash frozen in liquid nitrogen, and brain regions (ipsi- and contralateral) were isolated, weighed, and stored at −80°C before homogenization and extraction. Tissue was homogenized in formamide, and EB was extracted from brain tissue by incubating in formamide (Sigma-Aldrich) at 37°C overnight. Samples were then centrifuged at 4000g at 4°C for 10 min. The concentration of EB was measured in the supernatant with a spectrophotometer at 620 nm. A linear standard curve of EB in formamide was used to calculate brain-tissue EB concentration (μg/mL EB) and was then normalized to tissue weight in grams. Data are expressed as μg/mL EB/g of brain tissue.
Tissue collection
After decapitation, the brain was removed from the skull, rapidly sprayed by Richard-Allan Scientific Cytocool II (Thermo Scientific, Waltham, MA), and dipped into liquid nitrogen for approximately 6–10 sec. Using a prefrozen standard adult rodent brain slicer matrix (Zivic Instruments, Pittsburgh, PA), the brain was positioned and cut using three razor blades pressed into slice channels at 4, 8, and 13 mm from the tip of the frontal cortex to excise out a 4-mm width of prefrontal cortex and a 5-mm width of the injured area. A fourth razor blade was pressed at mid-line to separate the ipsilateral (injured) from the contralateral (uninjured) region, and a 5-mm width of uninjured cortex was collected. Brain tissues were collected at 24 h post-TBI and stored at −80°C for further analyses.
Real-time quantitative polymerase chain reaction analysis
Messenger RNA (mRNA) expression of IL-1β, IL-6, chemokine (C-C motif) ligand 2 (CCL2), TNF-α, COX2, and gp91phox (nicotinamide adenine dinucleotide phosphate [NADPH] oxidase; NOX2) was measured 24 h post-TBI at the site of injury. Total RNA was extracted from brain tissue using an RNeasy Plus Universal Mini Kit (Qiagen, Valencia, CA), according to the manufacturer's instructions. Total RNA was reverse transcribed using the TaqMan Reverse Transcription Reagent kit (Life Technologies Corporation, Carlsbad, CA). Primer sequences (Integrated DNA Technologies, Coralville, IA) for IL-1β, IL-6, TNF-α, and CCL2 used in this study are listed in Table 1. COX2 and NOX2 primers were purchased from SA Biosciences (Valencia, CA), and these sequences remain proprietary. Primer concentrations used were 500 nM. The RT2 SYBR Green FAST Mastermix (Qiagen) was used for real-time polymerase chain reaction (PCR). All reactions were performed on a CFX96 system (Bio-Rad Laboratories, Hercules, CA). Quantitative reverse-transcriptase PCR data were analyzed using the ΔΔCT method. Target genes were compared with RPS13 and normalized to control values. RPS13 was chosen as the endogenous control to normalize gene expression because it was stably expressed based on a meta-analysis of 13,629 gene array samples.44
Table 1.
qRT-PCR Primer Sequences for IL-1β, IL-6, CCL2, TNF-α, and RSP13 (Housekeeping Gene)
| Target | Forward primer | Reverse primer |
|---|---|---|
| IL-1β | agcagctttcgacagtgaggagaa | tctccacagccacaatgagtgaca |
| IL-6 | aagccagagtcattcagagc | gtccttagccactccttctg |
| CCL2 | tgctgtctcagccagatgcagtta | tacagcttctttgggacacctgct |
| TNF-α | ccaacaaggaggagaagttcccaa | gagaagatgatctgagtgtgaggg |
| RSP13 | gacgtgaaggaacaaatttacaagttggcc | Gaatcacacctatctgggaaggagtca |
qRT-PCR, quantitative reverse-transcriptase polymerase chain reaction; IL, interleukin; CCL2, chemokine (C-C motif) ligand 2; TNF-α, tumor necrosis factor alpha; RSP13, ribosomal protein 13.
Western blot analysis
Fresh frozen brain tissue from the ipsilateral cortex was powdered and then homogenized in RIPA buffer containing 50 mM of Tris HCL (pH 8), 150 mM of NaCL, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS) with Halt protease, and phosphatase inhibitor cocktail (Pierce Thermo Scientific, Rockford, IL). Protein concentrations from brain tissue lysates were determined using a bicinchoninic acid assay (Bio-Rad). Equal amounts of protein (60 ug) from each sample were separated on 4–20% gradient SDS/polyacrylamide gel electrophoresis gels and then transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA). Membranes were then blocked, incubated with anti-COX2 (1:200; Abcam, Cambridge, MA), anti-NOX2 (1:1000; Abcam), and anti-β-actin (1:1000; Cell Signaling Technology, Beverly, MA) primary antibodies (Abs) overnight at 4°C, and then incubated with secondary Abs conjugated with horseradish peroxidase (1:2500; Cell Signaling technology) for 1 h at room temperature. Bands were visualized using Chemiluminescence Reagent Plus (PerkinElmer Life Science, Boston, MA), and densitometry was used to quantify protein expression using Carestream molecular imaging software (Carestream Health, Inc., Rochester, NY). β-actin was used as a loading control on all membranes.
Immunohistochemistry
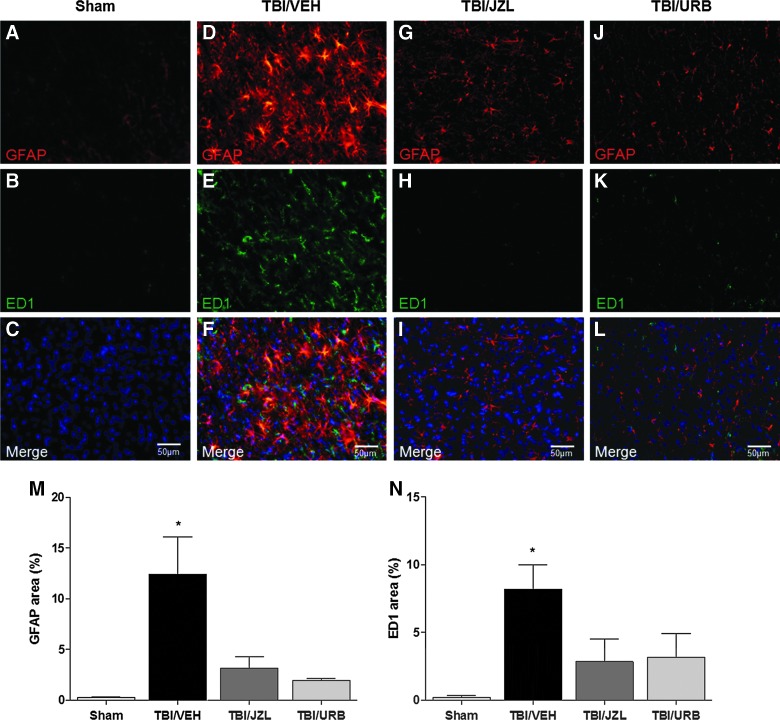
Perfusion-fixed brains were sliced at 35-μm thickness, and sections were mounted on glass slides before staining. Sections were permeabilized with 0.3% Triton-X 100 in phosphate-buffered saline (PBS) and blocked in 1% bovine serum albumin and 2% normal donkey serum for 1 h at room temperature. Sections were incubated with two primary Abs: rabbit anti-glial fibrillary acid protein (GFAP; 1:200; Abcam) and mouse anit-ED1 (1:200; Abcam) for 24 h at 4°C. Sections were then washed 3×5 min with PBS and incubated in a mixture of secondary Abs: Alexa Fluor 555 donkey anti-rabbit (1:200; by Life Technologies, Carlsbad, CA) and Alexa Fluor 488 donkey anti-mouse (1:200; by Life Technologies) for 2 h at room temperature. Slides were washed 3×5 min with PBS, dried, and cover-slipped using antifade mounting media with 4′,6-diamidino-2-phenylindole (ProLong Gold; Life Technologies). Sections were observed under a Nikon Eclipse TE2000-U (Nikon, Tokyo, Japan), and images were captured using NIS Elements (Version 3.22.11; Nikon). Images were then quantified using ImageJ software (NIH, Bethesda, MD) at 40× magnification. Values are expressed as percent area of positive staining. At least two pictures were taken of three sections, for a total of six to nine pictures per animal, and 3 animals were analyzed per group.
Statistical analysis
All data are expressed as mean±standard error of the mean (SEM) with the number of animals per group indicated in the figure legends. Statistical analysis of differences in outcome measures was determined by one-way analysis of variance (ANOVA) and two-way ANOVA with repeated measures using GraphPad Prism 5.0 statistical software (Graphpad Software Inc., La Jolla, CA). Pair-wise multiple comparisons were determined using Tukey's test for one-way ANOVA and Bonferroni's test for two-way ANOVA. Specific tests used for analysis are stated in the table (Tables 1 and 2) and figure legends. Statistical significance was set at p<0.05.
Table 2.
Body Temperature Measured in Pre-TBI and 2 and 24 h Post-TBI
| Body temperature (°C) | |||
|---|---|---|---|
| Treatment | Pre-TBI | 2 h | 24 h |
| SHAM | 37.64±0.16 | 37.51±0.13 | 37.49±0.13 |
| TBI/VEH | 37.41±0.11 | 37.48±0.16 | 37.21±0.23 |
| TBI/JZL | 37.40±0.11 | 37.28±0.3 | 36.94±0.38 |
| TBI/URB | 37.23±0.22 | 37.51±0.17 | 37.76±0.61 |
Data are presented as total mean score±SEM (SHAM, n=18; TBI/VEH, n=20; TBI/JZL, n=18; TBI/URB, n=19).
TBI, traumatic brain injury.
Results
Impact of traumatic brain injury on apnea, righting reflex, and respiratory rate
TBI produced significant apnea (17±5 sec; p<0.05) and a delayed righting reflex (479±21 sec; p<0.05). Respiratory rate was significantly reduced immediately following TBI (61±2 breaths/min; p<0.05), when compared to shams (73±2 breaths/min).
Impact of inhibition of endocannabinoid degradation on neurological severity and neurobehavioral scores after traumatic brain injury
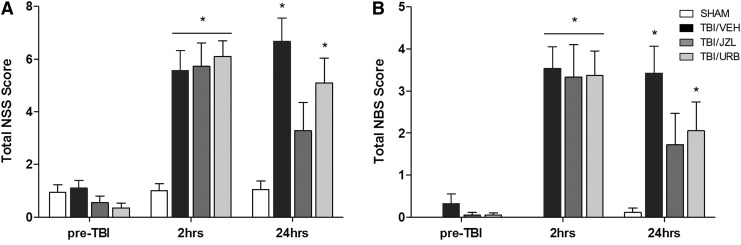
Animals' performance on NSS (Fig. 1A) and NBS (Fig. 1B) was tested pre-TBI and 2 and 24 h post-TBI. There was a significant main effect of treatment (two-way ANOVA: F(3,71)=6.68; p=0.0005) and time (two-away ANOVA: F(2,142)=60.33; p<0.0001) and a significant interaction (two-way ANOVA: F(6,142)=8.62; p<0.0001), as indicated by a marked increase in the NSS at 2 h post-TBI (TBI/VEH: 5.6±0.8, p<0.01; TBI/JZL: 5.8±0.9, p<0.01; TBI/URB: 6.1±0.6, p<0.01), when compared to shams. NSS scores remained significantly elevated in TBI/VEH (6.7±1.2; p<0.01) and TBI/URB (5.1±0.9; p<0.01) animals at 24 h post-TBI, when compared to shams. Animals treated with JZL184 had reduced NSS at 24 h (3.3±1.1). The NBS was also increased in TBI animals 2 h post-TBI (TBI/VEH, 3.5±0.6; TBI/JZL, 3.3±0.8; TBI/URB, 3.4±0.6), when compared to shams, and revealed a significant main effect of treatment (two-way ANOVA: F(3,71)=7.70; p=0.0002) and time (two-way ANOVA: F(2,142)=36.54; p<0.0001) and a significant interaction (two-way ANOVA: F(6,142)=4.69; p=0.0002).
FIG. 1.
Neurological severity scores (NSS) (A) and neurobehavioral scores (NBS) (B) assessed pre-TBI and 2 and 24 h post-TBI. Endocannabinoid degradation inhibitors, JZL184 and URB597, were given intraperitoneally 30 min post-TBI. Data are presented as total mean score±SEM (SHAM, n=18; TBI/VEH, n=20; TBI/JZL, n=18; TBI/URB, n=19) and were analyzed using two-way ANOVA with repeated measures. *p<0.05 versus time-matched shams. TBI, traumatic brain injury.
Protection of blood–brain barrier integrity with inhibition of endocannabinoid degradation
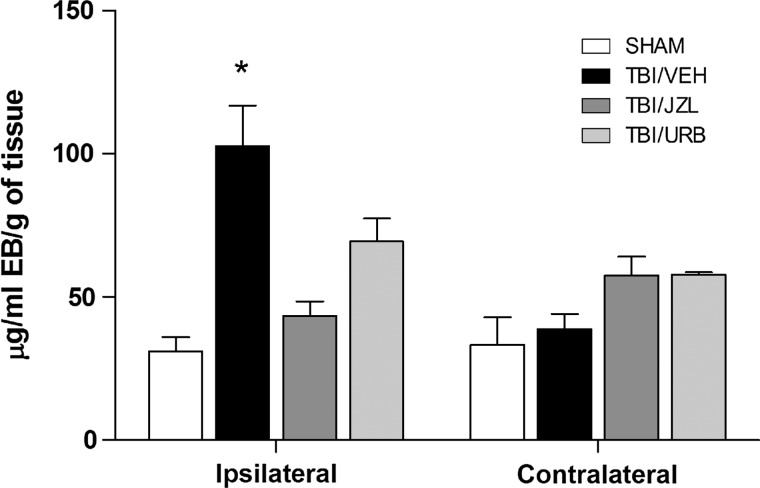
TBI (TBI/VEH) significantly disrupted BBB integrity, as reflected by the increased leak (103±14; p<0.05), when compared to shams (31±5; one-way ANOVA: F(3,36)=6.98; p=0.0008) at 24 h post-TBI (Fig. 2). Treatment with either JZL184 or URB597, 30 min post-TBI, was effective at minimizing BBB dysfunction (TBI/JZL: 43±5, not significant [NS]; TBI/URB: 69±8, NS) at 24 h (Fig. 2). BBB integrity remained intact on the contralateral side in the sham and TBI/VEH groups, and treatment with either JZL184 or URB597 did not affect BBB integrity on the contralateral brain region (Fig. 2).
FIG. 2.
Blood–brain barrier integrity was assessed in ipsilateral (injured) and contralateral (uninjured) brain regions at 24 h post-TBI. Endocannabinoid degradation inhibitors, JZL184 and URB597, were given intraperitoneally 30 min post-TBI. Data are presented as mean±SEM (SHAM, n=9; TBI/VEH, n=18; TBI/JZL, n=7; TBI/URB, n=6) and were analyzed using a one-way ANOVA. *p<0.05 versus time-matched shams. EB, Evans Blue; TBI, traumatic brain injury.
Effects of inhibiting endocannabinoid degradation on cytokine messenger RNA expression
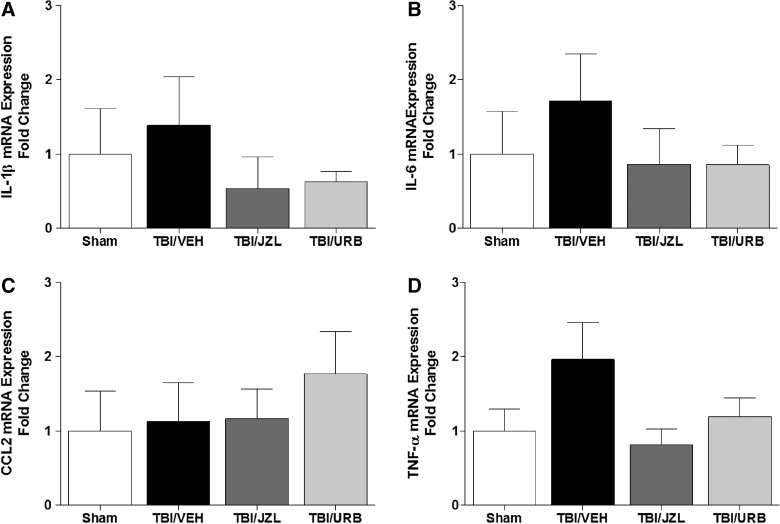
mRNA expression of IL-1β, IL-6, CCL2, and TNF-α was measured at 24 h post-TBI (Fig. 3A–D). No statistically significant differences were detected at 24 h post-TBI in mRNA expression of any of the measured cytokines (one-way ANOVA: IL-1β, F(3,22)=0.51, NS; IL-6, F(3,22)=0.62, NS; CCL2, F(3,22)=0.39, NS; TNF-α, F(3,22)=1.80, NS).
FIG. 3.
Brain tissue from the site of injury was analyzed for mRNA expression of interleukin (IL)-6 (A), IL-1β (B), CCL2 (C), and TNF-α (D) by quantitative reverse-transcriptase polymerase chain reaction 24 h post-TBI. Endocannabinoid degradation inhibitors, JZL184 and URB597, were given intraperitoneally 30 min post-TBI. Data are presented as mean±SEM (SHAM, n=6; TBI/VEH, n=9; TBI/JZL, n=5; TBI/URB, n=6) and were analyzed by one-way ANOVA. IL, interleukin; mRNA, messenger RNA; CCL2, chemokine (C-C motif) ligand 2; TNF-α, tumor necrosis factor alpha; TBI, traumatic brain injury.
Effects of inhibiting endocannabinoid degradation on oxidative-stress–related protein and messenger RNA expression
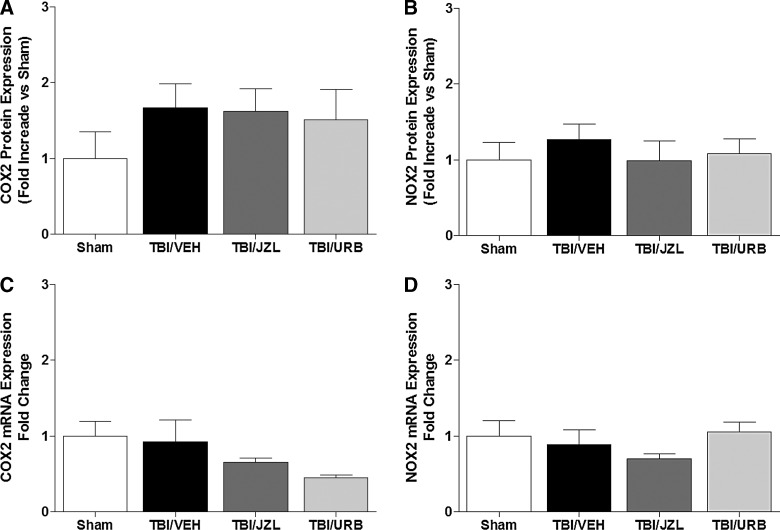
Protein and mRNA expression of COX2 and NOX2 were measured at 24 h post-TBI (Fig. 4A–D). No significant differences in protein (one-way ANOVA: COX2, F(3,12)=0.79, NS; NOX2, F(3,12)=0.33, NS) and mRNA (one-way ANOVA: COX2, F(3,24)=2.56, NS; NOX2, F(3,24)=1.56, NS) expression of COX2 and NOX2 were detected in any of the experimental groups.
FIG. 4.
Brain tissue from the site of injury was analyzed for protein and mRNA expression of COX2 (A and C) and NOX2 (B and D) by quantitative reverse-transcriptase polymerase chain reaction 24 h post-TBI. Endocannabinoid degradation inhibitors, JZL184 and URB597, were given intraperitoneally 30 min post-TBI. Data are presented as mean±SEM (protein, n=4/group; mRNA, SHAM, n=6; TBI/VEH, n=9; TBI/JZL, n=5; TBI/URB, n=6) and were analyzed by one-way ANOVA. COX2, cyclooxygenase 2; NOX2, nicotinamide adenine dinucleotide phosphate oxidase; mRNA, messenger RNA; TBI, traumatic brain injury.
Inhibiting endocannabinoid degradation increased astrocyte activation
Brain slices were double-stained with GFAP and ED1 to determine protein expression levels 24 h post-TBI (Fig. 5A–N). Astrocyte-specific GFAP immunoreactivity post-TBI was significantly increased (12.4±3.7; p<0.05) above sham levels (0.3±0.1; one-way ANOVA: F(3,8)=7.82; p=0.009), indicating increased astrocyte activation, and treatment with either JZL184 (3.2±1.1) or URB597 (1.9±0.2) inhibited the increased astrocyte activation observed in brains from TBI/VEH animals (Fig. 5M). In addition to activation, TBI alone induced visible astrocyte hypertrophy, as compared to sham, TBI/JZL, or TBI/URB animals (Fig. 5A,D,G,J). Expression of ED1, a marker of microglial activation, was significantly increased in TBI/VEH animals (8.2±1.8; p<0.05), when compared to shams (0.2±1.4; one-way ANOVA: F(3,8)=4.87; p=0.03), and this activation was abolished in both TBI/JZL (2.8±1.7) and TBI/URB (3.2±1.7) animals (Fig. 5B,E,H,K,N).
FIG. 5.
Perfusion-fixed brains at 24 h post-TBI were double-stained with GFAP and ED1 and counterstained with 4′,6-diamidino-2-phenylindole for sham (A–C), TBI/VEH (D–F), TBI/JZL (G–I), and TBI/URB (J–L). Endocannabinoid degradation inhibitors, JZL184 and URB597, were given intraperitoneally 30 min post-TBI. All images were captured at 40× magnification. Data are summarized as positive staining area percent for GFAP (M) and ED1 (N). Data are presented as mean±SEM (n=3/group) and were analyzed by one-way ANOVA. *p<0.05 versus time-matched shams. GFAP, glial fibrillary acidic protein; TBI, traumatic brain injury. Color image is available online at www.liebertpub.com/neu
Discussion
The present study examined the effects of EC degradation inhibition on TBI outcomes. Previous studies have demonstrated potential neuroprotection with exogenous administration of ECs.21,45 Therefore, we hypothesized that inhibiting the degradation of endogenously released ECs, 2-AG, or AEA with JZL184 or URB597, respectively, would extend EC signaling, provide neuroprotection, and improve outcomes after TBI. Our results demonstrate that inhibition of MAGL with JZL184 (30 min post-TBI) improved neurological and -behavioral function at 24 h, whereas inhibition of FAAH with UBR597 did not improve neurological and -behavioral function at 24 h. In addition, TBI-induced BBB dysfunction was reduced with inhibition of both MAGL and FAAH. mRNA expression of proinflammation cytokines IL-1β, IL-6, CCL2, and TNF-α were unchanged across groups at 24 h post-TBI. In addition, oxidative-related protein and mRNA expression of COX2 and NOX2 were unchanged. Finally, increased expression of GFAP, a marker of astrocyte activation, was observed in TBI/VEH that was reversed with both JZL184 and URB597 treatment. These results suggest that the selective EC degradation inhibitor, JZL184, is extremely effective at improving neurological and -behavioral impairment as well as protecting BBB integrity post-TBI, which is associated with increased astrocyte activation.
The neuroprotective effects of cannabinoid agonists and synthetic cannabinoids have been reported by several investigators.28–30 Mauler and colleagues demonstrated, with BAY 38-7271, a CRA, neuroprotection in a rat model of TBI.29 In addition, cannabinoid receptor 2 agonist, 0-1966, significantly reduced brain edema and improved locomotor performance in mice when given after cortical contusion impact injury. Moreover, treatment using a synthetic cannabinoid, HU-211, improved motor function recovery, which was accompanied by reduced BBB dysfunction and edema, in a model of closed head injury in rats.28 Interestingly, these experimental findings were confirmed in one clinical trial using HU-211 for treatment after severe closed head injury, but these improvements were not evident in another clinical trial using HU-211 for treatment after TBI.32,46 Although some experimental studies have shown neuroprotection, exogenous EC administration can impair learning and executive function as a result of their psychoactive effects and the CB1-dependent behavioral alterations (hypomotility, analgesia, and catalepsy).47 Alternatively, use of selective EC degradation inhibitors could avoid these behavioral alterations while increasing the effective time of endogenously released ECs in response to neuronal injury and promoting an intrinsic neuroprotective response through an activated EC system.24 In these studies, the model of TBI induced by LFP produced immediate apnea and a delayed righting reflex. In addition, TBI significantly impaired somatomotor and cognitive function (NSS) and neurobehavior (NBS) as early as 2 h post-TBI and this impairment persisted to 24 h. These deficits were reversed by post-TBI treatment with JZL184 and partially improved with URB597. Notably, JZL184 was most effective at improving somatomotor and cognitive function as well as improving neurobehavior.
TBI-induced brain damage results from direct and indirect events. The direct (immediate or primary) injury results from mechanical disruption of brain tissue. This is followed by an acute inflammatory response, breakdown of the BBB, edema formation, and swelling. Our findings show that inhibiting EC degradation with either JZL184 or URB597 was effective at protecting the BBB integrity by attenuating EB extravasation in the area of injury (ipsilateral) after TBI at 24 h. These findings are consistent with previous studies using exogenous administration of 2-AG after closed head injury and ischemia.21,45,48
After the BBB breakdown, infiltration of peripheral blood cells with activation of immunocompetent cells leads to intrathecal release of numerous immune mediators, such as cytokines and chemokines. The neuroinflammatory cascade characterized by activation of astrocytes and microglia, increased production of immune mediators, along with excitotoxic and oxidative responses, has been proposed as the principal underlying mechanisms of cell injury and has been confirmed by postmortem pathology in experimental and clinical studies.17,49 Although the early inflammatory response plays an important role in recovery from injury, its sustained duration contributes both to the acute pathological processes after TBI, including cerebral edema, and the longer-term neuronal damage and cognitive impairment.9,50 Inhibition of proinflammatory responses has been identified as a potential mechanism responsible for the improved outcome.21 Thus, timely modulation of neuroinflammation becomes critical in not interfering with the reparative contribution of activated glia. The current results were unable to detect any significant increase in mRNA expression of IL-6, IL-1β, CCL2, or TNF-α in TBI animals, when compared to time-matched shams at 24 h post-TBI. We believe this is because of the time point measured given that our previous studies have demonstrated increased IL-6 and CCL2 at 6 h post-TBI, which are not significantly different from sham values at 24 h.40 Earlier time points may reveal an increase in cytokine mRNA expression, which is no longer detectable at 24 h, and is therefore a limitation of these studies. Though these results are in contrast with more-robust and sustained neuroinflammatory changes after TBI, it is possible that differences in the severity of the injury, or time frame when we performed our measures, can explain these differences. In addition to the proinflammatory cytokines, we examined two key OS-related factors, COX2 and NOX2, at the level of protein and mRNA expression.
Cyclooxygenase and NADPH oxidases are two primary generators of ROS, and increased OS has been proposed to be a mediator of TBI-associated tissue injury.51–53 Classically, COX2 is an inducible enzyme involved in the formation of prostaglandins from arachidonic acid, which are potent mediators of cytotoxic inflammation during pathological conditions and is the predominant COX isoform found in inflammatory cells and the brain.54 Previous studies have demonstrated an increased expression of brain COX2 post-TBI, and administration of COX2 inhibitors has been shown to improve neurological reflexes and memory, as well as reduce inflammation after lateral cortical concussion in rats.55,56 In this study, we did not observe any changes in COX2 expression at 24 h post-TBI. Similar to the lack of significant increase in inflammatory cytokine expression at this time point, it is possible that we did not observe significant up-regulation in COX2 expression owing to a more modest injury severity in our studies, compared to that used in other studies.55 NOX2 is one isoform of the catalytic subunit of NADPH oxidase enzyme, and NOX2 deficiency in mice has been shown to be protective in ischemic stroke.57 The role NOX2 plays in TBI has not been fully explored, but a recent study using gp91phox−/− mice showed reduced ROS production and contusion area, when compared to wild-type mice.58 Although we did not observe increases in either NOX2 protein or mRNA expression at 24 h post-TBI, we believe this may be owing to species difference and/or injury severity.
Resident glial cells, including astrocytes and microglia in the brain, have been shown to play a role in the inflammatory process after injury.59 Reactive astrogliosis involves hypertrophy and proliferation of astrocytes, which alters morphology, and expression of a structural protein, GFAP.60 Following injury, astrocytes are activated, which induces increased GFAP expression along with cell projection hypertrophy.61 In addition to activation of astrocytes, microglia can be activated and migrate to the area of injury. Activation of microglia involves altered cell morphology, whereby microglia transform from a ramified shape to an amoeboid shape and concomitantly increase expression of ED1, a specific marker on lysosomal membranes. The increased expression of ED1 in microglia serves as a marker of activation. Our results showed increased expression of both GFAP and ED1 in the ipsilateral cortex of TBI/VEH animals, indicating activated astrocytes and microglia, respectively. Treatment with either JZL184 or URB597 was effective at reversing astrocyte activation, which may contribute to maintenance of the BBB and improved neurological and -behavioral recovery in this study. Cannabinoid receptors are present on both astrocytes and microglia, which may explain the lack of astrocyte and microglia activation in both treatment groups.62,63 The role astrocyte and microglia activation play in BBB dysfunction is not fully understood, but some evidence indicates inflammatory mediators release by activated astrocytes modulate cell migration through the BBB.64
Taken together, the results from the present study suggest that inhibition of EC degradation, primarily by JZL184, is effective in attenuating BBB dysfunction and neurological and -behavioral impairments, which are associated with increased astrocyte and microglia activation post-TBI. These findings provide additional support for the potential therapeutic benefit of EC modulation with the use of selective inhibitors of EC degradation after TBI. Whether the protective effects are sustained or improved with additional drug dosing after the initial 24-h post-TBI remains to be examined.
Acknowledgments
The authors thank Drs. Nicole LeCapitaine and Robert Siggins for their scientific guidance, Jane Schexnayder for her technical assistance, as well as Rebecca Gonzales for her editorial support. This work was supported by the Department of Defense (DOD-W81XWH-11-2-0011) and the National Institute on Alcohol Abuse and Alcoholism (NIAAA-007577 and NIAAA-19587).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Warden D. (2006). Military TBI during the Iraq and Afghanistan wars. J. Head Trauma Rehabil. 21, 398–402 [DOI] [PubMed] [Google Scholar]
- 2.Terrio H., Brenner L.A., Ivins B.J., Cho J.M., Helmick K., Schwab K., Scally K., Bretthauer R., and Warden D. (2009). Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J. Head Trauma Rehabil. 24, 14–23 [DOI] [PubMed] [Google Scholar]
- 3.Mayorga M.A. (1997). The pathology of primary blast overpressure injury. Toxicology 121, 17–28 [DOI] [PubMed] [Google Scholar]
- 4.McKee A.C., Cantu R.C., Nowinski C.J., Hedley-Whyte E., Gavett B.E., Budson A.E., Santini V.E., Lee H.S., Kubilus C.A., and Stern R.A. (2009). PMC2945234; chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 68, 709–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faul M., Xu L., Wald M.M., and Coronado V.G. (2010). Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths, 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA [Google Scholar]
- 6.Elder G.A., and Cristian A. (2009). Blast-related mild traumatic brain injury: mechanisms of injury and impact on clinical care. Mt. Sinai J. Med. 76, 111–118 [DOI] [PubMed] [Google Scholar]
- 7.Okie S. (2005). Traumatic brain injury in the war zone. N. Engl. J. Med. 352, 2043–2047 [DOI] [PubMed] [Google Scholar]
- 8.Riggio S. (2011). Traumatic brain injury and its neurobehavioral sequelae. Neurol. Clin. 29, 35–47, vii [DOI] [PubMed] [Google Scholar]
- 9.Lenzlinger P.M., Morganti-Kossmann M., Laurer H.L., and McIntosh T.K. (2001). The duality of the inflammatory response to traumatic brain injury. Mol. Neurobiol. 24, 169–181 [DOI] [PubMed] [Google Scholar]
- 10.Werner C., and Engelhard K. (2007). Pathophysiology of traumatic brain injury. Br. J. Anaesth. 99, 4–9 [DOI] [PubMed] [Google Scholar]
- 11.Schmidt O.I., Heyde C.E., Ertel W., and Stahel P.F. (2005). Closed head injury—an inflammatory disease? Brain Res. Brain Res. Rev. 48, 388–399 [DOI] [PubMed] [Google Scholar]
- 12.Chen Y., Constantini S., Trembovler V., Weinstock M., and Shohami E. (1996). An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J. Neurotrauma 13, 557–568 [DOI] [PubMed] [Google Scholar]
- 13.Lloyd E., Somera-Molina K., Van Eldik L.J., Watterson D.M., and Wainwright M.S. (2008). PMC2483713; suppression of acute proinflammatory cytokine and chemokine upregulation by post-injury administration of a novel small molecule improves long-term neurologic outcome in a mouse model of traumatic brain injury. J. Neuroinflammation 5, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain K.K. (2008). Neuroprotection in traumatic brain injury. Drug Discov. Today 13, 1082–1089 [DOI] [PubMed] [Google Scholar]
- 15.Loane D.J., and Faden A.I. (2010). Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol. Sci. 31, 596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cernak I., and Noble-Haeusslein L.J. (2010). Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J. Cereb. Blood Flow Metab. 30, 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitkänen A., Longhi L., Marklund N., Morales D.M., and McIntosh T.K. (2005). Neurodegeneration and neuroprotective strategies after traumatic brain injury. Drug Discov. Today Dis. Mech. 2, 409–418 [Google Scholar]
- 18.Di Marzo V., Bifulco M., and De Petrocellis L. (2004). The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 3, 771–784 [DOI] [PubMed] [Google Scholar]
- 19.Mackie K., and Stella N. (2006). Cannabinoid receptors and endocannabinoids: evidence for new players. AAPS J. 8, E298–E306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mechoulam R., Panikashvili D., and Shohami E. (2002). Cannabinoids and brain injury: therapeutic implications. Trends Mol. Med. 8, 58–61 [DOI] [PubMed] [Google Scholar]
- 21.Panikashvili D., Shein N.A., Mechoulam R., Trembovler V., Kohen R., Alexandrovich A., and Shohami E. (2006). The endocannabinoid 2-AG protects the blood-brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol. Dis. 22, 257–264 [DOI] [PubMed] [Google Scholar]
- 22.Wilson R.I., and Nicoll R.A. (2002). Endocannabinoid signaling in the brain. Science 296, 678–682 [DOI] [PubMed] [Google Scholar]
- 23.Savinainen J.R., Jarvinen T., Laine K., and Laitinen J.T. (2001). Despite substantial degradation, 2-arachidonoylglycerol is a potent full efficacy agonist mediating CB(1) receptor-dependent G-protein activation in rat cerebellar membranes. Br. J. Pharmacol. 134, 664–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang J., Adamson C., Butler D., Janero D.R., Makriyannis A., and Bahr B.A. (2010). Enhancement of endocannabinoid signaling by fatty acid amide hydrolase inhibition: a neuroprotective therapeutic modality. Life Sci. 86, 615–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu M., Ives D., and Ramesha C.S. (1997). Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J. Biol. Chem. 272, 21181–21186 [DOI] [PubMed] [Google Scholar]
- 26.Kozak K.R., Rowlinson S.W., and Marnett L.J. (2000). Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J. Biol. Chem. 275, 33744–33749 [DOI] [PubMed] [Google Scholar]
- 27.Kozak K.R., Prusakiewicz J.J., and Marnett L.J. (2004). Oxidative metabolism of endocannabinoids by COX-2. Curr. Pharm. Des. 10, 659–667 [DOI] [PubMed] [Google Scholar]
- 28.Nadler V., Biegon A., Beit-Yannai E., Adamchik J., and Shohami E. (1995). 45Ca accumulation in rat brain after closed head injury; attenuation by the novel neuroprotective agent HU-211. Brain Res. 685, 1–11 [DOI] [PubMed] [Google Scholar]
- 29.Mauler F., Horvath E., De Vry J., Jager R., Schwarz T., Sandmann S., Weinz C., Heinig R., and Bottcher M. (2003). BAY 38-7271: a novel highly selective and highly potent cannabinoid receptor agonist for the treatment of traumatic brain injury. CNS Drug Rev. 9, 343–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biegon A. (2004). Cannabinoids as neuroprotective agents in traumatic brain injury. Curr. Pharm. Des. 10, 2177–2183 [DOI] [PubMed] [Google Scholar]
- 31.Tchantchou F., and Zhang Y. (2013). Selective inhibition of alpha/beta-hydrolase domain 6 attenuates neurodegeneration, alleviates blood brain barrier breakdown and improves functional recovery in a mouse model of traumatic brain injury. J. Neurotrauma 30, 565–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knoller N., Levi L., Shoshan I., Reichenthal E., Razon N., Rappaport Z.H., and Biegon A. (2002). Dexanabinol (HU-211) in the treatment of severe closed head injury: a randomized, placebo-controlled, phase II clinical trial. Crit. Care Med. 30, 548–554 [DOI] [PubMed] [Google Scholar]
- 33.Shohami E., Cohen-Yeshurun A., Magid L., Algali M., and Mechoulam R. (2011). Endocannabinoids and traumatic brain injury. Br. J. Pharmacol. 163, 1402–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Correa F., Mestre L., Molina-Holgado E., Arevalo-Martin A., Docagne F., Romero E., Molina-Holgado F., Borrell J., and Guaza C. (2005). The role of cannabinoid system on immune modulation: therapeutic implications on CNS inflammation. Mini Rev. Med. Chem. 5, 671–675 [DOI] [PubMed] [Google Scholar]
- 35.Downer E.J. (2011). Cannabinoids and innate immunity: taking a toll on neuroinflammation. ScientificWorldJournal 11, 855–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dixon C.E., Lyeth B.G., Povlishock J.T., Findling R.L., Hamm R.J., Marmarou A., Young H.F., and Hayes R.L. (1987). A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 67, 110–119 [DOI] [PubMed] [Google Scholar]
- 37.McIntosh T.K., Vink R., Noble L., Yamakami I., Fernyak S., Soares H., and Faden A.L. (1989). Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28, 233–244 [DOI] [PubMed] [Google Scholar]
- 38.Thompson H.J., Lifshitz J., Marklund N., Grady M.S., Graham D.I., Hovda D.A., and McIntosh T.K. (2005). Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma 22, 42–75 [DOI] [PubMed] [Google Scholar]
- 39.Ling G.S.F., Lee E.Y., and Kalehua A.N. (2004). Traumatic brain injury in the rat using the fluid-percussion model. Curr. Protoc. Neurosci. Chapter 9, Unit 9.2 [DOI] [PubMed] [Google Scholar]
- 40.Teng S.X., and Molina P.E. (2014). Acute alcohol intoxication prolongs neuroinflammation without exacerbating neurobehavioral dysfunction following mild traumatic brain injury. J. Neurotrauma 31, 378–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyko M., Ohayon S., Goldsmith T., Novack L., Novack V., Perry Z.H., Gruenbaum B.F., Gruenbaum S.E., Steiner O., Shapira Y., Teichberg V.I., and Zlotnik A. (2011). Morphological and neuro-behavioral parallels in the rat model of stroke. Behav. Brain Res. 223, 17–23 [DOI] [PubMed] [Google Scholar]
- 42.Euser A.G., Bullinger L., and Cipolla M.J. (2008). Magnesium sulphate treatment decreases blood-brain barrier permeability during acute hypertension in pregnant rats. Exp. Physiol. 93, 254–261 [DOI] [PubMed] [Google Scholar]
- 43.Xu Q., Qaum T., and Adamis A.P. (2001). Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest. Ophthalmol. Vis. Sci. 42, 789–794 [PubMed] [Google Scholar]
- 44.de Jonge H.J., Fehrmann R.S., de Bont E.S., Hofstra R.M., Gerbens F., Kamps W.A., de Vries E.G., van der Zee A.G., te Meerman G.J., and ter Elst A. (2007). Evidence based selection of housekeeping genes. PLoS One 2, e898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panikashvili D., Simeonidou C., Ben-Shabat S., Hanus L., Breuer A., Mechoulam R., and Shohami E. (2001). An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature 413, 527–531 [DOI] [PubMed] [Google Scholar]
- 46.Maas A.I., Murray G., Henney H., Kassem N., Legrand V., Mangelus M., Muizelaar J., Stocchetti N., and Knoller N. (2006). Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. 5, 38–45 [DOI] [PubMed] [Google Scholar]
- 47.Cravatt B.F., Demarest K., Patricelli M.P., Bracey M.H., Giang D.K., Martin B.R., and Lichtman A.H. (2001). Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. U. S. A. 98, 9371–9376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagayama T., Sinor A.D., Simon R.P., Chen J., Graham S.H., Jin K., and Greenberg D.A. (1999). Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J. Neurosci. 19, 2987–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Povlishock J.T. (1992). Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathol. 2, 1–12 [PubMed] [Google Scholar]
- 50.Lloyd E., Somera-Molina K., Van Eldik L.J., Watterson D.M., and Wainwright M.S. (2008). Suppression of acute proinflammatory cytokine and chemokine upregulation by post-injury administration of a novel small molecule improves long-term neurologic outcome in a mouse model of traumatic brain injury. J. Neuroinflammation 5, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewen A., Matz P., and Chan P.H. (2000). Free radical pathways in CNS injury. J. Neurotrauma 17, 871–890 [DOI] [PubMed] [Google Scholar]
- 52.Tyurin V.A., Tyurina Y.Y., Borisenko G.G., Sokolova T.V., Ritov V.B., Quinn P.J., Rose M., Kochanek P., Graham S.H., and Kagan V.E. (2000). Oxidative stress following traumatic brain injury in rats: quantitation of biomarkers and detection of free radical intermediates. J. Neurochem. 75, 2178–2189 [DOI] [PubMed] [Google Scholar]
- 53.Pratico D., Reiss P., Tang L.X., Sung S., Rokach J., and McIntosh T.K. (2002). Local and systemic increase in lipid peroxidation after moderate experimental traumatic brain injury. J. Neurochem. 80, 894–898 [DOI] [PubMed] [Google Scholar]
- 54.Dubois R.N., Abramson S.B., Crofford L., Gupta R.A., Simon L.S., Van De Putte L.B., and Lipsky P.E. (1998). Cyclooxygenase in biology and disease. FASEB J. 12, 1063–1073 [PubMed] [Google Scholar]
- 55.Strauss K.I., Barbe M.F., Marshall R.M., Raghupathi R., Mehta S., and Narayan R.K. (2000). Prolonged cyclooxygenase-2 induction in neurons and glia following traumatic brain injury in the rat. J. Neurotrauma 17, 695–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gopez J.J., Yue H., Vasudevan R., Malik A.S., Fogelsanger L.N., Lewis S., Panikashvili D., Shohami E., Jansen S.A., Narayan R.K., and Strauss K.I. (2005). Cyclooxygenase-2-specific inhibitor improves functional outcomes, provides neuroprotection, and reduces inflammation in a rat model of traumatic brain injury. Neurosurgery 56, 590–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Z., Wei X., Liu K., Zhang X., Yang F., Zhang H., He Y., Zhu T., Li F., Shi W., Zhang Y., Xu H., Liu J., and Yi F. (2013). NOX2 deficiency ameliorates cerebral injury through reduction of complexin II-mediated glutamate excitotoxicity in experimental stroke. Free Radic. Biol. Med. 65, 942–951 [DOI] [PubMed] [Google Scholar]
- 58.Dohi K., Ohtaki H., Nakamachi T., Yofu S., Satoh K., Miyamoto K., Song D., Tsunawaki S., Shioda S., and Aruga T. (2010). Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J. Neuroinflammation 7, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ridet J.L., Malhotra S.K., Privat A., and Gage F.H. (1997). Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 20, 570–577 [DOI] [PubMed] [Google Scholar]
- 60.Pekny M., and Nilsson M. (2005). Astrocyte activation and reactive gliosis. Glia 50, 427–434 [DOI] [PubMed] [Google Scholar]
- 61.Eng L.F., Ghirnikar R.S., and Lee Y.L. (2000). Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem. Res. 25, 1439–1451 [DOI] [PubMed] [Google Scholar]
- 62.Stella N. (2009). Endocannabinoid signaling in microglial cells. Neuropharmacology 56, Suppl 1, 244–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stella N. (2010). Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 58, 1017–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiss J.M., Downie S.A., Lyman W.D., and Berman J.W. (1998). Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood-brain barrier. J. Immunol. 161, 6896–6903 [PubMed] [Google Scholar]