Abstract
Most HIV-1 replication occurs in secondary lymphoid tissues in T cells within B cell follicles. Mechanisms underlying the accumulation of HIV-1-producing cells at these sites are not understood. Antiapoptotic proteins such as Bcl-2 could promote follicular CD4+ T cell survival, contributing to sustained virus production. Tonsils obtained from subjects without known HIV infection were disaggregated and analyzed for Bcl-2 expression in follicular (CXCR5+) and extrafollicular (CXCR5−) CD3+CD4+ cells by flow cytometry. Additional tonsil cells were cultured with phytohemagglutinin (PHA) and interleukin-2 (IL-2) for 2 days, infected with either CCR5(R5) or CXCR4-tropic (X4) GFP reporter viruses, and analyzed for Bcl-2 expression. In freshly disaggregated CD3+CD4+ tonsil cells, mean florescence intensity (MFI) for Bcl-2 was higher in CXCR5+ (median, 292) compared to CXCR5− cells (median, 194; p=0.001). Following in vitro stimulation with PHA and IL-2, Bcl-2 MFI was higher in both CXCR5+ cells (median, 757; p=0.03) and CXCR5− cells (median, 884; p=0.002) in uninfected cultures compared to freshly isolated tonsil cells. Bcl-2 MFI was higher in GFP+CD3+CD8− R5-producing cells (median, 554) than in X4-producing cells (median, 393; p=0.02). Bcl-2 MFI was higher in R5-producing CXCR5+ cells (median, 840) compared to all other subsets including R5-producing CXCR5− cells (median, 524; p=0.04), X4-producing CXCR5+ cells (median, 401; p=0.02), and X4-producing CXCR5− cells (median, 332; p=0.008). Bcl-2 expression is elevated in R5 HIV-1-producing CXCR5+ T cells in vitro, which may contribute to propagation of R5 virus in B cell follicles in vivo.
Introduction
In the absence of antiretroviral therapy, HIV-1 replication persists and is largely concentrated in T cells located in secondary lymphoid tissues.1–4 While the majority of CD4+ T cells are found within the T cell zones (extrafollicular areas) of lymphoid tissues, most HIV-1-producing cells are CD4+ T cells located within B cell follicles in early and intermediate stages of HIV disease.5–9 In untreated HIV-1-infected individuals without AIDS, follicular CD4+ cells are 40 times more likely to be productively infected than extrafollicular CD4+ cells.9 Mechanisms underlying the concentration of HIV-1 replication in follicular CD4+ T cells are not well understood.
CD4+ T cells found in B cell follicles are a distinct subset of antigen-experienced CD4+ T cells that express the follicular homing molecule CXCR5.10,11 CXCL13 is produced within B cell follicles by stromal cells and follicular dendritic cells (FDC)12,13 and promotes chemotaxis of CXCR5+CD4+ cells into follicles.11,14 Subsets of CXCR5+ cells include T follicular helper cells, which express high levels of PD-1, interact with both FDC and B cells within germinal centers, and provide signals that facilitate B cell affinity maturation and differentiation.14–16 Follicular CD4+ T cells receive costimulatory signals from FDC and B cells, which potentiate their activation and HIV-1 replication.14,15,17–19 Replication of HIV-1 by follicular CD4+ T cells may be further promoted by a relative paucity of antiretroviral proteins and virus-specific CD8+ T cells within B cell follicles.9,20 Whether CXCR5+ cells or follicular CD4+ T cells have intrinsic characteristics that enhance their ability to survive and propagate HIV-1 is unknown.
Bcl-2 is an antiapoptotic factor that plays a prominent role in interleukin (IL)-7 and IL-2-mediated lymphocyte survival in HIV-1-infected and -uninfected cells.21–25 Low levels of Bcl-2 are found in peripheral blood lymphocytes of untreated HIV-1-infected individuals and correlate with higher levels of apoptosis in these cells compared to cells from uninfected individuals.26 HIV-1 proteins have been reported to modulate Bcl-2 with both higher27 and lower Bcl-2 levels reported.28–31 Recently, transduction of Bcl-2 into primary CD4+ T cells has been utilized to establish an effective model of latent infection through increasing cell survival in vitro and restoring T cell homeostasis.32 Whether Bcl-2 expression is upregulated within CXCR5+CD4+ T cells, thereby promoting survival through reduction in apoptosis and accumulation of virus-producing cells in follicular areas, is unknown. The present study was designed to evaluate two hypotheses: (1) CXCR5+ CD4+ lymphoid tissue cells express more Bcl-2 than CXCR5− cells and (2) HIV-1-producing follicular cells express more Bcl-2 than HIV-1-producing CXCR5− cells.
Materials and Methods
Clinical specimens
Tonsils were obtained from discarded pathologic specimens of children without known HIV-1 infection undergoing elective tonsillectomies at Children's Hospital Denver in accordance with local IRB regulations. Tonsils were first visually inspected, necrotic material was removed, and specimens were mechanically disaggregated in sterile phosphate-buffered saline (PBS, Mediatech, Manassas, VA). The cell suspension was filtered through a 70-μm filter (Fisher Scientific, Denver, CO) and washed with PBS.
In vitro infection with HIV-1 green fluorescent protein (GFP) reporter viruses
The HIV-1 NL4-3-based CXCR4-tropic (X4) GFP reporter virus NLENG1-IRES and CCR5-tropic (R5) GFP reporter virus NLYUV3-GFP have been described elsewhere.19,33 Virus stocks were generated by transfection of 293T cells using Effectene (Qiagen, Valencia, CA), and p24 concentrations were determined by ELISA (PerkinElmer, Shelton, CT). Freshly isolated tonsil cells were cultured with 5 μg/ml of phytohemagglutinin (PHA; Sigma-Aldrich, St. Louis, MO) at a concentration of 2 million cells/ml for 48 to 72 h in R10 medium consisting of RPMI (Mediatech), 1% l-glutamine, 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA), 1% penicillin, 1% streptomycin, and 10 units of IL-2/ml (Roche, Nutley, NJ). Cells were pelleted and resuspended in fresh medium at a concentration of 1×107 cells/ml. Then 0.5 ml to 1.5 ml of either R5-tropic reporter virus stock (ranging from 400 to 1,050 ng of p24 antigen/ml) or X4-tropic reporter virus stock (ranging from 380 to 1,050 ng p24 antigen/ml) was added for 2 h at 37°C. Samples were diluted to 2×106 cells/ml in R10 medium and cultured for an additional 48 h.
Flow cytometric analyses
Cells were stained with antibodies including CD3-Pacific Orange (Invitrogen, Camarillo, CA), CD4-APC-Cy7, CD8-Pacific Blue, and CXCR5-AF647 [all from Becton Dickinson (BD) Biosciences, San Diego, CA] for 30 min, then washed and fixed with 2% paraformaldehyde (Sigma) solution. To characterize Bcl-2 expression, cells were stained with the above antibodies, fixed for 15 min in 100 μl of solution A (Fix & Perm, Invitrogen), washed, and resuspended in 100 μl of solution B (Invitrogen). Following this, cells were incubated for 30 min with unconjugated Bcl-2 antibody (Epitomics, Burlingame, CA), washed, treated with goat anti-rabbit-PE (Invitrogen) for 30 min, then washed and fixed prior to flow cytometry. Data were acquired on a FACS Aria (BD, San Jose, CA) and analyzed using FlowJo (Tree Star, Ashland, OR). GFP+ cells were detected in the FITC channel. This antibody panel was optimized by methods described previously.34 Spectral overlap was determined to be no greater than 45%. A fluorescence minus one or FMO was used to identify gating for CXCR5 and Bcl-2 using uninfected cells. Percentages of antibody-staining cells were determined with the exception of Bcl-2, which was evaluated by using the geometric mean fluorescence intensity (MFI). In all populations analyzed, the MFI of Bcl-2 in the FMO was subtracted from the measured MFI of Bcl-2. Both the percentage and MFI (geometric mean) of GFP were determined for GFP+ cells.
Statistical analysis
Nonparametric statistical tests were used due to small sample sizes. Wilcoxon-signed rank two tailed t-tests were used to evaluate paired observations and the Mann–Whitney U test was used for unpaired observations. For determining correlations, Spearman's correlation was used. A p value <0.05 was considered statistically significant. Data were analyzed using Graphpad Prism (La Jolla, CA).
Results
Bcl-2 expression was elevated in CXCR5+CD4+ T cells in human tonsils
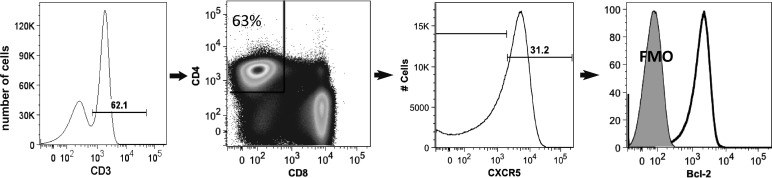
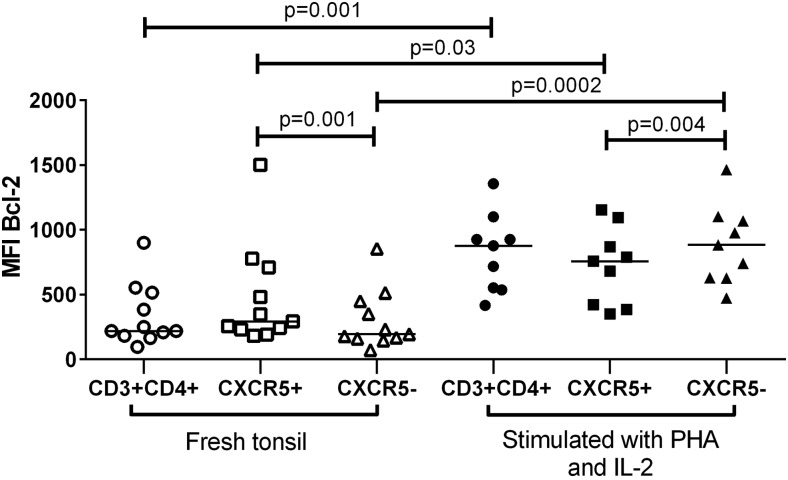
Tonsils were obtained from 20 children with a median age of 10 years (range, 2 to 16 years). Figure 1 illustrates the gating strategy for identifying CXCR5+ cells and characterizing Bcl-2 expression. Flow cytometry analyses of disaggregated tonsil cells revealed that a median of 26% (range, 8% to 58%) of CD3+CD4+ cells expressed CXCR5. In freshly isolated tonsil cells, MFI of Bcl-2 was 50% higher in CXCR5+ (median, 292) compared to CXCR5− CD4+ T cells (median, 194) (Fig. 2).
FIG. 1.
Representative flow cytometry plot demonstrating the gating strategy for CXCR5 and Bcl-2.
FIG. 2.
Bcl-2 expression in CD3+CD4+, follicular (CXCR5+), and extrafollicular (CXCR5) CD4+ T cells isolated from tonsils of subjects at low risk for HIV-1 infection before and after stimulation with phytohemagglutinin (PHA) and interleukin-2 (IL-2).
Bcl-2 expression increased in CD4+ T cells from tonsils following in vitro stimulation
PHA+IL-2 stimulation for 48 h resulted in a 4-fold increase in Bcl-2 expression on tonsil CD4+ T cells compared with freshly isolated cells (Fig. 2). Similarly, Bcl-2 expression in both CXCR5+ and CXCR5− subsets of PHA and IL-2-stimulated cells was higher than in freshly isolated tonsil cell subsets (median MFI, 757 versus 292 and 884 versus 194, respectively). In contrast to freshly isolated tonsil cells, CXCR5+ cells harbored significantly less Bcl-2 compared to CXCR5-CD3+CD4+ cells following in vitro stimulation with PHA and IL-2. The percentage of CXCR5+ cells did not significantly differ between stimulated and unstimulated CD3+CD4+ cells (medians, 26% vs. 32%, respectively; p=0.9).
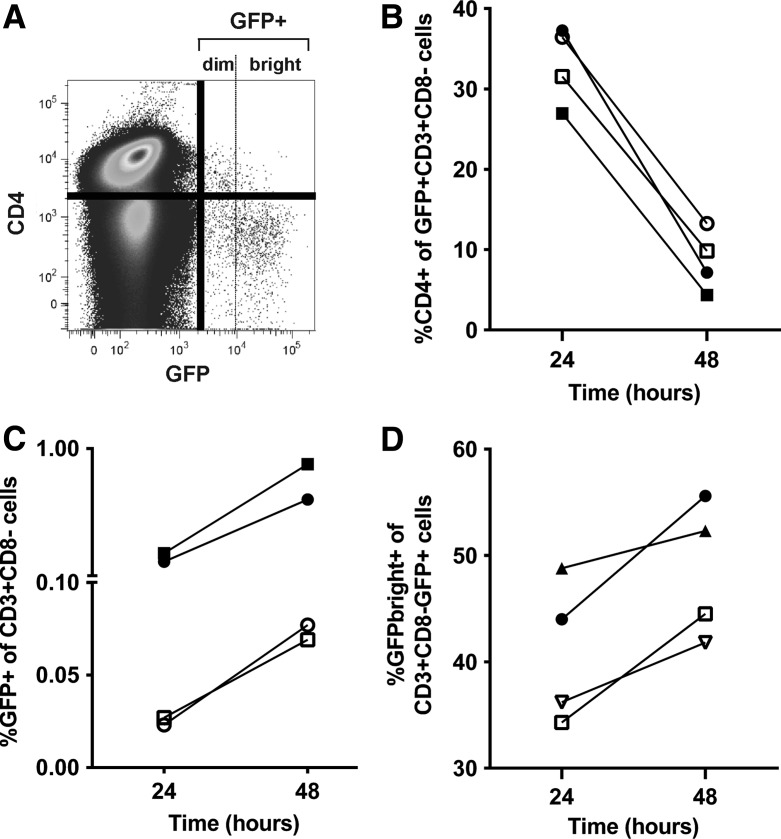
Infection of tonsil cells with GFP reporter viruses resulted in a time-related decrease in cell surface CD4 expression, accumulation of GFP+ cells, and accumulation of GFPbright cells
Disaggregated tonsil cells were stimulated with PHA and IL-2 for 2 days, then infected with HIV-1 GFP reporter viruses and monitored for GFP expression. HIV-1 Nef, Env, and Vpu downregulate CD4 expression on the surface of productively infected cells35,36 and we have previously demonstrated that CD4 is downregulated on PHA-stimulated peripheral blood mononuclear cells (PBMCs) productively infected with HIV-1 R5 and X4 reporter viruses.19 Infection of PHA-stimulated tonsil cells similarly resulted in downregulation of CD4 on the majority of GFP+ cells, as shown in a representative flow cytometry plot in Fig. 3A. Percentages of CD4+ GFP+ cells decreased between 24 and 48 h after infection with R5 and X4-tropic reporter viruses (Fig. 3B), whereas percentages of CD3+CD8-GFP+ cells (Fig. 3C) increased between 24 and 48 h of culture. We then divided cells into GFPbright and GFPdim populations to evaluate the accumulation of viral proteins (i.e., GFPbright cells) at 24 and 48 h of culture. As shown in Fig. 3D, the proportion of GFPbright cells increased between 24 and 48 h. Collectively, these findings of decreased CD4 expression and increased proportions of GFPbright cells over time are consistent with time-related accumulation of virally expressed proteins. Because of the downregulation of CD4 on GFP+ cells, the CD3+CD8− cell population was gated in subsequent flow cytometry analyses when evaluating GFP+ cells, as we previously described.19
FIG. 3.
Flow cytometry analysis of tonsil cells from two subjects infected ex vivo with R5 and X4 green fluorescent protein (GFP) reporter viruses. (A) Representative flow cytometry plot demonstrating downregulation of CD4 on GFP+ cells 48 h after infection with X4 virus. Dotted line indicates the separation between GFPbright and GFPdim cells. (B) Percentages of CD4+ T cells, (C) GFP expression in CD3+CD8−, and (D) GFPbright cells within the CD3+CD8−GFP+ population at 24 and 48 h after infection. The solid shapes represent X4 infection and the open shapes represent R5 infections.
PHA and IL-2-stimulated CXCR5+ cells did not demonstrate preferential replication of either R5 or X4 HIV-1 reporter viruses
As seen in Fig. 3C, percentages of GFP+ cells in the CD3+CD8− population after 48 h were consistently higher in wells infected with X4 (median, 0.59%; range 0.11 to 0.89%) compared to those infected with R5 HIV-1 reporter virus (median 0.026%, range 0.006 to 0.077%; p=0.01), as reported previously in PBMCs.19 MFI of GFP did not differ significantly between R5 (median, 12,655; range 8,943–15,199) and X4 HIV-1 infections (median, 13,258; range 10,412–16,567; p=0.4). There was no significant difference in percentages of CXCR5+ cells in the GFP+CD3+CD8− compared to the GFP−CD3+CD8− populations in either R5 (median, 14% versus 34%, respectively; p=0.3) or X4 (median, 36% versus 37%, respectively; p=0.3) infections. MFI of GFP did not differ significantly between CXCR5+ and CXCR5− GFP+ cells in either R5 (median, 8,589 and 11,951, respectively; p=0.4) or X4 HIV-1 infections (median, 12,489 and 13,775, respectively; p=0.2).
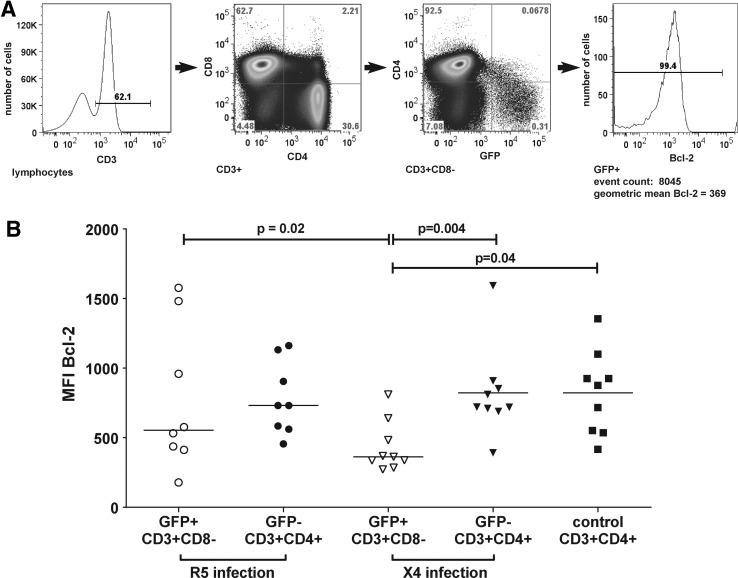
Bcl-2 expression was elevated in R5 compared to X4 HIV-1-producing cells
The gating strategy utilized to determine Bcl-2 expression is shown in Fig. 4A. Bcl-2 expression in GFP+ and GFP− cells from R5 and X4 infections and uninfected control cells is shown in Fig. 4B. We compared Bcl-2 expression in GFP+CD3+CD8− cells with GFP−CD3+CD4+ cells (termed “bystander” cells) based on the rationale that all GFP+ cells were originally CD4+. In R5 infections, the differences in Bcl-2 expression between GFP+CD3+CD8− cells and bystander cells was not statistically significant, nor were the differences between either of these subsets and uninfected control CD3+CD4+ cells. In X4 infections, MFI for Bcl-2 was lower in GFP+CD3+CD8− cells (median, 363) compared to bystander cells (median, 721) and controls (median, 877). No significant correlation was seen between percentages of GFP+ cells or MFI of GFP and MFI of Bcl-2 in GFP+CD3+CD8− cells in either R5 or X4 infections (data not shown). MFI for Bcl-2 was significantly higher in GFP+CD3+CD8− cells producing R5 virus (median, 554) compared to those producing X4 virus (median, 363). There was no statistically significant difference in MFI of Bcl-2 between bystander cells in R5 compared to X4 infections (702 vs. 721; p=1.0).
FIG. 4.
(A) Representative flow cytometry plot demonstrating the gating strategy utilized to identify Bcl-2 expression in GFP+ cells. (B) Bcl-2 expression in GFP+ and GFP− cells infected with R5 and X4 HIV-1 reporter viruses and control cells. Only statistically significant comparisons are shown. All other p values were>0.05.
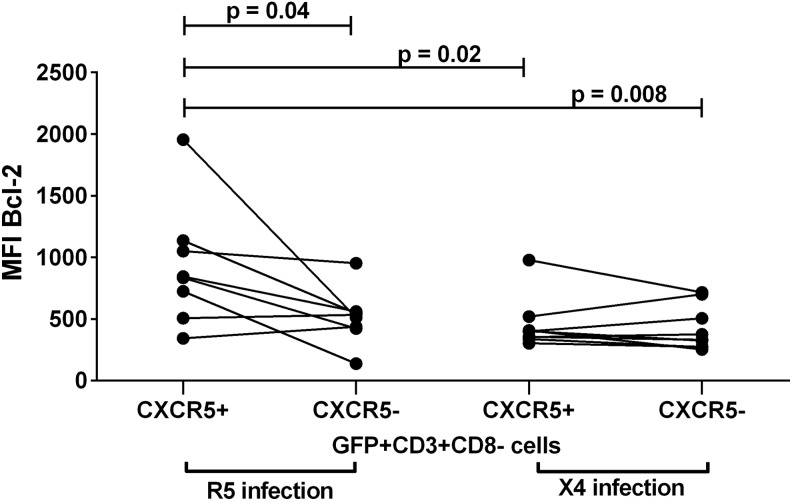
Bcl-2 expression was distinctly elevated in R5-producing follicular cells compared to X4-producing follicular cells
To investigate Bcl-2 expression in virus-producing follicular cells, we compared Bcl-2 expression among CXCR5+ and CXCR5− subsets of R5 and X4 GFP+ cells (Fig. 5). Bcl-2 MFI was significantly higher in R5-producing CXCR5+ cells (median, 840) compared to R5-producing CXCR5− cells (median, 524), X4-producing CXCR5+ cells (median, 401), and X4-producing CXCR5− cells (median, 332). In X4 infections, MFI of Bcl-2 in CXCR5+GFP+ cells did not differ significantly from that in CXCR5−GFP+ cells. Bcl-2 MFI in uninfected control CXCR5+ cells (median, 757; Fig.2) was similar to that in R5-infected CXCR5+GFP+ cells (median, 840; p=0.3) and higher than that in X4-infected CXCR5+GFP+ cells (median, 401), although differences were not statistically significant (p=0.2). Bcl-2 MFI in uninfected control CXCR5− cells (median, 884; Fig. 2) was significantly higher than that in CXCR5−GFP+ cells in both R5 (p=0.008) and X4 infections (p=0.02).
FIG. 5.
Bcl-2 expression in CXCR5+ and CXCR5− subsets of GFP+ cells in R5 and X4 infection. Only statistically significant comparisons are shown. All other p values were>0.05.
Discussion
This is the first study to evaluate the relationship between Bcl-2 expression and HIV-1 replication in lymphoid tissue cells, as well as the first to compare Bcl-2 levels between R5 and X4 HIV-1-producing T cells. We found that freshly isolated CXCR5+ CD4+ T lymphocytes from human tonsils express higher levels of Bcl-2 compared to CXCR5−CD4+ T cells. Conversely, after stimulation with PHA and IL-2, Bcl-2 levels were higher in CD4+CXCR5− cells compared to CD4+CXCR5+ cells. After infection with HIV-1 reporter viruses ex vivo, preserved levels of Bcl-2 were demonstrated within R5-producing T cells, whereas levels were reduced in X4-producing cells. Bcl-2 levels were higher in the CXCR5+ R5-producing subset compared to CXCR5− R5-producing cells and both X4-producing subsets. This is the first report of differences in Bcl-2 expression in HIV-1-producing cells related to virus tropism. In light of the well-established relationship between elevated levels of Bcl-2 expression and promotion of cell survival,23,25,26 these findings suggest that R5-producing CXCR5+ cells, which include the follicular subset, may be more resistant to apoptotic stimuli than R5-producing CXCR5− cells or X4-producing cells, thereby promoting R5 propagation in B cell follicles in vivo.
Previous studies have established that Bcl-2 is upregulated in T cells upon immune activation.25,37,38 Our findings that Bcl-2 expression was elevated in CXCR5+CD4+ T cells are consistent with this as lymphoid tissue CXCR5+ cells constitute a subset of recently antigen-stimulated cells.14,16 Also consistent with prior observations,39–41 stimulation with PHA and IL-2 resulted in higher levels of Bcl-2 in tonsil cells compared to unstimulated cells (Fig. 2). In PHA and IL-2-stimulated tonsil cells, Bcl-2 expression was significantly higher in CXCR5− cells than in CXCR5+ cells. We did not observe a change in the percentage of CXCR5+ cells, suggesting that elevated Bcl-2 in CXCR5− cells was not due to downregulation of CXCR5. While both PHA and IL-239–41 promote Bcl-2 expression within T lymphocytes, IL-2 may be a more potent inducer of Bcl-2.42 It is possible that CXCR5− cells express more IL-2R and are more sensitive to IL-2-mediated changes in Bcl-2.43 Importantly, the finding that Bcl-2 expression was higher in CXCR5− compared to CXCR5+ cells after treatment with PHA and IL-2 indicates that CXCR5 expression and Bcl-2 expression are not directly linked.
Bcl-2 was expressed at significantly higher levels in R5- compared to X4-infected GFP+ cells primarily due to preserved levels of Bcl-2 in R5-producing CXCR5+ cells. It cannot be determined from this study whether HIV-1 caused the reductions in levels of Bcl-2 in R5-infected CXCR5− cells or X4-producing subsets or whether cells with low levels of Bcl-2 preferentially replicated HIV. The failure of MFI of GFP to correlate with Bcl-2, however, weighs somewhat against the latter explanation. Crosslinking of the CD4 receptor by gp120 results in decreased Bcl-2 expression in vitro.44 HIV-1 tat has also been associated with decreased Bcl-2 expression in one in vitro model.28 Engagement of CXCR4 by X4 gp120 results in upregulation of the Bcl-2 interacting mediator of death extralong isoform (BimEL), which reduces Bcl-2 levels.32,45 Thus, multiple mechanisms could account for the decrease in Bcl-2 expression observed in R5-producing CXCR5− T cells and both subsets of X4-producing cells. Mechanisms to account for relatively preserved levels of Bcl-2 in R5-producing CXCR5+ T cells, however, are unexplained. Triggering of the CCR5 receptor could promote Bcl-2 expression and associated enhanced cell survival, a finding that has been demonstrated in lung tumor cells where lack of CCR5 expression was associated with a reduction in Bcl-2.46 Engagement and coreceptor signaling could also lead to increased IL-2 production as reductions in IL-2 are seen in T lymphocytes from CCR5–/– knockout mice47 and healthy donors with the CCR5 Δ32 allele.47 The density and percentage of CCR5 expression on CXCR5+ compared to CXCR5− cells are unknown and this is clearly an area of future study. While the causal factors remain uncertain, it appears that CXCR5+ cells, many of which are follicular cells, are uniquely primed to maintain Bcl-2 levels in the context of R5 HIV-1 infection, which could foster cell survival and subsequent viral persistence.
The distinct patterns in Bcl-2 expression in HIV-1-producing lymphoid tissue cells related to virus tropism observed in this study could potentially explain the enigmatic preference for R5 virus replication in early and mid-stages of HIV-1 infection.48 We have previously reported that follicular CD4+ T cells are 40 times more likely to be productively infected than extrafollicular CD4+ T cells in lymph nodes of individuals without AIDS during chronic HIV-1 infection.9 In all instances in which virus tropism has been determined in these subjects they were infected with R5 tropic virus.8 It is unknown whether a similar follicular concentration of virus exists in X4 infections. Intriguingly, studies of SIV-infected rhesus macaques acutely coinfected with R5 and X4 viruses demonstrated that the preference for R5 replication does not exist during acute infection, but only emerges several weeks after infection coincident with the development of virus-specific cytotoxic T cells (CTL),49 providing support for the hypothesis that it is the adaptive immune response that favors R5 infection over X4 infection. It is possible that preserved Bcl-2 expression in R5-producing CXCR5+ cells confers some resistance to apoptotic stimuli from CTL, which are not present during acute disease either in vitro or in vivo.
Our findings of distinct patterns in Bcl-2 expression relating to virus tropism, particularly in CXCR5+ T cells, provide additional support for an idea originally proposed by our group that B cell follicles are a sanctuary site for HIV-1 replication.9,20 Several avenues for future investigations are suggested by these results. First, these data are based on results from a single R5 and X4 reporter virus. It would be important to verify that similar findings occur in the presence of clinical isolates of R5 and X4 viruses. Second, these experiments were performed in the context of cells stimulated with PHA and IL-2, which may have altered results, as discussed above, and the numbers of events were limited. New techniques to infect lymphoid tissue cells without exogenous stimulation have been developed,50 and it would be important to determine whether similar differences in Bcl-2 expression related to virus tropism are seen in the context of infection of unstimulated cells. Third, in our in vitro experiments, we were not able to evaluate whether elevated Bcl-2 conferred a survival advantage. Additional experiments employing proapoptotic stimuli would be important to demonstrate whether differences in Bcl-2 expression between R5- and X4-producing cells truly confer a differential survival advantage. Finally, investigation of the mechanisms underlying differential expression of Bcl-2 in lymphoid tissue cells related to virus tropism merits further study. A better understanding of the ways in which virus tropism is related to cell survival could provide important insight into the immunopathogenesis of HIV-1 infection and suggest new targets for antiviral therapies that could be used to eliminate the follicular reservoir of HIV-1 replication.
Acknowledgments
The authors thank Rick Schlichtemeier and Tina Powell for providing expertise regarding flow cytometry, Elizabeth Kelly-McKnight for assistance in performing the flow cytometry, and Brodie Miles for his thoughtful review of this manuscript. This work was supported by grants from the National Institutes of Health including R56 AI080418, R01 AI096966, T32 AI07447, and P30 AI054907.
Part of this work was presented at the Conference on Retroviruses and Opportunistic Infections (CROI), February 27, 2011–March 3, 2011, Boston, MA.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Pantaleo G, Graziosi C, Demarest JF, et al. : HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 1993;362:355–358 [DOI] [PubMed] [Google Scholar]
- 2.Embretson J, Zupancic M, Ribas JL, et al. : Massive covert infection of helper T lymphocytes and macrophages by HICV during the incubation period of AIDS. Nature 1993;362:359–362 [DOI] [PubMed] [Google Scholar]
- 3.Tamalet C, Lafeuillade A, Fantini J, et al. : Quantification of HIV-1 viral load in lymphoid and blood cells: Assessment during four-drug combination therapy. AIDS 1997;11(7):895–901 [DOI] [PubMed] [Google Scholar]
- 4.Yang OO, Ferbas JJ, Hausner MA, et al. : Effects of HIV-1 infection on lymphocyte phenotypes in blood versus lymph nodes. J Acquir Immune Defic Syndr 2005;39(5):507–518 [PubMed] [Google Scholar]
- 5.Spiegel H, Herbst H, Niedobitek G, et al. : Follicular dendritic cells are a major reservoir for human immunodeficiency virus type 1 in lymphoid tissues facilitating infection of CD4+T-helper cells. Am J Pathol 1992;140(1):15–22 [PMC free article] [PubMed] [Google Scholar]
- 6.Tenner-Racz K. and Racz P: Follicular dendritic cells initiate and maintain infection of the germinal centers by human immunodeficiency virus. Curr Top Microbiol Immunol 1995;201:141–159 [DOI] [PubMed] [Google Scholar]
- 7.Hufert FT, Lunzen JV, Janossy G, et al. : Germina centre CD4+T cells are an important site of HIV replication in vivo. AIDS 1997;11:849–857 [DOI] [PubMed] [Google Scholar]
- 8.Meditz AL, Folkford JM, Lyle NH, et al. : CCR5 expression is reduced in lymph nodes of HIV-1 infected women compared to men but does not mediate sex differences in viral loads. J Infect Dis 2014;209(6):922–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folkvord JM, Armon C, and Connick E: Lymphoid follicles are sites of heightened human immunodeficiency virus type 1 (HIV-1) replication and reduced antiretroviral effector mechanisms. AIDS Res Hum Retroviruses 2005;21(5):363–370 [DOI] [PubMed] [Google Scholar]
- 10.Legler DF, Loetscher M, Roos RS, et al. : B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med 1998;187(4):665–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breitfeld D, Ohl L, Kremmer E, et al. : Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles and support immunoglobulin production. J Exp Med 2000;192(11):1545–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansel KM, Ngo VN, Hyman PL, et al. : A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 2000;406:309–314 [DOI] [PubMed] [Google Scholar]
- 13.Gunn MD, Ngo VN, Ansel KM, et al. : A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature 1998;391:779–803 [DOI] [PubMed] [Google Scholar]
- 14.Schaerli P, Willimann K, Lang AB, et al. : CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med 2000;192(11):1553–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer BE. Naomi B, and Fontenot AP: Functional and phenotypic characterization of CD57+CD4+T cells and their association with HIV-1-induced T cell dysfunction. J Immunol 2005;175:8415–8423 [DOI] [PubMed] [Google Scholar]
- 16.Kim CH, Rott LS, Clark-Lewis I, et al. : Subspecialization of CXCR5+T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+T cells. J Exp Med 2001:1373–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bégaud E, Chartier L, Marechal V, et al. : Reduced CD4 T cell activation and in vitro susceptibility to HIV-1 infection in exposed uninfected Central Africans. Retrovirology 2006;3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koning FA, Otto SA, Hazenberg MD, et al. : Low-level CD4+T cell activation is associated with low susceptibility to HIV-1 infection. J Immunol 2005;175(9):6117–6122 [DOI] [PubMed] [Google Scholar]
- 19.Meditz AL, Haas MK, Folkvord JM, et al. : HLA-DR+CD38+CD4+T lymphocytes have elevated CCR5 expression and produce the majority of R5-tropic HIV-1 RNA in vivo. J Virol 2011;85(19):10189–10200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connick E, Mattila T, Folkvord JM, et al. : CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J Immunol 2007;178(11):6975–6983 [DOI] [PubMed] [Google Scholar]
- 21.Akashi K, Kondo M, Freeden-Jeffry V, et al. : Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell 1997;89(7):1033–1041 [DOI] [PubMed] [Google Scholar]
- 22.Maraskovsky E, O'Reilly L, Teepe M, et al. : Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell 1997;89(7):1011–1089 [DOI] [PubMed] [Google Scholar]
- 23.Kim K, Lee C-k, Sayers TJ, et al. : The trophic action of IL-7 on pro-T cells: Inhibition of apoptosis of pro-T1, -T2, and -T3 cells correlates with Bcl-2 and Bax levels and is independent of Fas and p53 pathways. J Immunol 1998;160(12):5735–5741 [PubMed] [Google Scholar]
- 24.Akbar AN, Borthwick NJ, Wickremasinghe G, et al. : Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: Selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur J Immunol 1996;26(2):294–299 [DOI] [PubMed] [Google Scholar]
- 25.Mueller D, Seiffert S, Fang W, and Behrens TW: Differential regulation of bcl-2 and bcl-x by CD3, CD28, and the IL-2 receptor in cloned CD4+helper T cells. A model for the long-term survival of memory cells. J Immunol 1996;156(5):1764–1771 [PubMed] [Google Scholar]
- 26.Ledru E, Lecoeur H, Garcia S, et al. : Differential susceptibility to activation-induced apoptosis among peripheral Th1 subsets: Correlation with Bcl-2 expression and consequences for AIDS pathogenesis. J Immunol 1998;160(7):3194–3206 [PubMed] [Google Scholar]
- 27.Zauli G, Gibellini D, Caputo A, et al. : The human immunodeficiency virus type-1 Tat protein upregulates Bcl-2 gene expression in Jurkat T-cell lines and primary peripheral blood mononuclear cells. Blood 1995;86(10):3823–3834 [PubMed] [Google Scholar]
- 28.Sastry K, Marin M, Nehete PM, et al. : Expression of human immunodeficiency virus type I tat results in down-regulation of bcl-2 and induction of apoptosis in hematopoietic cells. Oncogene 1996;13(3):487–493 [PubMed] [Google Scholar]
- 29.Gougeon M-L: To kill or be killed: How HIV exhausts the immune system. Cell Death Differ 2005;12:845–854 [DOI] [PubMed] [Google Scholar]
- 30.Rasola A, Gramaglia D, Boccaccio C, and Comoglio PM: Apoptosis enhancement by the HIV-1 Nef protein. J Immunol 2001;166(1):81–88 [DOI] [PubMed] [Google Scholar]
- 31.Ndolo T, Dhillon NK, Nguyen H, et al. : Simian immunodeficiency virus Nef protein delays the progression of CD4+T cells through G1/S phase of the cell cycle. J Virol 2002;76(8):3587–3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trushin SA, Carena AA, Bren GD, et al. : SDF-1alpha degrades whereas glycoprotein 120 upregulates Bcl-2 interacting mediator of death extralong isoform: Implications for the development of T cell memory. J Immunol 2012;189(4):1835–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kutsch O, Benveniste EN, Shaw GM, and Levy DN: Direct and quantitative single-cell analysis of human immunodeficiency virus type 1 reactivation from latency. J Virol 2002;76(17):8776–8786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahnke YD. and Roederer M: Optimizing a multi-colour immunophenotyping assay. Clin Lab Med 2007;27(3):469-v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guy B, Kieny MP, Riviere Y, et al. : HIV F/3′ orf encodes a phosphorylated GTP-binding protein resembling an oncogene product. Nature 1987;330(6145):266–269 [DOI] [PubMed] [Google Scholar]
- 36.Garcia JV. and Miller AD: Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 1991;350:508–511 [DOI] [PubMed] [Google Scholar]
- 37.Marrack P. and Kappler J: Control of T cell viability. Annu Rev Immunol 2004;22:765–787 [DOI] [PubMed] [Google Scholar]
- 38.Song J, So T, and Croft M: Activation of NF-kappaB1 by OX40 contributes to antigen-driven T cell expansion and survival. J Immunol 2008;180(11):7240–7248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyazaki T, Liu Z-J, Kawahara A, et al. : Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell 1995;81(2):2223–2231 [DOI] [PubMed] [Google Scholar]
- 40.Yamada K, Yamakawa M, Imai Y, and Tsukamoto M: Expression of cytokine receptors on follicular dendritic cells. Blood 1997;90(12):4832–4841 [PubMed] [Google Scholar]
- 41.Adachi Y, Oyaizu N, Than S, et al. : IL-2 rescues in vitro lymphocyte apoptosis in patients with HIV infection: Correlation with its ability to block culture-induced down-modulation of Bcl-2. J Immunol 1996;157(9):4184–4193 [PubMed] [Google Scholar]
- 42.Reed JC, Tsujimoto Y, Alpers JD, et al. : Regulation of Bcl-2 proto-oncogene expression during normal human lymphocyte proliferation. Science 1987;236(4806):1295–1299 [DOI] [PubMed] [Google Scholar]
- 43.Pepper M, Pagán AJ, Igyártó BZ, et al. : Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity 2011;35(4):583–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hashimoto F, Oyaizu N, Kalyanaraman VS, and Pahwa S: Modulation of Bcl-2 protein by CD4 cross-linking: A possible mechanism for lymphocyte apoptosis in human immunodeficiency virus infection and for rescue of apoptosis by interleukin-2. Blood 1997;90(2):745–753 [PubMed] [Google Scholar]
- 45.Trushin SA, Algeciras-Schimnich A, Vlahakis SR, et al. : Glycoprotein 120 binding to CXCR4 causes p38-dependent primary T cell death that is facilitated by, but does not require cell-associated CD4. J Immunol 2007;178(8):4846–4853 [DOI] [PubMed] [Google Scholar]
- 46.Lee NJ, Choi DY, Song JK, et al. : Deficiency of C-C chemokine receptor 5 suppresses tumor development via inactivation of NF-{kgreen}B and inhibition of monocyte chemoattractant protein-1 in urethane-induced lung tumor model. Carcinogenesis 2012;33(12):2520–2528 [DOI] [PubMed] [Google Scholar]
- 47.Camargo JF, Quinones MP, Mummidi S, et al. : CCR5 expression levels influence NFAT translocation, IL-2 production, and subsequent signalling events during T lymphocyte activation. J Immunol 2009;182(1):171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuitemaker H, Koot M, Kootstra NA, et al. : Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: Progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol 1992;66(3):1354–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harouse JM, Buckner C, Gettie A, et al. : CD8+T cell-mediated CXC chemokine receptor 4-simian/human immunodeficiency virus suppression in dually infected rhesus macaques. Proc Natl Acad Sci USA 2003;100(19):10977–10982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doitsh G, Cavrois M, Lassen KG, et al. : Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell 2010;143(5):789–801 [DOI] [PMC free article] [PubMed] [Google Scholar]