Abstract
Traumatic brain injury (TBI) can cause sleep-wake disturbances and excessive daytime sleepiness. The pathobiology of sleep disorders in TBI, however, is not well understood, and animal models have been underused in studying such changes and potential underlying mechanisms. We used the rat lateral fluid percussion (LFP) model to analyze sleep-wake patterns as a function of time after injury. Rapid-eye movement (REM) sleep, non-REM (NREM) sleep, and wake bouts during light and dark phases were measured with electroencephalography and electromyography at an early as well as chronic time points after LFP. Moderate TBI caused disturbances in the ability to maintain consolidated wake bouts during the active phase and chronic loss of wakefulness. Further, TBI resulted in cognitive impairments and depressive-like symptoms, and reduced the number of orexin-A-positive neurons in the lateral hypothalamus.
Key words: : electroencephalography, lateral fluid percussion, orexin, sleep-wake disturbances, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a leading cause of mortality and morbidity, affecting approximately 5.3 million people in the United States alone.1 There are growing concerns about prevalence of sleep-wake disturbances among survivors of TBI that exacerbate the resulting long-term behavioral dysfunction.2
Sleep plays an important role in neurosynaptic plasticity and neurogenesis.3,4 A wide range of sleep disorders has been reported after TBI in humans. Clinical studies have observed increases in hypersomnia such as excessive daytime sleepiness (EDS) in approximately 25% of patients with TBI.5,6 Other retrospective studies indicate that approximately half of the patients experience some form of EDS with half of those having severe hypersomnia.5,6 Further, changes in the frequency and duration of rapid-eye movement (REM) have been observed in 25% of patients with TBI.7
Because polysomnograms from humans may be difficult to obtain, most studies rely on surveys that assess sleep-wake disturbances. Moreover, only a few animal studies have investigated the effects of TBI on sleep-wake behavior.8–13 Sleep-wake behavior plays a role in the consolidation and retention of memories within the hippocampus, which indicates that TBI-induced cognitive deficits might be partially attributed to altered sleep patterns.14,15 Changes in sleep-wake behavior are also caused by down-regulation of neurotransmitters within the hippocampus after TBI.16,17 Therefore, further investigation of sleep-wake behavior in terms of wakefulness, non-REM (NREM), and REM duration are needed to elucidate the underlying mechanisms that lead to such disturbances and may further exacerbate the functional deficits.
Orexin (ORX), also known as hypocretin, is a modulator of sleep-wake behavior.18,19 Expression of ORX is localized in the lateral hypothalamus, with projections to various sleep-wake nuclei in the brain.20 Loss of ORX-A–positive neurons results in inability to maintain arousal.21 Further, sleep-wake disturbances in other neurodegenerative disorders, such as Alzheimer disease, have been linked to altered expression of ORX-A.22
In this study, we used a well-established rat lateral fluid percussion (LFP) model23,24 to assess acute as well as chronic sleep-wake disturbances over a period of 30 days after TBI. The sleep-wake activity was evaluated in terms of total time in REM, NREM, and wakefulness in both light and dark phases. TBI-induced cognitive impairments and depressive-like behavior were performed using functional tasks. The changes in ORX-A levels in the lateral hypothalamus were examined using unbiased stereological techniques.
Methods
LFP brain injury
All surgical procedures and experiments were performed in accordance with protocols and guidelines approved by the Institutional Animal Care and Use Committee at the University of Maryland. All animals were single-housed in the animal facility with ad libitum access to food and water. Adult male Sprague-Dawley (SD) rats (310–330 g) were anesthetized using isoflurane (induction: 4%, maintenance: 2–2.5%; supply gas: 70% compressed air+30% oxygen). A sharp dissection was made followed by craniotomy (4 mm) located midway between lambda and bregma sutures over the left parietal cortex. A luer-loc adaptor was cemented over the craniotomy site and attached to our custom-built microprocessor-driven LFP device, which when triggered produced a pressure pulse causing a deformation of the underlying brain. The rats underwent moderate LFP brain injury (1.9–2.2 atmospheres) or sham operation following approved experimental guidelines, as described previously.23,24
Implantation of radiotelemetry transmitter
Immediately after the injury, a radiotelemetry transmitter (F40-EET, Data Science International, St. Paul, MN) was subcutaneously implanted on the left side of the abdominal region of the rats. A 1-inch parasagittal incision was made starting at the caudal aspect of the rib cage and proceeding toward the tail. Using blunt scissors, directed downward away from the incision, a subcutaneous pocket was made for the transmitter. The leads from the transmitter were fed through the trochar to the skull. After the implantation of the transmitter, the insulated electroencephalogram (EEG) leads (Data Science International) were secured around the screws (1.5 mm anterior and 1.0 mm left of the bregma; 0.7 mm anterior and 1.0 mm right of lambda), and the leads were secured with dental cement avoiding the craniotomy.
Implantation of electromyogram (EMG) electrodes
Biopotential EMG leads were placed in contact with the dorsal muscles of the neck to monitor motor activity. The lead wires were placed in direct contact with the muscle 1–2 mm apart along the same bundle of cervical trapezius muscles in the dorsal region of the neck. A 21-gauge needle was inserted through approximately 3 mm of the muscle tissue. A bare lead wire was passed into the lumen of the needle, the needle was withdrawn, and the lead wire was left embedded in the muscle. A suture tie was placed securely around the insulation of both leads. The incision was closed with surgical staples.
EEG and EMG analysis
Acquisition of EEG and EMG waveforms for recording of sleep patterns
Sleep-wake behavior assessment was performed on post-injury days (PIDs) 6, 19, and 29, so as not to interfere with behavioral assessments (Fig. 1). The complete light and dark phase was recorded starting at 8 pm and ending at 8 am on the following days 7, 20, and 30. The implanted telemetry device transmitted to a plate receiver (RPCI, Data Sciences International) located under the home cage of each individually housed animal. All recordings were performed in a designated room shielded from noise or other background disturbances. EEG and EMG waveform data were acquired using the Dataquest ART 4.0 software (DSI, St. Paul MN) to set a continuous sampling mode of 500 Hz. The analog signal was converted to a digital signal and stored for analysis.
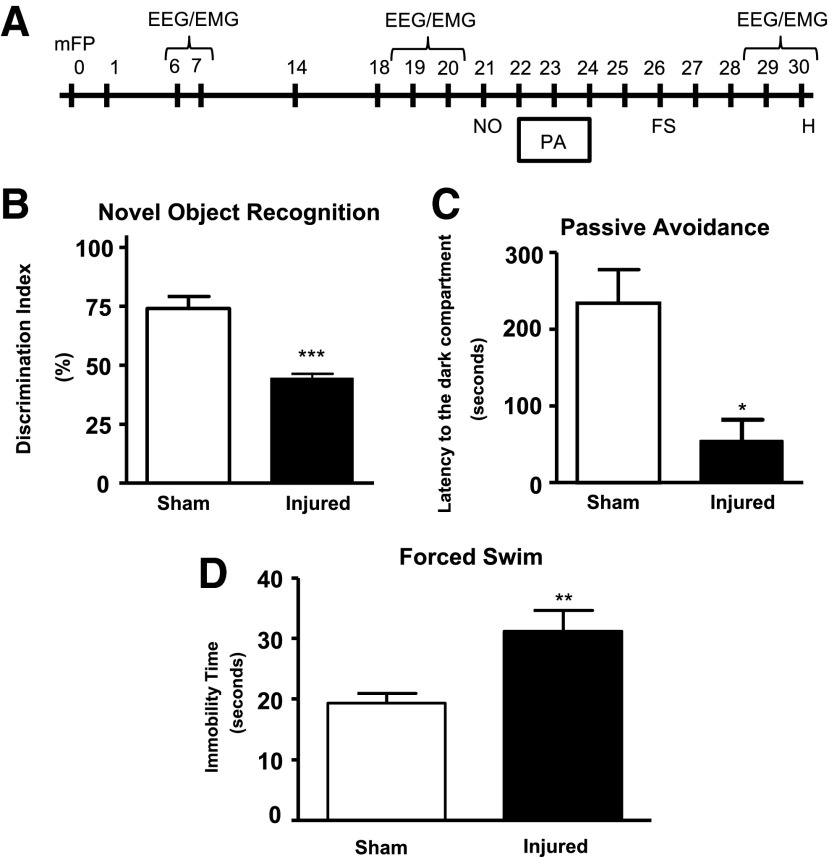
FIG. 1.
Behavioral assessment. (A) Timeline of behavioral studies and electroencephalogtram/electromyogram (EEG/EMG) recordings after rat lateral fluid percussion (LFP) injury. (B) Novel object recognition (NOR) demonstrated an inability to retain intact memory (***p<0.001, vs. sham). Analysis by two-tailed Mann-Whitney U t test. Mean±standard error of the mean (SEM; n=8–11/group. (C) Passive avoidance (PA) demonstrated a lack of fear-based conditioning of injured animals (*p<0.05 vs. sham). (D) Forced swim (FS) for depression showed an increase (**p<0.01 vs. sham) in immobility time of injured animals compared with sham animals. (Analysis by two-tailed unpaired Student t test (C-D). Mean±SEM; n=8–11/group.
Sleep scoring and data analysis
All animals were scored manually using NeuroScore software (Data Sciences International) into 10-sec epochs. Power bands were defined as delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), sigma (12–16 Hz), and beta (16–24 Hz).
Light and dark phases were separated into 1, 2, 3, 4, 6, and 12 h bins to further investigate changes in sleep-wake activity. Zeitberger time (ZT) was used throughout this study where ZT=0 corresponds to 8 am (lights on) and ZT=12 corresponds to 8 pm (lights off). Total time, average bout length, and number of bouts were calculated for each bin. Customized software developed under MATLAB (Mathworks, Natick, MA) was used to perform all sleep-wake state permutations, blind the investigator from animal group, and calculate power band analysis.
Wakefulness was defined as low amplitude (<150 μV), high frequency EEG combined with high amplitude EMG RMS (>10 μV). NREM was defined as having a high amplitude (>120 μV), low frequency EEG in the form of the theta-delta ratio between 0.5 and 1, combined with low EMG tone (<10 μV). REM was defined as low amplitude (<150 μV), high frequency EEG in the form of the theta-delta ratio (>1) combined with muscle atonia (low EMG RMS; <10 μV) with occasional muscle twitches.
Behavioral assessment
Novel object recognition for retention memory function
The novel object recognition (NOR) test (sham: n=8; LFP: n=11) was used to assess retention or intact memory function on PID 21. An open field (40 cm×20 cm×60 cm) with two adjacently located imaginary circular zones was used, as previously designed.24,25 The zones were equally spaced from the sides in the center of the square and designated as “old object” and “novel object” zones using the AnyMaze video tracking system (Stoelting Co., Wood Dale, IL). The old object used was square-shaped, whereas the novel object was L-shaped, assembled by building blocks from LEGO® toys, and were clearly distinct in shape and appearance.
On PID 20, all animals were placed in the open field without objects for 5 min each for habituation. Two 5-min trials were performed on PID 21: the first (training) trial with two old objects in both zones and the second (testing) phase with one old object and one novel object present in the respective zones of the open field, with an intertrial interval of 60 min. The time spent in novel and old object zones was assessed manually as well as by using AnyMaze software. Retention memory was determined by “Discrimination Index” (D.I.) for the second trial that was calculated as follows24,25:
% D.I.=Time spent in novel object zone×100.
(Time spent in old object zone+Time spent in novel object zone)
Passive avoidance test for fear-based cognitive function
The passive avoidance test (sham: n=8; LFP: n=11) was used to evaluate nonspatial fear-based amygdala-dependent contextual and emotional memory on PIDs 23–24, as previously described24 using one-trial step-through PACS-30 passive avoidance apparatus (Columbus Instruments, Columbus, OH) adjusted to an electric foot shock setting of 0.5 mA for 3 sec with a 24-h lag between training and testing. The cognitive impairment was assessed in terms of latency to the dark compartment in the testing phase.
Forced swim test for depressive-like symptoms
The forced swim test (sham: n=8; LFP: n=11) was used to evaluate depressive-like behavior.24,26 On PID 26, rats were individually forced to swim inside a vertical rectangular container, containing 30 cm of water maintained at 24–25°C for a period of 6 min. The total duration of immobility versus struggle was recorded manually using a timer.
Immunocytochemistry
Tissue processing
Immediately after the EEG and EMG recording on PID 29 and approximately 5 h into the active phase, the rats were anesthetized and transcardially perfused with saline, followed by 4% buffered paraformaldehyde solution (Fisher Scientific, Pittsburgh, PA) containing 2.5% acrolein (Sigma Aldrich, St. Louis, MO). The brains were removed, post-fixed in paraformaldehyde for 24 h and protected in 30% sucrose.
Brains were serially cut in the coronal plane on a cryostat and stored in a cryoprotectant solution (ethylene glycol/glucose in phosphate buffer) at −20°C until processed.5 Free-floating sections were rinsed in potassium phosphate buffered saline to remove cryoprotectant, and the sections were then incubated for 30 min in 0.5% sodium borohydride to neutralize the acrolein fixative. The sections were stained for ORX-A using rabbit anti-ORX-A primary immunoglobulin G (IgG) antibody (1:20,000; Phoenix Pharmaceuticals, Belmont, CA) for 48 h followed by incubation with biotinylated antirabbit IgG antibody (Vector Laboratories, Burlingame, CA) and processed further as described previously.
Quantification of ORX-A was performed by unbiased stereology using a Nikon Eclipse E600 microscope and Stereo Investigator software (MBF Biosciences, Williston, VT). A total of five anatomically matched sections of the hypothalamus were used for analysis. Sections analyzed were from a one-in-four series (adjacent sections were separated by 120 μm) roughly between the bregma coordinates −2.8 mm and −3.3 mm, based on the Paxinos and Watson (1998) rat brain atlas. The lateral hypothalamus was defined by a 1200 μm×600 μm box with horizontal alignment at the most ventral portion of the fornix and vertical alignment at 600 μm from either direction of the fornix for both hemispheres of the brain. The optical dissector had a size of 50 μm×50 μm in the x- and y-axis, respectively, with a dissector height of 10 μm and guard zone height of 4 μm from the top of the section. Grid spacing of 400 μm in the x-axis and 400 μm in the y-axis was used.
The volume of the region of interest was measured using the Cavalieri estimator method with grid spacing of 150 μm for the cortex. The estimated number of ORX-A–positive neurons in each field was divided by the volume of the region of interest to obtain the cellular density expressed in counts/mm3.
Statistical analysis
The quantification of data was expressed as mean±standard error of the mean. Analysis of sleep-wake states was performed using repeated-measures one-way analysis of variance followed by multiple pairwise comparisons using the Student-Newman-Keuls post-hoc test. The data for forced swim, passive avoidance test, and ORX-A expression were statistically analyzed by two-tailed unpaired Student t test. The data for cognitive impairments in novel object recognition were analyzed by two-tailed Mann-Whitney U t test. The functional and stereological data were analyzed using SigmaPlot 12 (Systat Software, San Jose, CA) or GraphPad Prism Version 4.0 (GraphPad Software, San Diego, CA). MATLAB (Mathworks) code created by Clauset and colleagues27 was used to determine the two-sample Kolmogorov-Smirnov test of the cumulative distribution probability. A p<0.05 was considered statistically significant.
Results
LFP results in cognitive deficits and depressive-like symptoms
Retention or intact memory function was evaluated using the NOR test24,25,28; LFP caused significant memory impairment (Fig. 1B; ***p<0.001 vs. sham). Nonspatial contextual/emotional memory was assessed using the passive avoidance test.29 LFP caused significant cognitive impairments compared with the sham group (Fig. 1C; *p<0.05 vs. sham). LFP also induced depressive-like symptoms in rats as assessed by the forced swim test24,26 (Fig. 1D; **p<0.01 vs. sham).
LFP results in alterations in sleep-wake behavior
Sleep-wake behavior at PIDs 6, 19, and 29 provided a longitudinal evaluation of the effects of TBI on the ability of animals to remain awake or asleep. Recordings with EEG and EMG telemetry before and after the behavioral tests enabled the measurement of sleep-wake behavior that coincided with the emergence of chronic behavior deficits. Visual inspection of hypnograms of the dark phase at PID 6, 19, and 29 of sham (Fig. 2A,C,E) and injured (Fig. 2B,D,F) suggested that LFP-injured animals had attenuated wake bouts during the active (dark) phase. This observation indicated that LFP resulted in wake fragmentation that was investigated further by performing a quantitative analysis of the sleep-wake architecture, in terms of total time in wake, NREM, and REM, number of episodes, and mean episode duration.
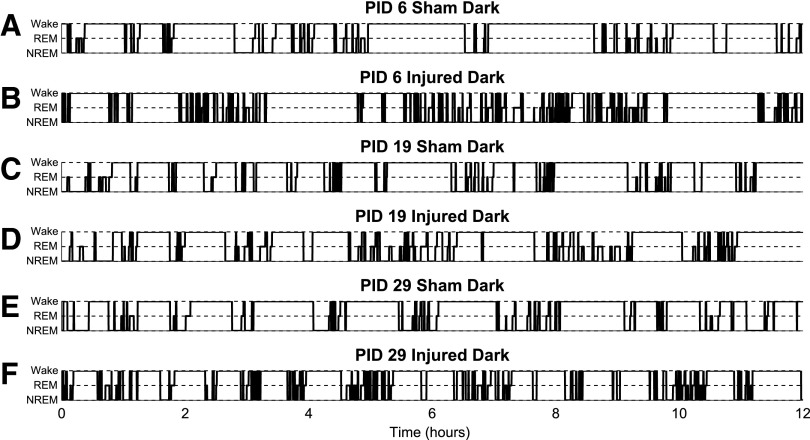
FIG. 2.
Hypnograms of sleep-wake bouts during dark (active) phase. Rats are nocturnal and are most active during the dark phase. Hypnograms representing the transitions of sleep-wake states during the 12-h dark (active) phase at post-injury day (PID) 6, 19, and 29. Fragmentation of wake bouts was observed throughout the study. (A) At PID 6, wake bouts of shams consisted of wake bout >1 h. (B) Injured animals at PID 6 were able to maintain wake bouts similar to shams but sustained non-rapid eye movement (NREM) and REM bouts were minimal. (C) and (D) Hypnograms of sham and injured animals appeared to be similar at PID 19 and are consistent with the quantitative results in Figures 3, 4, and 5. Differences in groups were not observed and might be indicative of a recovery or transition phase before chronic sleep-wake deficits. (E) The sham animal example has multiple sustained wake bouts >30 min throughout the entire period. (F) The injured animal example has fragmented wake bouts interrupted by NREM.
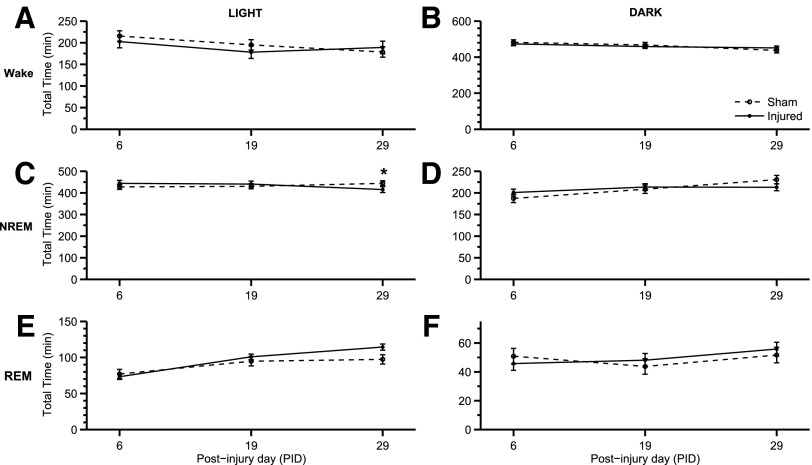
Interestingly, the total time in the states of wake, NREM, and REM was not significantly different between sham and injured animal groups during both light and dark phases. The total time spent in each state based on PID during the light phase did change, however: wake (Fig. 3A; F(2,28)=3.545; p=0.042), NREM (Fig. 3C; F(2,28)=3.969; p=0.030), and REM (Fig. 3E; F(2,28)=16.118; p<0.001). During the light phase, there was a significant interaction among animal group and PID (Fig. 3C; F(2,28)=3.379; p=0.048) for NREM. Taken together, these results suggest that injured animals exhibited an inability to maintain arousal during the active phase.
FIG. 3.
Total time of sleep-wake states. Few changes in total time in wake, non-rapid eye movement (NREM), and REM were observed throughout the study. (A) The total time in wake in the light phase changed based on the post-injury day (PID) (F(2,28)=3.545; p=0.042). (B) Total time in wake, however, was consistent throughout the study in the dark phase. (C) During the light phase, total time in NREM changed based on PID (F(2,28)=3.969; p=0.030). At PID 29, there was a slight decrease (*p<0.05, vs. sham) in the total time injured animals were in NREM during the light phase when the animals sleep the most. (D) This decrease in NREM, however, is not observed in the dark phase. (E) During the light phase, total time in REM did change based on PID (F(2,28)=16.118; p<0.001). (F) There was no significant change in total time in REM during the dark phase. Analysis by one-way (groups) repeated measures (trials/time) analysis of variance (A-F) followed by Student-Newman-Keuls post hoc test. Mean±standard error of the mean; n=11/injured group, n=8 (sham). Sham is open circle with dashed line. Moderate injury is solid circle and solid line.
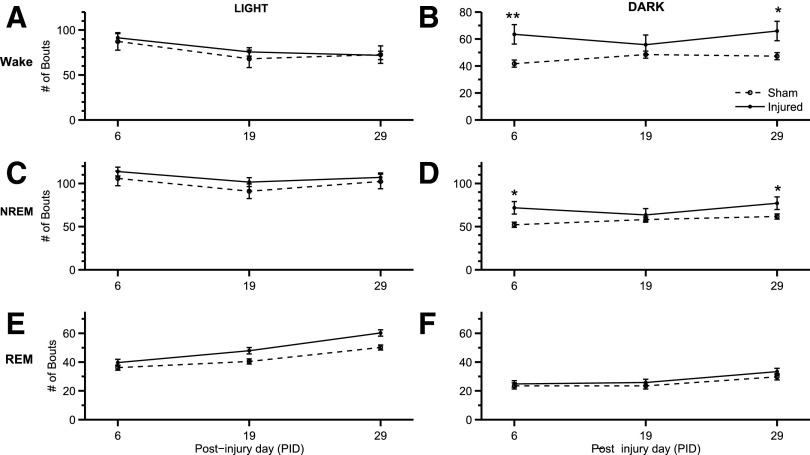
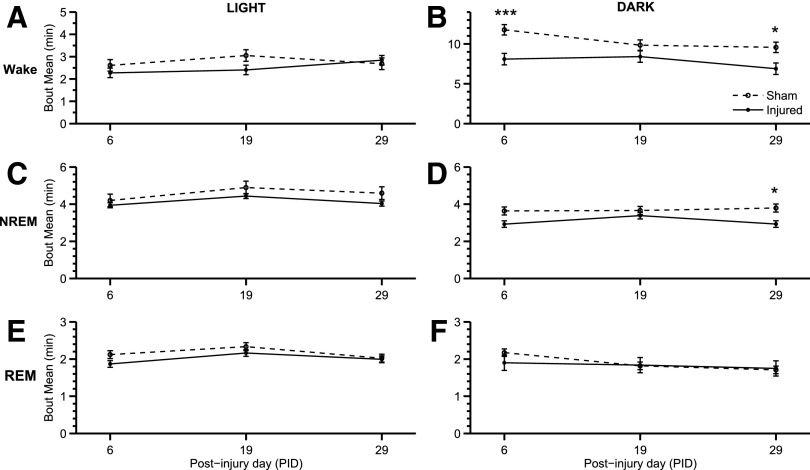
The number of bouts (Fig. 4) was inversely related to mean bout lengths (Fig. 5) throughout the study. There was an increase in number of wake bouts in the dark phase (Fig. 4B) at PID 6 (p=0.005) and PID 29 (p=0.015). In addition, there was also an increase in the number of NREM bouts during the dark phase (Fig. 4D) at PID 6 (p=0.010) and PID 29 (p=0.041). There were no observed significant differences during the light phase. LFP-injured animals exhibited significantly shorter wake (Fig. 5B; F(1,28)=21.848; p<0.001) and shorter NREM bouts (Fig. 5D; F(1,28)=12.183; p=0.004) during the dark phase. Similarly, the number of wake and NREM bouts increased in injured animals during the dark phase (Fig. 4B,D). Wake and sleep bouts were not altered during the light phase for either number of bouts and bout length.
FIG. 4.
Number of bouts of sleep-wake states. The number of bouts for wake, non-rapid eye movement (NREM), and REM were assessed during the light and dark phases at post-injury day (PID) 6, 19, and 29. Significant increases in wake and NREM bouts were found during the dark (active) phase. (A) The amount of wake bouts changed based on PID (F(2,28)=5.679; p=0.009). (B) During the dark phase, there was an increase by the injured group in the number of wake bouts (F(2,28)=16.590; p=0.001). Injured animals showed an increase in wake bouts at PID 6 (**p<0.01 vs. sham) and 29 (*p<0.05 vs. sham). (C) No significance difference was found in NREM bouts during the light phase. (D) Injured animals showed an increased in the number of NREM bouts during the dark phase based on the experimental group (F(1,28)=8.961; p=0.010). Injured animals demonstrated an increase in the number of bouts at PID 6 (*p=0.010 vs. sham) and 29 (*p=0.041 vs. sham). (E) During the light phase, the number of REM bouts changed based on PID (F(2,28)=13.230; p<0.001). (F) In addition, during the dark phase, the number of REM bouts changed based on PID (F(2,28)=9.292; p<0.001). Analysis by one-way (groups) repeated measures (trials/time) analysis of variance (A-F) followed by Student-Newman-Keuls post hoc test. Mean±standard error of the mean; n=11/injured group, n=8 (sham). Sham is open circle with dashed line. Moderate injury is solid circle and solid line.
FIG. 5.
Mean bout length of sleep-wake states. Changes in bout lengths were assessed for wake, non-rapid eye movement (NREM), and REM during the light and dark phases at PID 6, 19, and 29. Significant decreases in wake and NREM bout length were found during the dark (active) phase. (A) There was no significant change in wake bout length during the light phase. (B) Bout length of wake during the dark phased decreased (F(1,28)=21.848; p<0.001) in injured animals. The length of wake bouts was significantly shorter at PID 6 (***p<0.001 vs. sham) and 29 (*p<0.05 vs. sham). (C) During the light phase, there was not a significant change in NREM bout length. (D) Similar to wake bouts, injured animals had NREM bout lengths significantly shorter (F(1,28)=12.183; p<0.01) than sham animals in the dark phase. Lateral fluid percussion resulted in shorter NREM bout lengths at PID 29 (*p<0.05 vs. sham). (E) and (F) There was no significant difference in REM bout lengths during the light and dark phase, respectively. Analysis by one-way (groups) repeated measures (trials/time) analysis of variance (A-F) followed by Student-Newman-Keuls post hoc test. Mean±standard error of the mean; n=11/injured group, n=8 (sham). Sham is open circle with dashed line. Moderate injury is solid circle and solid line.
At PID 19, post hoc analysis showed no significant changes related to wake, NREM bout length, or number of bouts. At PID 29, however, bout lengths of wake (Fig. 5B; p=0.007) and NREM (Fig. 5D; p=0.019) were significantly shorter. The changes in sleep-wake behavior and architecture are illustrated in Figure 2 by the fragmentation of wake bouts. During the last EEG/EMG recording at PID 29, sleep-wake states demonstrated behavior similar to PID 6. In addition, NREM was significantly decreased during the light phase of PID 29 (Fig. 3C). The dark phase exhibited increases in the number of bouts and mean bout length for both the wake and NREM.
LFP impacts the distribution of sleep-wake bouts
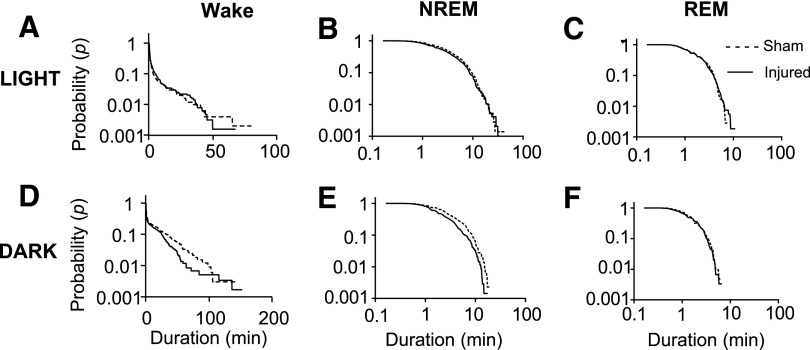
The dynamics of sleep-wake transitions were further investigated by calculating the probability of a bout of a certain duration by plotting the cumulative distribution on a semi-log scale for wake bouts (Fig. 6A,D) and the log-log scale for sleep bouts (Fig. 6B,C,E,F). Statistical significance was determined by Kolmogorov-Smirnov along with parametric estimations for the power law and exponential curve-fit to verify goodness-of-fit.
FIG. 6.
Cumulative distribution of sleep-wake states at PID 29. Sleep-wake behavior remained fragmented at PID 29 demonstrated by plotting the probability of a bout of a certain length. (A) During the light phase, there was a decrease in probability in wake bouts of injured animals greater than approximately 50 min (p<0.001). (B) and (C) There was no significant difference in the distribution of non-rapid eye movement (NREM) and REM bouts during the light phase, respectively. (D) The wake bouts during the dark phase of injured animals decreased at intermediate bout lengths from approximately 30 to 100 min (p<0.001). (E) During the dark phase, there was a decrease in NREM bout lengths greater than approximately 1 min in injured animals (p=0.0011). (F) In the dark phase, REM bout lengths were not different between sham and injured animals. The Kolmogorov-Smirnov test was used to determine statistical significance.
The number of bouts and mean bout length only described average changes, whereas the cumulative distribution curves demonstrate the probability of a bout of a certain length occurring within either the light or dark phase. Interestingly, both light and dark phases for all post-injury days of wake was highly significant (p<0.001; only PID 29 shown). The distribution of NREM bouts was significantly shorter during the dark phase (p=0.0011; Fig. 6E) but not the light phase (Fig. 6B). Another significant difference between sham and injured animals was an increase in REM bouts at PID 6 during the dark phase (p=0.0082; not shown).
LFP causes reduction in ORX-A–positive neurons in the lateral hypothalamus
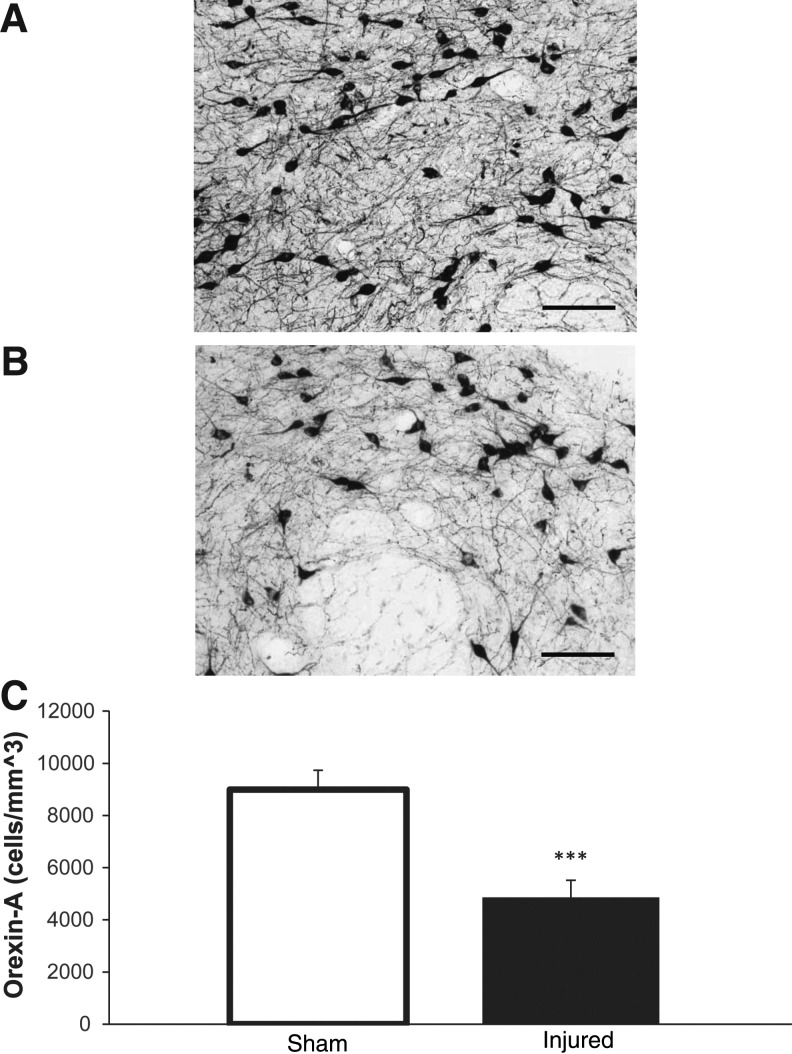
Quantitative assessment of ORX-A-positive neurons in the perifocal area of the lateral hypothalamus showed that LFP caused a significant decrease (4862±656 vs. 8995±7 43 counts/mm3, p<0.001, vs. sham) in the density of ORX-A-positive neurons (Fig. 7).
FIG. 7.
Orexin-A (ORX-A) in the perifornical region of the lateral hypothalamus. ORX-A positive cells were quantified bilaterally in the perifornical region of the lateral hypothalami. (A) Sham 20X, (B) injured 20X. (C) There was significant decrease (***p<0.001, vs. sham) in ORX-A positive cells in injured animals. Error bars for A and B are 100 μm. Analysis by two-tailed unpaired Student t test (C). Mean±standard error of the mean; n=6/group.
Discussion
TBI causes EDS in many patients.30 Diagnosis and assessment of sleep disorders in patients with TBI rely heavily on patient diaries31,32 and questionnaires such as the Pittsburgh Sleep Quality Index33 and Epworth sleepiness scale.34 These assessments, although widely used, are limited by patient subjectivity. Clinical studies are also problematic in that they include varying injury severities and limited population size. Analysis of sleep cycles with polysomnograms is needed to determine specific sleep disorders, but it may overlook daytime activity or wakefulness, and sleep behavior is usually only monitored for a short duration (i.e., one night). Continuous polysomnograms may be difficult to perform in patients with moderate to severe TBI. Fatigue may also be a cause of sleepiness after TBI,35 and comorbidities such as anxiety and depression can be confounding.36
Studies suggest that sleep disorders associated with TBI may also exacerbate cognitive deficits.2 Further investigations using experimental models, however, are needed to understand underlying mechanisms that exacerbate the functional impairments. Limited diagnostic tools and potential relationship to long-term behavioral dysfunction, such as cognitive deficits, underscores the need for experimental modeling.2 The rat LFP model simulates many pathobiological features of clinical contusive head trauma.23,37–39 It produces both focal and diffuse injury with vascular disruption, neuronal cell death, and glial proliferation.23,37–39
In this study, we investigated the chronic disruptions in sleep-wake behavior along with cognitive impairments and depression after LFP injury. Trauma caused significant impairments in memory retention as well as fear-based contextual and emotional memory functions, as indicated by the cognitive decline observed in novel object recognition and passive avoidance tasks, respectively. Further, LFP-injured animals demonstrated depressive-like behavior in the forced swim test.
Sleep-wake behavior was assessed in terms of degree of wakefulness, REM, and NREM sleep patterns. Fragmentation in the wake bouts was observed during the dark phase, but no changes were found in light phase sleep-wake behavior except for the total time in NREM during PID 29 (Fig. 3C). In addition, no differences in the REM total time, mean bout length, and number of bouts were observed (Fig. 3,4, 5), which has been previously reported in a focal TBI model of controlled cortical impact.12 We did not observe wake to REM transitions indicative of narcolepsy. There was fragmentation of NREM bouts during the dark phase, however. Notably, our study is the first to explore and determine such sleep-wake disturbances up to a month after experimental TBI.
Fragmentation of wake bouts was observed throughout the study. Wake bouts in sham animals consisted of wake bout >1 h. In contrast, the injured animals were able to maintain wake bouts similar to shams, but with minimal sustained NREM and REM bouts at 6 days after TBI. Although the hypnograms of sham and injured animals appeared to be similar at 19 days after TBI, injured animals showed fragmented wake bouts interrupted by NREM at 1 month.
There were no significant differences between groups in light and dark phases with respect to total time in wake, NREM, and REM. Significant increases in the number of wake and NREM bouts were observed in the TBI group during the dark (active) phase at 6 and 19 days after injury, however. The number of bouts was observed to be inversely proportional to the bout length throughout the study. Injured animals showed significant decreases in wake and NREM bout lengths at acute as well as chronic time points, when compared with the sham group.
To further understand the sleep-wake architecture, we calculated the probability of a bout of certain duration within either phase by plotting a cumulative distribution on a semi-log scale for wake bouts and log-log scale for sleep bouts. There were no differences between the two groups at earlier time points. There was a decrease in probability of wake bouts of injured animals in the light phase, however, 1 month after TBI, as well as a decrease in NREM bout lengths. Taken together, our findings indicate that LFP resulted in an inability to maintain consolidated wake bouts, particularly in the active phase.
TBI causes dynamic changes with regard to pathobiology and behavior, some of which may be multiphasic. In part, this may reflect the complex secondary injury processes initiated by trauma, including both neurodestructive and neurorestorative mechanisms. In our study, sleep-wake behavior was altered after TBI on PID 6 and 29, with minimal changes at PID 19. This underscores the importance of assessing multiple post-injury time points, as done here, in contrast to most previous clinical and pre-clinical assessments. Further sleep-wake behavior studies are needed to elucidate the mechanisms underlying the fragmentation of sleep-wake behavior as a function of time post-injury, and to correlate with biochemical and histological changes.
LFP injury caused significant decreases in ORX-A–positive cells in the lateral hypothalamus, which is believed to stabilize the transitions between wakefulness and sleep by innervating neurons throughout the central nervous system that are important to arousal. Previous studies demonstrated a slight increase in ORX-A-positive neurons up to a week after injury,12,40 with subsequent decreases near the injury site associated with loss of efferent connectivity by ORX-positive neurons in the hypothalamus.40
We found significant reduction in numbers of ORX-A-positive cells in the lateral hypothalamus 1 month after TBI, associated with the chronic loss of arousal. Our data are consistent with the previous clinical finding that the loss of wakefulness correlates with a decrease in ORX levels after TBI.16,41–43 Cortical excitability decreases after TBI, suggesting a decrease in orexinergic input.44 The lack of ability to maintain a normal bout length is consistent with lesion and knockout studies of ORX.45,46 In our study, in addition to shorter wake bouts, we observed shorter NREM bouts during the dark (active) phase.
Conclusion
Our study demonstrates polyphasic changes of sleep-wake cycles in a rodent TBI model. There was fragmentation of sleep-wake behavior, suggesting a chronic loss of arousal, which was associated with reduced ORX-A levels in the hypothalamus. Our findings provide further evidence that TBI leads to chronic sleep-wake disruptions, associated with disruptions of the orexinergic system. TBI-induced sleep-wake disorders may also exacerbate cognitive and depressive-like changes that commonly occur after injury and that were observed in our animal model. Use of pharmacotherapy to modulate ORX levels may therefore not only serve to improve wakefulness abnormalities after TBI, but may secondarily also influence post-traumatic cognitive or affective changes.
Acknowledgments
We thank Michael Makley, Adam Puche, and Cecilia Diniz Behn for their help with the sleep-wake analysis and the National Institutes of Health (5 T32 GM 75767-5) for their support.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Marr A.L., and Coronado V.G., eds (2004). Central Nervous System Injury Surveillance Data Submission Standards—2002. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control [Google Scholar]
- 2.Wiseman-Hakes C., Murray B., Moineddin R., Rochon E., Cullen N., Gargaro J., and Colantonio A. (2013). Evaluating the impact of treatment for sleep/wake disorders on recovery of cognition and communication in adults with chronic TBI. Brain Inj. 27, 1364–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meerlo P., Mistlberger R.E., Jacobs B.L., Heller H.C., and McGinty D. (2009). New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med. Rev. 13, 187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker M.P., Stickgold R., Alsop D., Gaab N., and Schlaug G. (2005). Sleep-dependent motor memory plasticity in the human brain. Neuroscience 133, 911–917 [DOI] [PubMed] [Google Scholar]
- 5.Baumann C.R., Werth E., Stocker R., Ludwig S., and Bassetti C.L. (2007). Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain 130, 1873–1883 [DOI] [PubMed] [Google Scholar]
- 6.Masel B.E., Scheibel R.S., Kimbark T., and Kuna S.T. (2001). Excessive daytime sleepiness in adults with brain injuries. Arch. Phys. Med. Rehabil. 82, 1526–1532 [DOI] [PubMed] [Google Scholar]
- 7.Verma A., Anand V., and Verma N.P. (2007). Sleep disorders in chronic traumatic brain injury. J. Clin. Sleep Med. 3, 357–362 [PMC free article] [PubMed] [Google Scholar]
- 8.Rowe R.K., Harrison J.L., O'Hara B.F., and Lifshitz J. (2014). Recovery of neurological function despite immediate sleep disruption following diffuse brain injury in the mouse: clinical relevance to medically untreated concussion. Sleep 37, 743–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hazra A., Macolino C., Elliott M.B., and Chin J. (2014). Delayed thalamic astrocytosis and disrupted sleep-wake patterns in a preclinical model of traumatic brain injury. J. Neurosci. Res. 92, 1434–1445 [DOI] [PubMed] [Google Scholar]
- 10.Rowe R.K., Harrison J.L., O'Hara B.F., and Lifshitz J. (2014). Diffuse brain injury does not affect chronic sleep patterns in the mouse. Brain Inj. 28, 504–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowe R.K., Striz M., Bachstetter A.D., Van Eldik L.J., Donohue K.D., O'Hara B.F., and Lifshitz J. (2014). Diffuse brain injury induces acute post-traumatic sleep. PloS One 9, e82507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willie J.T., Lim M.M., Bennett R.E., Azarion A.A., Schwetye K.E., and Brody D.L. (2012). Controlled cortical impact traumatic brain injury acutely disrupts wakefulness and extracellular orexin dynamics as determined by intracerebral microdialysis in mice. J. Neurotrauma 29, 1908–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petraglia A.L., Plog B.A., Dayawansa S., Chen M., Dashnaw M.L., Czerniecka K., Walker C.T., Viterise T., Hyrien O., Iliff J.J., Deane R., Nedergaard M., and Huang J.H. (2014). The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J. Neurotrauma 31, 1211–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rauchs G., Bertran F., Guillery-Girard B., Desgranges B., Kerrouche N., Denise P., Foret J., and Eustache F. (2004). Consolidation of strictly episodic memories mainly requires rapid eye movement sleep. Sleep 27, 395–401 [DOI] [PubMed] [Google Scholar]
- 15.Stickgold R. (2005). Sleep-dependent memory consolidation. Nature 437, 1272–1278 [DOI] [PubMed] [Google Scholar]
- 16.Baumann C.R., Bassetti C.L., Valko P.O., Haybaeck J., Keller M., Clark E., Stocker R., Tolnay M., and Scammell T.E. (2009). Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann. Neurol. 66, 555–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujiki N., and Nishino S. (2007). Neuropeptides as possible targets in sleep disorders: special emphasis on hypocretin-deficient narcolepsy. CNS Neurol. Disord. Drug Targets 6, 45–62 [DOI] [PubMed] [Google Scholar]
- 18.Lu J., Sherman D., Devor M., and Saper C.B. (2006). A putative flip-flop switch for control of REM sleep. Nature 441, 589–594 [DOI] [PubMed] [Google Scholar]
- 19.Saper C.B., Chou T.C., and Scammell T.E. (2001). The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 24, 726–731 [DOI] [PubMed] [Google Scholar]
- 20.Sakurai T. (2005). Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Med. Rev. 9, 231–241 [DOI] [PubMed] [Google Scholar]
- 21.Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R.M., Tanaka H., Williams S.C., Richarson J.A., Kozlowski G.P., Wilson S., Arch J.R., Buckingham R.E., Haynes A.C., Carr S.A., Annan R.S., McNulty D.E., Liu W.S., Terrett J.A., Elshourbagy N.A., Bergsma D.J., and Yanagisawa M. (1998). Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 [DOI] [PubMed] [Google Scholar]
- 22.Kang J.E., Lim M.M., Bateman R.J., Lee J.J., Smyth L.P., Cirrito J.R., Fujiki N., Nishino S., and Holtzman D.M. (2009). Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabadi S.V., Hilton G.D., Stoica B.A., Zapple D.N., and Faden A.I. (2010). Fluid-percussion-induced traumatic brain injury model in rats. Nat. Protoc. 5, 1552–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabadi S.V., Stoica B.A., Loane D.J., Luo T., and Faden A.I. (2014). CR8, a novel inhibitor of CDK, limits microglial activation, astrocytosis, neuronal loss, and neurologic dysfunction after experimental traumatic brain injury. J. Cereb. Blood Flow Metab. 34, 502–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo T., Wu J., Kabadi S.V., Sabirzhanov B., Guanciale K., Hanscom M., Faden J., Cardiff K., Bengson C.J., and Faden A.I. (2013). Propofol limits microglial activation after experimental brain trauma through inhibition of nicotinamide adenine dinucleotide phosphate oxidase. Anesthesiology 119, 1370–1388 [DOI] [PubMed] [Google Scholar]
- 26.Slattery D.A., and Cryan J.F. (2012). Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 7, 1009–1014 [DOI] [PubMed] [Google Scholar]
- 27.Clauset A., Shalizi C.R., and Newman M.E. (2009). Power-law distributions in empirical data. SIAM Rev. 51, 661–703 [Google Scholar]
- 28.Bevins R.A., and Besheer J. (2006). Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study “recognition memory.” Nat. Protoc. 1, 1306–1311 [DOI] [PubMed] [Google Scholar]
- 29.Zhao Z., Loane D.J., Murray M.G., 2nd, Stoica B.A., and Faden A.I. (2012). Comparing the predictive value of multiple cognitive, affective, and motor tasks after rodent traumatic brain injury. J. Neurotrauma 29, 2475–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verma A., Anand V., and Verma N.P. (2007). Sleep disorders in chronic traumatic brain injury. J. Clin. Sleep Med. 3, 357–362 [PMC free article] [PubMed] [Google Scholar]
- 31.Parcell D.L., Ponsford J.L., Rajaratnam S.M., and Redman J.R. (2006). Self-reported changes to nighttime sleep after traumatic brain injury. Arch. Phys. Med. Rehabil. 87, 278–285 [DOI] [PubMed] [Google Scholar]
- 32.Ponsford J.L., Parcell D.L., Sinclair K.L., Roper M., and Rajaratnam S.M.W. (2013). Changes in sleep patterns following traumatic brain injury: a controlled study. Neurorehabil. Neural Repair 27, 613–621 [DOI] [PubMed] [Google Scholar]
- 33.Fichtenberg N.L., Zafonte R.D., Putnam S., Mann N.R., and Millard A.E. (2002). Insomnia in a post-acute brain injury sample. Brain Inj. 16, 197–206 [DOI] [PubMed] [Google Scholar]
- 34.Wiseman-Hakes C., Victor J.C., Brandys C., and Murray B.J. (2011). Impact of post-traumatic hypersomnia on functional recovery of cognition and communication. Brain Inj. 25, 1256–1265 [DOI] [PubMed] [Google Scholar]
- 35.Schönberger M., Herrberg M., and Ponsford J. (2014). Fatigue as a cause, not a consequence of depression and daytime sleepiness: a cross-lagged analysis. J. Head Trauma Rehabil. 29, 427–431 [DOI] [PubMed] [Google Scholar]
- 36.Fogelberg D.J., Hoffman J.M., Dikmen S., Temkin N.R., and Bell K.R. (2012). Association of sleep and co-occurring psychological conditions at 1 year after traumatic brain injury. Arch. Phys. Med. Rehabil. 93, 1313–1318 [DOI] [PubMed] [Google Scholar]
- 37.Graham D.I., Raghupathi R., Saatman K.E., Meaney D., and McIntosh T.K. (2000). Tissue tears in the white matter after lateral fluid percussion brain injury in the rat: relevance to human brain injury. Acta Neuropathol. (Berl.) 99, 117–124 [DOI] [PubMed] [Google Scholar]
- 38.Thompson H.J., Lifshitz J., Marklund N., Grady M.S., Graham D.I., Hovda D.A., and McIntosh T.K. (2005). Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma 22, 42–75 [DOI] [PubMed] [Google Scholar]
- 39.Dixon C.E., Lighthall J.W., and Anderson T.E. (1988). Physiologic, histopathologic, and cineradiographic characterization of a new fluid-percussion model of experimental brain injury in the rat. J. Neurotrauma 5, 91–104 [DOI] [PubMed] [Google Scholar]
- 40.Mihara Y., Dohi K., Yofu S., Nakamachi T., Ohtaki H., Shioda S., and Aruga T. (2011). Expression and localization of the orexin-1 receptor (OX1R) after traumatic brain injury in mice. J. Mol. Neurosci. MN 43, 162–168 [DOI] [PubMed] [Google Scholar]
- 41.Ponsford J.L., Ziino C., Parcell D.L., Shekleton J.A., Roper M., Redman J.R., Phipps-Nelson J., and Rajaratnam S.M. (2012). Fatigue and sleep disturbance following traumatic brain injury—their nature, causes, and potential treatments. J. Head Trauma Rehabil. 27, 224–233 [DOI] [PubMed] [Google Scholar]
- 42.Kempf J., Werth E., Kaiser P.R., Bassetti C.L., and Baumann C.R. (2010). Sleep-wake disturbances 3 years after traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 81, 1402–1405 [DOI] [PubMed] [Google Scholar]
- 43.Baumann C.R., Stocker R., Imhof H.-G., Trentz O., Hersberger M., Mignot E., and Bassetti C.L. (2005). Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury. Neurology 65, 147–149 [DOI] [PubMed] [Google Scholar]
- 44.Nardone R., Bergmann J., Kunz A., Caleri F., Seidl M., Tezzon F., Gerstenbrand F., Trinka E., and Golaszewski S. (2011). Cortical excitability changes in patients with sleep-wake disturbances after traumatic brain injury. J. Neurotrauma 28, 1165–1171 [DOI] [PubMed] [Google Scholar]
- 45.Chemelli R.M., Willie J.T., Sinton C.M., Elmquist J.K., Scammell T., Lee C., Richardson J.A., Williams S.C., Xiong Y., Kisanuki Y., Fitch T.E., Nakazato M., Hammer R.E., Saper C.B., and Yanagisawa M. (1999). Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98, 437–451 [DOI] [PubMed] [Google Scholar]
- 46.Gerashchenko D., Blanco-Centurion C., Greco M.A., and Shiromani P.J. (2003). Effects of lateral hypothalamic lesion with the neurotoxin hypocretin-2-saporin on sleep in Long-Evans rats. Neuroscience 116, 223–235 [DOI] [PubMed] [Google Scholar]