Abstract
Ethanol increases the interstitial dopamine (DA) concentration in the nucleus accumbens (NAcc) of experimental animals, but positron emission tomography (PET) studies using the single-bolus protocol of the [11C]-raclopride competition paradigm have yielded conflicting results in humans. To resolve disparate previous findings, we utilized the bolus-plus-infusion (B/I) method, allowing both baseline and intervention quantification of [11C]raclopride binding during a single 105-minute PET scan, to investigate possible ethanol-induced DA release in nine healthy male subjects. A 25-minute intravenous ethanol (7.6%) infusion, resulting in a 1.3 g/L mean blood ethanol concentration, was administered using masked timing during the PET scan. Automated region-of-interest analysis testing the difference between baseline (40–50 minutes) and intervention (60–85 minutes) revealed an average 12.6% decrease in [11C]raclopride binding in the ventral striatum (VST, P=0.003) including the NAcc. In addition, a shorter time interval from the start of ethanol infusion to the first subjective effect was associated with a greater binding potential decrease bilaterally in the VST (r=0.92, P=0.004), and the feeling of pleasure was associated with a decrease in binding potential values in both the caudate nucleus (r=−0.87, P=0.003) and putamen (r=−0.74; P=0.02). These results confirm that ethanol induces rapid DA release in the limbic striatum, which can be reliably estimated using the B/I method in one imaging session.
Keywords: [11C] raclopride, alcohol, bolus-plus-infusion, dopamine, ethanol, positron emission tomography
Introduction
There is plenty of evidence indicating that both forced and voluntary ethanol administration induces dopamine (DA) release in the ventral striatum (VST) of experimental animals (for a review see the study by Pierce and Kumaresan1). Positron emission tomography (PET) imaging with the DA D2/D3 receptor antagonist ligand [11C]raclopride provides a non-invasive tool for experimentally measuring the induced endogenous DA release in vivo in humans. According to the competition principle, [11C]raclopride competes with DA in binding the same DA D2/D3 receptors and, thus, an experimentally induced increase in the endogenous DA release results in decreased [11C]raclopride binding. The competition principle has been applied in numerous studies that have shown increases in striatal DA levels in humans as a response to psychoactive substances (for a review see the study by Laruelle2).
Previous PET studies exploring the effects of acute alcohol intervention on DA neurotransmission in humans have yielded conflicting results. The first [11C]raclopride PET study applying oral alcohol administration did not show any effect,3 but in a later study decreased [11C]raclopride binding was shown, suggesting an increased DA concentration in the VST, although the effect varied considerably among the subjects.4 Two studies using prolonged stable intravenous ethanol infusion during PET data acquisition failed to detect changes in striatal [11C]raclopride binding.5, 6 However, in another study, the same group reported that intravenous ethanol without alcohol-related cues increased, but alcohol-related cues alone (without ethanol intervention) decreased DA concentration in the limbic striatum.7 A recent study applying oral alcohol administration, found a systematic decrease in [11C]raclopride binding in all striatal subregions, suggesting a quite non-specific DA release in male subjects.8 The dopaminergic effects of ethanol could not, however, be differentiated from the influence of expectation or sensory effects related to drinking alcohol.
All the studies mentioned above utilized study designs involving two separate PET experiments: one during ethanol intervention and another during the control condition. Changes in endogenous DA concentration during a quantification period violate the equilibrium assumption of the conventional models, and the entire scan is usually utilized as the outcome measure in studies using single-bolus [11C]raclopride measurements.3, 4, 5, 6, 7 This could result in an underestimation of intervention-induced BPND decrease, which in combination with a small effect size and inter-subject variability could conceal a decrease in BPND owing to DA release.9 Furthermore, the time resolution of the single-bolus method may not be suitable for measuring short-term changes in DA concentration. Finally, optimal timing of the intervention in relation to the PET scan is difficult to define.6, 10
The main problems encountered in the repeated single-bolus protocol can be avoided using a bolus-plus-infusion protocol (B/I), where [11C]raclopride is administered as a bolus followed by a constant infusion, which leads to a sustained equilibrium of radioligand levels in the blood and brain (for a review see the study by Carson11). The B/I method has been applied in one PET study on ethanol, but it utilized the repeated measurement protocol and only one quantification during the PET scan.8 However, the B/I method also enables double quantification during a single PET scan.12, 13 Using this type of methodology, the effects on [11C]raclopride binding, whether transient or longer lasting, induced by ethanol intervention can be quantified after baseline quantification. A short interval between baseline and intervention measurements reduces variation and the simple design increases the sensitivity in detecting weak changes in DA concentration.
The aim of the present study was to examine, in a single B/I scan design, whether a psychoactive dose of ethanol administered using a brief intravenous infusion would transiently decrease the binding of [11C]raclopride in the functional subdivisions of striatum, which would suggest an increased DA release. An automated region-of-interest (ROI) analysis and independent voxel-based receptor mapping analysis, with superior spatial accuracy, were both performed. Subjective responses to ethanol were recorded and correlated with baseline BPND values as well as with ethanol-induced changes in the BPND values.
Materials and methods
Subjects
The study was reviewed and approved by the Ethics committee of the Hospital District of Southwest Finland (Turku, Finland) and was conducted in accordance with the Declaration of Helsinki. Twelve healthy, non-smoking, Caucasian volunteer male subjects were enrolled after providing written informed consent and undergoing a thorough medical examination. The subjects were social alcohol drinkers (mean alcohol consumption: 4.2 U/week, range: 1–10 U, 1 U equaling 12 g of 100% ethanol); both total abstainers and excessive alcohol drinkers were excluded from the study. Excessive alcohol drinking was defined as the subject consuming more than 21 U of alcohol per week or more than 7 U in a single day. PET scans of two subjects failed because of a technical problem with tracer administration; one PET scan was interrupted because the subject experienced a headache during the scan. These three subjects were excluded from the analyses; thus, the sample size was nine and included only subjects with successful experiments. The age, weight, and height of the remaining nine subjects were 21±3 years, 77±8 kg, and 182±6 cm (mean±s.d.), respectively. To exclude organic brain changes and for anatomical reference, a magnetic resonance image of the brain was acquired from all subjects. Imaging was performed with a 1.5 T device (GE Signa, GE Medical Systems, Milwaukee, WI, USA). An axial T1 3D (fast spoiled gradient echo) and axial T2 weighted sequences were obtained.
Study Design
This was a non-randomized, open-label, single-group study with a within-subject design. Each subject underwent one [11C]raclopride B/I PET scan that included baseline and intervention quantification in a fixed order. The subjects were told that they would receive ethanol during PET scanning, but they were masked to the timing of ethanol administration to eliminate the effect of ethanol anticipation.
Ethanol Intervention
The ethanol solution (7.6%) was prepared by diluting 95% ethanol infusion concentrate (Alkohol-Konzentrat, B.Braun, Melsungen, Germany) with isotonic NaCl infusion solution (Fresenius Kabi, Bad Homburg, Germany). The ethanol solution was warmed to body temperature and administered using continuous intravenous infusion controlled by a volumetric infusion pump (IVAC), starting 50 minutes after the beginning of the scan and l asting for 25 minutes. To ensure that the subjects were masked to the timing of the intervention, the infusion pump and route were set up before the PET scanning and the ethanol intervention was started silently and remotely. The ethanol dose (A), targeted to result in a 0.8 g/L blood ethanol concentration in equilibrium, was individually calculated utilizing total body water (TBW) and using an equation by Watson et al14: A=(TBW/0.8) × C, where TBW is 2.447—(age (years) × 0.09516)+(height (cm) × 0.1074)+(body weight (kg) × 0.3362), and C is the equilibrated blood ethanol concentration. The target equilibrium blood ethanol concentration of 0.8 g/L was chosen because such an ethanol blood concentration typically induces psychomotoric effects in human subjects15 and comparable blood ethanol concentrations have been shown to promote a transient DA release in the rat nucleus accumbens (NAcc).16, 17 Moreover, such a mild ethanol exposure does not pose healthy subjects to any increased risk of medical hazard. Blood samples for ethanol blood concentration were collected before ethanol infusion, at the end of infusion and at 10, 20, and 30 minutes post infusion.
Behavioral Assessments
The subjective effects were assessed with a battery of questions based on subjective mood assessment scales.18 During the PET scan, subjects were asked to verbally rate, at 15-minute time intervals, the following items: feeling of intoxication (scale 0 to 10), depression versus high (−10 to 10), anxiety versus relaxed feeling (−10 to 10), unpleasant mood versus feeling of pleasure (−10 to 10). In addition, the subjects were asked to verbally rate a feeling of pain (0 to 10) and urge to urinate (0 to 10). Before the PET scan, the subjects were instructed to verbally indicate the first subjective feeling they regarded as an ethanol effect. The time interval between the start of ethanol infusion and the first subjective ethanol-related sensation was recorded.
Preparation of [11C]Raclopride
The precursor O-desmethylraclopride free base and raclopride were obtained from ABX Advanced Biochemical Compounds, Radeberg, Germany. [11C]Methane was produced at the Accelerator Laboratory of Åbo Akademi with a 103-cm isochronous Efremov cyclotron using the 14N(p,α)11C reaction. High specific radioactivity [11C]methyl iodide was prepared from [11C]methane.19, 20 The preparation of [11C]raclopride from [11C]methyl triflate was performed according to a published procedure.21 The radiochemical purity, exceeding 99.5%, and the specific radioactivity of the product were determined using high-performance liquid chromatography and ultraviolet-detection at 214 nm. The volume of the final product solution was calculated by weighing the sterile product vessel before and after sterile filtration and then dividing the weight by the density of the sterile solvent.
PET Scanning
PET studies were carried out using an ECAT EXACT HR+ scanner (Siemens/CTI, Knoxville, TN, USA) in three-dimensional list mode. This camera yields 63 slices of 2.43 mm thickness (axial FOV 155 mm), and has a spatial resolution of 4.4 mm in the horizontal plane and 4.1 mm axially (full width at half maximum). Head fixation was accomplished using a special head holder and vacuum hood supporting the head and neck. Two laser beams parallel to canthomeatal and sagittal lines were used to position the heads. Before each experiment, a 10-minute transmission scan was performed with 68Ge rod sources.
The right antecubital vein was cannulated for injection of [11C]raclopride. A B/I rate ratio (Kbol) of 105 minutes was selected based on an optimization study12 and earlier studies.13, 22 As the scan duration of 105 minutes was chosen, the magnitude of the bolus component of the total volume of the tracer was 50%, but because of decay, the bolus accounted for 77% of the total radioactive dose. An intravenous bolus of [11C]raclopride (214±41 MBq, mean±s.d.) was given and then a saline flush was carried out. The infusion component (215±36 MBq) of [11C]raclopride was started immediately after the bolus and was controlled by an infusion pump (Perfusor fm, B Braun Melsungen AG; Germany) during the scan. The total injected dose, specific radioactivity at the end of synthesis and the injected mass were 429±76 MBq, 281±191 MBq/nmol, and 0.90±1.63 μg, respectively. Imaging data were reconstructed to 21 5-minute frames.
Quantification of PET Data
During the equilibrium condition, the BPND23 representing specific binding of [11C]raclopride can be obtained from the radioactivity concentration of the target region and a cerebellar reference region using the formula BPND=([target]−[reference])/[reference].24, 25
Here the term BPND (unitless) has the same meaning as the V3” utilized in some earlier studies, e.g., refer the study by Martinez et al.26 The relationship between BPND and receptor parameters is described by BPND=f2Bmax/KDapp where f2 is the free fraction in nondisplaceable distribution volume, Bmax is the number of available binding sites (receptor density), and KDapp is the apparent affinity of [11C]raclopride in the presence of competition from endogenous DA. The baseline BPND was quantified using a 40–50-minute interval; previous studies have suggested this interval to be optimal.12, 13 The intervention lasting 25 minutes was started at 50 minutes, and the gradually rising blood ethanol concentration increases DA release and finally changes [11C]raclopride BPND with some latency. Theoretically, true equilibrium during a quantification interval cannot be assumed if DA activation is transient. To capture the whole DA surge for all subjects, the duration of the intervention quantification period matched the length of intervention, but started with 10 minutes of latency; thus, quantification was made during 60–85 minutes. If DA concentration is not at steady-state during quantification, then the measured ΔBPND becomes a time-weighted average of the instantaneous ΔBPND, which may decrease effect size and increase inter-subject variability in ΔBP.9
Changes in BPND were calculated as ΔBPND=(BPND intervention−BPND baseline) so that ΔBPND directly indicates the direction of change. Simulation studies derived from simultaneous [11C]raclopride PET and microdialysis experiments in monkeys indicate that a decrease in BPND between baseline and intervention quantification is proportional to the integral of the intervention-induced DA release.24, 27
Automated Region-of-Interest Analysis
An automated ROI analysis was performed to obtain the regional BPND values of [11C]raclopride. As this method is based on a common stereotaxic space, i.e., spatial normalization of images, the operator-induced errors in defining ROIs individually for each subject can be avoided and the objectivity of analysis can be secured. Automated ROI analysis was performed using general principles described previously in detail.28, 29 In brief, parametric images representing [11C]raclopride BPND at the voxel level during baseline and intervention were calculated using Matlab 6.5 for Windows (Math Works, Natick, MA, USA) with the formula mentioned above and an average regional radioactivity in the cerebellar reference region, which was defined using Imadeus software (version 1.50; Forima, Turku, Finland). Parametric images were spatially normalized with parameters estimated using Statistical Parametric Mapping30 software version 2002 (SPM2), summated [11C]raclopride images, and a ligand-specific template for [11C]raclopride.31 The striatal ROIs were defined on the magnetic resonance image template image, representing brain anatomy in accordance with Montreal Neurologic Institute space (MNI space; Montreal Neurologic Institute database), and they were situated bilaterally on the dorsal caudate nucleus, dorsal putamen, and VST, including the NAcc.
The ROIs on the dorsal striatum were defined on transaxial slices and ROIs on VST were defined on coronal slices because it enables more accurate positioning of ROIs22 and better test–retest reproducibility.32 The average regional values of [11C]raclopride BPND were calculated using these ROIs from spatially normalized parametric images and were subjected to statistical analysis.
Voxel-Based Receptor Mapping Analysis
To enable confirmation as well as more detailed visualization and localization of the results of the automated ROI analysis, a voxel-based receptor mapping analysis was conducted. This methodology is fully automated and performed independently of ROI analysis. It is not hampered by errors in anatomical positioning of ROIs. SPM analysis was performed in accordance with a procedure published earlier,28 with minor modifications required by the tracer, its administration method, and updated software versions. Before the analysis, the parametric images calculated and processed as described above were smoothed using a 9-mm (full width at half maximum) Gaussian filter. The confirmatory SPM analysis was performed using small volume correction and was confined to the VST including the NAcc and the surrounding region. The volumes of interest defining the analysis search volume were drawn using Imadeus (version 1.50; Forima) on the magnetic resonance image template image,representing the common stereotactic space in accordance with the MNI space.28 The volumes of interest were converted to the binary image representing the search volume in MNI space. This image was used as the explicit mask in the SPM analysis.
Statistical Analyses
All statistical analyses were conducted using SPSS for Windows (SPSS, release 16; SPSS Inc., Chicago, IL, USA) except voxel-based analysis, which was performed using SPM2. To statistically test the effects of ethanol intervention on regional outcome measures, baseline BPND was compared with the intervention BPND using a repeated-measures analysis of variance (rmANOVA) model with two within-subject factors: measurement (baseline, intervention) and hemisphere (left, right). To examine possible associations between baseline BPND, intervention-induced change in BPND, and subjective responses, correlation analyses were performed. In correlation analyses of baseline BPND values, the left and right side were analyzed separately.
In the analysis correlating changes in BPND values, to minimize the number of independent statistical tests and decrease the random variation of variables, the left and right side were averaged when a measurement-by-hemisphere interaction in rmANOVA indicated that there were no side differences. Spearman's rank correlation coefficients were used in analysis with subjective ratings, as the scale of ratings cannot be assumed to be continuous. Pearson's coefficients were used in correlation testing associations between continuous variables: change in BPND values and ethanol concentration or time. The alpha level was set to 0.05 in all statistical analyses on ROI-based BPND values. Because of the explorative nature of this study, a multiple comparison correction was not utilized in the analyses on ROI-based BPND values. Data are given as the mean±s.d. if not otherwise stated.
To test ethanol-induced changes in [11C]raclopride uptake at the voxel level, the SPM2 analysis was performed using paired t-test subtraction analysis and t contrast. As statistical tests are made independently for all voxels in the search volume, SPM's inherent correction for multiple comparisons based on Random Field Theory was utilized. A height threshold of t=3.40 was used at the voxel level. A corrected P-value <0.05 was considered statistically significant.
Results
The ethanol intervention was well-tolerated and none of the subjects reported pain or discomfort in the cannulated arm during ethanol infusion. The average blood ethanol concentration was 1.3 g/L (range 0.9–1.6) at the end of infusion, 1.1 g/L (0.9–1.2) 10 minutes later, and 0.9 g/L (0.8–1.0) 30 minutes after the end of ethanol infusion. The average latency between the start of ethanol infusion and the first subjective ethanol-related sensation was 4 minutes 57 seconds in seven subjects (range 2 minutes 30 seconds–8 minutes). One subject reported an ethanol effect before the ethanol intervention was started and one subject did not report an ethanol effect at all.
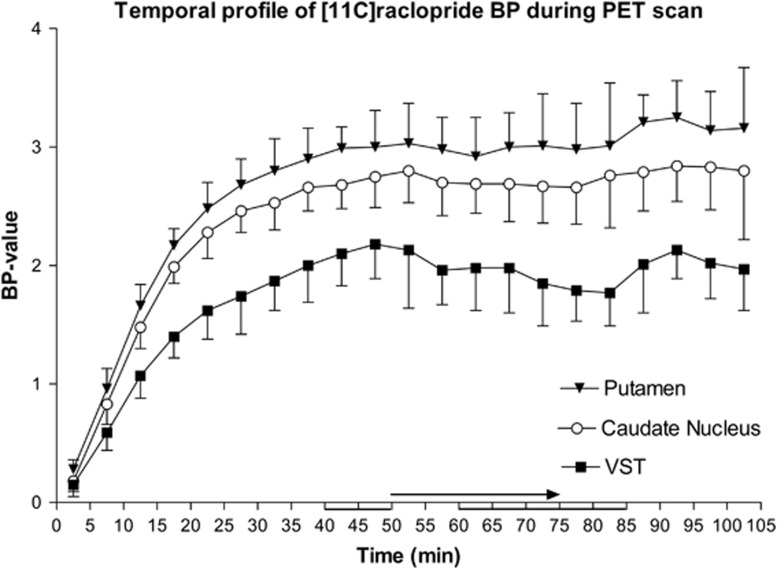
Observed [11C]raclopride time–activity curves for all regions are presented in Figure 1. Regional analysis of the [11C]raclopride BPND in the VST showed a 12.6% decrease during intervention in comparison with the baseline (F=37.91; P<0.001 for the main effect of measurement). The measurement-by-side interaction indicated that the effect was not lateralized (F=0.19; P=0.67). The change in BPND both in the caudate nucleus and putamen was <1% and was clearly non-significant (P>0.7). The results of rmANOVA and regional estimates of BPND values are presented in Table 1. Temporal profiles of the BPND values during a PET scan indicated that baseline quantification was made during the equilibrium period in all regions; the decrease in BPND in the VST started to recover 30 minutes after the start of the intervention (i.e., 80 minutes after the start of the scanning). Mean BPND curves during the PET scans in all studied regions are presented in Figure 2. An independent voxel-based receptor parametric mapping analysis confirmed the results of the ROI analysis by showing a bilateral decrease in [11C]raclopride BPND in the VST. The effect was located in immediate proximity to the NAcc bilaterally, and the effect was slightly more intensive in the left side (T value=6.88; cluster-level P-value 0.007) than in the right side (T value=4.77; cluster-level P-value=0.017). The visualization of the results of the voxel-based analysis is presented in Figure 3.
Figure 1.
Observed [11C]raclopride time–activity curves for all regions. x-axis represents time (minutes) after the start of the scan and y-axis represents radioactivity concentration (Bq/mL).
Table 1. The regional BPND values of [11C]raclopride, ethanol-induced mean change in BPND, and results of rmANOVA.
|
Binding estimates |
Mean change | s.d. | Range |
rmANOVA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Baseline |
Intervention |
Repetion |
Side |
Side by repetition |
|||||||||
| Mean | s.d. | Mean | s.d. | F-value | sig. | F-value | sig. | F-value | sig. | ||||
| Putamen | |||||||||||||
| Right | 3.02 | 0.25 | 3.01 | 0.39 | 0.46% | 9.45% | −20.50% 10.11% | 0.24 | 0.88 | 5.2 | 0.05 | 0.001 | 0.98 |
| Left | 2.98 | 0.22 | 2.96 | 0.37 | |||||||||
| Caudate nucleus | |||||||||||||
| Right | 2.75 | 0.22 | 2.74 | 0.31 | 1.0% | 8.85% | −16.05% 12.84% | 0.14 | 0.72 | 5.87 | 0.04 | 2.46 | 0.16 |
| Left | 2.69 | 0.21 | 2.64 | 0.28 | |||||||||
| VST | |||||||||||||
| Right | 2.26 | 0.27 | 2.00 | 0.27 | 12.6% | 6.38% | −21.39% −3.82% | 37.91 | 0.000 | 17.60 | 0.003 | 0.19 | 0.67 |
| Left | 2.03 | 0.30 | 1.75 | 0.28 | |||||||||
rmANOVA, repeated-measures analysis of variance; sig., significance.
BPND values of both hemispheres are presented separately as the mean±s.d. F and P values of rmANOVA testing the main effects (repetition and side) and repetition-by-side interaction are also presented. The change in BPND is presented as the mean of the left and right hemisphere as the repetition-by-side interaction was non-significant in all regions.
Figure 2.
Temporal profiles of the average regional BPND during positron emission tomography scan. X-axis represents time (minutes) after injection of [11C]raclopride and y-axis shows BPND values. Baseline and ethanol intervention values were calculated using data from 40 to 50 minutes and 60 to 85 minutes, respectively (lines below time axis). The binding achieved equilibrium during the baseline phase. Intravenous ethanol was administered during 50–75 minutes (arrow above time axis). The largest decrease in binding in the limbic striatum takes place during 75–85 minutes, and thereafter started to recover close to baseline level.
Figure 3.
Visualization of the results of voxel-based receptor mapping analysis. Statistical parametric map of T value of the analysis testing the decrease in [11C]raclopride BPND during ethanol intervention in comparison to the baseline. The effect in the VST is localized in the NAcc bilaterally. The map is visualized on the magnetic resonance image template image, representing common stereotactic Montreal Neurologic Institute space and presented in accordance with the radiological convention (right is left). The color bar represents the T value according to the numerical scale.
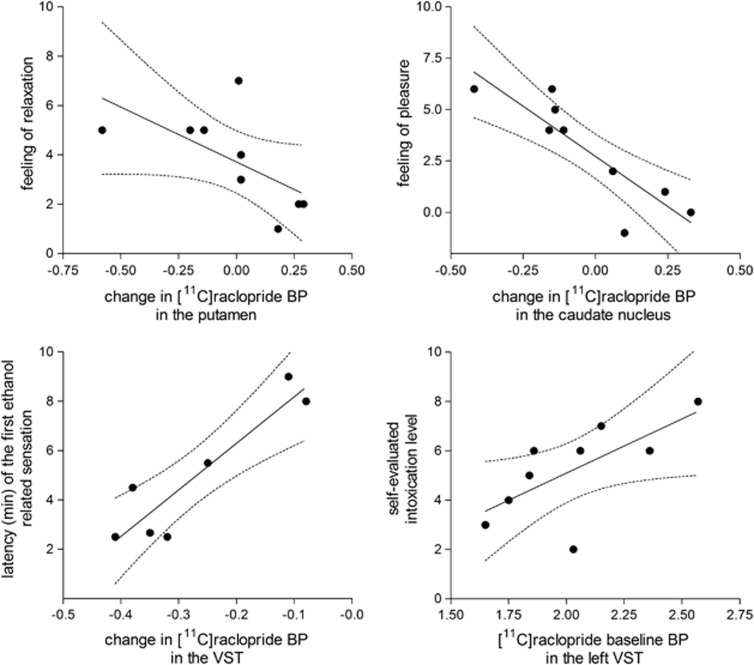
In the correlation analyses, testing the associations between subjective responses and binding-related variables, a high self-rated intoxication level was associated with a high baseline BPND value in the left VST (r=0.76, P=0.02) but not in the right (r<0.3; P>0.6). The feeling of pleasure during intervention was associated with a decrease in BPND values both in the caudate nucleus (r=−0.87, P=0.003) and putamen (r=−0.74, P=0.02). In addition, the decrease in BPND values in the putamen was associated with an increased feeling of relaxation (r=−0.82, P=0.007) and a high (r=−0.77, P=0.02). Both of these correlations showed that an intensive subjective response (pleasure and relaxation) was associated with a decrease in BPND, indicating an increased release of endogenous DA. Moreover, the time interval from the start of ethanol infusion to the first subjective ethanol-related sensation was associated with a change in BPND in the VST (r=0.92, P=0.004), which means that the quicker the subjective response to ethanol the more intense the BPND decrease. There were no significant correlations between changes in regional BPNDs and ethanol blood concentrations or flow rates of ethanol intervention (P>0.6 for all regions). The scatter plots describing the most relevant correlations are presented in Figure 4.
Figure 4.
Scatter plots describing the associations between ethanol-induced subjective responses and [11C]raclopride BPND values. Upper panels: correlations between reported feeling of relaxation (left panel) and pleasure (right panel) versus change in [11C]raclopride BPND in the putamen (left panel) and caudate nucleus (right panel). Correlation analysis testing revealed a significant correlation between the feeling of relaxation and change in [11C]raclopride BPND in the putamen (left panel, r=−0.82, P=0.007) and between the feeling of pleasure and change in [11C]raclopride BPND in the caudate nucleus (right panel, r=−0.87, P=0.003). Lower panels: correlations between latency of the first reported ethanol-related sensation and change in [11C]raclopride BPND in the ventral striatum (VST; left panel, r=0.92, P=0.004) and between reported maximal intoxication level during the positron emission tomography scan and in [11C]raclopride baseline BPND in the left VST (right panel, r=0.76, P=0.02). Changes in BPND were calculated as (BPND intervention−BPND baseline), and thus negative values indicate intervention-induced decrease in BPND, suggesting increased dopamine concentration.
Discussion
In the present study, ethanol intervention decreased regional BPND values of [11C]raclopride by 12.6% in the VST, but no change in the dorsal caudate nucleus or putamen was observed. According to the competition principle, [11C]raclopride competes with DA in binding the same DA D2/D3 receptors; thus an experimentally induced increase in endogenous DA concentration displaces [11C]raclopride from receptor binding sites, which leads to a decrease in [11C]raclopride binding when a new equilibrium is achieved (for a review see the study by Laruelle2). In addition to competition in binding, agonist-mediated receptor internalization, polymerization, and some other mechanisms may contribute to the decreased binding of [11C]raclopride, but also these mechanisms are mediated by an increased DA concentration.2, 33 The temporal profiles of the BPND values during the PET scan (Figure 1) indicated that the observed decrease in BPND in the VST started to recover 10 minutes after the end of ethanol infusion, which supports the interpretation that the decrease in BPND was caused by temporarily increased DA release. In addition, the regional specificity of the effect in the limbic striatum is in line with preclinical evidence (for a review see the study by Pierce and Kumaresan1).
Correlation analyses revealed interesting associations between subjective effects and [11C]raclopride measurements. The latency from the start of ethanol infusion to the first subjective ethanol-related sensation correlated positively with the bilateral change in BPND in the VST (r=0.92, P=0.004). Thus, the more quickly the subject experienced a sensation attributed to ethanol, the stronger the decrease in BPND, suggesting a more intense DA release in these regions. Although, the present data do not indicate any systematic ethanol-induced decrease in [11C]raclopride BPND in dorsal striatal regions, the changes in BPND values both in the putamen and caudate nucleus were associated with the feeling of pleasure during intervention, suggesting that individual changes in DA concentration in the dorsal striatum is related to the experience of pleasure. Moreover, an increased feeling of relaxation was associated with a decrease in BPND values in the putamen. These associations suggest that DA has a functional role in subjective feelings regarding ethanol administration in the dorsal striatum although no significant mean change at the group-level exists. However, the correlations should be interpreted as preliminary and with caution because of the relatively small number of study subjects and small changes in BPs.
In this study, a self-rated intoxication level did not correlate with the change in [11C]raclopride BPND in any brain region, which is in accordance with the negative finding reported earlier4, 7, but is not fully in line with a more recent study where subjectively evaluated activation correlated with the magnitude of BPND decrease in VST in men.8 However, we found that the self-evaluated intoxication level correlated with baseline BPND values in the left VST, but there was no association in the right side. This lateralization of the association is in agreement with a recent finding, showing that a peak perceived intoxication score correlated with [11C]raclopride baseline BPND in the left NAcc.5 Although this replication of findings supports the reliability of this association, the interpretation is not unequivocal because the baseline BPND value depends on both Bmax and apparent affinity of the tracer. In other words, high BPND values might have been caused by an increased receptor density, increased affinity, or by a combination of these processes or by a low DA concentration.
The results from earlier PET studies exploring the effects of acute alcohol intervention on DA neurotransmission in humans are conflicting. The first study utilized early PET technology in which the VST could not be quantified and yielded negative results.3 The second study showed that oral alcohol induced a decrease in [11C]raclopride binding in the VST, which is consistent with increased DA release, but the effect varied considerably among the subjects.4 The lack of positive findings in two studies using intravenous ethanol intervention might have been caused by methodological limitations, e.g., a fixed order of repeated PET scans, differences in intervention timing,5 or insufficient sensitivity.6 In the next study, the same group showed that intravenous ethanol administration with neutral (non-alcohol-related) cues induced a 12% decrease in BPND in the left NAcc, suggesting ethanol-induced increase in DA concentration.7 The magnitude of the effect found in the present study (12.6%) is in line with this, although the effect was not lateralized.
The methodological weaknesses related to [11C]raclopride single-bolus studies can be avoided by using the B/I method.34 The accuracy and precision of D2 receptor quantification with the [11C]raclopride B/I method in the striatal regions including VST has been shown to be appropriate for the study of DA transmission using endogenous competition techniques.22 The B/I methodology has also been successfully utilized in studies on dopaminergic effects of drugs, e.g., amphetamine.13 The same group recently utilized the same methodology to study the effects of pre-scan oral alcohol administration and found a statistically significant decrease in [11C]raclopride binding in all striatal subregions in male subjects with an overall gender effect.8 In both sexes, the decrease in [11C]raclopride binding in the VST was significant, but in men, it was twofold larger (12.1%) than in women (6.2%). In men, the decrease in binding in the dorsal striatum during an alcohol scan was between 7.3% and 8.5%. In the present study, the magnitude of the effect in the VST (12.6%) is in line with the effect found in men, but our data showed only a 1% non-significant decrease in BPND in the caudate nucleus and putamen. As the preclinical evidence suggests that ethanol induces DA release preferentially in the ventral striatum,1, 35 a systematic decrease in binding in all striatal regions8 might not be directly related to the neurobiological effects of ethanol. Consistently, parameters related to alcohol-induced activation and drinking history correlated with the [11C]raclopride binding decrease in the VST only. As the independent effect of scan order was highly significant (P<0.001) and the order of conditions in both groups was unbalanced, the order effect might have been one confounding factor.8 Thus, methodological factors might at least partially explain the discrepancies.
To induce a distinct effect, we utilized as rapid and short-lasting intravenous ethanol administration as possible, using the maximum ethanol concentration that was well-tolerated by the subjects. The administration procedure for ethanol used in this study might induce a different dopaminergic response than oral pre-scan administration3, 4, 8 or intravenous ‘clamping administration' targeting a constant blood alcohol concentration.5, 6, 7 The average blood ethanol concentration was 1.3 g/L (range 0.9–1.6) at the end of the infusion, and it decreased to 0.9 g/L (range 0.8–1.0) 30 minutes later. The between-subject variation and a few relatively high outset values were most likely caused by individual variation in the early ethanol distribution in the body water, as the range tapered considerably over 30 minutes. The measured blood ethanol concentration values at the 30-minute time point somewhat exceeded the calculated target concentration of 0.8 g/L, but fell below the ethanol concentrations reported in one study,6 as the breath blood ethanol concentration of 80 mg% approximately corresponds to a blood ethanol concentration of 1.7 g/L.
There are a few limitations in the present study. First, a general limitation of the B/I method is that an appropriate equilibrium must be established during the quantification periods. Although 105 minutes was suggested to be the most optimal12 Kbol value and was thus utilized in the present study, this Kbol value might not be the most appropriate for all subjects. It has been suggested that a Kbol of 105 minutes is associated with a decrease in [11C]raclopride binding of ~10%/h during a 40- to 90-minute interval22; to achieve complete equilibrium, the Kbol should be slightly reduced. However, more recent results suggest that this issue has a negligible impact.13 The baseline BPND was quantified using a time interval of 40–50 minutes as previous studies have suggested this to be adequate.9, 12, 13 However, individual time–activity curves (Figure 1) suggest that in the caudate nucleus and putamen true equilibrium conditions at baseline were not reached. It is possible that the non-equilibrium reduced the sensitivity to detect intervention-induced changes in BPND in these brain regions. Although equilibrium is crucial to the reliability of quantification, non-equilibrium would have led to an underestimation of the DA surge effect on BPND9 and, consequently, does not undermine the validity of the present results regarding ethanol-induced decrease in BPND in the VST.
Second, all conventional PET methods, the simplified reference tissue model and Logan graphical reference method, assume that endogenous DA concentration does not change during the quantification period. This limitation also concerns the equilibrium analysis routinely utilized in the quantification of B/I studies, as in the present study. If DA concentration changes during the quantification, it might lead to underestimation of the change in BPND, increasing inter-subject variability and reducing effect size.9 The comparison of different quantification intervals between 60 and 85 minutes for the present data revealed that the differences and variability in BPND changes were minimal, and the average variability was the smallest using a full time span 60–85 minutes (data not shown). Moreover, dorsal striatal changes were non-significant with all intervals. However, the chosen intervention quantification interval (60–85 minutes) might have led to an underestimation of the true change in BPND. This might partially explain why significant correlations with behavioral measurements were observed in the dorsal striatum, although the average changes in BPND were minimal. Although, changes in BP can be robustly measured at the group-level with simple quantification, the linear variant of the ntPET model could provide better tools for exploration of the timing and the highest DA surge at the individual level.35
Our study was not placebo controlled. In studies on the neurobiology of alcohol, the comparison condition is, however, extremely problematic. Alcohol-related cues alone have been shown to decrease DA concentration7, suggesting that ethanol anticipation is a dopaminergically deactivating condition. In addition, psychological effects associated with a placebo depend on the order of ethanol administration.8 Our study used the B/I method with double quantification. The conditioned effects of alcohol drinking and the effects of alcohol anticipation were eliminated by using intravenous ethanol infusion and by blinding the subjects regarding the timing of ethanol infusion. Thus, the expectation and other possible dopaminergic effects related to a conventional placebo condition prevailed during the baseline quantification, and an ethanol-induced decrease in [11C]raclopride binding could be shown. The double quantification enabling the baseline and the intervention measurement in one PET scan is a clear advantage of the B/I paradigm. It could be utilized to obtain additional evidence by conducting a repeated B/I experiment, where saline is given to the subjects as a placebo comparison condition.
Conclusions
Intravenous ethanol administration induces a decrease in BPND of [11C]raclopride, suggesting a transient DA release in the limbic striatum, particularly in the NAcc, but ethanol-induced changes in DA neurotransmission also in the dorsal striatum are associated with subjective effects of ethanol. These results also indicate that the B/I method of administration of [11C]raclopride with double quantification is a feasible method to quantify small and transient intervention-induced dopaminergic effects.
Acknowledgments
The authors thank the personnel of Turku PET Centre, Turku, Finland for competent technical support. The first author thanks Miia Vahlsten for her valuable comments on the manuscript.
The authors declare no conflict of interest.
Footnotes
The study was financially supported by the Academy of Finland (project #111879).
References
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse. Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Salonen I, Hietala J, Laihinen A, Lehikoinen P, Leino L, Någren K, et al. A PET study on the acute effect of ethanol on striatal D2 dopamine receptors with [11C]raclopride in healthy males. Hum Psychopharmacol. 1997;12:145–152. [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, et al. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Kareken DA, Seyoum RA, O'Connor SJ, Wang C, Zheng QH, et al. Dopamine D(2) receptor availability is associated with subjective responses to alcohol. Alcohol Clin Exp Res. 2005;29:965–970. doi: 10.1097/01.alc.0000171041.32716.42. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Constantinescu CC, Kareken DA, Normandin MD, Cheng TE, O'Connor SJ, et al. Heterogeneous effects of alcohol on dopamine release in the striatum: a PET study. Alcohol Clin Exp Res. 2007;31:965–973. doi: 10.1111/j.1530-0277.2007.00390.x. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Morris ED, Constantinescu CC, Cheng TE, Normandin MD, O'Connor SJ, et al. When what you see isn't what you get: alcohol cues, alcohol administration, prediction error, and human striatal dopamine. Alcohol Clin Exp Res. 2009;33:139–149. doi: 10.1111/j.1530-0277.2008.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NB, Kegeles LS, Slifstein M, Xu X, Martinez D, Sakr E, et al. Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [(1)(1)C]raclopride. Biol Psychiatry. 2010;68:689–696. doi: 10.1016/j.biopsych.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Kim SJ, Cosgrove KP, Morris ED. Limitations of SRTM, Logan graphical method, and equilibrium analysis for measuring transient dopamine release with [(11)C]raclopride PET. Am J Nucl Med Mol Imaging. 2013;3:247–260. [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Wang C, Morris ED. Change in binding potential as a quantitative index of neurotransmitter release is highly sensitive to relative timing and kinetics of the tracer and the endogenous ligand. J Nucl Med. 2004;45:903–911. [PubMed] [Google Scholar]
- Carson RE. PET physiological measurements using constant infusion. Nucl Med Biol. 2000;27:657–660. doi: 10.1016/s0969-8051(00)00138-4. [DOI] [PubMed] [Google Scholar]
- Watabe H, Endres CJ, Breier A, Schmall B, Eckelman WC, Carson RE. Measurement of dopamine release with continuous infusion of [11C]raclopride: optimization and signal-to-noise considerations. J Nucl Med. 2000;41:522–530. [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33:27–39. doi: 10.1093/ajcn/33.1.27. [DOI] [PubMed] [Google Scholar]
- Brasser SM, McCaul ME, Houtsmuller EJ. Alcohol effects during acamprosate treatment: a dose-response study in humans. Alcohol Clin Exp Res. 2004;28:1074–1083. doi: 10.1097/01.alc.0000130802.07692.29. [DOI] [PubMed] [Google Scholar]
- Bustamante D, Quintanilla ME, Tampier L, Gonzalez-Lira V, Israel Y, Herrera-Marschitz M. Ethanol induces stronger dopamine release in nucleus accumbens (shell) of alcohol-preferring (bibulous) than in alcohol-avoiding (abstainer) rats. Eur J Pharmacol. 2008;591:153–158. doi: 10.1016/j.ejphar.2008.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JB, Conrod P, Vassileva J, Gianoulakis C, Pihl RO. Differential effects of naltrexone on cardiac, subjective and behavioural reactions to acute ethanol intoxication. J Psychiatry Neurosci. 2006;31:386–393. [PMC free article] [PubMed] [Google Scholar]
- Larsen PUJ, Dahlström K, Jensen M. Synthesis of [11C]methyl iodide by iodination of [11C]methane. Appl Radiat Isot. 1997;48:153–157. [Google Scholar]
- Någren KTP, Helin S, Amir A, Halldin C. Production of high specific radioactivity [C-11]FLB 457 from target produced [C-11]methane. Eur J Nucl Med. 2003;30:S217. [Google Scholar]
- Langer O, Någren K, Dolle F, Lundkvist C, Sandell J, Swahn C-G, et al. Precursor synthesis and radiolabelling of the dopamine D2 receptor ligand [11C]raclopride from [11C]methyl triflate. J Labelled Comp Radiopharm. 1999;42:1183–1193. [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Endres CJ, Kolachana BS, Saunders RC, Su T, Weinberger D, Breier A, et al. Kinetic modeling of [11C]raclopride: combined PET-microdialysis studies. J Cereb Blood Flow Metab. 1997;17:932–942. doi: 10.1097/00004647-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Carson RE, Breier A, de Bartolomeis A, Saunders RC, Su TP, Schmall B, et al. Quantification of amphetamine-induced changes in [11C]raclopride binding with continuous infusion. J Cereb Blood Flow Metab. 1997;17:437–447. doi: 10.1097/00004647-199704000-00009. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Endres CJ, Carson RE. Assessment of dynamic neurotransmitter changes with bolus or infusion delivery of neuroreceptor ligands. J Cereb Blood Flow Metab. 1998;18:1196–1210. doi: 10.1097/00004647-199811000-00006. [DOI] [PubMed] [Google Scholar]
- Aalto S, Bruck A, Laine M, Nagren K, Rinne JO. Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: a positron emission tomography study using the high-affinity dopamine D2 receptor ligand [11C]FLB 457. J Neurosci. 2005;25:2471–2477. doi: 10.1523/JNEUROSCI.2097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aalto S, Scheinin NM, Kemppainen NM, Nagren K, Kailajarvi M, Leinonen M, et al. Reproducibility of automated simplified voxel-based analysis of PET amyloid ligand [11C]PIB uptake using 30-min scanning data. Eur J Nucl Med Mol Imaging. 2009;36:1651–1660. doi: 10.1007/s00259-009-1174-1. [DOI] [PubMed] [Google Scholar]
- Friston KJHA, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Penttila J, Kajander J, Aalto S, Hirvonen J, Nagren K, Ilonen T, et al. Effects of fluoxetine on dopamine D2 receptors in the human brain: a positron emission tomography study with [11C]raclopride. Int J Neuropsychopharmacol. 2004;7:431–439. doi: 10.1017/S146114570400450X. [DOI] [PubMed] [Google Scholar]
- Alakurtti K, Aalto S, Johansson JJ, Nagren K, Tuokkola T, Oikonen V, et al. Reproducibility of striatal and thalamic dopamine D2 receptor binding using [11C]raclopride with high-resolution positron emission tomography. J Cereb Blood Flow Metab. 2011;31:155–165. doi: 10.1038/jcbfm.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Dewey SL, Volkow ND, Gatley SJ. A consideration of the dopamine D2 receptor monomer-dimer equilibrium and the anomalous binding properties of the dopamine D2 receptor ligand, N-methyl spiperone. J Neural Transm. 2001;108:279–286. doi: 10.1007/s007020170073. [DOI] [PubMed] [Google Scholar]
- Carson RE, Channing MA, Blasberg RG, Dunn BB, Cohen RM, Rice KC, et al. Comparison of bolus and infusion methods for receptor quantitation: application to [18F]cyclofoxy and positron emission tomography. J Cereb Blood Flow Metab. 1993;13:24–42. doi: 10.1038/jcbfm.1993.6. [DOI] [PubMed] [Google Scholar]
- Normandin MD, Schiffer WK, Morris ED. A linear model for estimation of neurotransmitter response profiles from dynamic PET data. NeuroImage. 2012;59:2689–2699. doi: 10.1016/j.neuroimage.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]