Abstract
Cannabinoids (CBs) show promise as neuroprotectants with some agents already licensed in humans for other conditions. We systematically reviewed CBs in preclinical stroke to guide further experimental protocols. We selected controlled studies assessing acute administration of CBs for experimental stroke, identified through systematic searches. Data were extracted on lesion volume, outcome and quality, and analyzed using random effect models. Results are expressed as standardized mean difference (SMD) with 95% confidence intervals (CIs). In all, 144 experiments (34 publications) assessed CBs on infarct volume in 1,473 animals. Cannabinoids reduced infarct volume in transient (SMD −1.41 (95% CI −1.71), −1.11) P<0.00001) and permanent (−1.67 (−2.08, −1.27), P<0.00001) ischemia and in all subclasses: endocannabinoids (−1.72 (−2.62, −0.82), P=0.0002), CB1/CB2 ligands (−1.75 (−2.19, −1.31), P<0.00001), CB2 ligands (−1.65 (−2.09, −1.22), P<0.00001), cannabidiol (−1.20 (−1.63, −0.77), P<0.00001), Δ9-tetrahydrocannabinol (−1.43 (−2.01, −0.86), P<0.00001), and HU-211 (−2.90 (−4.24, −1.56), P<0.0001). Early and late neuroscores significantly improved with CB use (−1.27 (−1.58, −0.95), P<0.00001; −1.63 (−2.64, −0.62), P<0.002 respectively) and there was no effect on survival. Statistical heterogeneity and publication bias was present, median study quality was 4 (range 1 to 6/8). Overall, CBs significantly reduced infarct volume and improve functional outcome in experimental stroke. Further studies in aged, female and larger animals, with other co-morbidities are required.
Keywords: cannabinoid, meta-analysis, neuroprotection, preclinical, stroke
Introduction
Components of the endocannabinoid system (ECS) are altered after ischemic stroke. The expression of cannabinoid (CB)1 and CB2 receptors is upregulated in the rat brain after cerebral ischemia,1, 2 indicating that the ECS may have an important role in the endogenous response to stroke, though the relevance of these changes is not known. Human and animal in vivo data have shown increases in neurolonal levels of anandamide (AEA), oleoylethanolamide (OEA), and palmitoylethanolamide (PEA), with 2-arachidonoylglycerol levels either unchanged or increased.3, 4, 5, 6, 7, 8 Preclinical stroke studies have derived neuroprotective qualities from a range of approaches to manipulate the ECS. For example, CB2 ligands can modify the poststroke inflammatory response, and CB1 activation can initiate a chemical hypothermia, with both processes resulting in a decrease in stroke infarct volume. 9, 10 Activation of CB2 receptors has only showed protective effects and the role of CB1 activation is less clear with studies demonstrating efficacy of both CB1 agonists and antagonists.11, 12
Cannabinoids can be divided into three categories: endocannabinoids, phytocannabinoids, and synthetic CBs. Anandamide and 2-arachidonoylglycerol (both CB1/2 agonists) are the best-studied endocannabinoids, but other chemically similar compounds have been suggested as endocannabinoids or endocannabinoid-like compounds, including OEA, PEA, lauroylethanolamide, and linoleoylethanolamide. Endocannabinoids also display activity at non-CB1/2 receptor sites, including TRPV1 (transient receptor potential cation channel subfamily V1), peroxisome proliferator-activated receptor (PPAR)α/γ, 5HT1A, and GPR55.13 Phytocannabinoids are derived from the cannabis plant, a unique source of over 60 different compounds, with Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) already in clinical use to treat spasticity in multiple sclerosis (Sativex). Δ9-tetrahydrocannabinol is a partial agonist for CB1 and CB2 receptors, while CBD displays low affinity for CB receptors.14 Synthetic CB compounds have been developed, some of which exhibit high potency at CB1 (arachidonyl-2'-chloroethylamide) or CB2 (JWH-133, O-1966, and O-3853) receptors, activate both CB1/2 (CP 55940, HU-210, TAK-937, and WIN 55212-2), or activate non-CB receptors (e.g., HU-211, a proposed NMDA antagonist).
Given the accumulating preclinical evidence for the use of CBs in stroke, as well as the expansion in the use of CB-based medicines in other disorders, a systematic review of the currently available preclinical literature is warranted. While it is clear that there are many studies describing the benefit of administering CBs for experimental stroke, a number of unanswered questions remain before the transition is made into ‘bedside' testing. It is unclear as to whether the optimal time of administration and dose of the various CB classes have been established, and whether the body of evidence is reliable and consistent. The aim of this study, therefore, is to systematically review and meta-analyze the effects of exogenous CB administration on infarct volume, functional outcome, and survival in animal models of ischemic stroke.
Materials and methods
Search Criteria
Experimental (nonhuman) studies in evaluating the effect of CBs on focal acute stroke were searched up to December 2013 in PubMed, Medline, Embase, ScienceDirect, and Web Of Science. Search keywords included were ‘stroke,' ‘ischemia,' ‘cannabinoid,' ‘cannabidiol,' ‘delta-9-tetrahydrocannabinoid,' ‘WIN 55212-2,' ‘2-Arachidonoy glycerol,' ‘endocannabinoids,' ‘CB1 receptors,' and ‘CB2 receptors.' References from included studies and conference proceedings were also searched. There was no protocol per se, although prespecified exclusion criteria were used to prevent bias and studies were included if the following were met: (1) a focal ischemic stroke model, not global; (2) treatment was given for an acute model (within 48 hours), not chronic; (3) only CB ligands were given; (4) there was a control group; (5) there were measures of infarct size, functional outcome, or survival; and (6) data were from an original article, not a review article. If an article was only available as an abstract, then it was not included.
Data Acquisition
Data on total infarct size, measured in percentage (%) or volume (mm3) were extracted from included papers. Volumes corrected for edema were chosen instead of uncorrected data. When all data were not available, authors were contacted for the exact numbers of animals used in each group for each experiment. If authors were unable to provide necessary information, then the lowest number of animals within the range given was used. The Grab application (version 1.5) was used to obtain values from figures given in published articles if no values were stated within the text. Similarly, information on vital status, weight (grams), Rotarod test (time spent on Rotarod expressed in seconds or percentage compared with baseline), and neurologic score were collected. If published articles used multiple groups (e.g., to assess time response relationships) with one control group, then the number of animals per control group was divided into the number of comparison groups. Since different procedures were used in different experiments, the total dose of drug given throughout a complete experiment was taken instead of a single dose. When drugs were given at more than one point of time, the earliest time of administration was used.
Quality
Methodological quality was assessed using an eight-point criteria derived from STAIR,15 as used previously,16, 17 with 1 point given to evidence of the following: the presence of randomization, monitoring temperature throughout the experiment, masked outcome measurement, assessment of outcome at days 1 to 3, assessment of outcome at days 7 to 30, assessment of outcome other than just infarct size, dose-response relationship conducted, and therapeutic time window relationship of a particular agonist conducted.
Data Analysis
Data were grouped before analysis by (1) model type (permanent or transient ischemia); (2) species; (3) time to treatment; (4) total dose, and (5) CB type. Data from each of these groups were analyzed as forest plots using the Cochrane Review Manager software (version 5.2, Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration, 2012) and Stata (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX, USA), as used in the previous animal meta-analyses.17 Since heterogeneity was expected between study protocols (different species, stroke models, dose, and time), random-effect models were used. The results of continuous data are expressed as standardized mean difference (SMD), with 95% confidence intervals (CIs), which allows data measured on different scales and in different species to be merged. The results of binary data (survival) are expressed as odds ratios with 95% CI. Studies were weighted by sample size and statistical significance was set at P<0.05. PRISM 6 (GraphPad, Software, La Jolla, CA, USA) was used to compare the dose- and time-response relationship between drug classes. Infarct volume data acquired can be accessed at http://dx.doi.org/10.6084/m9.figshare.1228070.
Results
Design of the Studies
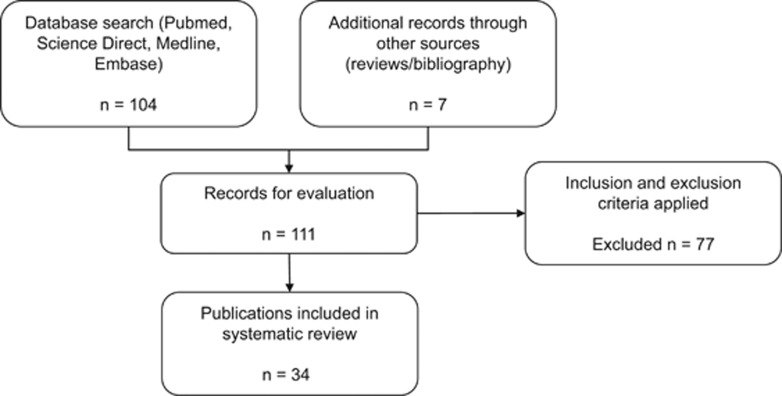
The initial search for studies identified 111 relevant publications. Once the prespecified inclusion criteria were applied, a total of 34 publications were chosen for analysis (Figure 1; Table 1). These came from 18 laboratories in 9 countries (USA, Israel, Italy, Japan, Spain, Denmark, Germany, UK, and China). Studies were excluded if they examined global ischemia, neonatal animals, did not measure infarct volume or functional outcome, did not administer a CB receptor ligand, were review articles (not original articles), where induction of injury was by methods other than ischemia/reperfusion or if the data were unobtainable.
Figure 1.
Record identification process.
Table 1. Description of included studies.
| Study | Species | Model | Drug | Total dose | Route | Time of administration | Unit of infarct volume | Time of assessment | STAIR score |
|---|---|---|---|---|---|---|---|---|---|
| Ahmad 2012 18 | Wistar rats | T 2 hours | PEA | 10 mg/kg | IP | 1 and 6 hours post | mm3 | 24 hours | 4 |
| Bonfils 2006 19 | Wistar rats | T 0.5 hours | WIN 55212-2 | 9 mg/kg/h | IV | 0.5 hours post until 22 hours | mm3 | 7 days | 2 |
| Berger 2004 20 | Wistar rats | Pa | SR141716 | 1 mg/kg | IV | 30 minutes post onset | mm3 | 5 hours | 3 |
| Belayev 1995a 21 | Wistar rats | T 1.5 hours | HU-211 | 4 mg/kg | IV | 70 minutes post onset | mm3 | 72 hours | 2 |
| Belayev 1995b 22 | Wistar rats | P | HU-211 | 4 mg/kg | IV | 30 minutes post onset | n/a | n/a | 2 |
| Garg 2010 23 | |||||||||
| Experiments 1–4 | Spr-Dawley rats | T 1.5 hours | PEA | 10 mg/kg | IP | Pre, 0, 2 or 3 hours post | % | 24 hours | |
| Garg 2011 24 | |||||||||
| Experiment 1 | Spr-Dawley rats | T 1.5 | Lauroylethanolamide | 10 mg/kg | IP | Pre onset | % | 24 hours | 4 |
| Experiments 2 and 3 | Linoleoylethanolamide | 10 or 20 mg/kg | |||||||
| Hayakawa 2004 36 | |||||||||
| Experiment 1 | ddY mice | T 4 hours | CBD | 6 mg/kg | IP | Pre onset | mm3 | 24 hours | 3 |
| Experiment 2 | THC | 20 mg/kg | |||||||
| Hayakawa 2007a 12 | ddY mice | T 4 hours | THC | 20 mg/kg | IP | Pre onset | mm3 | 24 hours | 3 |
| Hayakawa 2007b 39 | |||||||||
| Experiments 1–3 | ddY mice | T 4 hours | THC | 2, 6, 20 mg/kg | IP | Pre onset | mm3 | 24 hours | 3 |
| Experiments 4–6 | CBD | 0.2, 2, 6 mg/kg | |||||||
| Experiment 7 | SR141716 | 1 mg/kg | |||||||
| Hayakawa 2007c 38 | |||||||||
| Experiments 1–3 | ddY mice | T 4 hours | CBD | 0.2, 2, 6 mg/kg | IP | Pre onset | mm3 | 24 hours | 5 |
| Experiments 4–6 | THC | 2, 6, 20 mg/kg | Pre onset | 24 hours | |||||
| Experiments 7 and 9 | CBD | 6 mg/kg | Pre-onset | 24 and 72 hours | |||||
| Experiments 8 and 15 | THC | 20 mg/kg | Pre onset | 24 and 72 hours | |||||
| Experiments 10–14 | CBD | 3 mg/kg | Pre onset, 3 hours post, at reperfusion, 1 hours or 2 hours post reperfusion | 24 hours | |||||
| Experiments 16–18 | THC | 10 mg/kg | Pre onset, 3 hours, reperfusion | 24 hours | |||||
| Experiment 19 | SR141716 | 1 mg/kg | Pre onset | ||||||
| Hayakawa 2008 37 | |||||||||
| Experiments 1–3 | ddY mice | T 4 hours | CBD | 0.1, 1, 3 mg/kg | IP | Pre onset | mm3 | 24 hours | 4 |
| Experiment 4 | SR141716 | 1 mg/kg | |||||||
| Hayakawa 2009 35 | |||||||||
| Experiments 1–3 | ddY mice | T 4 hours | CBD | 3 mg/kg | IP | Days 1, 3, or 5 | n/a | - | 4 |
| Hu 2010 25 | |||||||||
| Experiments 1–3 | Spr-Dawley rats | T 2 hours | WIN 55212-2 | 1, 3 or 5 mg/kg | IP | Pre onset 1, 3, or 5 days | % | 72 hours | 6 |
| Lavie 2001 26 | |||||||||
| Experiments 1–3 | Hypertensive rats | P | HU-211 | 4.5 mg/kg | IV | 1, 3, or 6 hours post onset | % | 24 hours | 6 |
| Experiments 4–6 | 1, 3 or 6 hours post onset | 30 days | |||||||
| Leker 1999 27 | Hypertensive rats | P | HU-211 | 4 mg/kg | IV | 1 hour post onset | % | 24 hours | 2 |
| Leker 2003 10 | |||||||||
| Experiments 1–4 | Sp-Dawley rats | P | HU-210 | 5, 10, 30, 45 μg/kg | IV | 1 hour post onset | % | 72 hours | 5 |
| Experiments 5–8 | 45 μg/kg | 1, 2, 4, or 6 hours post onset | |||||||
| Mishima 2005 3 | |||||||||
| Experiments 1–4 | ddY mice | T 4 hours | CBD | 0.2, 2, 6, or 20 mg/kg | IP | At pre onset | mm3 | 24 hours | 4 |
| Experiments 5 and 6 | Abnormal CBD | 6 or 20 mg/kg | |||||||
| Experiments 7 and 8 | AEA | 6 or 20 mg/kg | |||||||
| Experiments 9 and 10 | Methanandamide | 6 or 20 mg/kg | |||||||
| Muthian 2004 5 | |||||||||
| Experiments 1–3 | Wistar rats | T 2.5 hours | WIN 55212-2 | 0.1, 0.3, or 1 mg/kg | IV | 5 minutes pre onset | mm3 | 24 hours | 5 |
| Experiments 4–6 | SR141716 | 0.1, 0.3, or 1 mg/kg | |||||||
| Experiment 7 | LY320153 | 6 mg/kg | |||||||
| Murakami 2013 28 | |||||||||
| Experiments 1–6 | Rats | P | TAK-937 | 30, 100 mcg/kg/h | IV | 3, 5, or 8 hours post, until 24 hours | % | 48 hours | 4 |
| Experiments 7 and 8 | Aged rats | Pa | 100 mcg/kg/h | 1 hour post until 24 hours | |||||
| Experiment 9 | Pa | 1 hour post until 24 hours | |||||||
| Murikinati 2010 9 | C57BL/6 mice | P | JWH-133 | 1 mg/kg/day | IV | 4 hour pre onset | mm3 | 3 d | 2 |
| Nagayama 1999 29 | |||||||||
| Experiments 1–4 | Spr-Dawley rats | P | WIN 55212-2 | 1 mg/kg | IP | 30 minutes pre-, 30, 60, or 120 minutes post- | mm3 | 24 hours | 4 |
| Experiment 5 | SR141716 | 1 mg/kg | |||||||
| Schomacher 2008 30 | |||||||||
| Experiments 1 and 2 | Wistar rats | T 1.5 hours | PEA | 30 or 10 mg/kg | IP | 30 minutes post onset | mm3 | 24 hours | 5 |
| Experiment 3 | AEA | 10 mg/kg | |||||||
| Sun 2007 40 | C57BL/6 Mice | T 2 hours | OEA | 10 mg/kg/day | IP | −3, −2 and −1 days pre onset | mm3 | 48 hours | 1 |
| Sun 2013a 31 | |||||||||
| Experiments 1 and 2 | Spr-Dawley rats | P | WIN 55212-2 | 1 or 9 mg/kg | IV | 2 hours post onset | % | 24 hours | 5 |
| Sun 2013b 32 | |||||||||
| Experiments 1–5 | Spr-Dawley rats | P | WIN 55212-2 | 1, 3 or 9 mg/kg | IV | 2 hours | % | 24 hours | 4 |
| Experiments 6 and 7 | SR141716 | 1 or 2 mg | |||||||
| Suzuki 2012a 45 | |||||||||
| Experiments 1–4 | Spr-Dawley rats | T 2 hours | TAK-937 | 3, 10, 30 or 100 μg/kg | IV | At reperfusion | % | 24 hours | 4 |
| Experiment 5 | Cynomolgus monkeys | T 0.5 hour | 2 μg/kg | 30 minutes post reperfusion | |||||
| Suzuki 2012b 33 | Spr-Dawley rats | T 2 hours | TAK-937 | 100 mcg/kg/h | IV | 2 hours (on reperfusion) for 24 hours | mm3 | 24 hours | 2 |
| Teichner 2003 34 | |||||||||
| Experiments 1 and 2 | Hypertensive rats | P | HU-211 | 4.5 mg/kg | IV | 1 hour | % | 1 and 30 days | 4 |
| Zarruk 2012 41 | |||||||||
| Experiments 1 and 3 | Mice | P | JWH-133 | 0.5, 1.5 or 5 mg/kg | IP | 10 minutes post onset | % | 48 hours | 6 |
| Experiments 4 and 5 | 1.5 mg/kg | 10 minutes or 3 hours post onset | |||||||
| Zhang 2007 43 | |||||||||
| Experiments 1 and 3 | Mice | T 1 hour | O-3853 | 1 mg/kg | IV | 1 hour pre- or 10 minutes post- | mm3 | 24 hours | 3 |
| Experiments 2 and 4 | O-1966 | 1 mg/kg | 1 hour pre- or 10 minutes post- | ||||||
| Zhang 2008 11 | |||||||||
| Experiments 1 and 2 | Mice | T 1 hour | O-1966 | 1 mg/kg | IV | 1 hour pre onset | % | 24 hours | 2 |
| Experiments 3 and 4 | SR141716 | 5, 20 mg/kg | |||||||
| Experiments 5 and 6 | SR144528 | 5, 20 mg/kg | |||||||
| Zhang 2009 42 | |||||||||
| Experiments 1–3 | Mice | T 1 hour | O-1966 | 1, 5, or 10 mg/kg | IP | 1 hour pre onset | % | 24 hours | 5 |
| Experiments 4–6 | 5 mg/kg | 1 hour pre onset, 1 hour or 3 hours post reperfusion | |||||||
| Zhou 2012 44 | |||||||||
| Experiments 1–9 | Kunming mice | T 1.5 hours | OEA | 10, 20, 40 mg/kg | Oral | 3 days pre onset or 30, 60, 90, 150 minutes post onset | mm3 | 24 hours | 5 |
AEA, anandamide; CBD, cannabidiol; IP, intraperitoneal; IV, intravenous; MCAO, middle cerebral artery occlusion; OEA, oleoylethanolamide; P, permanent MCAO; PEA, palmitoylethanolamide; T, transient MCAO; THC, Δ9-tetrahydrocannabinol.
Photothrombotic model.
In all, 19 of 34 publications studied 786 rats5, 10, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 and 14 studied 673 mice;3, 9, 11, 12, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 1 article studied rats and primates.45 Twenty-four articles examined greater than one experimental paradigm (total number of experiments 144). Transient ischemic models were used in 21 publications, with vessel occlusion time ranging between 30 minutes and 4 hours. Permanent models of ischemia were used in 13 articles (photothrombotic n=2). Drugs were administered intravenously (n=17) or via the peritoneum (n=16), and one study used the oral route.44 Time of administration ranged from preischemia up to 5 days after middle cerebral artery occlusion (MCAo). Median study quality was 4 (range 1 to 6).
Infarct Volume
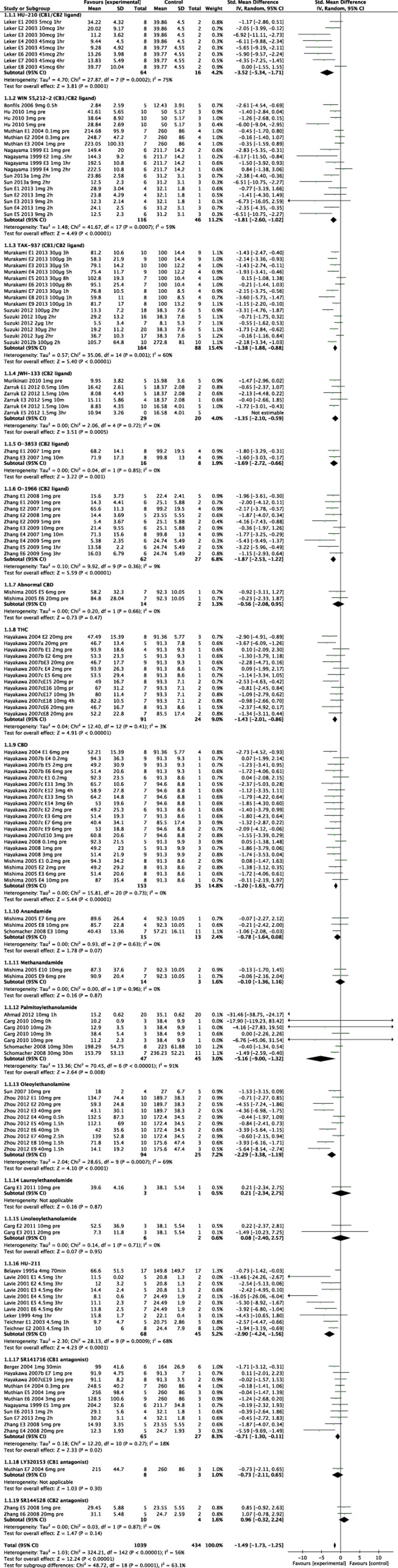
Overall, administration of CB receptor ligands reduced infarct volume in comparison with vehicle; SMD −1.49, 95% CI, −1.73 to −1.25, P<0.00001 (Figure 2; Table 2). If we only include the 18 publications (75 experiments, 767 animals) reporting absolute lesion volume in the analysis, then the weighted mean difference between groups was −28.3 mm3 (95% CI −32.4, −24.2, P<0.00001) in favor of CBs; equivalent to an SMD of −1.27 (95% CI −1.58, −0.97, P<0.00001).
Figure 2.
Forest plot of the effects of cannabinoids on experimental infarct volume subdivided by drug treatment. Each subgroup is ordered by increasing dose. Time of administration is given where ‘pre' represents administration before stroke onset and ‘h' the number of hours after. For full view of this figure please see the HTML version.
Table 2. Change in infarct volume (according to stroke model, species, and drug class), motor impairment and survival after administration of any cannabinoid in experimental stroke.
| No. of experiments | No. of animals | SMD (95% CI) | P value | |
|---|---|---|---|---|
| Lesion volume | ||||
| Stroke model | ||||
| Transient | 90 | 945 | −1.41 (−1.71, −1.11) | <0.00001 |
| Permanent | 54 | 519 | −1.67 (−2.08, −1.27) | <0.00001 |
| Species | ||||
| Rats | 69 | 786 | −1.75 (−2.15, −1.35) | <0.00001 |
| Mice | 74 | 673 | −1.34 (−1.61, −1.06) | <0.00001 |
| Monkeys | 1 | 14 | −0.55 (−1.63, 0.53) | 0.32 |
| Drug class | ||||
| Endocannabinoids | 25 | 268 | −1.72 (−2.62, −0.82) | 0.0002 |
| Synthetic cannabinoids | ||||
| Mixed CB1/CB2 ligands | 41 | 494 | −1.75 (−2.19, −1.31) | <0.00001 |
| CB2 ligands | 18 | 162 | −1.65 (−2.09, −1.22) | <0.00001 |
| Abnormal CBD | 2 | 16 | −0.56 (−2.08, 0.95) | 0.47 |
| HU-211 | 10 | 113 | −2.90 (−4.24, −1.56) | <0.0001 |
| CB1 antagonists | 12 | 103 | −0.70 (−1.22, −0.18) | 0.009 |
| CB2 antagonists | 2 | 14 | 0.96 (−0.32, 2.24) | 0.14 |
| Phytocannbinoids | ||||
| THC | 13 | 115 | −1.43 (−2.01, −0.86) | <0.00001 |
| CBD | 21 | 188 | −1.20 (−1.63, −0.77) | <0.00001 |
| Motor impairment | ||||
| Early (24–72 hours) neuro-score | 55 | 590 | −1.27 (−1.58, −0.95) | <0.00001 |
| Late (2–4 weeks) neuro-score | 8 | 126 | −1.63 (−2.64, −0.62) | 0.002 |
| Rotarod (24 hours after IS) | 10 | 86 | 6.09 (0.7, 11.48)a | 0.03 |
| Survival | ||||
| Transient ischemia | 7 | 154 | 2.09 (0.39, 11.3)b | 0.39 |
CB, cannabinoid; CBD, cannabidiol; CI, confidence interval; IS, ischemic stroke; THC, Δ9-tetrahydrocannabinol; SMD, standardized mean difference.
Weighted mean difference (seconds).
Odds ratio.
Infarct volume was significantly reduced in rats and mice, SMD −1.75, (95% CI −2.15 to −1.35, P<0.00001) and −1.34 (95% CI −1.61 to −1.06, P<0.00001), respectively. The only study involving primates revealed nonsignificant infarct volume reduction upon administration of TAK-937, a CB1/CB2 receptor ligand (SMD −0.55, 95% CI −1.62 to 0.53, P=0.32).45
When grouped by drug class, synthetic agonists (mixed CB1/CB2 ligands (P<0.00001), CB2 ligands (P<0.00001), HU-211 (P<0.0001)), phytocannabinoids (THC and CBD (both P<0.00001)), and endocannabinoids (P=0.002) all reduced infarct volume significantly (see Table 2). The breakdown by individual compound can be seen in Figure 2; the most profound infarct volume reduction is seen with HU-210, a synthetic CB1/CB2 ligand (n=8 experiments, 80 animals (SMD −3.52, 95% CI −5.34 to −1.71).10 Methanandamide, lauroylethanolamide, and linoleoylethanolamide were all neutral in their effect, whereas AEA (n=3, 28 animals) showed borderline significant infarct volume reduction (SMD −0.78, 95% CI −1.64 to 0.08, P=0.07).
Individual studies of the CB1 antagonist SR141716 had a neutral effect on lesion volume except when used at a very high dose (20 mg/kg11) leading to a significant reduction in lesion size (SMD −5.59 [95% CI −9.69, −1.49], P=0.008). Trends to harm were seen using the CB2 antagonist SR144528 (SMD 0.96, 95% CI −0.32 to 2.24 P=0.14).
There was significant statistical heterogeneity (I2 56%, P<0.00001, Figure 2) in the all study analysis.
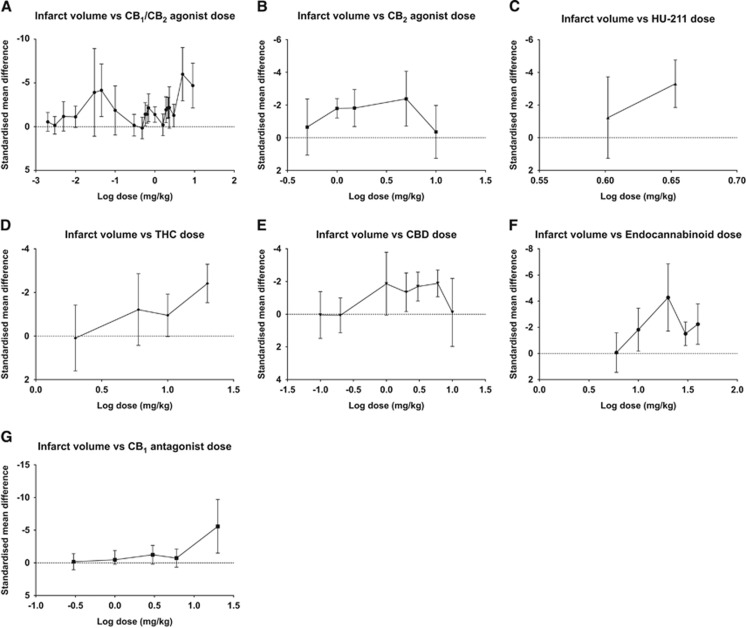
Drug Dose
The effect of drug class dose on infarct volume was analyzed to help establish whether there was a dose-response relationship with infarct volume reduction for each class of CB (Figure 3).
Figure 3.
The effect of cannabinoid (CB) drug dose on experimental infarct volume subdivided by drug class. The standardized mean difference (SMD) in infarct volume is plotted against log [dose] for each drug subgroup (A–G). Error bars represent 95% confidence intervals (CI) and values are not significant where they cross zero. CBD, cannabidiol; THC, Δ9-Tetrahydrocannabinol.
The CB1/CB2 agonists were significantly effective at numerous doses and showed a bimodal distribution of maximum effect with peaks at 45 mcg/kg (HU-210, SMD −4.16, 95% CI −7.17 to −1.16, P=0.007, n=5 experiments, 50 animals)10 and 5 mg/kg (WIN 55212-2, SMD −6.0, 95% CI −9.04 to −2.95, P=0.0001, n=1, 13 animals,25 Figure 3A). Significant statistical heterogeneity was present (I2 63%, P<0.00001).
The CB2 ligands were tested between total doses 0.5 and 10 mg/kg with peak effect at 5 mg/kg (JWH-133 and O-1966, SMD −2.38, 95% CI −4.06 to −0.71, P=0.005, n=5, 37 animals, Figure 3B).41, 42 There was no significant statistical heterogeneity.
Δ9-Tetrahydrocannabinol significantly reduced infarct volume at two doses, 10 and 20 mg/kg (SMD −0.95, 95% CI −1.92 to −0.02, P=0.05, n=3, 27 animals; and SMD −2.41, 95% CI −3.29 to −1.53, P<0.00001, n=6, 59 animals, respectively; Figure 3D). A dose-response relationship was observed with CBD, the greatest lesion volume reduction using 6 mg/kg (SMD −1.89, 95% CI −2.7 to −1.07, P<0.00001, n=6, 57 animals).36, 38, 39 No effect was seen at the greater dose of 10 mg/kg (n=1, 9 animals) (Figure 3E).9
Peak effect with administration of endocannabinoids was seen at 20 mg/kg (SMD −4.28, 95% CI −6.85 to −1.71, P=0.001, n=2, 16 animals24, 44) with significant but less potent effects seen with 30 and 40 mg/kg (Figure 3F). Statistical heterogeneity was evident in the endocannabinoid analysis (I2 78%, P<0.00001) but not for THC and CBD.
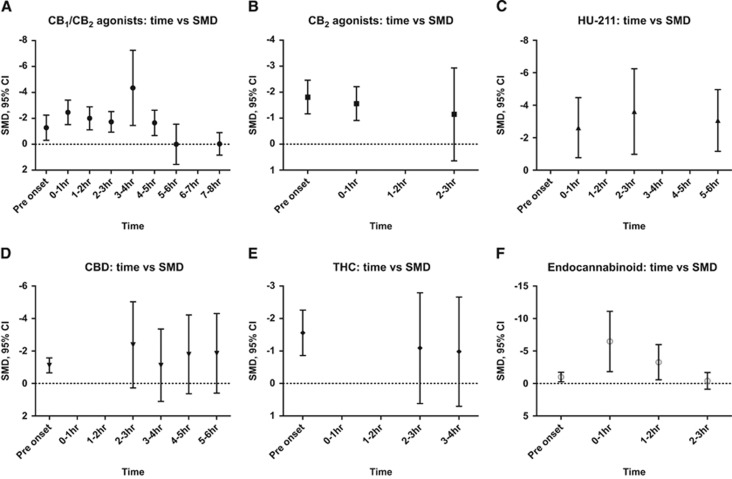
Time of Administration
The CB1/CB2 agonists, assessed up to 8 hours after stroke, revealed a gradual decline in effect size over time, with significant effects seen up to 4 to 5 hours after insult (Figure 4A). A similar pattern was seen for endocannbinoids but with loss of significant effect as soon as 2 to 3 hours after stroke (Figure 4F). HU-211 produced significant infarct volume reduction as late as 6 hours after ictus (Figure 4C). Both CBD (up to 6 hours) and THC (up to 4 hours) showed trends to infarct reduction with later administration but there were too few studies to produce significant values at these later time points (Figures 4D and 4E); 17 of 23 experiments using CBD (and 11 of 13 for THC) administered the drug before stroke onset.
Figure 4.
The effect of time of administration on experimental infarct volume subdivided by drug class. The standardized mean difference (SMD) in infarct volume is plotted against time of administration for each drug subgroup (A–F). Error bars represent 95% confidence intervals (CIs) and values are not significant where they cross zero. CB, cannabinoid.
Functional Outcome and Survival
Early neurologic outcome improved significantly when evaluated in 55 experiments (590 animals), SMD −1.27 95% CI −1.58 to −0.95, P<0.00001. Late neurologic impairment was only assessed in 8 experiments (126 animals) but this still resulted in a significantly improved outcome (P=0.002, Table 2). No effect was seen on survival in 7 experiments (154 animals).
Quality
In all, 10 of 34 publications used randomization in their design, 4 reported blinding of outcome assessments, 21 monitored temperature during surgery, 33 measured outcome at 1 to 3 days and 4 at 7 to 30 days, 28 measured outcomes other than lesion size, 12 assessed a time window for administration and 16 established dose-response effects.
There was no relationship between quality score and lesion volume effect size, Spearman's rho coefficient −0.113, P=0.18. Likewise, there were no significant differences in effect size when comparing individual components of the scale such as randomization and blinding of outcome assessment.
Publication Bias
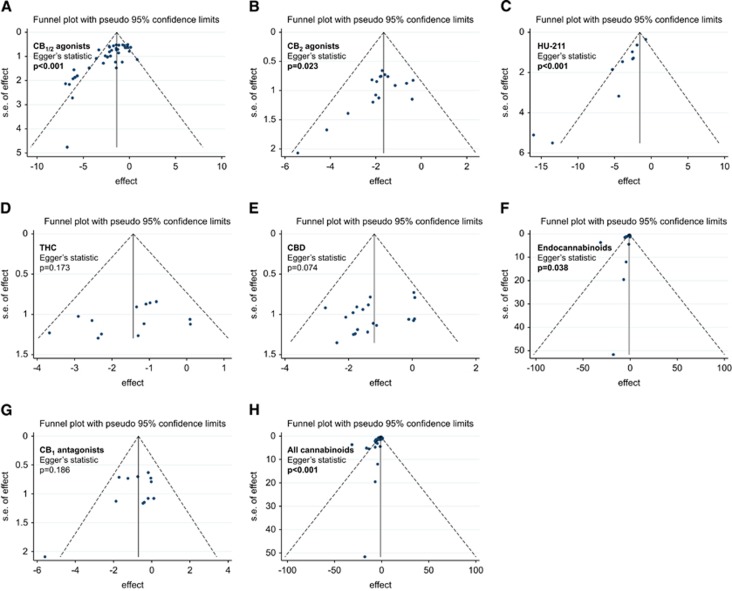
Begg's funnel plots were visually analyzed to determine the presence or absence of publication bias. For all studies, significant bias was present (Egger's statistic P<0.001, Figure 5H).46 A significant bias was present in the subgroups CB1/2 agonists (P<0.001, Figure 5A), CB2 agonists (P=0.023, Figure 5B), HU-211 (P<0.001, Figure 5C), and endocannabinoids (P=0.038, Figure 5F).
Figure 5.
Funnel plots for all studies (A) and each cannabinoid (CB) subgroup (B–H) evaluating publication bias. Standard error of the standardized mean difference (SE (SMD), y axes) for each study is plotted against its effect size (SMD, horizontal axes)). CBD, cannabidiol; THC, Δ9-Tetrahydrocannabinol.
Discussion
This extensive meta-analysis has determined that CBs significantly reduce infarct volume in both transient and permanent models of ischemia, and improve both early and late functional outcome. Almost twice as many animals were studied in transient (n=945) than in permanent (n=519) models and greater infarct volume reductions were seen in permanent models (SMD −1.67 versus −1.41). HU-210, a CB1/CB2 agonist, showed the greatest infarct volume reduction and the CB1/CB2 agonist group was effective when administered as late as 5 hours after stroke onset. HU-211, a proposed NMDA antagonist and enantiomer of HU-210, was effective up to 6 hours after onset.
The mechanisms of action responsible for the effects of CBs in the preclinical setting are multiple but not well understood or always explored within these studies. The CB1 receptors are primarily located in the central nervous system, with activation known to decrease excessive glutamate release,38 allied excitotoxicity,47 and enhance cerebral blood flow.48 Δ9-Tetrahydrocannabinol,36 TAK-937,33 WIN 55212-2,19 and HU-21010 are protective through CB1-mediated hypothermia, an effect abolished by warming. The CB2 receptors are expressed predominantly by cells of the immune system but they also display central nervous system presence, in particular, microglial cells activated during the course of inflammation express CB2 receptors.49 Activation of CB2 receptors results in a decrease in the release of pro-inflammatory cytokines, neutrophil recruitment,9, 41 and leukocyte adhesion to cerebral vessels.43
Cannabinoid-induced neuroprotection is also likely to be mediated through other receptor targets, though the only proven sites include CB1,36, 38 CB2,9, 11, 41 and 5HT1A.3, 39 For example, the effects of CBD are not inhibited by CB1, CB2, or TRPV1 (capsaicin receptor) antagonism, but its ability to decrease infarct volume and enhance cerebral blood flow appear to be mediated, at least in part, through 5HT1A.3, 39 Other mechanisms, such as anti-inflammatory effects, are yet to be linked to a particular target site, and other known CB target sites of action such as TRPs and PPARs require further exploration. The endocannabinoid PEA is associated with reduced cell death, edema, and inflammation,18 and OEA is thought to mediate its infarct-reducing effects through PPARα, as the protective effects of OEA were absent in PPARα−/− mice40 and inhibited by a PPARα antagonist.44
Our systematic review has highlighted many deficiencies in the existing literature that warrants further investigation. It is not apparent that CB2 antagonists have been tested against mixed CB1/CB2 ligands, which is important considering that CB2 activation is a potential therapeutic target. Furthermore, expression of CB2 receptors decreases in the first 3 hours after MCAo and then gradually increases by 24 hours.11 It may, therefore, be of benefit to stimulate CB2 at later time points; our time-to-treatment analysis only showed a trend to infarct volume reduction at 2 to 3 hours with CB2 agonists and there were no experiments extending drug delivery beyond 3 hours. In this review, other CBs show promise with later administration causing significant infarct volume reduction, including CB1/CB2 receptor agonists (up to 5 hours) and HU-211 (up to 6 hours). Cannabidiol may also be beneficial at later time points with trends to reduce infarct volume as late as 6 hours but there were too few studies to show a significant effect; in one study, animal survival was significantly increased even when CBD was administered 3 days after stroke.35
The optimal dose of administration for each drug class also remains unclear. It was generally seen that higher doses resulted in a greater degree of infarct volume reduction. Furthermore, questions are raised with regard to the role of CB1 antagonism; CB1 agonists mediate their positive effects through the various mechanisms described but CB1 antagonism with SR141716 used at a high dose (20 mg) also appeared to be beneficial (it was neutral at lower doses). The mechanisms of such an effect are not understood. If CB1 agonism was detrimental in stroke, then the effects of the mixed CB1/CB2 agonists should be less than that of the CB2-specific drugs, although this was not observed. It is more likely that beneficial effects of the CB1 antagonist at high doses are off-target effects, non-CB1 mediated responses, as previously suggested for SR141716A.50
Further data are also required exploring the effects of CBs in stroke in animals with other comorbidities, as would occur in humans. For example, only one group have also observed the effects of CBs (TAK-937) in aged and female rats and larger species.28, 45 Moreover, TAK-937 is the only compound that has examined coadministration with thrombolysis,28 essential with regard to safety since some neuroprotectants can enhance the risks of recombinant tissue plasminogen activator-associated hemorrhage.51 Hypertensive rats have been studied using HU-211 only, 26, 27, 34 but largely data are absent on the effects of CBs in animals with comorbidities relevant to stroke.52 It is also clear that neurologic assessments of functional outcome at later time points are lacking with only 8 of 144 experiments (HU-211, CBD, and TAK-937) measuring late neuroscores.26, 35, 45 This is important considering the outcomes in future clinical trials will be related to functional outcome and safety.
There are limited data regarding the safety of CBs in humans and none in the stroke population. Sativex, licenced for use in treating spasticity secondary to Multiple Sclerosis, containing THC and CBD in a 1:1 ratio (2.7 mg:2.5 mg per 100 μL), is generally well tolerated but commonly causes transient dizziness, depression, euphoria, gastro-intestinal upset, and altered appetite; uncommonly it is associated with palpitations, tachycardia, hallucinations, and suicidal ideation.53 In a 14-week open label study of 339 patients, 5% discontinued Sativex secondary to treatment-related side effects.54 The psychotropic side effects appear to be mediated through THC CB1 stimulation and studies using CBD alone, however, indicate that it is very well tolerated; in three small studies CBD did not affect heart rate and blood pressure using a single 600 mg dose,55, 56, 57 and in regular use for epilepsy (200 to 300 mg), no specific adverse events were reported (4 randomized studies of poor quality, total n=48).58
Our paper has a number of limitations affecting interpretation of results, issues that confound many meta-analyses. First, significant heterogeneity is present secondary to the variability in design of individual studies. This is accounted for, in part, by using a random-effect model of analysis. Moreover, further heterogeneity is introduced by organizing compounds into subgroups; although we have classed the drugs by mechanisms of action, it is likely that many will act on other target sites not identified and therefore statements on efficacy could be an underestimate or overestimate. Second, caution must also be taken due to the presence of significant publication bias;59 our search strategy may have missed publications in less well-known journals; the noninclusion of some studies means that the estimated treatment effects could be inaccurate. Third, the results also depend on study quality that can also impact report precision; 10 of 34 publications used randomization in their design and only 4 reported blinding of outcome assessments. The impact of various quality items on reported efficacy has been previously assessed;60 the presence or absence of randomization to a treatment group, blinding of drug allocation and blinding of outcome assessments were the most powerful determinants of outcome. In contrast, this review did not find any relationship between study quality and efficacy, even when individual components of quality were analyzed. However, the absence of some of the parameters in our ‘quality' score does not necessarily mean that the experiment was performed to a poor standard; for example, evaluating timing of outcome assessments is simply expanding the cohort of evidence rather than improving the study quality. It may be that some studies did not report specific components such as randomization, which could explain why we found no relationship between quality and efficacy. Fourth, many publications would often use an inadequate number of animals in the control arms of the experiments involving multiple comparisons (e.g., comparing several dose arms with one control group) resulting in smaller control groups in the meta-analysis (it is important not to count the control animals more than once). Moreover, the small group sizes produce imprecise estimates of the variance and, therefore, the SMD. Standardized mean difference, and not weighted mean difference, was used to merge different scales measuring the same parameter; of 34 publications, 14 measured infarct volume as percentage and 18 used absolute volume (mm3). Interpretation of SMD is less intuitive but it has allowed us to include significantly more studies within the analysis.
The failure of multiple neuroprotective agents to be translated into the clinical setting has been extensively highlighted in the literature, 15, 61 hence, evaluating preclinical data thoroughly and systematically before progressing to design human clinical trials is of great importance. Indeed, before moving novel experimental ideas into clinical trials, it is proposed that multicenter phase III-type preclinical studies are performed (www.dcn.ed.ac.uk/multipart). There are no previous clinical trials using CBs in stroke but positive data from trials using CBs in other neurologic diseases already exist.62 The pleiotropic effects of CBs on the ischemic penumbra and cerebral vasculature after stroke, combined with their excellent tolerability, make them promising candidates for future treatment.
The authors declare no conflict of interest.
References
- Jin KL, Mao XO, Goldsmith PC, Greenberg DA. CB1 cannabinoid receptor induction in experimental stroke. Ann Neurol. 2000;48:257–261. [PubMed] [Google Scholar]
- Ashton JC, Rahman RM, Nair SM, Sutherland BA, Glass M, Appleton I, et al. Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neurosci Lett. 2007;412:114–117. doi: 10.1016/j.neulet.2006.10.053. [DOI] [PubMed] [Google Scholar]
- Mishima K, Hayakawa K, Abe K, Ikeda T, Egashira N, Iwasaki K, et al. Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism. Stroke. 2005;36:1077–1082. doi: 10.1161/01.STR.0000163083.59201.34. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Giuffrida A, Berger C, Aschoff A, Schwaninger M, Schwab S, et al. Release of fatty acid amides in a patient with hemispheric stroke: a microdialysis study. Stroke. 2002;33:2112–2114. doi: 10.1161/01.str.0000023491.63693.18. [DOI] [PubMed] [Google Scholar]
- Muthian S, Rademacher DJ, Roelke CT, Gross GJ, Hillard CJ. Anandamide content is increased and CB1 cannabinoid receptor blockade is protective during transient, focal cerebral ischemia. Neuroscience. 2004;129:743–750. doi: 10.1016/j.neuroscience.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Franklin A, Parmentier-Batteur S, Walter L, Greenberg DA, Stella N. Palmitoylethanolamide increases after focal cerebral ischemia and potentiates microglial cell motility. J Neurosci. 2003;23:7767–7775. doi: 10.1523/JNEUROSCI.23-21-07767.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degn M, Lambertsen KL, Petersen G, Meldgaard M, Artmann A, Clausen BH, et al. Changes in brain levels of N-acylethanolamines and 2-arachidonoylglycerol in focal cerebral ischemia in mice. J Neurochem. 2007;103:1907–1916. doi: 10.1111/j.1471-4159.2007.04892.x. [DOI] [PubMed] [Google Scholar]
- Naccarato M, Pizzuti D, Petrosino S, Simonetto M, Ferigo L, Grandi FC, et al. Possible Anandamide and Palmitoylethanolamide involvement in human stroke. Lipids Health Dis. 2010;9:47. doi: 10.1186/1476-511X-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murikinati S, Juttler E, Keinert T, Ridder DA, Muhammad S, Waibler Z, et al. Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J. 2010;24:788–798. doi: 10.1096/fj.09-141275. [DOI] [PubMed] [Google Scholar]
- Leker RR, Gai N, Mechoulam R, Ovadia H. Drug-induced hypothermia reduces ischemic damage: effects of the cannabinoid HU-210. Stroke. 2003;34:2000–2006. doi: 10.1161/01.STR.0000079817.68944.1E. [DOI] [PubMed] [Google Scholar]
- Zhang M, Martin BR, Adler MW, Razdan RK, Ganea D, Tuma RF, et al. Modulation of the balance between cannabinoid CB(1) and CB(2) receptor activation during cerebral ischemic/reperfusion injury. Neuroscience. 2008;152:753–760. doi: 10.1016/j.neuroscience.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Nozako M, Hazekawa M, Ogata A, Fujioka M, et al. Delta9-tetrahydrocannabinol (Delta9-THC) prevents cerebral infarction via hypothalamic-independent hypothermia. Life Sci. 2007;80:1466–1471. doi: 10.1016/j.lfs.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2) Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta(9)-tetrahydrocannabinol, cannabidiol and Delta(9)-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAIR Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Gray LJ, Bath PM, Murphy SP. Progesterone for the treatment of experimental brain injury; a systematic review. Brain. 2008;131:318–328. doi: 10.1093/brain/awm183. [DOI] [PubMed] [Google Scholar]
- England TJ, Gibson CL, Bath PM. Granulocyte-colony stimulating factor in experimental stroke and its effects on infarct size and functional outcome: a systematic review. Brain Res Rev. 2009;62:71–82. doi: 10.1016/j.brainresrev.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Genovese T, Impellizzeri D, Crupi R, Velardi E, Marino A, et al. Reduction of ischemic brain injury by administration of palmitoylethanolamide after transient middle cerebral artery occlusion in rats. Brain Res. 2012;1477:45–58. doi: 10.1016/j.brainres.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Bonfils PK, Reith J, Hasseldam H, Johansen FF. Estimation of the hypothermic component in neuroprotection provided by cannabinoids following cerebral ischemia. Neurochem Int. 2006;49:508–518. doi: 10.1016/j.neuint.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Berger C, Schmid PC, Schabitz WR, Wolf M, Schwab S, Schmid HH, et al. Massive accumulation of N-acylethanolamines after stroke. Cell signalling in acute cerebral ischemia. J Neurochem. 2004;88:1159–1167. doi: 10.1046/j.1471-4159.2003.02244.x. [DOI] [PubMed] [Google Scholar]
- Belayev L, Busto R, Zhao W, Ginsberg MD.HU-211, a novel noncompetitive N-methyl-D-aspartate antagonist, improves neurological deficit and reduces infarct volume after reversible focal cerebral ischemia in the rat Stroke 1995262313–2319.discussion 2319-20. [DOI] [PubMed] [Google Scholar]
- Belayev L, Busto R, Watson BD, Ginsberg MD. Post-ischemic administration of HU-211, a novel non-competitive NMDA antagonist, protects against blood-brain barrier disruption in photochemical cortical infarction in rats: a quantitative study. Brain Res. 1995;702:266–270. doi: 10.1016/0006-8993(95)01127-9. [DOI] [PubMed] [Google Scholar]
- Garg P, Duncan RS, Kaja S, Koulen P. Intracellular mechanisms of N-acylethanolamine-mediated neuroprotection in a rat model of stroke. Neuroscience. 2010;166:252–262. doi: 10.1016/j.neuroscience.2009.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg P, Duncan RS, Kaja S, Zabaneh A, Chapman KD, Koulen P, et al. Lauroylethanolamide and linoleoylethanolamide improve functional outcome in a rodent model for stroke. Neurosci Lett. 2011;492:134–138. doi: 10.1016/j.neulet.2011.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Wang Q, Chen Y, Du J, Zhu X, Lu Y, et al. Neuroprotective effect of WIN 55212-2 pretreatment against focal cerebral ischemia through activation of extracellular signal-regulated kinases in rats. Eur J Pharmacol. 2010;645:102–107. doi: 10.1016/j.ejphar.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Lavie G, Teichner A, Shohami E, Ovadia H, Leker RR. Long term cerebroprotective effects of dexanabinol in a model of focal cerebral ischemia. Brain Res. 2001;901:195–201. doi: 10.1016/s0006-8993(01)02356-3. [DOI] [PubMed] [Google Scholar]
- Leker RR, Shohami E, Abramsky O, Ovadia H. Dexanabinol; a novel neuroprotective drug in experimental focal cerebral ischemia. J Neurol Sci. 1999;162:114–119. doi: 10.1016/s0022-510x(98)00301-3. [DOI] [PubMed] [Google Scholar]
- Murakami K, Suzuki M, Suzuki N, Hamajo K, Tsukamoto T, Shimojo M, et al. Cerebroprotective effects of TAK-937, a novel cannabinoid receptor agonist, in permanent and thrombotic focal cerebral ischemia in rats: therapeutic time window, combination with t-PA and efficacy in aged rats. Brain Res. 2013;1526:84–93. doi: 10.1016/j.brainres.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Nagayama T, Sinor AD, Simon RP, Chen J, Graham SH, Jin K, et al. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J Neurosci. 1999;19:2987–2995. doi: 10.1523/JNEUROSCI.19-08-02987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomacher M, Muller HD, Sommer C, Schwab S, Schabitz WR. Endocannabinoids mediate neuroprotection after transient focal cerebral ischemia. Brain Res. 2008;1240:213–220. doi: 10.1016/j.brainres.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Sun J, Fang Y, Chen T, Guo J, Yan J, Song S, et al. WIN55212-2 promotes differentiation of oligodendrocyte precursor cells and improve remyelination through regulation of the phosphorylation level of the ERK 1/2 via cannabinoid receptor 1 after stroke-induced demyelination. Brain Res. 2013;1491:225–235. doi: 10.1016/j.brainres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Sun J, Fang YQ, Ren H, Chen T, Guo JJ, Yan J, et al. WIN55212-2 protects oligodendrocyte precursor cells in stroke penumbra following permanent focal cerebral ischemia in rats. Acta Pharmacol Sin. 2013;34:119–128. doi: 10.1038/aps.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Suzuki M, Hamajo K, Murakami K, Tsukamoto T, Shimojo M, et al. Contribution of hypothermia and CB1 receptor activation to protective effects of TAK-937, a cannabinoid receptor agonist, in rat transient MCAO model. PLoS ONE. 2012;7:e40889. doi: 10.1371/journal.pone.0040889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichner A, Ovadia H, Lavie G, Leker RR. Combination of dexanabinol and tempol in focal cerebral ischemia: is there a ceiling effect. Exp Neurol. 2003;182:353–360. doi: 10.1016/s0014-4886(03)00083-9. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Irie K, Sano K, Watanabe T, Higuchi S, Enoki M, et al. Therapeutic time window of cannabidiol treatment on delayed ischemic damage via high-mobility group box1-inhibiting mechanism. Biol Pharmaceutical Bull. 2009;32:1538–1544. doi: 10.1248/bpb.32.1538. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Abe K, Hasebe N, Takamatsu F, Yasuda H, et al. Cannabidiol prevents infarction via the non-CB1 cannabinoid receptor mechanism. Neuroreport. 2004;15:2381–2385. doi: 10.1097/00001756-200410250-00016. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Irie K, Hazekawa M, Mishima S, Fujioka M, et al. Cannabidiol prevents a post-ischemic injury progressively induced by cerebral ischemia via a high-mobility group box1-inhibiting mechanism. Neuropharmacology. 2008;55:1280–1286. doi: 10.1016/j.neuropharm.2008.06.040. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Nozako M, Hazekawa M, Irie K, Fujioka M, et al. Delayed treatment with cannabidiol has a cerebroprotective action via a cannabinoid receptor-independent myeloperoxidase-inhibiting mechanism. J Neurochem. 2007;102:1488–1496. doi: 10.1111/j.1471-4159.2007.04565.x. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Nozako M, Ogata A, Hazekawa M, Liu AX, et al. Repeated treatment with cannabidiol but not Delta9-tetrahydrocannabinol has a neuroprotective effect without the development of tolerance. Neuropharmacology. 2007;52:1079–1087. doi: 10.1016/j.neuropharm.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Sun Y, Alexander SP, Garle MJ, Gibson CL, Hewitt K, Murphy SP, et al. Cannabinoid activation of PPAR alpha; a novel neuroprotective mechanism. Br J Pharmacol. 2007;152:734–743. doi: 10.1038/sj.bjp.0707478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarruk JG, Fernandez-Lopez D, Garcia-Yebenes I, Garcia-Gutierrez MS, Vivancos J, Nombela F, et al. Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke. 2012;43:211–219. doi: 10.1161/STROKEAHA.111.631044. [DOI] [PubMed] [Google Scholar]
- Zhang M, Adler MW, Abood ME, Ganea D, Jallo J, Tuma RF, et al. CB2 receptor activation attenuates microcirculatory dysfunction during cerebral ischemic/reperfusion injury. Microvasc Res. 2009;78:86–94. doi: 10.1016/j.mvr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Martin BR, Adler MW, Razdan RK, Jallo JI, Tuma RF, et al. 2) receptor activation decreases cerebral infarction in a mouse focal ischemia/reperfusion model. J Cereb Blood Flow Metab. 2007;27:1387–1396. doi: 10.1038/sj.jcbfm.9600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yang L, Ma A, Zhang X, Li W, Yang W, et al. Orally administered oleoylethanolamide protects mice from focal cerebral ischemic injury by activating peroxisome proliferator-activated receptor alpha. Neuropharmacology. 2012;63:242–249. doi: 10.1016/j.neuropharm.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Suzuki M, Murakami K, Hamajo K, Tsukamoto T, Shimojo M, et al. Cerebroprotective effects of TAK-937, a cannabinoid receptor agonist, on ischemic brain damage in middle cerebral artery occluded rats and non-human primates. Brain Res. 2012;1430:93–100. doi: 10.1016/j.brainres.2011.10.044. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Piser TM, Seybold VS, Thayer SA. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci. 1996;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier-Batteur S, Jin K, Mao XO, Xie L, Greenberg DA. Increased severity of stroke in CB1 cannabinoid receptor knock-out mice. J Neurosci. 2002;22:9771–9775. doi: 10.1523/JNEUROSCI.22-22-09771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Ward SJ. CB. (1)-independent mechanisms of Delta(9)-THCV, AM251 and SR141716 (rimonabant) J Clin Pharm Ther. 2012;37:260–265. doi: 10.1111/j.1365-2710.2011.01284.x. [DOI] [PubMed] [Google Scholar]
- Zechariah A, ElAli A, Hermann DM. Combination of tissue-plasminogen activator with erythropoietin induces blood-brain barrier permeability, extracellular matrix disaggregation, and DNA fragmentation after focal cerebral ischemia in mice. Stroke. 2010;41:1008–1012. doi: 10.1161/STROKEAHA.109.574418. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Electronic Medicines Compendium Sativex: Summary of Product Charactreristics http://www.medicines.org.uk/emc/medicine/23262 .
- Langford RM, Mares J, Novotna A, Vachova M, Novakova I, Notcutt W, et al. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol. 2013;260:984–997. doi: 10.1007/s00415-012-6739-4. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Allen P, Bhattacharyya S, Crippa JA, Mechelli A, Borgwardt S, et al. Modulation of effective connectivity during emotional processing by Delta 9-tetrahydrocannabinol and cannabidiol. Int J Neuropsychopharmacol. 2010;13:421–432. doi: 10.1017/S1461145709990617. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Allen P, Bhattacharyya S, Fusar-Poli P, Crippa JA, Seal ML, et al. Neural basis of Delta-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biol Psychiatry. 2008;64:966–973. doi: 10.1016/j.biopsych.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Bergamaschi MM, Queiroz RH, Chagas MH, de Oliveira DC, De Martinis BS, Kapczinski F, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology. 2011;36:1219–1226. doi: 10.1038/npp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014;3:Cd009270. doi: 10.1002/14651858.CD009270.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8:e1000344. doi: 10.1371/journal.pbio.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley NA, Sena E, Goehler J, Horn J, van der Worp B, Bath PM, et al. Empirical evidence of bias in the design of experimental stroke studies: a metaepidemiologic approach. Stroke. 2008;39:929–934. doi: 10.1161/STROKEAHA.107.498725. [DOI] [PubMed] [Google Scholar]
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW, et al. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006. pp. 467–477. [DOI] [PubMed]
- Collin C, Ehler E, Waberzinek G, Alsindi Z, Davies P, Powell K, et al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res. 2010;32:451–459. doi: 10.1179/016164109X12590518685660. [DOI] [PubMed] [Google Scholar]