Abstract
Although growth factors and anti-apoptotic peptides have been shown to be neuroprotective in stroke models, translation of these experimental findings to clinic is hampered by limited penetration of peptides to the brain. Here, we show that a large peptide like the basic fibroblast growth factor (bFGF) and a small peptide inhibitor of caspase-3 (z-DEVD-FMK) can effectively be transported to the brain after systemic administration by incorporating these peptides to brain-targeted nanoparticles (NPs). Chitosan NPs were loaded with peptides and then functionalized by conjugating with antibodies directed against the transferrin receptor-1 on brain endothelia to induce receptor-mediated transcytosis across the blood–brain barrier (BBB). Pre-ischemic systemic administration of bFGF- or z-DEVD-FMK-loaded NPs significantly decreased the infarct volume after 2-hour middle cerebral artery occlusion and 22-hour reperfusion in mice. Co-administration of bFGF- or z-DEVD-FMK-loaded NPs reduced the infarct volume further and provided a 3-hour therapeutic window. bFGF-loaded NPs were histologically detected in the brain parenchyma and also restored ischemia-induced Akt dephosphorylation. The neuroprotection was not observed when receptor-mediated transcytosis was inhibited with imatinib or when bFGF-loaded NPs were not conjugated with the targeting antibody, which enables them to cross the BBB. Nanoparticles targeted to brain are promising drug carriers to transport large as well as small BBB-impermeable therapeutics for neuroprotection against stroke.
Keywords: cerebrovascular disease/stroke, caspases, growth factors/cytokines, nanoparticles, neuroprotection
Introductıon
Recent research has disclosed that cell death in acute as well as chronic neurodegenerative diseases involves common mechanisms such as caspase activation.1, 2, 3, 4, 5 Therefore, inhibitors of caspases (e.g., benzyloxycarbonyl-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-fluoromethylketone (z-DEVD-FMK)) appear to be attractive therapeutic targets for a wide range of neurologic diseases including stroke.6, 7, 8 Unfortunately, most of the inhibitor agents are small peptides that cannot cross the blood–brain barrier (BBB). However, growth factors (e.g., basic fibroblast growth factor (bFGF)) suppress cell death by acting at several points on death pathways and, additionally, promote regeneration.9, 10, 11 These features make them promising agents for treatment of stroke and other neurodegenerative diseases. Unfortunately, these large peptides too cannot penetrate the brain tissue when systemically administrated. Although it has been proposed that opening of the BBB after ischemia could allow large molecules such as bFGF to enter the brain, recent studies examining the BBB leakage topographically have shown that early leakage is only seen in the deeply ischemic core areas but not in the potentially salvageable penumbra.12, 13, 14
Promoting cell survival while inhibiting death effectors is a potentially promising neuroprotective approach that may provide additive synergy between these two groups of drugs. Indeed, Ma et al15 demonstrated that both z-DEVD-FMK and bFGF could reduce infarct size when given intracerebroventricularly and that their combined administration had a synergistic effect; not only the infarct reduction was greater but also the therapeutic time window was prolonged compared with their individual use. Therefore, the development of an effective brain delivery system for peptides is needed to translate these promising experimental achievements to clinic.
Brain-targeted nanoparticles (NPs) can transport large amounts of BBB-impermeable agents across the BBB on systemic administration.16, 17 We previously demonstrated that chitosan NPs loaded with z-DEVD-FMK and conjugated with antitransferrin receptor-1 monoclonal antibody (TfRMAb) rapidly crossed the BBB upon systemic administration to mice by way of transferrin receptor-mediated transcytosis and provided neuroprotection against focal cerebral ischemia.18 However, loading large peptides such as bFGF to NPs and their transport to the brain in an active form and sufficient concentrations remained a challenge. In the present study, we have first developed the conditions to load bFGF to chitosan NPs and then showed that they reduced ischemic injury on systemic administration. Next, we investigated if we could obtain an additive protective activity by combined administration of z-DEVD-FMK and bFGF-loaded NPs and whether this approach could provide a prolonged therapeutic window. We have also demonstrated activation of the downstream pathways in bFGF signaling to confirm that bFGF was transported in sufficient concentrations similar to the inhibition of caspase-3 activity with z-DEVD-FMK-loaded NPs as we previously showed.18 Finally, we verified that neuroprotection was mediated by transcytosis of peptide-loaded NPs across the endothelium by inhibiting transcytosis with imatinib.19 Importantly, the targeted brain delivery of bFGF-loaded NPs provided not only neuroprotection but also ~300 times reduction in the amount of the peptide administered to the mouse compared with the systemic administration of plain bFGF, offering a great advantage to reduce systemic adverse effects.20
Materials and Methods
Preparation and characterization of the TfRMAb-conjugated or unconjugated chitosan NPs and evaluation of z-DEVD-FMK and bFGF release from them are given elsewhere.11 Briefly, NPs were prepared by loading either peptide to chitosan–polyethyleneglycol–biotin graft polymers in a process aided by reticulation with pentasodium tripolyphosphate. Polyethyleneglycol chains were added to the NP surface to reduce clearance by the reticuloendothelial system, whereas biotin was used to conjugate streptavidinylated TfRMAb by way of streptavidin–biotin binding. These procedures yielded NPs in sizes of 715±32 nm and 747±42 nm when z-DEVD-FMK or bFGF were loaded, respectively.
The experiments were performed on 75 Swiss albino, male mice (25–30 g). The study protocol was approved by the Hacettepe University Animal Experiments Local Ethics Committee (2008/78-1). Focal cerebral ischemia was induced with the intraluminal filament middle cerebral artery occlusion (MCAo) method as described previously.21 Briefly, animals were anesthetized with 2% isoflurane and maintained on 1.5% isoflurane and 30% oxygen. After ligating the right common carotid and external carotid arteries, a nylon filament (8-0) inserted into the common carotid artery was advanced to the origin of MCA. The distal 3 mm of filaments was coated with silicone resin/hardener mixture. For reperfusion, the filament was withdrawn. The regional cerebral blood flow in ischemic area was measured by laser-Doppler flowmetry. Rectal temperature was maintained at 37.0°C±0.5°C with a homeothermic blanket. Systolic blood pressure was measured noninvasively. Tissue oxygen saturation was monitored from the paw by a pulse oximeter (V3304 Tabletop Pulse Oximeter; Surgivet, Waukesha, WI, USA).
Seven groups of mice were subjected to 2 hours proximal MCAo and 22 hours reperfusion. Animals were randomly allocated to study groups in blocks. Fifteen years of experience of our lab shows that this stroke model in Swiss albino mice results in an infarct volume of 50±6 mm3 (mean±s.d.). Based on a previous study15 demonstrating neuroprotection by intracerebroventricular administration of caspase inhibitors and bFGF against 2 hours MCA occlusion in mice, we anticipated a decrease in infarct size by at least 20% in our model. Accordingly, sample size calculation indicated that six animals were required per group for 80% power and an α of 0.05. The control group received an equal mixture of nonfunctionalized (TfRMAb-free) NPs loaded with z-DEVD-FMK (240 ng) or bFGF (17 ng). Treatment groups received functionalized (TfRMAb-conjugated) NPs loaded with z-DEVD-FMK (240 ng) or bFGF (17 ng) or an equal mixture of both. Nanoparticles were administered just before inducing ischemia in these groups. The combined treatment was given to another group 3 hours post-ischemia. Nanoparticles were administered intraperitoneally in 0.1 mL ultrapure water in the above groups. To compare the efficacy of intravenous and intraperitoneal administrations, another group was treated intravenously with bFGF-loaded NPs in 0.1 mL ultrapure water pre-ischemia. Finally, mice treated with imatinib (150 mg/kg twice a day for 2 days) before the day of the MCAo were administered functionalized bFGF-loaded NPs (intraperitoneally) pre-ischemia. Details of the groups and treatments are given in Table 1.
Table 1. Experimental groups.
| Groups | Administration time and route | Treatmenta |
|---|---|---|
| (1) TfRMAb-free, control | Pre-ischemia, intraperitoneally | Equal mixture of TfRMAb-free NPs loaded with z-DEVD-FMK or bFGF |
| (2) z-DEVD | Pre-ischemia, intraperitoneally | TfRMAb-conjugated NPs loaded with z-DEVD-FMK |
| (3) bFGF | Pre-ischemia, intraperitoneally | TfRMAb-conjugated NPs loaded with bFGF |
| (4) bFGF and z-DEVD pre | Pre-ischemia, intraperitoneally | Equal mixture of TfRMAb-conjugated NPs loaded with z-DEVD-FMK or bFGF |
| (5) bFGF and z-DEVD post | 3 hours post-ischemia, intraperitoneally | Equal mixture of TfRMAb-conjugated NPs loaded with z-DEVD-FMK or bFGF |
| (6) bFGF, intravenously | Pre-ischemia, intravenously | TfRMAb-conjugated NPs loaded with bFGF |
| (7) bFGF+imatinib | Imatinib; 150 mg/kg intraperitoneally twice per day for 2 days before the experiment NPs; pre-ischemia, intraperitoneally | TfRMAb-conjugated NPs loaded with bFGF |
bFGF, basic fibroblast growth factor; NP, nanoparticle; TfRMAb, anti-transferrin receptor-1 monoclonal antibody; z-DEVD-FMK, benzyloxycarbonyl-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-fluoromethylketone.
Groups consist of six mice except groups 4 and 7 (n=5 in each).
z-DEVD-FMK (240 ng) or basic fibroblast growth factor (17 ng) loaded nanoparticles were administered in 0.1 mL ultrapure water.
Motor functions were scored as described previously.22 Twenty-two hours after reperfusion, before sacrifice, clinical motor functions were assessed and scored in each animal as: grade 0: no observable motor deficit (normal); grade I: failure to extend left forepaw on lifting the whole body by the tail (mild); grade II: circling to the contralateral side (moderate); and grade III: leaning to the contralateral side at rest or no spontaneous motor activity (severe). One of the two evaluators was masked about the study groups and only the values agreed on by both investigators were analyzed.
Mice were then anesthetized with a lethal dose of chloral hydrate (1 g/kg, intraperitoneally), transcardially perfused with 100 mL of heparinized saline (10 IU/mL) followed by 4% paraformaldehyde and then decapitated. Brains were stored in 4% paraformaldehyde for 24 hours, after which 2-mm thick five brain slabs were serially cut in coronal plane and embedded in paraffin. Five μm-thick sections were obtained from the posterior surface of each slab and stained with hematoxylin and eosin. To calculate the infarct volume, the boundary of infarct area, characterized by reduced eosin staining on hematoxylin and eosin-stained coronal sections under light microscopy, was outlined with image analysis software (ImageJ 1.44o, National Institute of Health, Bethesda, MD, USA). The size of infarcted area in each section was calculated by subtracting the noninfarcted region in the ischemic hemisphere from the total surface area of the contralateral hemisphere. The infarct volume was then calculated by multiplying each sequential infarct area by the distance between sections (2 mm) and by 5 (the number of sections from each brain).
In another set of animals, brains were examined to illustrate the NPs penetrated into parenchyma. Ten naïve mice were given functionalized bFGF-loaded NPs (intraperitoneally or intravenously) 30 minutes (n=2), 1 hour (n=2), and 3 hours (n=6) before sacrifice. Mice administered nonfunctionalized bFGF-loaded NPs served as controls (n=3). NPs were also searched in brain sections of four mice subjected to 2 hours ischemia and 1 hour reperfusion, and received functionalized (n=2) or nonfunctionalized (n=2) bFGF-loaded NPs. Two mice given the vehicle of NP suspensions (0.1 mL ultrapure water, intraperitoneally) served as sham controls. Nanoparticles in sections were detected by either labeling free biotin molecules on the NP surface with streptavidin–horseradish peroxidase or immunolabeling against the human bFGF loaded to NPs.
For conjugating streptavidin to biotin molecules decorating the NP surface, 4 to 5 μm-thick coronal sections were prepared from the paraffin-embedded blocks. Sections were deparaffinized at 60°C overnight and then in xylene for 1 hour. Sections were hydrated in graded alcohol solutions, washed in phosphate-buffered saline (PBS) for 10 min, and then treated with streptavidin–horseradish peroxidase complex (Reagent C of Acu-Stain Mouse+Rabbit HRP Kit, Genemed Biotechnologies, San Francisco, CA, USA) for 1 hour at room temperature. After three 10-minute washes with PBS, sections were treated with filtered diaminobenzidine (Genemed Biotechnologies) for 1 hour at room temperature. Sections were cover-slipped with Hoechst 33258 after three 10-minute washes with PBS.
For immunolabeling, paraffin-embedded 4 to 5 μm-thick sections were deparaffinized and hydrated in xylol and graded alcohol solutions. Endogenous peroxidase activity was blocked with 3% hydrogen peroxidase solution. Antigen retrieval was performed with 10 mmol/L citrate buffer (pH=6) in a pressure cooker for 20 minutes. The sections were then rinsed with PBS (pH 7.4) at room temperature and nonspecific binding was blocked by using 3% bovine serum albumin (Sigma, St Louis, MO, USA) in PBS for 20 minutes. Next, the sections were incubated with antihuman-FGF-2/basic FGF polyclonal antibody (Millipore, Upstate, Billerica, MA, USA; 1:100 in PBS) at room temperature for 1 hour. Immunodetection of bFGF was performed using the conventional streptavidin–horseradish peroxidase technique (Dako cytomation kit, Dako, Carpinteria, CA, USA). Filtered diaminobenzidine (Sigma) was used as a chromogen. Negative controls were carried out by omitting the primary antibody.
In another set of animals, brain samples of the MCA area from mice subjected to 2 hours of ischemia and 22 hours of reperfusion and received (intravenously) either functionalized NPs (n=6) or nonfunctionalized NPs loaded with bFGF (n=4) as well as from sham-operated naïve mice (n=4) were used for the blotting procedure. Samples obtained by wedge resection of the MCA area were placed on ice and lysed in radioimmunoprecipitation assay buffer (25 mM Tris–HCl (pH 7.6), 150 mM NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate) with protease (Sigma) and phosphatase inhibitors (Sigma) by using a ultrasonic homogenizer (Bandelin Sonopuls, Berlin, Germany). The lysates were centrifuged at 14,000 r.p.m. for 15 minutes at 4°C. The supernatants were collected and protein concentrations of the samples were determined by Qubit Protein Assay Kit (Invitrogen, Eugene, OR, USA). Equal amounts of protein were loaded to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (4% to 12% Invitrogen) and subsequently transferred to polyvinylidene difluoride membranes (Invitrogen) by iBlot dry blotting system (Invitrogen). Nonspecific protein binding was blocked by incubating the polyvinylidene difluoride membranes in 3% bovine serum albumin (Sigma) in Tris buffered saline with Tween 20 for 1 hour at 4 °C, and then the membranes were incubated with antirabbit phospho-Akt (Ser473) antibody (1:1,000, Cell Signaling Technology, Danvers, MA, USA) and antirabbit Akt antibody (1:1,000, Cell Signaling Technology) overnight at 4 °C. Next day, after washing with Tris buffered saline with Tween 20, the membranes were incubated with horseradish peroxidase-conjugated anti-rabbit immunoglobulin-G secondary antibody (1:3000, Cell Signaling Technology) for 1 hour at room temperature. The protein bands were visualized by chemiluminescence (Super Signal West-Femto; Pierce, Rockford, IL, USA). Images were captured by Kodak 4000 MM image station (Kodak, Japan). ImageJ was used for data analysis.
Statistical Analysis
Data are expressed as mean±s.e.m. Blood pressure, regional cerebral blood flow, tissue oxygen saturation, motor function scores, infarct volumes, and p-Akt/Akt ratios of groups were compared with Kruskal–Wallis test. Statistically significant data were further analyzed with Mann–Whitney U-test. The infarct volumes (parametric values) were correlated with the neurologic deficit scores (nonparametric values) by using Spearman's test. A P value of <0.05 was regarded as statistically significant. P values were corrected for multiple comparisons.
Results
Systemically Administered Peptide-Loaded Nanoparticles Provide Neuroprotection
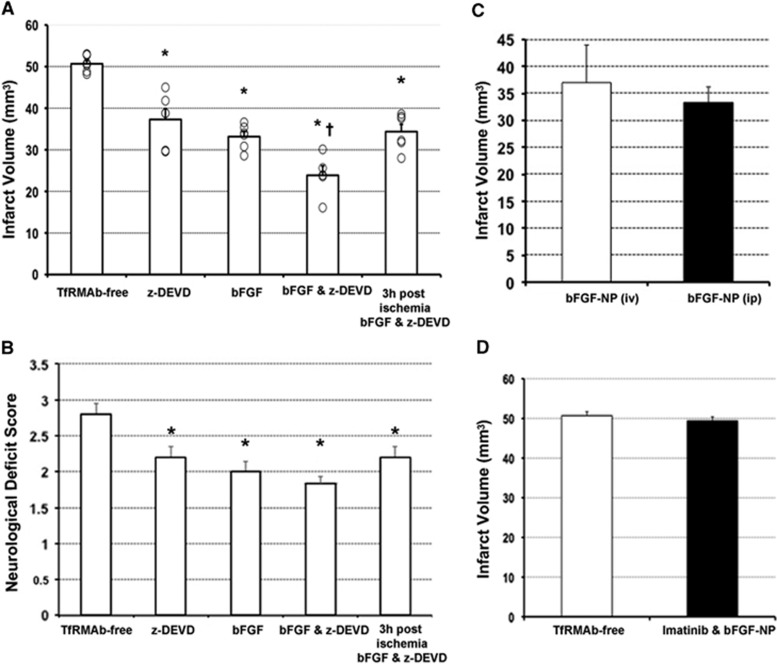
For neuroprotection study, mice were randomly allocated to seven groups of six animals to avoid any selection bias. The mean (±s.e.m.) infarct volume of the control group receiving nonfunctionalized NPs loaded with an equal mixture of bFGF or z-DEVD-FMK, which can release bFGF and z-DEVD-FMK to the plasma but are unable to cross BBB, was 51±1 mm3 after 2 hours of MCAo and 22 hours of reperfusion (Figure 1A). In contrast, functionalized bFGF-loaded NPs significantly decreased the infarct volume when administered either intravenously (37±3 mm3) or intraperitoneally (33±1 mm3) (Figures 1A and 1C). z-DEVD-FMK-loaded, functionalized NPs (intraperitoneally) also reduced the infarct size (37±3 mm3) similarly to when they were administered intravenously, as previously reported.18 Administration of an equal mixture of bFGF- or z-DEVD-FMK-loaded, functionalized NPs further reduced the infarct volume to 24±2 mm3 (n=5, one animal from this group was excluded because we detected ischemic changes also in the contralateral hemisphere). To test if the neuroprotection observed with the pre-ischemic treatments above might have clinical significance, an equal mixture of bFGF- and z-DEVD-FMK-loaded NPs was administered 3 hours after stroke (1 hour after reperfusion), which also significantly reduced the infarct volume to 34±2 mm3. Treatments with NPs improved motor function deficit scores parallel to the reductions in infarct size although the decreases in deficit scores were less striking possibly because some sensorimotor fibers ascending to or descending from the recovered cortex could not escape damage in the ischemic subcortical area (Figure 1B). However, we found a moderate but statistically significant correlation (r=0.59, P=0.001, n=29) between the infarct volumes and the neurologic deficit scores.
Figure 1.
The infarct volumes and motor function deficit scores of the control and treatment groups. Data are presented as mean±s.e.m. 'o' denotes individual values. (A) After 2 hours of ischemia and 22 hours of reperfusion, infarct volumes of the groups receiving functionalized nanoparticles (NPs) loaded with benzyloxycarbonyl-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-fluoromethylketone (z-DEVD-FMK), basic fibroblast growth factor (bFGF), or an equal mixture of bFGF and z-DEVD-FMK decreased significantly compared with the group receiving nonfunctionalized NPs loaded with an equal mixture of bFGF and z-DEVD-FMK (anti-transferrin receptor-1 monoclonal antibody (TfRMAb) free, control). Combined treatment was more effective and had a 3-hour therapeutic window (last 2 columns, respectively). (B) Changes in motor function scores paralleled the infarct volume changes. (C) Intraperitoneally or intravenously administration of functionalized NPs loaded with bFGF was equally effective in decreasing the infarct volume. (D) When the endothelial transcytosis was inhibited with imatinib treatment, no neuroprotection was obtained with bFGF-loaded functionalized NPs. *P<0.05 compared with the TfRMAb-free group. †P<0.05 compared with the bFGF or Z-DEVD-FMK-treated groups. The bFGF-NP (intraperitoneally) group in C and TfRMAb-free group in D were reproduced from the data illustrated in A for comparison.
To confirm that the neuroprotection provided by NPs is mediated by peptides transported into brain parenchyma, we treated another group of mice with imatinib, which inhibits the receptor-mediated endothelial transcytosis, 2 days before administering NPs.19 Imatinib treatment suppressed the neuroprotective effect of bFGF-loaded functionalized NPs; the infarct volume of this group (49±2 mm3, n=5, one mouse allocated to this group died during surgery) was not significantly different than the control group receiving nonfunctionalized NPs (Figure 1D). These mice slightly lost weight (3±0 g in 2 days); however, we did not detect any histological abnormalities in brain endothelia or other vascular structures or in parenchyma in nonischemic hemispheres by light microscopy as reported.23
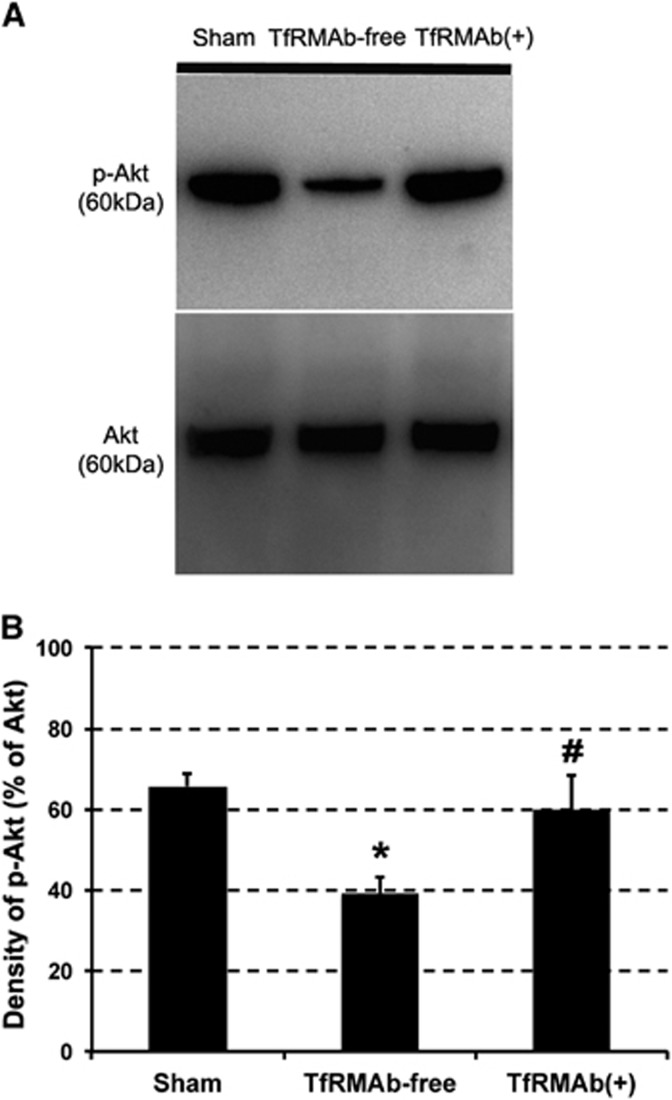
Since no significant differences in regional cerebral blood flow changes, arterial blood pressure, and tissue oxygen saturation were observed between groups during ischemia (Table 2), we attributed the reduction in infarct volume and motor function to neuroprotective action of the brain-delivered peptides but not to any potential hemodynamic or systemic changes induced by circulating NPs. Moreover, to verify that effective concentrations of bFGF in the parenchyma were achieved, we measured the phosphorylation level of Akt (a downstream step in bFGF signaling) in wedge-shaped ischemic MCA territory specimens in 14 mice. p-Akt/Akt ratio significantly dropped to 39%±4% in mice subjected to 2 hours of ischemia and 22 hours of reperfusion and treated with nonfunctionalized NPs (n=4) compared with 66%±3% in nonischemic, sham-operated mice brains (n=4, P<0.05). Treatment with functionalized NPs restored the p-Akt/Akt ratio in ischemic brain specimens near to sham-operated brain levels (60%±9%, n=6, P=0.61; Figure 2). Of note, effective inhibition of caspase-3 activity in ischemic mouse brain with z-DEVD-FMK-loaded NPs given at the same dose used in the present study had previously been shown.18
Table 2. Physiologic parameters of groups.
| Groups | Blood pressure (mm Hg)a | rCBF during ischemia (%) | Tissue oxygen saturation (%) |
|---|---|---|---|
| TfRMAb-free, control | 93±7 (n=4) | 29±3 | 96±1 |
| z-DEVD | 95±4 (n=3) | 27±2 | 94±2 |
| bFGF | 90±5 (n=5) | 28±3 | 97±1 |
| bFGF and z-DEVD (pre-ischemia) | 83±10 (n=4) | 30±3 | 96±2 |
| bFGF and z-DEVD (3 hours post-ischemia) | 94±5 (n=3) | 25±4 | 95±3 |
| bFGF (intravenously) | 98±8 (n=5) | 26±3 | 96±2 |
| bFGF+imatinib | 94±3 (n=3) | 28±4 | 96±1 |
bFGF, basic fibroblast growth factor; rCBF, regional cerebral blood flow; TfRMAb, anti-transferrin receptor-1 monoclonal antibody; z-DEVD, benzyloxycarbonyl-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-fluoromethylketone.
Values are given as mean±s.e.m. Group means were not significantly different (P>0.05).
Blood pressure could not be measured in some mice for technical reasons. n=5–6 for rCBF and tissue oxygen saturation values. Physiologic parameters were recorded during the first 30 minutes of ischemia before terminating anesthesia.
Figure 2.
p-Akt/Akt western blots of the middle cerebral artery (MCA) area of mice subjected to 2 hours of ischemia and 22 hours of reperfusion. Akt phosphorylation (p-Akt/Akt ratio) decreased in the ischemic MCA area of the group treated with nonfunctionalized nanoparticles (NPs) (anti-transferrin receptor-1 monoclonal antibody (TfRMAb) free). Treatment with functionalized NPs (TfRMAb+) restored the p-Akt/Akt ratio close to the sham-operated brain levels. *, P=0.029 and #, P=0.61 compared to the sham-operated group. The representative samples illustrated in A derived from the same blot. The samples from three groups (B) were loaded to two gels and processed in parallel.
Detection of Nanoparticles in Brain Parenchyma
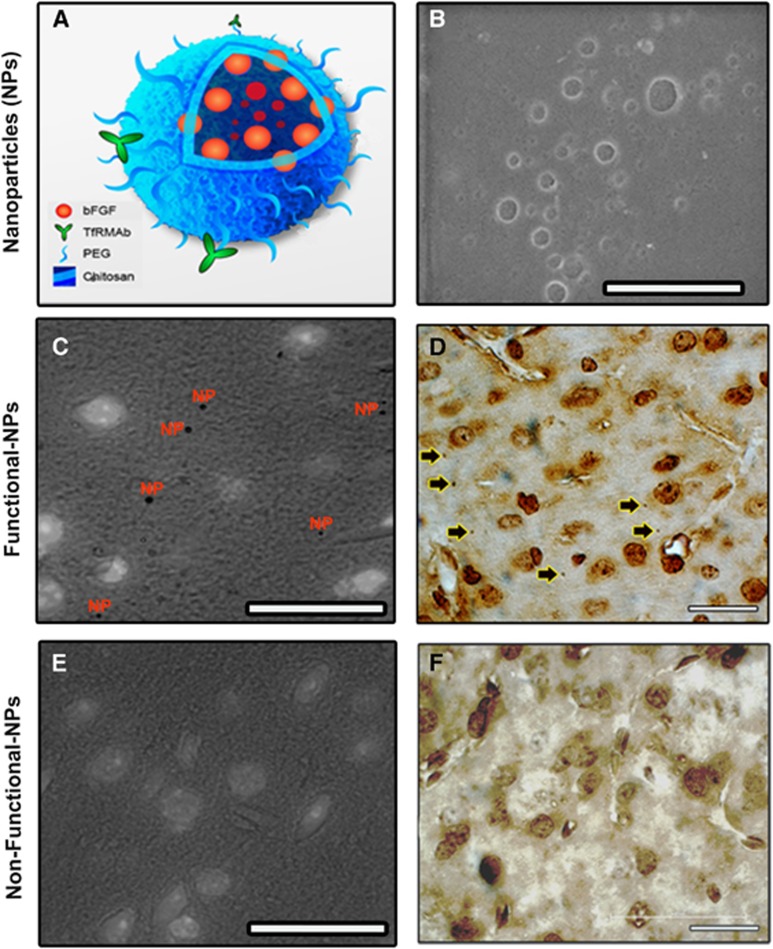
Investigators masked to the treatment evaluated the brain sections to detect NPs in parenchyma in 19 mice (Figure 3). NPs were scattered throughout the brain parenchyma and as the mice were thoroughly perfused before sacrifice none was found inside vessel lumens except a few adhered to the endothelium. Numerous parenchymal NPs were detected 30 minutes and 1 hour after administration, decreasing by 3 hours. This observation conforms to the time profile of NP penetrance to the brain parenchyma by way of transferrin receptor-mediated transcytosis as detected with in vivo brain imaging in intact mice under anesthesia.18 Since NPs could unambiguously be detected only at × 1,500 magnification, quantification of their distribution across 5 μm-thick sections was not feasible and, hence, was limited by sampling error; nevertheless, NPs appeared to be similarly distributed in ischemic as well as nonischemic hemispheres with visual screening. In contrast, we did not observe any NPs in brain parenchyma (including the ischemic hemisphere) of the mice given nonfunctionalized NPs, excluding the possibility that NPs could penetrate the brain through the ischemia-damaged BBB in accordance with the neuroprotection and biochemistry data. NPs had a clearly distinguishable spherical shape (<1 μm) when visualized with the streptavidin–horseradish peroxidase technique exploiting the presence of free biotin molecules on the NP surface (Figure 3A). The specificity of this labeling was further confirmed by immunostaining of bFGF peptides loaded to the NPs with anti-bFGF antibodies (Figure 3C). There was no evidence of inflammation or astrocyte end-feet swelling in the nonischemic brain parenchyma around the NPs, conforming with the idea that chitosan is a biocompatible polymer and does not induce tissue toxicity at microscopic level.24
Figure 3.
Numerous nanoparticles (NPs) were detected in the brain (cortex) parenchyma 1 hour after systemic administration of the functionalized NPs. NPs were prepared by loading peptides (here, basic fibroblast growth factor (bFGF)) to chitosan–polyethyleneglycol–biotin graft polymers and were functionalized by conjugating streptavidinylated antitransferrin receptor-1 monoclonal antibody with streptavidin–biotin binding (A). The transmission electron micrograph in B illustrates a group of NPs in suspension; scale bar, 2 μm. (C) NPs in brain tissue were detected by binding streptavidin–horseradish peroxidase complex to free biotin molecules on the NP surface (NPs). (D) Human bFGF molecules on the surface of NPs were also visualized with anti-human bFGF antibody (arrows). Nonfunctionalized NPs did not penetrate to parenchyma (E, F). Anti-human bFGF antibody has cross reactivity to the mouse bFGF, hence, labeled the cortical cells, especially their nuclei, and caused a light background staining (D, F). In C and E, bright field images illustrating diaminobenzidine (DAB) labeling were superimposed with fluorescent images exhibiting Hoechst 33258-labeled nuclei of the same area to show cells nearby NPs. Scale bars, 20 μm.
Discussion
In this study, we demonstrated that (1) a relatively large peptide like bFGF (17.2 kDa) can be successfully encapsulated into chitosan NPs and targeted to the brain as well as z-DEVD-FMK, a small peptide consisting of four amino acids; (2) systemically administered NPs can transport bFGF to the brain rapidly, efficiently, and importantly in an active form, which provides significant neuroprotection against stroke induced by 2 hours of MCAo; (3) this effect can totally be suppressed by inhibition of transcytosis across the BBB; (4) combined treatment with z-DEVD-FMK- and bFGF-loaded NPs provides additive neuroprotective effect, and is effective even when administered 3 hours post-ischemia.
The active substances encapsulated to NPs were composed of amino acids. Therefore, during preparation of NPs, we chose mild chemical conditions to preserve their three-dimensional structures and functional groups.25 Indeed, both peptides were found to be biologically active in the brain after systemic administration (see also ref. 18). Systemically administered bFGF-loaded NPs showed neuroprotection only when they were conjugated with antibodies against the TfR-1 located on the brain vascular endothelium. This excludes the possibilities that bFGF released from the NPs to circulation or direct access of bFGF-loaded NPs to the brain through the ischemia-damaged BBB may have provided neuroprotection and, strongly suggests that transcytosis of NPs mediated by the TfR-1 is the main mechanism transporting bFGF into the brain parenchyma. This idea was further supported by complete suppression of the neuroprotection after inhibiting endothelial transcytosis with imatinib.19, 26 Despite unambiguous microscopic and pharmacological (i.e., neuroprotection) evidence provided by previous studies,18, 26 we did not exclude the possibility that the infarct sparing effect might have been secondary to an endothelial action of the peptides (e.g., as proposed for systemically administrated bFGF27). The present study with imatinib, which allows penetration into the endothelium but disrupts transcytosis by inhibiting the exocytosis from the endothelium to parenchyma, convincingly shows that, for neuroprotection, NPs must be exocytosed from the endothelium to the brain parenchyma and that an endothelial action alone is not sufficient.19 We should emphasize that the total amount of bFGF that we administered to a mouse with NPs is too small (680 ng/kg) compared with the dose (200 μg/kg, intravenously) used in studies reporting neuroprotection with systemically administered bFGF to the mouse,20 supporting the idea that rather than bFGF spilled to plasma from NPs bFGF transported to the brain provides neuroprotection, possibly by achieving parenchymal concentrations comparable to that obtained with intracerebroventricular injection of 30 ng of bFGF in the study by Ma et al.15 Rich and selective expression of TfR-1 on brain vasculature unlike the TfR2 subtype expressed in other tissues may have facilitated preferential targeting of TfRMAb-conjugated NPs to the brain. Therefore, targeted delivery of bFGF to the brain not only provides neuroprotection but also reduces the possibility of systemic side effects by reducing the amount of bFGF administered ~300 times. Finally, we should note that the administration of NPs either before inducing ischemia when circulation was still intact or 1 hour after reperfusion when the ischemic tissue was at least partly reperfused must have allowed distribution and transport of NPs to all MCA territory. The collateral blood flow to the penumbra must have further sustained the delivery during 2 hours of MCA occlusion.
Earlier work from our group demonstrated that chitosan NPs loaded with small peptides were rapidly transported into the brain when they were functionalized with TfRMAb.18, 26 The neuroprotection observed and the activation of the brain bFGF–p-Akt pathway in the present study further illustrate that they can efficiently carry large peptides to the brain as well. This finding is important from a technological viewpoint because it shows that encapsulating large molecules does not unfavorably modify NP transport kinetics and that the preparation procedures used do not lead to loss of the peptide activity, which is extremely vulnerable to minor alterations in the three-dimensional structure or to modifications by small molecules. Importantly, the peptides were also neuroprotective when co-administered 3 hours after ischemia onset. This conforms to the 3-hour therapeutic window detected after their intracerebroventricular co-administration,15 and suggests that a significant amount of the loaded peptide must have been released from NPs in this time window. It is likely that, once inside the brain parenchyma, peptides are rapidly released from the NPs due to degradation of chitosan by enzymatically active forms of human chitinases such as the acidic mammalian chitinase, the di-N-acetylchitobiase, and chitotriosidase.24 Supporting this, histologically detectable NPs in parenchyma were decreased 3 hours after administration. The upper limit of the therapeutic time window remains to be detected by future studies, as the main aim of the present extensive study was to replicate the neuroprotection and synergy obtained with intracerebroventricular injection of bFGF and z-DEVD-FMK 3 hours post-ischemia with systemically administered NPs. The present study also shows that co-administration of NPs loaded with different agents is a viable strategy to obtain additive effects and, technically, is a more practical approach than incorporating multiple agents into the same NP. Therefore, NPs loaded with several other protease inhibitors and survival factors shown to be neuroprotective in stroke models can be combined for more effective suppression of cell death mechanisms without inducing systemic toxicity.28
In conclusion, chitosan NPs targeted to the brain appear to be promising drug carriers to transport large as well as small BBB-impermeable therapeutic agents. They are easy to prepare, rapidly transported to the brain, readily release their content without loss of activity, allow combination of different agents, and do not have any gross toxicity. Therefore, these exciting findings warrant further research to translate the developments to clinical applications.
The authors declare no conflict of interest.
Footnotes
Turgay Dalkara's work is supported by the Turkish Academy of Sciences. This study is supported by The Scientific and Technological Research Council of Turkey (TUBITAK, Project Number: 109S017).
References
- Asahi M, Hoshimaru M, Uemura Y, Tokime T, Kojima M, Ohtsuka T, et al. Expression of interleukin-1 beta converting enzyme gene family and bcl-2 gene family in the rat brain following permanent occlusion of the middle cerebral artery. J Cereb Blood Flow Metab. 1997;17:11–18. doi: 10.1097/00004647-199701000-00003. [DOI] [PubMed] [Google Scholar]
- Chen J, Nagayama T, Jin KL, Stetler RA, Zhu RL, Graham SH, et al. Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci. 1998;18:4914–4928. doi: 10.1523/JNEUROSCI.18-13-04914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, et al. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredesen DE. Programmed cell death mechanisms in neurological disease. Curr Mol Med. 2008;8:173–186. doi: 10.2174/156652408784221315. [DOI] [PubMed] [Google Scholar]
- Dericioglu N, Soylemezoglu F, Gursoy-Ozdemir Y, Akalan N, Saygi S, Dalkara T. Cell death and survival mechanisms are concomitantly active in the hippocampus of patients with mesial temporal sclerosis. Neuroscience. 2013;237:56–65. doi: 10.1016/j.neuroscience.2013.01.050. [DOI] [PubMed] [Google Scholar]
- Hara H, Friedlander RM, Gagliardini V, Ayata C, Fink K, Huang Z, et al. Inhibition of interleukin 1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Natl Acad Sci USA. 1997;94:2007–2012. doi: 10.1073/pnas.94.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Schulz JB. Caspases as treatment targets for neurodegenerative diseases. J Neurochem. 1999;73:S57–S57. doi: 10.1002/1531-8249(199904)45:4<421::aid-ana2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: A play in three akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Dawbarn D, Allen SJ. Neurotrophins and neurodegeneration. Neuropathol Appl Neurobiol. 2003;29:211–230. doi: 10.1046/j.1365-2990.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- Caban S, Capan Y, Couvreur P, Dalkara T. Preparation and characterization of biocompatible chitosan nanoparticles for targeted brain delivery of peptides. Methods Mol Biol. 2012;846:321–332. doi: 10.1007/978-1-61779-536-7_27. [DOI] [PubMed] [Google Scholar]
- Taheri S, Candelario-Jalil E, Estrada EY, Rosenberg GA. Spatiotemporal correlations between blood-brain barrier permeability and apparent diffusion coefficient in a rat model of ischemic stroke. PloS ONE. 2009;4:e6597. doi: 10.1371/journal.pone.0006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowland D, Arac A, Sekiguchi KJ, Hsu M, Lutz SE, Perrino J, et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82:603–617. doi: 10.1016/j.neuron.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- Ma J, Qiu J, Hirt L, Dalkara T, Moskowitz MA. Synergistic protective effect of caspase inhibitors and bfgf against brain injury induced by transient focal ischaemia. Br J Pharmacol. 2001;133:345–350. doi: 10.1038/sj.bjp.0704075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47:65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- Nicolas J, Mura S, Brambilla D, Mackiewicz N, Couvreur P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev. 2013;42:1147–1235. doi: 10.1039/c2cs35265f. [DOI] [PubMed] [Google Scholar]
- Karatas H, Aktas Y, Gursoy-Ozdemir Y, Bodur E, Yemisci M, Caban S, et al. A nanomedicine transports a peptide caspase-3 inhibitor across the blood-brain barrier and provides neuroprotection. J Neurosci. 2009;29:13761–13769. doi: 10.1523/JNEUROSCI.4246-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Huang Z, Chen K, Huang PL, Finklestein SP, Moskowitz MA. bFGF ameliorates focal ischemic injury by blood flow-independent mechanisms in eNOS mutant mice. Am J Physiol. 1997;272:H1401–H1405. doi: 10.1152/ajpheart.1997.272.3.H1401. [DOI] [PubMed] [Google Scholar]
- Gursoy-Ozdemir Y, Bolay H, Saribas O, Dalkara T. Role of endothelial nitric oxide generation and peroxynitrite formation in reperfusion injury after focal cerebral ischemia. Stroke. 2000;31:1974–1980. doi: 10.1161/01.str.31.8.1974. [DOI] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- Venalis P, Maurer B, Akhmetshina A, Busch N, Dees C, Sturzl M, et al. Lack of inhibitory effects of the anti-fibrotic drug imatinib on endothelial cell functions in vitro and in vivo. J Cell Mol Med. 2009;13:4185–4191. doi: 10.1111/j.1582-4934.2008.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev. 2010;62:3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Amidi M, Romeijn SG, Borchard G, Junginger HE, Hennink WE, Jiskoot W. Preparation and characterization of protein-loaded n-trimethyl chitosan nanoparticles as nasal delivery system. J Control Release. 2006;111:107–116. doi: 10.1016/j.jconrel.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Aktas Y, Yemisci M, Andrieux K, Gursoy RN, Alonso MJ, Fernandez-Megia E, et al. Development and brain delivery of chitosan-peg nanoparticles functionalized with the monoclonal antibody OX26. Bioconjug Chem. 2005;16:1503–1511. doi: 10.1021/bc050217o. [DOI] [PubMed] [Google Scholar]
- Rosenblatt S, Irikura K, Caday CG, Finklestein SP, Moskowitz MA. Basic fibroblast growth factor dilates rat pial arterioles. J Cereb Blood Flow Metab. 1994;14:70–74. doi: 10.1038/jcbfm.1994.11. [DOI] [PubMed] [Google Scholar]
- Kilinc M, Gursoy-Ozdemir Y, Gurer G, Erdener SE, Erdemli E, Can A, et al. Lysosomal rupture, necroapoptotic interactions and potential crosstalk between cysteine proteases in neurons shortly after focal ischemia. Neurobiol Dis. 2010;40:293–302. doi: 10.1016/j.nbd.2010.06.003. [DOI] [PubMed] [Google Scholar]