Abstract
The effects of hydrogen sulfide (H2S) on blood–brain barrier (BBB) and brain edema after cardiac arrest (CA) and cardiopulmonary resuscitation (CPR) remain poorly understood. We investigated the effects of exogenous 80-p.p.m. H2S gas on BBB, brain water content, neurologic outcome, and survival rate after CA and CPR. Cardiopulmonary resuscitation followed CA induced in rats by ventricular fibrillation for 6 minutes. Results show that inhalation of 80-p.p.m. H2S significantly reduced the permeability of the BBB in both in the cortex and hippocampus at 24 hours after resuscitation. Hydrogen sulfide also lessened brain edema in the cortex and hippocampus, ameliorated neurologic outcome as evaluated by neurologic deficit score and tape removal test, and improved the 14-day survival rate. Hydrogen sulfide also attenuated CA and CPR-induced increases of matrix metalloproteinase-9 (MMP-9) activity and vascular endothelial growth factor (VEGF) expression, and increased the expression of angiogenin-1 (Ang-1). These results indicate that inhalation of 80-p.p.m. H2S immediately after CPR attenuated BBB permeability and brain edema, and improved neurologic outcome and 14-day survival of rats after CA. The therapeutic benefits of H2S could be associated with suppression of MMP-9 and VEGF expression and increased expression of Ang-1.
Keywords: blood–brain barrier, brain recovery, cardiac arrest, cardiopulmonary resuscitation, hydrogen sulfide, matrix proteins
Introduction
Poor neurologic outcome after cardiac arrest (CA) and resuscitation is a serious public health problem around the world. Even when return of spontaneous circulation (ROSC) is achieved, more than half of the survivors suffer serious neurologic dysfunction.1, 2, 3 New therapeutic strategies are needed to improve patient survival and outcomes. Hydrogen sulfide (H2S) has been shown to have a variety of protective actions in various models of tissue and organ injury.4, 5, 6 However, the effect of H2S on neurologic outcome after CA and resuscitation, and the underlying mechanism, is not known.
Disruption of the blood–brain barrier (BBB) after ROSC is one of the major factors leading to permanent brain damage.7, 8 The BBB is a physical and metabolic barrier that maintains a constant environment around the neurons in the brain. Disruption of the BBB leads to extravasation of serum albumin, other macromolecular proteins, and cellular elements into the brain extracellular space, resulting in vasogenic brain edema, neuronal apoptosis, and cell death.8, 9 Matrix metalloproteinase-9 (MMP-9),10 vascular endothelial growth factor (VEGF),11 and angiogenin-1 (Ang-1)12 play important roles in maintaining the integrity of the BBB. Matrix metalloproteinase-9 promotes BBB damage and brain edema in the early period of cerebral ischemia by degrading the extracellular matrix of cerebral microvessel basal lamina. This matrix consists of collagen IV, laminin, fibronectin, and interendothelial tight junction proteins such as zonula occludens-1.13, 14 Angiogenin-1 can counteract VEGF-induced vascular permeability by regulating MMP-9 activity.15
We evaluated the effects of inhalation of H2S gas on neurologic outcome after CA and resuscitation, and examined whether its protective effects were associated with permeability of the BBB. We further examined whether changes in MMP-9, VEGF, and Ang-1 levels were associated with these effects.
Materials and Methods
Animal Surgical Procedures
This study was approved by the Institutional Animal Care and Use Committee of Harbin Medical University, Harbin, Heilongjiang, China, and followed national guidelines (Guidelines on Administration of Laboratory Animals in China and Guidelines on the Humane Treatment of Laboratory Animals in China) for the treatment of animals. Male Sprague-Dawley rats weighing 250 to 300 g were used in this study. Food and water were available ad libitum until morning of the experiment.
Animals were anesthetized with 1% to 2% halothane and 30% oxygen. Ventilation was controlled with a small animal ventilator (Inspira ASV, Harvard Apparatus, MA, USA) to maintain arterial pH at 7.35 to 7.45, PaCO2 at 35 to –45 mm Hg, and PaO2 over 90 mm Hg. Pericranial temperature was monitored using thermistor probes (BIOPAC Systems, Santa Barbara, CA, USA) placed adjacent to the skull and maintained at 37°C±0.5°C throughout the experiment. A standard lead II electrocardiogram was recorded continuously using subdermal needle electrodes placed in the limbs. The right femoral artery and vein were cannulated using PE-50 catheters (Clay Adams, Parsippany, NJ, USA) for continuous monitoring of blood pressure, measurement of blood gases, infusion of fluid, and drug administration. Physiologic data were monitored and recorded using a BIOPAC MP150 physiometer (BIOPAC Systems).
Cardiac Arrest and Resuscitation
After preparation and subsequent stabilization, ventricular fibrillation was induced using an esophageal electrode (5F, Bard Peripheral Vascular, Tempe, AZ, USA) with a 1-minute pulse of a 12 V, 50 Hz alternating current. If spontaneous defibrillation occurred, additional 20 seconds impulses were delivered.16 Cardiac arrest was confirmed by an abrupt decrease in mean arterial pressure to <15 mm Hg and absence of ventilation. After 6 minutes of CA, cardiopulmonary resuscitation was implemented by reconnecting the ventilator with FiO2=1.0, administration of epinephrine (0.02 mg/kg) and sodium bicarbonate (1 mEq/kg), and applying sternal compressions at a rate of 200 per minute. After 2 minutes of cardiopulmonary resuscitation, external defibrillation (biphasic, 2 J; HeartStart XL M4735A defibrillator, Philips Medical Systems, Andover, MA, USA) was performed. Cycles of chest compressions for 1 minute followed by defibrillation were repeatedly performed until the ROSC. Achievement of ROSC was confirmed by spontaneous cardiac rhythm in combination with a mean arterial pressure of 50 mm Hg or greater. If cardiopulmonary resuscitation exceeded 10 minutes without ROSC, the cardiopulmonary resuscitation was stopped. Resuscitated rats were randomly assigned to a CA group or a H2S group (FiO2=0.5, with or without 80-p.p.m. H2S gas for 1 hour using mechanical ventilation). Blood–gas analyses were performed at 15 and 30 minutes after ROSC, and appropriate ventilator adjustments were made. After ROSC for 1 hour, vascular catheters were removed. Surgical wounds were sutured and infiltrated with 0.25% bupivacaine (total 0.5 mg). Rats were then weaned from the ventilator, extubated, and placed in a chamber enriched in 50% oxygen with or without 80-p.p.m. H2S for another 1 hour. Animals were then returned to their cages with easily accessible food and water. Sham-treated animals underwent all procedures except ventricular fibrillation, resuscitation, and post-ROSC ventilation.
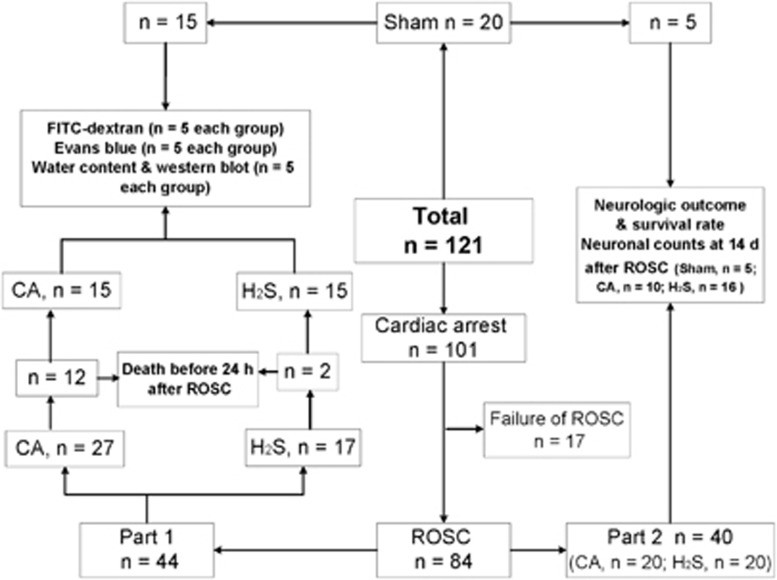
The whole experiment was divided into two parts. In part 1, BBB permeability evaluation, brain water content measurement, and western blot analyses were performed in rats survived for 24 hours after ROSC in sham, CA, and H2S groups. In part 2, 40 resuscitated rats were divided randomly into CA group (n=20) and H2S group (n=20). Neurologic outcome, survival rate, and neuronal counts were performed in sham, CA, and H2S groups. The number of animals and the flow diagram of the experimental groups were shown in Figure 1.
Figure 1.
Flow diagram of the experimental groups. CA, cardiac arrest and resuscitation group; H2S, H2S group; ROSC, return of spontaneous circulation; sham, sham group.
Evaluation of Blood–Brain Barrier Permeability using Fluorescein Isothiocyanate–Dextran and Evans Blue
Blood–brain barrier permeability was assessed in the brain cortex and hippocampus using fluorescein Isothiocyanate (FITC; Sigma, St Louis, MO, USA)–dextran17, 18 and Evans blue dye15 (EB; Sigma) at 24 hours after ROSC. Fluorescein isothiocyanate–dextran (0.5 mL, 30% solution in saline of 4 kDa polymers) or EB (2% solution of 0.96 kDa dye in saline) were administrated intravenously and allowed to circulate for 5 or 30 minutes, respectively. Rats were then administered transcardial saline to remove intravascular dextran or EB. The brains were removed and rinsed with PBS, and two 4-mm wide coronal slices (1.8 mm anterior to the bregma to 6.2 mm posterior to the bregma) were made. The cerebral cortex above the rhinal fissure from the first slice and the hippocampus from the second slice were dissected as shown according to previous studies.19 After weighing, the cortex and hippocampus were homogenized in 50% trichloroacetic acid. Samples were centrifuged at 10,000 g for 20 minutes, the supernatant was collected, and FITC–dextran fluorescence (ng/mL) was measured at 520 nm using 495 nm excitation (Tecan Trading AG, Mannedorf, Switzerland). Total fluorescence of each sample was calculated from concentrations of external standards (100 to 8,000 ng/mL) and presented as percentage of change from the sham group. For the EB measurement, the samples were centrifuged for 30 minutes at 21,000 g. Evans blue per weight of sample was quantified using the absorbance at 620 nm in the supernatant relative to a series of standard EB solution.
Determination of Brain Water Content
Cortical and hippocampal water content was determined 24 hours after ROSC. Brain tissue was immediately divided after decapitation into cortex and hippocampus and weighed to obtain the wet weight. The tissues were slow dried in an oven (105°C) for 72 hours and reweighed to determine the dry weight. Brain water content (%) was calculated as wet weight − dry weight/wet weight × 100%.20
Western Blot Analyses
The brain cortex and hippocampus were dissected and immediately frozen in liquid nitrogen and stored at −80°C for western blot analysis. Protein homogenates of brain samples were prepared by rapid homogenization in Tissue Extraction Reagent II (Invitrogen Corporation, Carlsbad, CA, USA), according to the manufacturer's instructions. Protein concentration was determined using a BCA protein assay kit (Bio-Rad, Hercules, CA, USA). The homogenates were diluted and boiled for 8 minutes in 1:1 sample: loading buffer. Forty micrograms of each sample was added to 10% sodium dodecyl sulfate–polyacrylamide gels, and separated by gel electrophoresis (Mini-Protean III, Bio-Rad, Hercules, CA, USA). The proteins were transferred onto a 0.45-μm polyvinylidene difluoride membrane (Millipore Corporation, Bedford, MA, USA). The membranes were blocked with 5% skimmed milk powder in Tris buffered saline Tween and then incubated overnight at 4°C with anti-VEGF (1:2000; Abcam, Cambridge, MA, USA), anti-Ang-1 (1:500; BIOS, Beijing, China) or antiactin (1:2000; Zhongshan Golden Bridge Biotechnology, Beijing, China). After washing with Tris buffered saline Tween, the membranes were incubated with horseradish peroxidase–conjugated antimouse or antirabbit antibodies (1:3000, Zhongshan Golden Bridge Biotechnology). Bands were detected using the enhanced chemiluminescence detection system (Beyotime, Shanghai, China), and protein band densities were digitally quantified and normalized to β-actin using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Zymography of Matrix Metalloproteinase-9
Matrix metalloproteinase-9 activities were measured using gelatin zymography as described previously.21 Samples were electrophoretically resolved on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis containing 0.1% gelatin as a substrate. At the end, the gel was incubated in 2.5% Triton X-100 (Sigma-Aldrich, St Louis, MO, USA) for 1 hour, rinsed in distilled water, and then incubated in an metalloproteinase activation buffer (50-mmol/L Tris-HCl, pH 7.4, containing 5-mmol/L CaCl2 and 1-μmol/L ZnCl2) for 20 hours at 37°C. The gels were stained in Coomassie blue R-250 (Amresco, Solon, OH, USA) for 40 minutes and then distained with destining solution (10% acetic acid and 40% methnol). The digested regions representing MMP-9 activity, as accessed by running prestained molecular weight markers, were quantified densitometrically using ImageJ software (NIH). The results are expressed as fold changes when compared with sham group.
Assessment of Neurologic Outcome and Survival Rate
Neurologic outcome and survival rate were determined at 1, 3, and 14 days after ROSC in all successfully resuscitated animals. Before the experiment, all animals were familiarized with the neurologic test for three consecutive days and evaluated to ensure normal neurologic function. All evaluations were performed by the same investigator who was masked to treatment.
Neurologic deficit score evaluations were performed using a system including consciousness and breathing, cranial nerves reflexes, motor function, sensory responses, and coordination (0% to 100% scale; 0%=normal, 100%=brain death) described by Neumar et al22 for this model.
A modified tape removal test described previously was conducted to evaluate sensorimotor integration outcome.23 Briefly, 10 mm by 12 mm adhesive tapes were affixed to both forepaws. The time to remove both adhesive tapes was recorded. The test was truncated at 180 seconds and all times >179 seconds were recorded as ‘180 seconds'.
Neuronal Counts
Fourteen days after ROSC, rats were deeply anesthetized with halothane, perfused through the heart with ~200 mL 0.1-mol/L phosphate-buffered saline and perfusion fixed with 4% paraformaldehyde. Brains were removed and fixed in 4% paraformaldehyde before paraffin embedding. Hematoxylin and eosin–stained sections of the hippocampus (corresponding to the bregma −3.3 cm according to Paxinos and Watson)24 were examined. Ischemic neurons were recognized by the pyknotic or karyorrhectic nuclei lacking a clear nucleolus. Viable neurons were defined as cells showing a distinct nucleus and nucleolus. One observer masked to the experimental protocol counted the number of normal-appearing pyramidal neurons per high-power field ( × 400). The number of neurons in the CA1 region of hippocampus was quantitated as the mean number from five sections (5-μm thickness, 80 μm apart) per rat (5 rats in sham group, 10 rats in CA group, and 16 rats in H2S group). In each section, the number of neurons was averaged from three random different vision fields in the CA1 region in each hemisphere under microscope ( × 400).25
Statistical Analysis
Data from the tape removal test and neurologic deficit score were presented as the median, 25th, and 75th percentiles and analyzed using the Kruskal–Wallis test. A Mann–Whitney U analysis was performed when the overall P value was significant. Other data were presented as the mean±s.d. and analyzed using a one-way analysis of variance with Bonferroni correction for post hoc comparison between multiple experimental groups. Kaplan–Meier survival curves were compared using log-rank testing. P<0.05 was considered statistically significant. Statistical analyses were performed using Statistical Product and Service Solutions software (Statistical Product and Service Solutions, Chicago, IL, USA).
Results
Hydrogen Sulfide Decreased Blood–Brain Barrier Permeability Evaluated by Fluorescein Isothiocyanate–dextran and Evans Blue
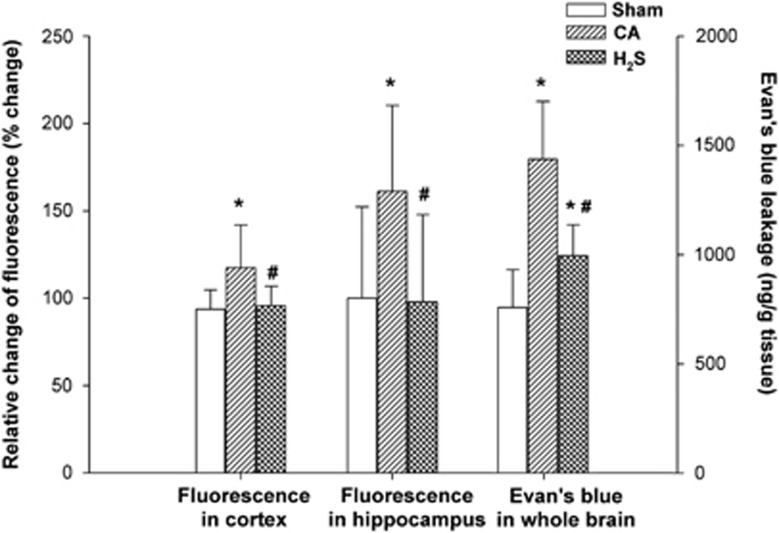
There was a significant increase in cortical permeability to 4 kDa FITC–dextran in the CA group compared with the sham group at 24 hours after ROSC (P<0.05). This increase was significantly attenuated in the H2S group (P<0.05) showing that H2S inhalation appeared to limit the extent of BBB permeability in the cortex after ROSC. Hippocampal permeability to FITC–dextran in the CA group was also increased at 24 hours compared with the sham group (P<0.05). This decreased was also suppressed in the H2S group (P<0.05), again, showing significant attenuation of BBB permeability by H2S (P<0.05; Figure 2).
Figure 2.
Blood–brain barrier permeability evaluated using FITC–dextran permeability in the cortex and hippocampus, and Evans blue in the whole brain at 24 hours after cardiac arrest and resuscitation (n=5 rats per group). Data are presented as mean±s.d. CA, cardiac arrest and resuscitation group; H2S, H2S group; sham, sham group. *P<0.05 versus sham group. #P<0.05 versus CA group.
Similar to dextran, EB dye extravasation in the whole brain at 24 hours after ROSC in the CA group was significantly greater than in the sham group (P<0.05) and was diminished relative to the CA group as a result of H2S inhalation (P<0.05).
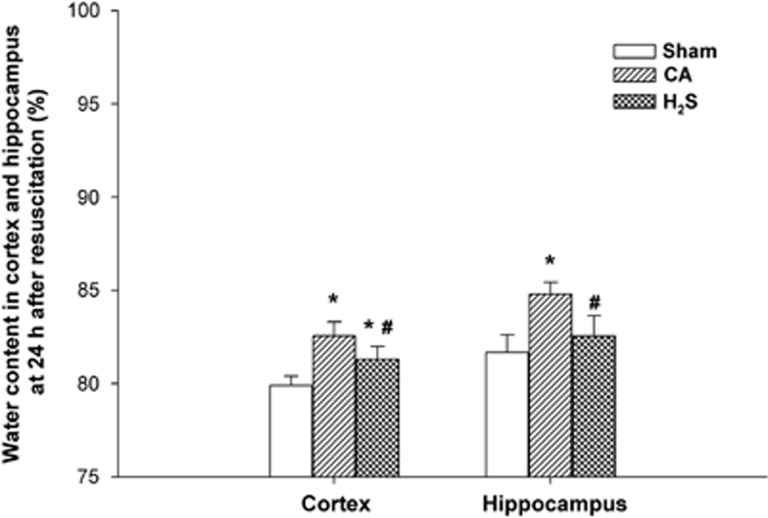
Hydrogen Sulfide Inhalation Diminished Brain Edema after Cardiac Arrest and Resuscitation
The water content of cortical tissue 24 hours after ROSC was 82.6%±0.8% in the CA group and 81.3%±0.7% in the H2S group (Figure 3). These were both significantly higher than the water content of the sham group (79.9%±0.5%, both P<0.05), but H2S inhalation had significantly limited the increase in cortical water content compared with levels measured in the CA group (P<0.05). The water conent of the hippocampus was significantly higher in the CA group than in the sham group 24 hours after ROSC (84.8%±0.6% versus 81.7%±0.9%, respectively, P<0.05). Water content of hippocampal tissues within the CA group was also higher than levels seen in the H2S group, suggesting H2S inhalation significantly suppressed an increase in water content after ROSC (82.5%±1.1%, compared with the CA group, P<0.05).
Figure 3.
Water content in the cortex and hippocampus at 24 hours after cardiac arrest and resuscitation in sham, CA, and H2S groups (n=5 rats per group). Data are presented as mean±s.d. CA, cardiac arrest and resuscitation group; H2S, H2S group; sham, sham group. *P<0.05 versus sham group. #P<0.05 versus CA group.
Hydrogen Sulfide Modulates Vascular Endothelial Growth Factor, Angiogenin-1 Expression, and Matrix Metalloproteinase-9 Activity after ROSC
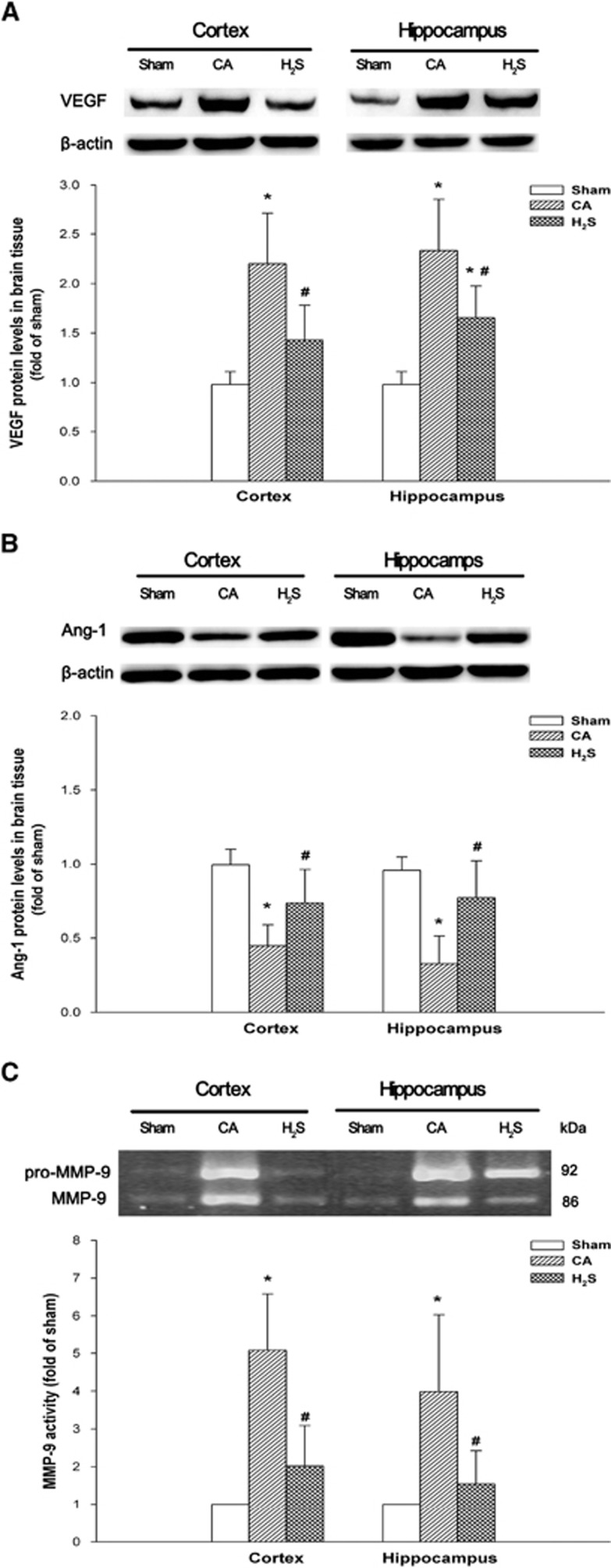
To elucidate the molecular mechanisms underlying the BBB alterations that occur after CA and resuscitation, we studied VEGF and Ang-1 protein expression, and MMP-9 activity in the brain cortex and hippocampus obtained at 24 hours after ROSC.
As shown in Figure 4A, a significant increase in VEGF protein was detected in the cortex (2.20±0.34-fold of sham, P<0.05) and hippocampus (2.33±0.52-fold of sham, P<0.05) at 24 hours after ROSC in the CA group when compared with the sham group. Importantly, H2S inhalation was associated with significantly lower levels of VEGF protein in the cortex (1.43±0.35-fold of sham, P<0.05) and hippocampus (1.65±0.32-fold of sham, P<0.05) at 24 hours compared with the CA group. Protein levels of Ang-1 in the cortex (0.45±0.14-fold of sham, P<0.05) and hippocampus (0.33±0.19-fold of sham, P<0.05) were decreased at 24 hours after resuscitation (CA group; Figure 4B). However, resuscitated animals receiving H2S inhalation had normal levels of Ang-1 in the cortex (0.76±0.21-fold of sham, P<0.05) and hippocampus (0.76±0.23-fold of sham, P<0.05) at 24 hours post-ROSC, which remained significantly higher than seen in the CA group. Significant increases in MMP-9 activity were seen in the CA group relative in the cortex (4.08±1.03-fold of sham, P<0.05) and hippocampus (4.45±1.77-fold of sham, P<0.05) to the sham-treated animals coinciding with the changes seen in VEGF (Figure 4C). Similarly, increased levels of MMP-9 activity were significantly attenuated in the H2S inhalation group in both the cortex (2.30±1.39-fold of sham, P<0.05) and hippocampus (2.15±1.63-fold of sham, P<0.05) at 24 hours after ROSC.
Figure 4.
Vascular endothelial growth factor (A), angiopoietin-1 (B) protein levels and MMP-9 (C) activities in the cortex and hippocampus at 24 hours after cardiac arrest and resuscitation (n=5 rats per group). Results are expressed as percentage of the sham group. Representative western blots for VEGF, angiopoietin-1, β-actin, and gelatin zymogram for MMP-9 are shown above the bars. Data are presented as mean±s.d. Ang-1, angiopoietin-1; CA, cardiac arrest and resuscitation group; H2S, H2S group; MMP-9=matrix metalloproteinase-9; sham, sham group; VEGF, vascular endothelial growth factor. *P<0.05 versus sham group. #P<0.05 versus CA group.
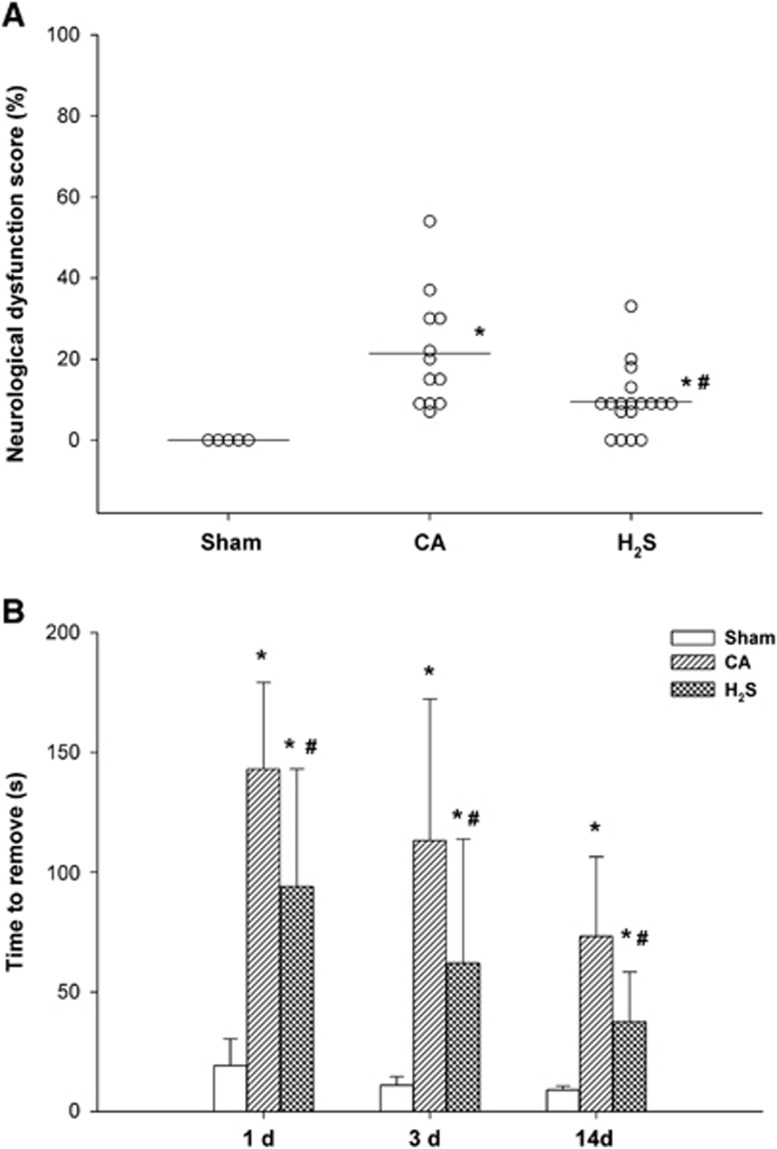
Neurologic Deficit Score, Tape Removal Test, and Overall Survival Rate after ROSC
As shown in Figure 5A, at 24 hours after ROSC, the neurologic deficit score of the H2S group was significantly better than that of the CA group (P<0.05). All animals in the CA and H2S groups showed a clear sensorimotor deficit after ROSC on both testing days compared with sham-treated animals. Similarly, time needed for tape removal was significantly lower in the H2S group than in the CA group on day 1 (99 seconds (21 to 180 seconds) versus 143 seconds (98 to 180 seconds), P<0.05 ), day 3 (61 seconds (17 to 180 seconds) versus 113 seconds (33 to 180 seconds), P<0.05), and day 14 (30 seconds (23 to 58 seconds) versus 62 seconds (47 to 105 seconds), P<0.05; Figure 5B).
Figure 5.
Neurologic deficit score at 24 hours (A) and tape removal test at days 1, 3, and 14 (B) after cardiac arrest and resuscitation. (A) Open circles indicate values for individual animals. Horizontal bars indicate median values. (B) Data are presented as mean±s.d. CA, cardiac arrest and resuscitation group; H2S, H2S group; sham, sham group. *P<0.05 versus sham group. #P<0.05 versus CA group.
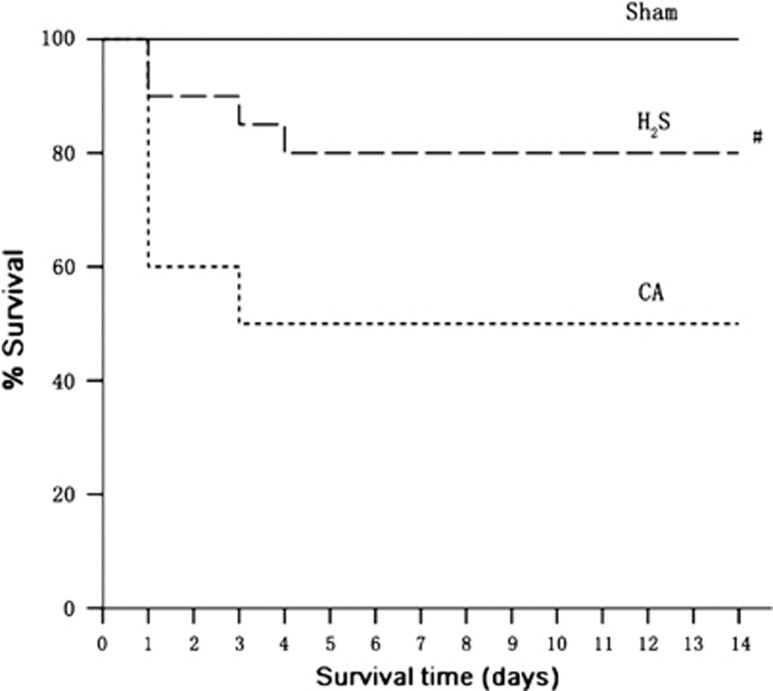
A significant benefit to survival was found with H2S treatment (Figure 6). Ten rats (50% (10 of 20)) in the CA group compared with 16 rats (80% (16 of 20)) in the H2S group survived until day 14 at the end of the experiment (P<0.05). The animals died 3 and 4 days after ROSC could be ascribed to the post-CA syndrome.
Figure 6.
Kaplan–Meier plot of cumulative survival 14 days after cardiac arrest and resuscitation in sham (n=5 rats), CA (n=20), and H2S (n=20) groups. CA, cardiac arrest and resuscitation group; H2S, H2S group; sham, sham group. #P<0.05 versus CA group.
Neuronal Counts
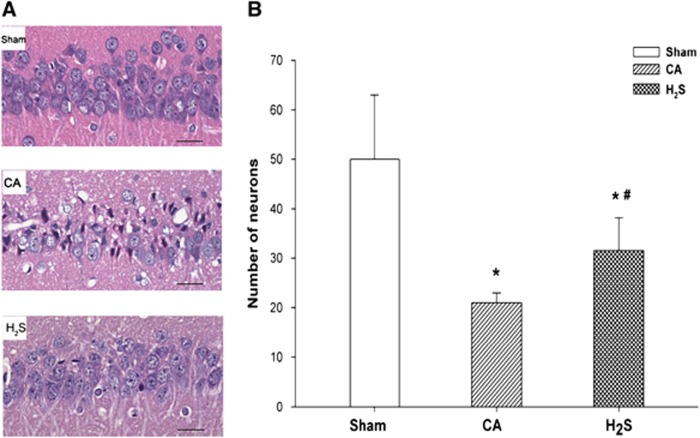
Fourteen days after ROSC, neuronal densities in the CA and H2S groups were much lower than those of the sham group (Figure 7). The numbers of viable CA1 region neurons per high-power field ( × 400) in the CA group (20±6) and H2S group (33±8) were significantly lower than those of the sham group, where neuronal death was rarely seen (53±10, both P<0.05). Nuclear pyknosis, karyorrhexis, and vacuolization were seen in the CA and H2S groups. However, neuronal density and cell morphologies were more well preserved in the H2S group, where the neuron count was significantly higher than that of the CA group (P<0.05).
Figure 7.
Representative histologic images of the hippocampus CA1 region, in sham (n=5), CA (n=10), and H2S (n=16) groups, 14 days after cardiac arrest and resuscitation (A). All images were captured at × 400 magnification. Scale bar indicates 100 μm. (B) Number of viable neurons in the CA1 region belonging to different groups. Data are presented as mean±s.d. CA, cardiac arrest and resuscitation group; H2S, H2S group; sham, sham group. *P<0.05 versus sham group. #P<0.05 versus CA group.
Discussion
As the third novel gasotransmitter, H2S can permeate cell membranes freely without specific transporters.26 Hydrogen sulfide has been shown to regulate synaptic activity, decrease metabolism, suppress oxidative stress, have antiinflammatory effects, and shown to be neuroprotective in ischemia/reperfusion injury models of the central nervous system.5, 27, 28, 29 We investigated whether inhalation of H2S was protective against CA and resuscitation-induced BBB disruption.
The present study showed that inhalation of 80-p.p.m. H2S decreased the permeability during the early period of CA and resuscitation. This beneficial effect was associated with an increased expression of Ang-1 and decreased expression of VEGF and MMP-9 activities. Hydrogen sulfide inhalation also diminished brain edema at 24 hours after ROSC, increased the number of normal neurons, improved the neurologic function, and increased the 14-day survival rate after CA and resuscitation.
Different sizes of molecules have been used to evaluate the magnitude of BBB openings. We assessed BBB permeability by measuring EB and FITC–dextran content in brain tissue. Evans blue binds to albumin and the EB–albumin complex has a net molecular weight of ~68 kDa. The extravasation of EB indicates there is passage of large molecular weight proteins through the BBB. The extravasation of FITC–dextran (4 kDa) is indicative of the entry of small molecules, such as solutes and ions.18, 30 We evaluated BBB disruption in the cortex and hippocampus because neuronal injury is most marked in the cortex and hippocampus after CA.8 In our CA and resuscitation model, we found permeability of the BBB to low (FITC–dextran) and high (EB–albumin) molecular weight markers at 24 hours after ROSC, similar to other studies.31, 32 Inhalation of 80-p.p.m. H2S decreased FITC–dextran and EB penetration, showing that BBB disruption was diminished during the early stages of ROSC. Pluta et al32 showed that the BBB opened in a biphasic manner in a CA model. The first stage started at the second minute after arrest until 1 hour, while the second phase occurred from the 6th to 24th hour after resuscitation.33, 34 Our data suggest that H2S inhalation decreased the permeability of the BBB after CA and resuscitation.
Previous studies have shown that extravasation of proteins into extracellular spaces is correlated with the development of vasogenic edema, which leads to increased intracranial pressure, decreased brain blood flow and higher mortality.30, 31 In accordance with these previous studies, severe brain edema was observed in the cortex and hippocampus at 24 hours after ROSC, when the BBB was significantly damaged according to the EB and FITC–dextran studies.
The expression and activity of MMP-9 is a well-established destructive mediator of BBB disruption both in cerebral ischemia and inflammation studies.29, 35, 36, 37, 38 Studies by Toft-Hansen et al37 revealed that metalloproteinase enzymes are implicated in leukocyte infiltration in neuroinflammation. Agrawal et al38 further stated that MMP-9-mediated selective cleavage of β-dystroglycan, which anchors astrocyte endfeet to the astroglial basement membrane, is critical for leukocyte penetration of the parenchymal basement membrane and the damage of BBB. Emerging studies indicate that H2S plays an important role in the regulation of MMPs, including MMP-9. It has been shown that decreased generation of H2S and increased levels of MMP-9 are associated with pathologic renal remodeling in diabetes.39 Tyagi et al21 showed that physiologic levels of H2S had a therapeutic effect on hyperhomocysteinemia-induced microvascular permeability by normalizing the MMP/tissue inhibitor of metalloproteinase ratio in the brain. A recent study by Wang et al36 found that 2-dithiole-3-thione, a H2S donor, significantly reduced BBB damage via suppressing ischemia-induced excessive activity of MMP-9 in the brain. This effect was also confirmed in a mice CA model study, in which Kida et al29 revealed that sodium sulfide, another H2S donor, markedly improved the neurologic outcome and survival of by inhibiting MMP-9 expression after CA and resuscitation. Similar to these investigations, we found that H2S inhalation decreased postresuscitation MMP-9 levels and BBB damage, suggesting that inhibition of MMP-9 expression or accumulation was at least partially involved in the protective effect of H2S.
Several recent researches have suggested that VEGF, Ang-1, and MMP-9 in brain tissue involved in the BBB disruption after cerebral ischemic reperfusion injury.11, 15, 40 In Pichiule et al's11 study about VEGF expression in the rat brain after CA and resuscitation, they found that there was a significant increase of VEGF in the cortex and hippocampus, and the increased VEGF expression mostly associated with astrocyte. Using the three-dimensional immunofluorescent analysis technology, Zhang et al40 revealed that the temporal and spatial coincidence between VEGF and Ang-1 in ischemic brain tissue and the BBB leakage. The upregulation of VEGF and downregulation of Ang-1 in the ischemic brain mediate the BBB leakage. Furthermore, in both brain ischemic in vivo and BBB in vitro models, the VEGF enhances BBB damage and MMP-9 activity. In contrast, Ang-1 leads to a decrease in vascular leakage.15 These results are consistent with our findings in this study. We found that H2S inhalation significantly diminished BBB disruption. This was associated with decreased expression of MMP-9 and VEGF, and increased expression of Ang-1 in the cortex and hippocampus. Taken together, it is likely that the protective effect of H2S against CA and resuscitation was through the induction of Ang-1 and inhibition of VEGF-mediated MMP-9-dependent BBB leakage. The inhalation of 80-p.p.m. H2S in the early CA setting could be used to mitigate BBB disruption and improve neurologic function.
The lack of pharmacological inhibition, knockout, or small-interfering RNA limits our ability to give a more powerful support to understand the pathway underlying the protective effects of H2S. This is a limitation of this study, and we will confirm these pathways and the detailed regulatory mechanisms in the further investigations.
In conclusion, inhalation of 80-p.p.m. H2S attenuated BBB permeability and brain edema, and improved neurologic outcome and 14-day survival rate after CA and resuscitation in rats. The therapeutic benefits of H2S were associated with decreased MMP-9 and VEGF expression, and increased of angiopoietin-1 expression.
The authors declare no conflict of interest.
Footnotes
This study was supported by grants from the Educational Commission of Heilongjiang Province, China (1251G041) and the National Natural Science Foundation of China (81372026 and 81000822).
References
- Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Böttiger BW, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79:350–379. doi: 10.1016/j.resuscitation.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Herlitz J, Andersson E, Bång A, Engdahl J, Holmberg M, lindqvist J, et al. Experiences from treatment of out-of-hospital cardiac arrest during 17 years in Göteborg. Eur Heart J. 2000;21:1251–1258. doi: 10.1053/euhj.2000.2150. [DOI] [PubMed] [Google Scholar]
- Young GB. Clinical practice. Neurologic prognosis after cardiac arrest. N Engl J Med. 2009;361:605–611. doi: 10.1056/NEJMcp0903466. [DOI] [PubMed] [Google Scholar]
- Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, et al. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation. 2013;127:1116–1127. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan A, Long D, Li J, Ji W, Zhang M, Hong L, et al. Hydrogen sulfide attenuates spatial memory impairment and hippocampal neuroinflammation in beta-amyloid rat model of Alzheimer's disease. J Neuroinflammation. 2012;9:202. doi: 10.1186/1742-2094-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. Hydrogen sulfide: the third gasotransmitter in biology and medicine. Antioxid Redox Signal. 2010;12:1061–1064. doi: 10.1089/ars.2009.2938. [DOI] [PubMed] [Google Scholar]
- Miclescu A, Sharma HS, Martijn C, Wiklund L. Methylene blue protects the cortical blood-brain barrier against ischemia/reperfusion-induced disruptions. Crit Care Med. 2010;38:2199–2206. doi: 10.1097/CCM.0b013e3181f26b0c. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Miclescu A, Wiklund L. Cardiac arrest-induced regional blood-brain barrier breakdown, edema formation and brain pathology: a light and electron microscopic study on a new model for neurodegeneration and neuroprotection in porcine brain. J Neural Transm. 2011;118:87–114. doi: 10.1007/s00702-010-0486-4. [DOI] [PubMed] [Google Scholar]
- Spatz M. Past and recent BBB studies with particular emphasis on changes in ischemic brain edema: dedicated to the memory of Dr. Igor Klatzo. Acta Neurochir Suppl. 2010;106:21–27. doi: 10.1007/978-3-211-98811-4_3. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA. Neurological diseases in relation to the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1139–1151. doi: 10.1038/jcbfm.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichiule P, Chávez JC, Xu K, LaManna JC. Vascular endothelial growth factor upregulation in transient global ischemia induced by cardiac arrest and resuscitation in rat brain. Brain Res Mol Brain Res. 1999;74:83–90. doi: 10.1016/s0169-328x(99)00261-2. [DOI] [PubMed] [Google Scholar]
- Xia YP, He QW, Li YN, Chen SC, Huang M, Wang Y, et al. Recombinant human sonic hedgehog protein regulates the expression of ZO-1 and occludin by activating angiopoietin-1 in stroke damage. PLoS One. 2013;8:e68891. doi: 10.1371/journal.pone.0068891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Yang G, Li G. Molecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol Dis. 2010;38:376–385. doi: 10.1016/j.nbd.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JH, Guo S, Lok J, Navaratna D, Whalen MJ, Kim KW, et al. Neurovascular matrix metalloproteinases and the blood-brain barrier. Curr Pharm Des. 2012;18:3645–3648. doi: 10.2174/138161212802002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, et al. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005;25:1491–1504. doi: 10.1038/sj.jcbfm.9600148. [DOI] [PubMed] [Google Scholar]
- Kwon WY, Suh GJ, Kim KS, Lee HJ, Jeong KY, Kwak YH, et al. Niacin suppresses the mitogen-activated protein kinase pathway and attenuates brain injury after cardiac arrest in rats. Crit Care Med. 2013;41:e223–e232. doi: 10.1097/CCM.0b013e31828a2394. [DOI] [PubMed] [Google Scholar]
- Willis CL, Meske DS, Davis TP. Protein kinase C activation modulates reversible increase in cortical blood-brain barrier permeability and tight junction protein expression during hypoxia and posthypoxic reoxygenation. J Cereb Blood Flow Metab. 2010;30:1847–1859. doi: 10.1038/jcbfm.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Bredno J, Wendland M, Derugin N, Ohara P, Wintermark M. High and low molecular weight fluorescein isothiocyanate (FITC)-dextrans to assess blood-brain barrier disruption: technical considerations. Transl Stroke Res. 2011;2:106–111. doi: 10.1007/s12975-010-0049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzsér G, Kis B, Bari F, Busija DW. Diazoxide preconditioning attenuates global cerebral ischemia-induced blood-brain barrier permeability. Brain Res. 2005;1051:72–80. doi: 10.1016/j.brainres.2005.05.064. [DOI] [PubMed] [Google Scholar]
- Dzialowski I, Weber J, Doerfler A, Forsting M, von Kummer R. Brain tissue water uptake after middle cerebral artery occlusion assessed with CT. J Neuroimaging. 2004;14:42–48. [PubMed] [Google Scholar]
- Tyagi N, Givvimani S, Qipshidze N, Kundu S, Kapoor S, Vacek JC, et al. Hydrogen sulfide mitigates matrix metalloproteinase-9 activity and neurovascular permeability in hyperhomocysteinemic mice. Neurochem Int. 2010;56:301–307. doi: 10.1016/j.neuint.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumar RW, Bircher NG, Sim KM, Xiao F, Zadach KS, Radovsky A, et al. Epinephrine and sodium bicarbonate during CPR following asphyxial cardiac arrest in rats. Resuscitation. 1995;29:249–263. doi: 10.1016/0300-9572(94)00827-3. [DOI] [PubMed] [Google Scholar]
- Albertsmeier M, Teschendorf P, Popp E, Galmbacher R, Vogel P, Böttiger BW. Evaluation of a tape removal test to assess neurological deficit after cardiac arrest in rats. Resuscitation. 2007;74:552–558. doi: 10.1016/j.resuscitation.2007.01.040. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.(eds). The Rat Brain in Stereotaxic Coordinates5th edn, Elsevier Academic Press; Amsterdam; 2005 [Google Scholar]
- Xiong WX, Zhou GX, Wang B, Xue ZG, Wang L, Sun HC, et al. Impaired spatial learning and memory after sevoflurane-nitrous oxide anesthesia in aged rats is associated with down-regulated cAMP/CREB signaling. PLoS One. 2013;8:e79408. doi: 10.1371/journal.pone.0079408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- Sutherland BA, Harrison JC, Nair SM, Sammut IA. Inhalation gases or gaseous mediators as neuroprotectants for cerebral ischaemia. Curr Drug Targets. 2013;14:56–73. doi: 10.2174/138945013804806433. [DOI] [PubMed] [Google Scholar]
- Hu LF, Lu M, Hon Wong PT, Bian JS. Hydrogen sulfide: neurophysiology and neuropathology. Antioxid Redox Signal. 2011;15:405–419. doi: 10.1089/ars.2010.3517. [DOI] [PubMed] [Google Scholar]
- Kida K, Minamishima S, Wang H, Ren J, Yigitkanli K, Nozari A, et al. Sodium sulfide prevents water diffusion abnormality in the brain and improves long term outcome after cardiac arrest in mice. Resuscitation. 2012;83:1292–1297. doi: 10.1016/j.resuscitation.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S. Blood-brain barrier permeability using tracers and immunohistochemistry. Methods Mol Med. 2003;89:133–144. doi: 10.1385/1-59259-419-0:133. [DOI] [PubMed] [Google Scholar]
- Meng F, Liu R, Gao M, Wang Y, Yu X, Xuan Z, et al. Pinocembrin attenuates blood-brain barrier injury induced by global cerebral ischemia-reperfusion in rats. Brain Res. 2011;1391:93–101. doi: 10.1016/j.brainres.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Pluta R, Lossinsky AS, Wiśniewski HM, Mossakowski MJ. Early blood-brain barrier changes in the rat following transient complete cerebral ischemia induced by cardiac arrest. Brain Res. 1994;633:41–52. doi: 10.1016/0006-8993(94)91520-2. [DOI] [PubMed] [Google Scholar]
- Metzger AK, Herman M, McKnite S, Tang W, Yannopoulos D. Improved cerebral perfusion pressures and 24-hr neurological survival in a porcine model of cardiac arrest with active compression-decompression cardiopulmonary resuscitation and augmentation of negative intrathoracic pressure. Crit Care Med. 2012;40:1851–1856. doi: 10.1097/CCM.0b013e318246b9ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Li X, Hu CL, Jiang L, Dai G, Wu GF, et al. The changes of brain water diffusion and blood flow on diffusion-weighted and perfusion-weighted imaging in a canine model of cardiac arrest. Resuscitation. 2012;83:645–651. doi: 10.1016/j.resuscitation.2011.10.017. [DOI] [PubMed] [Google Scholar]
- ElAli A, Doeppner TR, Zechariah A, Hermann DM. Increased blood-brain barrier permeability and brain edema after focal cerebral ischemia induced by hyperlipidemia: role of lipid peroxidation and calpain-1/2, matrix metalloproteinase-2/9, and RhoA overactivation. Stroke. 2011;42:3238–3244. doi: 10.1161/STROKEAHA.111.615559. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jia J, Ao G, Hu L, Liu H, Xiao Y, et al. Hydrogen sulfide protects blood-brain barrier integrity following cerebral ischemia. J Neurochem. 2014;129:827–838. doi: 10.1111/jnc.12695. [DOI] [PubMed] [Google Scholar]
- Toft-Hansen H, Buist R, Sun XJ, Schellenberg A, Peeling J, Owens T. Metalloproteinases control brain inflammation induced by pertussis toxin in mice overexpressing the chemokine CCL2 in the central nervous system. J Immunol. 2006;177:7242–7249. doi: 10.4049/jimmunol.177.10.7242. [DOI] [PubMed] [Google Scholar]
- Agrawal S, Anderson P, Durbeej M, van Rooijen N, Ivars F, Opdenakker G, et al. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. 2006;203:1007–1019. doi: 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu S, Pushpakumar SB, Tyagi A, Coley D, Sen U. Hydrogen sulfide deficiency and diabetic renal remodeling: role of matrix metalloproteinase-9. Am J Physiol Endocrinol Metab. 2013;304:E1365–E1378. doi: 10.1152/ajpendo.00604.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]