Abstract
Cerebrovascular dysfunction seen in Alzheimer's disease (AD) and vascular dementia (VaD) is multifaceted and not limited to the amyloid-β (Aβ) pathology. It encompasses structural alterations in the vessel wall, degenerating capillaries (string vessels), vascular fibrosis and calcification, features recapitulated in transgenic mice that overexpress transforming growth factor-β1 (TGF mice). We recently found that simvastatin rescued Aβ-mediated cerebrovascular and cognitive deficits in a transgenic mouse model of AD. However, whether simvastatin can counteract Aβ-independent deficits remains unknown. Here, we evaluated the effects of simvastatin in aged TGF mice on cerebrovascular reactivity and structure, and on cognitive performance. Simvastatin restored baseline levels of nitric oxide (NO), NO-, and KATP channel-mediated dilations and endothelin-1-induced contractions. Simvastatin significantly reduced vasculopathy with arteriogenic remodeling and string vessel pathology in TGF mice. In contrast, simvastatin did not lessen gliosis, and the cerebrovascular levels of pro-fibrotic proteins and calcification markers remained elevated after treatment. The TGF mice displayed subtle cognitive decline that was not affected by simvastatin. Our results show potent benefits of simvastatin on endothelial- and smooth muscle cell-mediated vasomotor responses, endothelial NO synthesis and in preserving capillary integrity. We conclude that simvastatin could be indicated in the treatment of cerebrovascular dysfunction associated with VaD and AD.
Keywords: aging, cerebrovascular fibrosis, cholesterol, neurovascular coupling, vascular dementia
Introduction
Alzheimer's disease (AD) and vascular dementia (VaD), the two most common forms of cognitive deterioration in the elderly, display substantial pathologic similarities.1, 2 Alzheimer's disease neuropathologic landmarks include senile plaques, neurofibrillary tangles, neuronal loss, and several alterations in the cerebrovascular bed, including the amyloid β (Aβ) pathology that leads to dysfunction through Aβ-mediated oxidative stress,3, 4 that compare well with those of VaD.1, 2 Indeed, structural changes, unrelated to the Aβ pathology, such as vascular fibrosis and thickened vessel walls, degenerating capillaries (string vessel pathology), and disruption of the neurovascular unit leading to reduced brain perfusion are common to both AD and VaD.5, 6 In this respect, transforming growth factor-β1 (TGF-β1), which is central to fibrotic responses in the brain vasculature,7 is increased in the brain, cerebrospinal fluid, or blood of AD and VaD patients.8, 9 Moreover, genetic variability or increased signaling in TGF-β1 may enhance the risk for VaD10 and AD,11 or appears as the main causal event in a form of small vessel disease known as cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephatopathy.12
Transgenic mice that overproduce a constitutively active form of TGF-β1 (TGF mice)7 display thickened vascular walls due to accumulation of extracellular matrix proteins, impaired dilatory function,13 cerebral hypoperfusion,14 neurovascular uncoupling,13 and cerebral microhemorrhages,15 similar to what is seen in AD and VaD.1, 2 These cerebrovascular alterations are unrelated to the Aβ pathology and may thus be highly relevant to the pathophysiology of VaD.
We recently found that simvastatin rescued, in an age-dependent manner, cerebrovascular and cognitive deficits in an AD mouse model of the Aβ pathology (amyloid precursor protein, APP mice).4 These findings suggested that simvastatin could have a therapeutic window in AD. Yet, clinical trials, although supporting an early use of simvastatin in AD, did not find clear benefits,16 possibly because the Aβ pathology does not faithfully recapitulate the multifaceted cerebrovascular alterations that may contribute to cognitive failure in AD and VaD.1, 2 Therefore, we tested the potential benefits of simvastatin in aged TGF mice with severe cerebrovascular deficits. We found that simvastatin restored cerebrovascular reactivity mediated by endothelial and smooth muscle cells (SMC), improved arteriogenesis and string vessel pathology, benefits that occurred independently from any significant countering effects on the fibrotic pathology. These findings point to substantial recoveries in cerebrovascular function and structural alterations associated with AD and VaD after simvastatin even at an advanced stage of the pathology.
Materials and methods
Animals and Simvastatin Treatment
About equal numbers of aged (~18 months old) male and female heterozygous transgenic mice overexpressing a constitutively active form of TGF-β1 under the glial fibrillary acidic protein (GFAP) promoter on a C57BL/6J background (TGF mice, line T64)17 and their wild-type (WT) littermate controls were used. Transgene expression was confirmed with touchdown PCR using tail-extracted DNA.13 Simvastatin (Enzo Life Science, Farmingdale, NY, USA) was activated by alkaline lysis, as per the manufacturer's protocol and added to the drinking water at a concentration of 0.04%, such that mice received ~40 mg/kg body weight per day.4 The TGF mice were randomized for gender and litters between simvastatin or control solution that was administered for a period of 8 weeks (~20 months at end point). Simvastatin was administered at 20 mg/kg per day for 3 days, increased to 30 mg/kg per day for 4 days and then kept at 40 mg/kg per day for the rest of the treatment. Controls received the same solution without simvastatin. Since we previously documented no difference in cognitive and cerebrovascular function in different cohorts of adult and aged WT controls compared with WT mice treated with a similar dose of simvastatin,4 simvastatin-treated WT mice were omitted from this study. Total blood cholesterol levels were measured in a subset of mice with Accutrend GC meter (Roche Diagnostic, Laval, QC, Canada), and were similar in all groups (mmol/L): WT (n=3) 4.15±0.085, TGF (n=4) 4.16±0.16, and TGF+simvastatin (n=5) 4.09±0.03, as in previous studies.4 Using western blot analysis, we confirmed increased TGF-β1 protein levels in TGF mouse brains compared with WT controls, and found that they were not altered by simvastatin (Supplementary Figure 1). All experiments were approved by the Animal Ethics Committee of the Montreal Neurological Institute and complied with the Canadian Council on Animal Care and the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines.
Morris Water Maze
Spatial memory was tested in the Morris water maze, as described before.4 Mice first received a 3-day habituation period requiring them to swim (1.4 m diameter pool, 17±1°C opaque water) to a visible platform (60 seconds). The wall cues and platform location were then switched, the platform submerged (1 cm) and 5 days of hidden-platform trials ensued (3 trials from different orientations per day, max 90 s/trial, 45 minutes intertrial interval) in which the mice had to find the hidden platform using distal visuo-spatial cues. Two days later (day 10), mice were given a probe trial (60 seconds) in which the percentage time spent and distance traveled in the target quadrant (where the platform used to be located) were recorded, along with the number of crossings above the previous platform location and swim speed. These parameters including the escape latency during the hidden platform training were recorded and analyzed (2020 Plus tracking system and Water 2020 software, HVS Image, Buckingham, UK).
Cerebrovascular Reactivity
Procedures were as described previously.13 Briefly, segments (~2 mm long) of the middle or posterior cerebral arteries were cannulated, pressurized (60 mm Hg), and superfused with a Krebs' solution in a chamber for online videomicroscopy. Dilatory responses to acetylcholine (ACh, 10−10 to 10−5 mol/L) and calcitonin gene-related peptide (CGRP, 10−10 to 10−6 mol/L) were tested on vessels preconstricted submaximally with phenylephrine or 5-hydroxytryptamine (2 × 10−7 mol/L). Contractile responses to endothelin-1 (ET-1, 10−10 to 10−6 mol/L) and the tonic production of the vasodilator nitric oxide (NO) were measured in vessels at basal tone, the latter after inhibition of NO synthase (NOS) with Nω-nitro-L-arginine (10−5 mol/L, 40 minutes). In some vessels (n=5/group), endothelin-1 type A (ETA) receptors were blocked by preincubation (30 minutes) with the ETA receptor antagonist BQ-123 (10−7 mol/L) before generating ET-1 dose–response curves.18 KATP channel function was tested with the KATP channel opener levcromakalim (LEV, 10−9 to 10−4 mol/L). In some preconstricted vessels from WT and TGF mice, a dose–response curve to simvastatin was tested with or without preincubation with the KATP channel inhibitor glibenclamide (10−5 mol/L).
Western Blotting
Protein changes were investigated in pial vessels (endothelial NOS, eNOS) and cortex (TGF-β1) by western blot.13 Brain tissues, major cerebral arteries (except middle or posterior cerebral arteries when used for reactivity experiments) and their branches in the pial membrane were collected from each mouse, frozen on dry ice and kept in −80°C until use. Vessels and brain samples were then sonicated in lysis buffer (20 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 0.1% Nonidet P-40, 1% glycerol, 0.2 mmol/L sodium vanadate, and protease inhibitor mixture from Roche Diagnostic) and cleared by centrifugation. Proteins (6 and 20 μg from vessel and brain extracts, respectively) were loaded into 10% SDS-PAGE, and transferred onto nitrocellulose membrane. The membrane was incubated overnight (4°C) with mouse anti-eNOS (1:1,000, BD Transduction Lab, Mississauga, ON, CA, USA), rabbit anti-TGF-β1 (1:1,000, Cell Science, Canton, MA, USA) or anti-β-actin (1:10,000, Sigma, St-Louis, MO, USA) to normalize for loading. Blots were incubated with horseradish peroxidase-conjugated secondary antibodies (1:2,000, 1 hour), and proteins visualized with ECL using phosphorImager (Scanner STORM 860; GE Healthcare, Mississauga, ON, Canada), followed by densitometric quantification.
Immunohistochemistry and Immunofluorescence
Mice were perfused (n=4 to 6/group) with 4% paraformaldehyde, their brains postfixed overnight (4% paraformaldehyde, 4°C): one hemibrain was processed for paraffin sections (5-μm-thick coronal sections) and the other half for sectioning on a freezing microtome (25 μm). Paraffin sections were dewaxed, treated with antigen retrieval (0.05% citraconic anhydride solution, pH=7.4, 20 minutes, 97°C), and incubated (overnight, room temperature) with goat anti-collagen I (SouthernBiotech, Birmingham, AL, USA, 1:300) or collagen IV (Millipore, Billerica, MA, USA 1:300), or with rabbit anti-Iba-1 (ionized calcium binding adaptor molecule-1, Wako, Richmond, VA, USA, 1:300) antibody, followed by biotinylated IgG (Vector Lab, Burlingame, CA, USA, 1 hour 30 minutes), and the avidin-biotin complex (ABC Kit, Vector Lab, 1 hour 15 minutes). The reaction was visualized with a 0.05% 3, 3′-diaminobenzidine-nickel solution. Free-floating 25-μm-thick sections were incubated with rabbit anti-GFAP (Dako, Burlington, ON, Canada, a marker of reactive astrocytes; 1:2,000), goat anti-RANKL (receptor activator of nuclear factor-κB (RANK) ligand, Santa Cruz, Dallas, TX, USA, 1:300, a regulator of vascular calcification seen in aging and pathologic conditions19 or goat anti-collagen IV (1:300, Millipore, for detecting string vessels). For double-immunohistochemical detection of endothelial cells (rat anti-CD31, 1:300, BD Biosciences, Mississauga, ON, CA, USA) and vascular basement membrane (collagen IV), CD31 was detected in the first position with the SG reagent (gray blue precipitate, Vector Labs), followed by collagen IV in second position using 3′-diaminobenzidine (brown precipitate). Double immunoflurorescence for endothelial cells (CD 31) and smooth muscle actin (rabbit anti alpha-actin, 1:500, Millipore) was detected with anti-rat cyanin 3-conjugated (red) and anti-rabbit cyanin 2-conjugated (green) secondary antibodies (Jackson Labs, West Grove, PA, USA), respectively. Sections (a minimum of 2 to 3 sections/mouse) were observed under light microscopy or epifluorescence on a Leitz Aristoplan microscope (Leica, Montréal, QC, Canada), digital pictures were taken and used for analysis. Staining specificity was confirmed by omitting the primary antibodies.
Histochemical Staining
Thioflavin-S staining
Perfusion- or immersion-fixed arteries from the circle of Willis (n=3 WT and 3 TGF mice) were mounted on gelatin-coated slices and stained (8 minutes) for possible cerebral amyloid angiopathy with a 1% Thioflavin-S solution4 and visualized under epifluorescence (FITC filter).
NADPH-diaphorase staining of intraparenchymal vessels
Free-floating 25 μm thick sections were rinsed and incubated (37°C) in 0.1 mol/L PBS (pH 7.4) containing 0.3% Triton X-100, 0.01% nitroblue tetrazolium chloride and 0.1% β-NADPH-diaphorase (NADPH-D) (ICN Biomedicals, Irvine, CA, USA) until the appearance of NADPH-D-positive vessels. Sections (a minimum of 2 to 3 sections/mouse) were mounted on gelatin-coated slices, dehydrated and defatted before observation under light microscopy. Digital pictures were taken and used for quantitative analysis.
Smooth Muscle Cell Cultures
Primary cultures of rat SMC were generated from brain intracortical microvessels, as described previously.18 After dissociation of the microvessels with type IV collagenase (1 mg/mL, 6 minutes at 37°C), cells were seeded onto 0.5% gelatin-coated culture plates containing 64% medium M199, 30% fetal bovine serum, peptone (0.05%), D-glucose (1%), BME amino acid (1 × ), BME Vitamin (1%), and antibiotics. After 2 to 3 weeks in culture, >85% of cells stained positively for smooth muscle α-actin (anti-α-actin 1:400, Sigma), as described in detail before.18 For activation of the p38 MAPK signaling cascade, cells were pretreated with TGF-β1 (3 ng/mL, 72 hours) and simvastatin (5 μmol/L, 72 hours) in serum-free medium and then in serum-free medium alone (2 hours) before stimulation with ET-1 (1 × 10−7 mol/L for 10 or 15 minutes).18
Data Analysis
Vascular responses (% change diameter from basal or preconstricted tone) were plotted as a function of agonist concentration or duration of NOS inhibition. Concentration-dependent and maximal (EAmax) responses and the agonist concentration eliciting half the EAmax (EC50 value or pD2=−log EC50) were used to compare agonist efficacy and potency, respectively. Low and high magnification digital pictures were used to measure the intensity of collagen I or collagen IV immunostaining in vessel walls or pial membrane using the Image J software (NIH, Bethesda, MD, USA). The number of abnormally large cortical NADPH-D-positive vessels, and the density of secondary vessels branching from the main trunk of these vessels were counted on high magnification digital pictures. Similarly, the number of collagen IV-immunostained string vessels in the frontoparietal cortex and hippocampus was counted blind by two independent observers directly under the microscope using high magnification ( × 400). The percent cortical or hippocampal area occupied by NADPH-D-positive vessels, GFAP, or RANKL immunoreactivity was measured on digital pictures with the MetaMorph 6.1r3 software (Universal Imaging, Downington, PA, USA). Data are expressed as mean±s.e.m., and were analyzed by one-way ANOVA followed by Newman–Keuls post hoc multiple comparison test or when indicated, by Student's t-test for two group comparisons (GraphPad Prism4, San Diego, CA, USA). A P<0.05 was considered as significant.
Results
Simvastatin Restored Cerebrovascular Reactivity in Transforming Growth Factor-β1 Mice
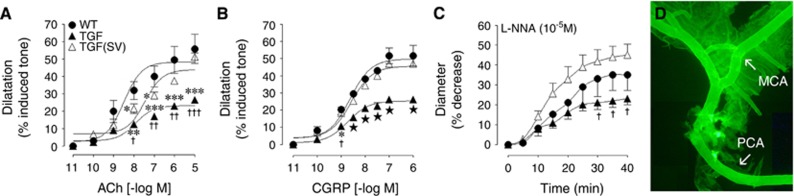
Cerebral arteries from TGF mice displayed impaired concentration-dependent and maximal dilations to ACh and CGRP compared with WT controls (Figures 1A and 1B, Supplementary Table 1), as previously documented.13 These deficits were not attributed to receptor desensitization since ACh and CGRP pD2 values did not differ between groups (Supplementary Table 1). The tonic production of the vasodilator NO, evaluated by inhibiting NOS activity with Nω-nitro-L-arginine, was also reduced compared with WT (Figure 1C, Supplementary Table 1). Simvastatin improved ACh-induced dilations, completely normalized those evoked by CGRP, and the basal NO production was increased to levels slightly but not significantly higher than those of WT control vessels (Figure 1). Despite the advanced age of TGF mice no deposits of Aβ peptide or other beta-pleated proteins visualized with thioflavin-S staining20 could be seen in the walls of the arteries used in reactivity experiments, as was the case in all main arteries from the circle of Willis and their small branches (Figure 1D).
Figure 1.
Simvastatin (SV) improved cerebrovascular reactivity in transforming growth factor-β1 (TGF) mice. The impaired dilatory responses of isolated arteries to acetylcholine (ACh) (A) and calcitonin gene-related peptide (CGRP) (B) in TGF mice (▴, n=5) relative to wild-type controls (WT, ●, n=3) were normalized in SV-treated TGF (△, n=5) mice. Similarly, the basal nitric oxide (NO) synthesis, measured through NO synthase (NOS) inhibition with Nω-nitro-L-arginine (L-NNA) (C, 10−5 mol/L) was fully restored by SV treatment (△). TGF mice did not display perivascular amyloid deposits as seen with Thioflavin-S staining, the brighter segments correspond to small pieces of the attached pial membrane (D). MCA, middle cerebral artery; PCA, posterior cerebral artery. *P<0.05, **P<0.01, ***P<0.001 compared with WT; †P<0.05, ††P<0.01, †††P<0.001 compared with SV-treated TGF mice; ★P<0.05 compared with WT and SV-treated TGF mice.
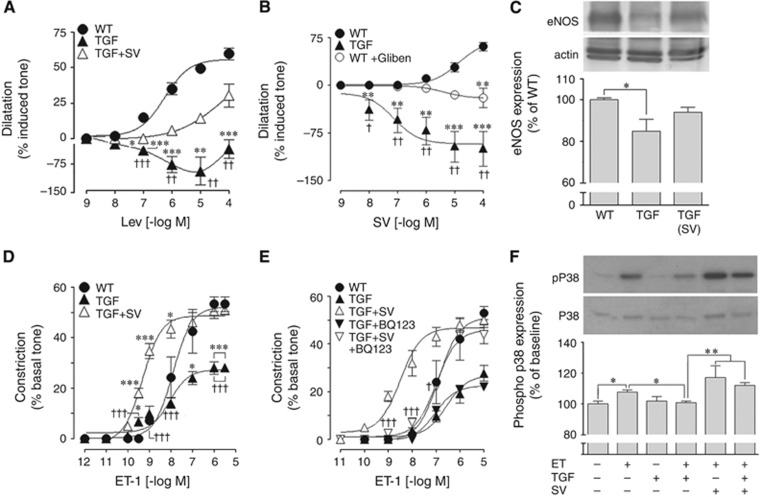
Calcitonin gene-related peptide mediates dilation in mouse cerebral arteries largely through opening of KATP channels, and simvastatin totally rescued Aβ-induced impairment of these channels in APP mice.4 Similarly, we found that the KATP channel opener levcromakalim induced dilations in WT vessels that were dramatically impaired in TGF vessels, being even reversed to contraction. After simvastatin treatment, KATP channel-induced dilations were greatly improved in TGF mice and did not significantly differ from WT controls (Figure 2A). Impaired KATP channel-induced dilations were also evidenced by in vitro application to isolated arterial segments of simvastatin, which can act as a KATP channel opener.21 Whereas simvastatin potently dilated WT vessels, it induced contractions in TGF vessels, a response that was partly reproduced in WT arteries pretreated with the KATP channel blocker glibenclamide (Figure 2B, Supplementary Table 1). Collectively, these data pointed to cerebrovascular KATP channel dysfunction in TGF mice being significantly improved by simvastatin therapy. In line with the recovered basal NO production (Figure 1C), western blot experiments showed that the decreased levels of eNOS protein in cerebral and pial vessel extracts from TGF mice (−16%, P<0.05) were increased by simvastatin to intermediate levels that did not differ from those in WT vessels (Figure 2C).
Figure 2.
Simvastatin (SV) improved KATP channel function, endothelial nitric oxide synthase (eNOS) protein levels, and potentiated endothelin-1 (ET-1) type A (ETA) receptor function in transforming growth factor-β1 (TGF) mice through activation of p38 MAP kinase. (A) KATP channels were impaired in TGF mice (▴, n=6) as shown with the channel opener levcromakalim (Lev). SV restored KATP channel function in treated TGF mice (△, n=4) to levels not significantly different from wild-type (WT) controls (●, n=4). (B) Impaired KATP channel function in TGF mice (▴, n=4) when activated by SV was mimicked in WT arteries incubated with the KATP channel blocker glibenclamide (Gliben, ○, n=6), vessels showing contraction to SV instead of dilation. (C) Western blot analysis of eNOS protein showed significantly reduced levels in vessels from TGF mice compared with WT controls, and SV increased these to levels not significantly different from WT (n=4 to 7 mice/group). (D) The impaired contractile response to ET-1 in TGF mice (▴, n=5) compared with WT controls (●, n=3) was potentiated beyond control levels in SV-treated TGF mice (△, n=4). This SV potentiating effect was completely blocked by the selective ETA receptor antagonist BQ123 (E), which had no effect in vessels from non-treated TGF mice. (F) The ET-1-induced phosphorylation of p38 MAP kinase in cerebral smooth muscle cell cultures was inhibited by TGF-β1, an effect prevented by preincubation with SV (n=3 to 6). *P<0.05; **P<0.01; ***P<0.001 compared with WT; †P<0.05; ††P<0.01; †††P<0.001 compared with SV-treated TGF mice, or WT+glibemclamide.
The contractile capacity of cerebral arteries is selectively compromised in aged TGF mice,18 as confirmed here by the reduced response to the potent constrictor ET-1 (Figure 2D). Simvastatin not only restored the ET-1 maximal contractile response (EAmax) in treated TGF mice to WT levels, but the ET-1 dose–response curve was shifted to the left, with a significantly higher affinity (pD2 value) for ET-1 (Figure 2D, Supplementary Table 1). This shift in affinity was completely blocked by preincubation of the vessels with the ETA receptor antagonist BQ123 (Figure 2E), indicating that simvastatin's potentiating effect on the ET-1-mediated contraction occurred through interaction with ETA receptors. To better assess how simvastatin facilitated ETA receptor-induced contractions, we tested its effects on cerebrovascular SMC exposed to TGF-β1 and stimulated with ET-1 in culture. We first confirmed that TGF-β1 impaired the ET-1-induced activation of the ETA receptor-mediated p38 MAPK signaling cascade, in agreement with our previous findings.18 Further, we found that simvastatin slightly, albeit not significantly, potentiated the ETA receptor signaling, whereas it significantly countered the inhibitory effect of TGF-β1 (Figure 2F). This observation indicated that simvastatin facilitated the ETA receptor downstream signaling cascade involved in ET-1-induced contractions. Together, these findings show that altered signaling is an important factor in the impaired cerebrovascular dilatory and contractile responses in vessels from TGF mice.
Effect of Simvastatin on Vascular Fibrosis and Receptor Activator of Nuclear Factor κB Ligand (RANKL) Expressing Vessels in Hippocampus
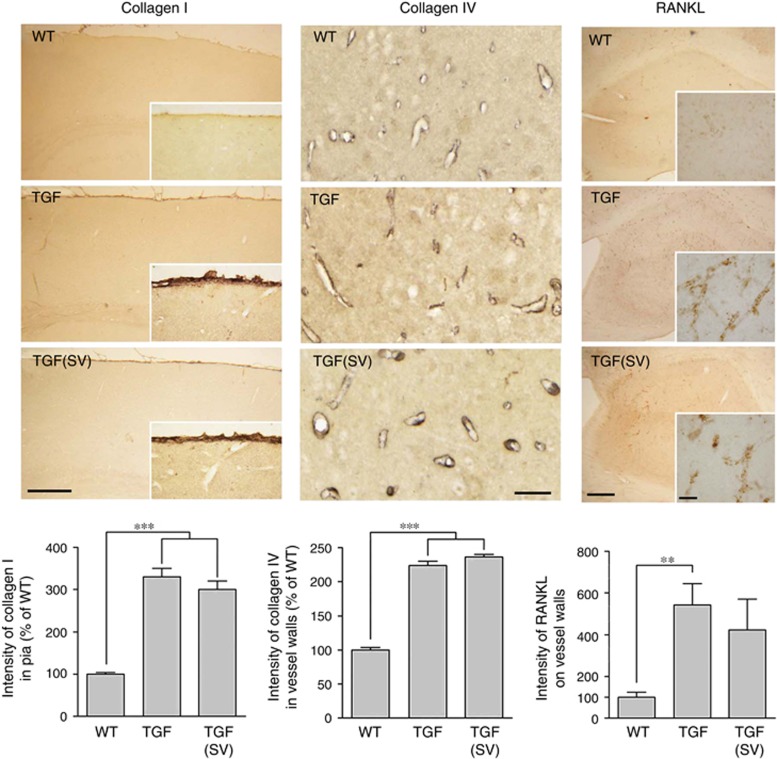
As expected from previous studies,13, 17 the pial membrane and cerebral vessels of TGF mice displayed a fibrotic phenotype characterized by accumulation of extracellular matrix proteins such as collagen I and collagen IV (Figure 3). In aged simvastatin-treated TGF mice with restored cerebrovascular function, these two proteins remained upregulated, as visualized by the large increase (twofold to threefold, P<0.001) in immunostaining intensity in the pia or intraparenchymal blood vessels (Figure 3). Similarly, TGF mice displayed cortical and hippocampal increases, though most prominent in the hippocampus (Figure 3), in vascular RANKL immunostaining, a marker of vascular calcification seen in aging, AD, and patients with diabetes and cardiovascular diseases.19 Consistent with vascular calcification occurring in normal aging, a few RANKL-immunopositive vessels were seen in the brain of WT mice. In contrast, numerous densely immunostained RANKL-positive vessels were found throughout the hippocampus of TGF mice, and simvastatin had limited effect on cerebrovascular RANKL upregulation (Figure 3).
Figure 3.
Simvastatin (SV) failed to normalize vascular fibrosis and slightly reduced vascular calcification. The intensity of immunostaining for collagen I (n=3 mice/group) in the pial membrane and of collagen IV (n=4 mice/group) in the walls of cortical microvessels in 5-μm-thick paraffin sections was increased in transforming growth factor-β1 (TGF) mice compared with wild-type (WT) controls, and SV exerted no reducing effect at this level. In 25-μm-thick hippocampal sections, the intensity of receptor activator of nuclear factor κB ligand (RANKL) immunopositive vessels was significantly increased in TGF mice (n=5) compared with WT controls (n=4), and SV (n=6) slightly reduced this intensity to levels not significantly different from WT controls. Bars: left panel=250 μm; middle panel=50 μm; and right panel=200 μm (inset=20 μm). **P<0.01; ***P<0.001.
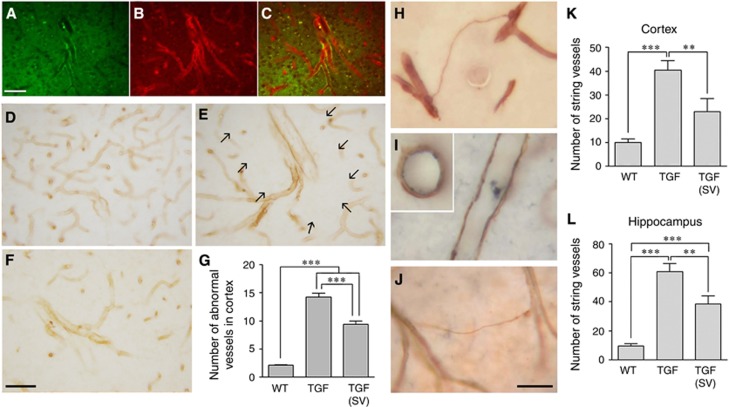
Simvastatin Reduced Large Intracortical Arterioles and String Vessel Pathology in Transforming Growth Factor-β1 Mice
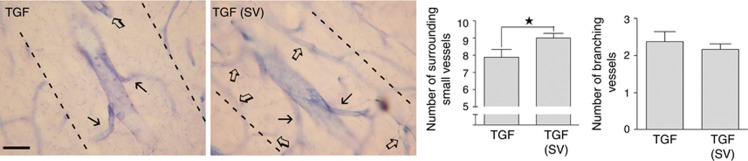
In contrast to WT controls, TGF mice displayed numerous large vessels across cortical layers (Figures 4A to 4C). Double immunofluorescence for endothelial cell marker CD31 and smooth muscle actin identified these vessels as arterioles (Figures 4A to 4C). Quantitative analysis of collagen IV-immunostained vessels showed close to a threefold increase in TGF mice in the number of these large arterioles that were typically surrounded by an area devoid of capillaries or branching vessels (Figure 4E, arrows). Simvastatin treatment slightly but significantly reduced the number of large vessels (Figures 4D to 4G). Similarly, the string vessel pathology in TGF mice, which corresponds to degenerating capillaries immunodetected as thin collagen IV ribbons without endothelial cells (Figures 4H and 4J) that line the lumen of normal capillaries (Figure 4I), was significantly reduced in both cortex and hippocampus in simvastatin-treated mice (Figures 4K to 4L). To explore further the avascular area surrounding the large cortical vessels in TGF mice (Figure 4E), we used NADPH-D histochemical staining of NOS to visualize the microcirculation, and we confirmed the paucity of small vessels (Figure 5). Remarkably, simvastatin significantly increased the density of small vessels in the vicinity of large arterioles (Figure 5). Yet, whereas the number of large vessels was reduced by simvastatin (Figure 4G), that of branching vessels from their main trunk was unaltered (Figure 5).
Figure 4.
Simvastatin (SV) reduced arteriogenic remodeling and string vessel pathology in transforming growth factor-β1 (TGF) mice. (A to C) Double immunofluorescence for the endothelial cell marker CD31 (A, green) and smooth muscle actin (B, red) showed the presence of abnormally large intracortical arterioles (C, merge). These large vessels immunopositive for collagen IV were rarely seen in the cortex of WT controls (D), but they were numerous in TGF mice (E and F). In most cases, these large vessels had few ramifications and were surrounded by an area devoid of capillaries (arrows in E, see also Figure 5). SV treatment slightly but significantly reduced this pathology (G) (n=3 mice/group). (H) String vessel pathology was revealed by collagen IV immunostaining in TGF mice. In contrast to WT capillaries that show endothelial CD31 immunostaining (I, blue) inside the collagen IV immunostained basement membrane (I, brown), the thin walls of string vessels only stained for collagen IV (H and J). SV treatment significantly reduced the number of string vessels in cortex and hippocampus of TGF mice (K and L) (n=7 to 9 mice/group). **P<0.01; ***P<0.001. Bars: (A to C)=50 μm, (D to F)=30 μm.
Figure 5.
Simvastatin (SV) slightly increased the number of capillaries around large intracortical arterioles in transforming growth factor-β1 (TGF) mice. Histochemical NADPH diaphorase staining of cortical blood vessels in TGF (n=8) and SV-treated (n=9) TGF mice showed that SV slightly but significantly increased the number of small vessels (open arrows) localized in the immediate vicinity (dotted lines) of large intracortical arterioles. However, SV did not improve the limited branching (black arrows) of these large vessels. ★P<0.05 by Student's t-test. Bar=30 μm.
Simvastatin Does Not Silence Reactive Glial Cells in Transforming Growth Factor-β1 Mice
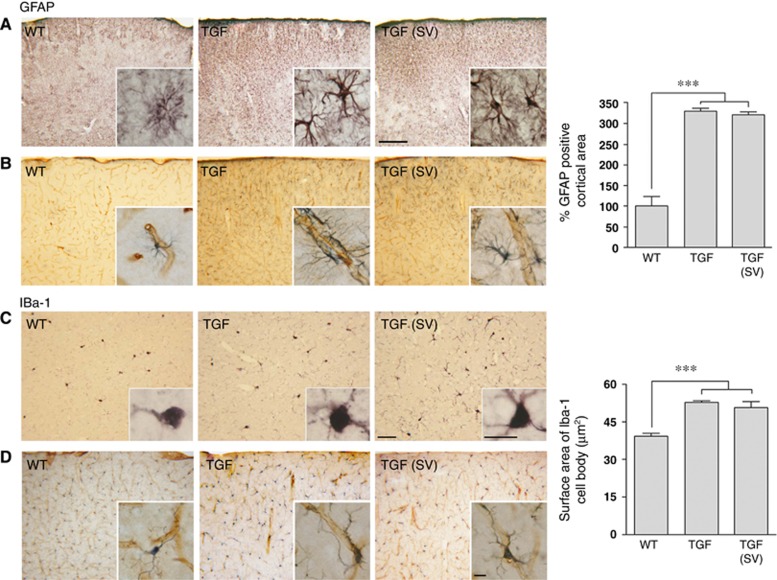
Astrocytes and microglial cells exhibited a reactive phenotype in the brain of TGF mice, as evidenced by increased GFAP and Iba-1 immunoreactivity (Figure 6). The GFAP-positive area in somatosensory cortex of TGF mice was greatly increased compared with WT mice. Astroglial cell bodies were enlarged and displayed thickened and more darkly stained processes, many of which contacted blood vessels (Figure 6, top panels and insets). Similarly, TGF mice exhibited Iba-1 immunopositive reactive microglial cells with enlarged cell bodies and more processes, including on local microvessels, compared with WT mice (Figure 6). Simvastatin had virtually no reducing effects on the activated state of glial cells in TGF mice (Figure 6, right panels).
Figure 6.
Simvastatin (SV) did not suppress astrogliosis and microgliosis in the cortex of transforming growth factor-β1 (TGF) mice. Glial fibrillary acidic protein (GFAP)-immunostained astrocytes in the cerebral cortex (A) and double immunostaining for intracortical microvessels and astrocytes (B, collagen IV, (brown: vessels) and GFAP (gray: astrocytes)) showed an activated astroglial phenotype with larger cell bodies and higher number of processes. The surface area occupied by GFAP-positive astrocytes was increased in TGF mice compared with WT controls, and SV has no effect at this level (top right panel) (n=4 to 6 mice/group). Similarly, Iba-1 immunopositive microglial cells displayed a reactive phenotype with increased processes (C), some of which associated with intracortical microvessels (D). SV did not reduce this phenotype (n=4 mice/group). Bars: top panel=200 μm and bottom=50 μm. Inserts, Bar=10 μm. ***P<0.001.
Cognitive Function in Aged Transforming Growth Factor-β1 Mice
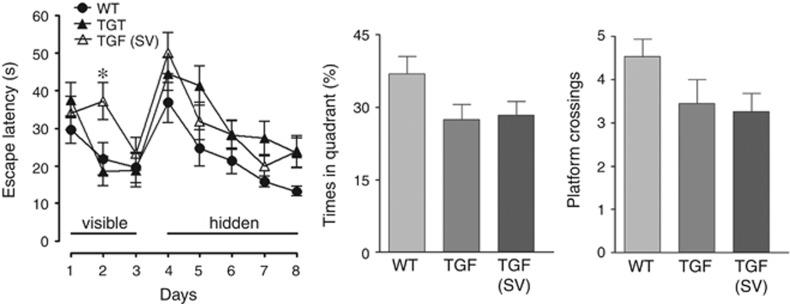
The TGF mice had no difficulty in finding the visible platform compared with WT controls, despite a significant longer escape latency on day 2 for simvastatin-treated TGF mice (Figure 7). During the hidden platform sessions, TGF mice spent slightly more time to find the platform than WT mice, but these differences did not reach significance (Figure 7). Similarly, despite comparable swim speed among all groups in the probe trial, TGF mice showed a trend toward spending less time in the target quadrant and performing a smaller number of platform crossings (Figure 7), the latter being indicative of a loss of precision during the search for the virtual platform. Simvastatin did not provide benefit, the performance in treated TGF mice being still slightly, albeit not significantly, poorer than WT littermate controls (Figure 7).
Figure 7.
Aged transforming growth factor-β1 (TGF) mice exhibited no significant deficit in the Morris water maze, and simvastatin (SV) had not effect on cognitive performance. TGF mice and SV-treated TGF mice did not significantly differ from wild-type (WT) controls in the time they needed to find the visible or hidden platform (left panel), despite the longer escape latency needed by SV-treated TGF mice on day 2 of the visible platform training. Similarly, TGF and SV-treated TGF mice were slightly but not significantly impaired in the probe trial (n=15 mice/group).
Discussion
The results show that simvastatin was extremely effective in improving cerebrovascular function and in counteracting TGF-β1-mediated vasculopathy with vascular remodeling and string vessel pathology, benefits associated with improved eNOS protein levels, KATP channel function, and p38 MAP kinase signaling. Simvastatin, however, was largely ineffective against vascular fibrosis and calcification, and did not affect the subtle cognitive decline seen in aged TGF mice. We conclude that simvastatin shows promise in curing cerebrovascular alterations associated with VaD and AD.
Simvastatin Rescues Cerebrovascular Reactivity and Signaling in Aged Transforming Growth Factor-β1 Mice
Simvastatin benefits can be attributable to its pleiotropic effects since they were unrelated to changes in cholesterol levels that were comparable between all groups. We found that normalized dilatory function in TGF brain arteries occurred together with improvement of eNOS protein levels and KATP channel function. This is supported by simvastatin's ability to increase eNOS activity22 and NO bioavailability,4, 23 and to restore KATP channel function in cerebral 4 or peripheral 21 blood vessels. Although not tested in this study, simvastatin likely also acted on endothelial TRPV4 channels recently found to be impaired in TGF mice and to mediate part of ACh-induced dilations.24 Additionally, simvastatin countered the deleterious effect of TGF-β1 on the ET-1 contractions mediated by smooth muscle ETA receptors, likely through normalization of the ET-1/ETA receptor p38 MAPK/HSP27 signaling cascade.18 Moreover, simvastatin induced a leftward shift, blocked by the ETA receptor antagonist BQ123, in the dose–response curve of ET-1 in TGF vessels, further pointing to a facilitating effect of simvastatin on ETA receptor signaling. Whereas mixed effects of statins on ETA receptors have been reported with reduced expression or upregulation,25, 26 our findings in brain vessels and SMC indicate that simvastatin rescues ETA receptor-mediated ET-1 contraction and restored the downstream p38 MAP kinase signaling cascade involved in this response.18 Statins have likewise been shown to stimulate p38 MAP kinase in endothelial cells.27 However, a direct effect of simvastatin on ET-1 levels in brain or brain vessels has not been investigated, and thus cannot be excluded. These results further indicate that, in contrast to APP mice,4 the antioxidant properties of simvastatin cannot be credited for its positive effects as dysfunctions in TGF brain vessels are unrelated to reactive oxygen species and free radicals.13, 24 We thus conclude that simvastatin's benefits on NO synthesis, ion channel function, and receptor signaling in the vessel wall likely underlie its capacity to restore dilatory and contractile responses in TGF mice. Whether such improvement would be matched by normalization of brain perfusion at rest and under neuronal activity-driven function hyperemia would deserve additional experiments.
Simvastatin Reduced Arteriogenic Remodeling and String Vessel Pathology in Aged Transforming Growth Factor-β1 Mice
We described a remodeling response in the cortical microcirculation of TGF mice exemplified by increased medium to large size arterioles with few surrounding capillaries, and increased number of string vessels likely representing degenerating capillaries. Simvastatin partly but significantly lessened these abnormalities despite persisting high levels of TGF-β1, showing that simvastatin can, to some extent, counter TGF-β1 remodeling effects. Enhanced TGF-β1 signaling through TGF-β type III receptor endoglin promotes arteriogenic remodeling induced by chronic cerebral hypoxia.28 Hence, the reported ability of statins to reduce endoglin expression29 may account for simvastatin-positive effects seen here in treated TGF mice. Additionally, chronic hypoperfusion, as displayed by TGF mice,14 reduces blood flow shear stress required for endothelial cell and capillary function, presumably leading to endothelial cell death and collapsing of capillaries; therefore, favoring string vessels formation.5 The increased eNOS protein levels and recovered baseline NO synthesis in brain vessels from simvastatin-treated TGF mice show improved endothelial cell function, which could oppose the string vessel pathology. Alternatively, reduced string vessel pathology could result from simvastatin capacity to rescue endothelial cells from apoptosis caused by overexpression of TGF-β130 or, as seen in diabetic retinopathy,31 protect the capillary bed by increasing circulating endothelial progenitor cells and serum NO levels.
Simvastatin Did Not Counter Cerebrovascular Fibrosis or Calcification
Arterial stiffness, a contributor to cognitive impairment in VaD and AD, can result from calcium or collagen depositing in the vessel wall, with calcium being the likely causal factor in increased vascular fibrosis.32 Specifically, RANKL binding to RANK induces vascular calcification.33 Whereas the low expression of RANKL in hippocampal vessels of WT mice supports modest vascular calcification in normal aging,19 prominent RANKL immunostained vessels in the hippocampus of aged TGF mice–compatible with TGF-β1 increasing RANKL in endothelial cells,34 indicated calcification of the brain vasculature in TGF mice. This is supported by findings in an inducible TGF mouse model of upregulation in genes involved in both vascular calcification and vascular fibrosis.35
The TGF-β1-mediated vascular fibrosis involves Smad3 signaling. However, despite the ability of simvastatin to abrogate Smad3 phosphorylation in dermal fibroblasts in culture,36 no decrease in cerebrovascular fibrosis could be measured in the present study. Similarly, although statins can enhance expression of RANKL decoy receptor osteoprotegerin through p38 MAP kinase,37 a pathway facilitated by simvastatin (Figure 1), a modest benefit of simvastatin on cerebrovascular RANKL was found. Consistent with the previous studies on statins,19 we conclude that simvastatin has limited benefits on cerebrovascular fibrosis or calcification in TGF mice. Interestingly, genes or proteins involved in vascular fibrosis or calcification are thought to account for thioflavin-S-positive material that does not correspond to Aβ peptide in brain microvessels from TGF mice.15, 20, 35 Therefore, despite cerebrovascular fibrosis and calcification, there is no amyloid pathology in TGF mice, in agreement with the lack of increased levels of APP,20, 35, 38 Aβ peptide,38 and thioflavin-S staining in brain arteries (this study).
Simvastatin Did Not Reduce Glial Activation
Consistent with the previous reports,13, 20, 39 aged TGF mice featured prominent gliosis, with activated GFAP-positive astrocytes and Iba-1-positive microglial cells throughout the cerebral cortex and hippocampus, several being located perivascularly. Whereas gliosis is linked to a neuroinflammatory response, the use of a GFAP promoter in transgenic TGF mice likely contributed to the chronic upregulation of GFAP-labeled astrocytes.7 Consequently, astrogliosis may have repercussion on microglial cells due to the tight interactions between these two cells in various processes of neuroinflammation or neurodegeneration.40 The failure of simvastatin to counter astroglial and microglial activation could possibly underlie the subtle albeit not significant lower cognitive performance in aged TGF mice compared with WT controls.
Transforming Growth Factor-β1 Mice and Cognitive Performance, Effect of Simvastatin
Whereas we confirmed previous findings of no obvious spatial learning and memory deficits in elderly (>18 months old) TGF mice,13 the present cohort exhibited a slightly poorer performance in remembering both the quadrant within which the hidden platform was located and the precise location of the platform within this quadrant. These trends, however, did not reach statistical significance, and simvastatin exerted no benefit on these slight impairments. Our findings, however, do not exclude that simvastatin might exert benefits in tests other than the Morris water maze. Indeed, despite subtle deficits in performance in the Morris water maze at 12 months39 and 20 months of age (this study), 16-month-old TGF mice displayed deficits in other memory tests like the novel object recognition test and the Y maze.15 It thus appears that cerebrovascular disease in TGF mice is insufficient to initiate clear cognitive decline until late in life.
Conclusions
These findings show cerebrovascular dysfunction in aged TGF mice associated with light cognitive deficits related to spatial memory. Most importantly, our results show that cerebrovascular function can be fully restored by simvastatin despite persisting vascular fibrosis, calcification, and perivascular gliosis. These remarkable benefits of simvastatin could be highly relevant for counteracting similar cerebrovascular alterations that characterize both VaD and AD patients.1, 2 In this regards, the possibility that a chronically compromised cerebral circulation as seen in TGF mice might precipitate cognitive failure when combined with risk factors for VaD or AD, such as cardiovascular diseases and diabetes,1, 2 deserves further investigation. Together, our findings support that targeting TGF-β1 signaling might have a therapeutic value in small vessel diseases.12
Acknowledgments
The authors thank Dr L Mucke (Gladstone Institute of Neurological Disease and Department of Neurology, UCSF, CA, USA) and the J David Gladstone Institutes for TGF transgenic mouse breeders. The authors also thank Dr Luqing Zhang for counting string vessels in tissue sections, Dr Clotilde Lecrux and Ms Jessika Royea for helpful comments on the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by grants from the Canadian Institutes of Health Research (CIHR, MOP-84275, and MOP-126001), and the Heart & Stroke Foundation of Québec (to EH).
Supplementary Material
References
- Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann M, Kiliaan AJ, Claassen JA. Vascular aspects of cognitive impairment and dementia. J Cereb Blood Flow Metab. 2013;33:1696–1706. doi: 10.1038/jcbfm.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, et al. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci. 1999;2:157–161. doi: 10.1038/5715. [DOI] [PubMed] [Google Scholar]
- Tong XK, Lecrux C, Hamel E. Age-dependent rescue by simvastatin of Alzheimer's disease cerebrovascular and memory deficits. J Neurosci. 2012;32:4705–4715. doi: 10.1523/JNEUROSCI.0169-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JM, Kwan J, Malek-Ahmadi M, Maarouf CL, Kokjohn TA, Belden C, et al. Morphological and pathological evolution of the brain microcirculation in aging and Alzheimer's disease. PLoS ONE. 2012;7:e36893. doi: 10.1371/journal.pone.0036893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Lin C, Sanan DA, Mucke L, Masliah E. Chronic overproduction of transforming growth factor-β1 by astrocytes promotes Alzheimer's disease-like microvascular degeneration in transgenic mice. Am J Pathol. 2000;156:139–150. doi: 10.1016/s0002-9440(10)64713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkowski E, Issa R, Sjogren M, Wallin A, Blennow K, Tarkowski A, et al. Increased intrathecal levels of the angiogenic factors VEGF and TGF-β in Alzheimer's disease and vascular dementia. Neurobiol Aging. 2002;23:237–243. doi: 10.1016/s0197-4580(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Malaguarnera L, Motta M, Di Rosa M, Anzaldi M, Malaguarnera M. Interleukin-18 and transforming growth factor-beta 1 plasma levels in Alzheimer's disease and vascular dementia. Neuropathlogy. 2006;26:307–312. doi: 10.1111/j.1440-1789.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- Peila R, Yucesoy B, White LR, Johnson V, Kashon ML, Wu K, et al. A TGF-beta1 polymorphism association with dementia and neuropathologies: the HAAS. Neurobiol Aging. 2007;28:1367–1373. doi: 10.1016/j.neurobiolaging.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Caraci F, Bosco P, Signorelli M, Spada RS, Cosentino FI, Toscano G, et al. The CC genotype of transforming growth factor-beta1 increases the risk of late-onset Alzheimer's disease and is associated with AD-related depression. Eur Neuropsychopharmacol. 2012;22:281–289. doi: 10.1016/j.euroneuro.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Fukutake T. Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL): from discovery to gene identification. J Stroke Cerebrovasc Dis. 2011;20:85–93. doi: 10.1016/j.jstrokecerebrovasdis.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Nicolakakis N, Aboulkassim T, Aliaga A, Tong XK, Rosa-Neto P, Hamel E. Intact memory in TGF-beta1 transgenic mice featuring chronic cerebrovascular deficit: recovery with pioglitazone. J Cereb Blood Flow Metab. 2011;31:200–211. doi: 10.1038/jcbfm.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertner RF, Wyss-Coray T, Von Euw D, Lesne S, Vivien D, Lacombe P. Reduced brain tissue perfusion in TGF-β1 transgenic mice showing Alzheimer's disease-like cerebrovascular abnormalities. Neurobiol Dis. 2005;19:38–46. doi: 10.1016/j.nbd.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Lifshitz V, Weiss R, Benromano T, Kfir E, Blumenfeld-Katzir T, Tempel-Brami C, et al. Immunotherapy of cerebrovascular amyloidosis in a transgenic mouse model. Neurobiol Aging. 2012;33:432.e1–432.e13. doi: 10.1016/j.neurobiolaging.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M, Bell KL, Galasko D, Galvin JE, Thomas RG, van Dyck CH, et al. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology. 2011;77:556–563. doi: 10.1212/WNL.0b013e318228bf11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Borrow P, Brooker MJ, Mucke L. Astroglial overproduction of TGF-b1 enhances inflammatory central nervous system disease in transgenic mice. J Neuroimmunol. 1997;77:45–50. doi: 10.1016/s0165-5728(97)00049-0. [DOI] [PubMed] [Google Scholar]
- Tong XK, Hamel E. Transforming growth factor-beta 1 impairs endothelin-1-mediated contraction of brain vessels by inducing mitogen-activated protein (MAP) kinase phosphatase-1 and inhibiting p38 MAP kinase. Mol Pharmacol. 2007;72:1476–1483. doi: 10.1124/mol.107.039602. [DOI] [PubMed] [Google Scholar]
- Wu M, Rementer C, Giachelli CM. Vascular calcification: an update on mechanisms and challenges in treatment. Calcif Tissue Int. 2013;93:365–373. doi: 10.1007/s00223-013-9712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe P, Mathews PM, Schmidt SD, Breidert T, Heneka MT, Landreth GE, et al. Effect of anti-inflammatory agents on transforming growth factor beta over-expressing mouse brains: a model revised. J Neuroinflammation. 2004;1:1–11. doi: 10.1186/1742-2094-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YJ, Zhao JL, You SJ, Wu YJ, Jing ZC, Gao RL, et al. Post-infarction treatment with simvastatin reduces myocardial no-reflow by opening of the KATP channel. Eur J Heart Fail. 2007;9:30–36. doi: 10.1016/j.ejheart.2006.04.013. [DOI] [PubMed] [Google Scholar]
- McGirt MJ, Lynch JR, Parra A, Sheng H, Pearlstein RD, Laskowitz DT, et al. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke. 2002;33:2950–2956. doi: 10.1161/01.str.0000038986.68044.39. [DOI] [PubMed] [Google Scholar]
- Abdel-Zaher AO, Elkoussi AE, Abudahab LH, Elbakry MH, Elsayed EA. Effect of simvastatin on the antihypertensive activity of losartan in hypertensive hypercholesterolemic animals and patients: role of nitric oxide, oxidative stress, and high-sensitivity C-reactive protein. Fundam Clin Pharmacol. 2014;28:237–248. doi: 10.1111/fcp.12020. [DOI] [PubMed] [Google Scholar]
- Zhang L, Papadopoulos P, Hamel E. Endothelial TRPV4 channels mediate dilation of cerebral arteries: impairment and recovery in cerebrovascular pathologies related to Alzheimer's disease. Br J Pharmacol. 2013;170:661–670. doi: 10.1111/bph.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland AJ, Nataatmadja MI, Walker PJ, Cuttle L, Garlick RB, West MJ. Vascular remodeling in the internal mammary artery graft and association with in situ endothelin-1 and receptor expression. Circulation. 2006;113:1180–1188. doi: 10.1161/CIRCULATIONAHA.105.582890. [DOI] [PubMed] [Google Scholar]
- Luo L, Zheng YF, Dai Y, Dai DZ. Hypercholesterolaemia induces early renal lesions characterized by upregulation of MMP-9 and iNOS and ET(A)R: alleviated by a dual endothelin receptor antagonist CPU0213 and simvastatin. J Pharm Pharmacol. 2009;61:775–780. doi: 10.1211/jpp/61.06.0010. [DOI] [PubMed] [Google Scholar]
- Dunoyer-Geindre S, Fish RJ, Kruithof EK. Regulation of the endothelial plasminogen activator system by fluvastatin. Role of Rho family proteins, actin polymerisation and p38 MAP kinase. Thromb Haemost. 2011;105:461–472. doi: 10.1160/TH10-07-0444. [DOI] [PubMed] [Google Scholar]
- Boroujerdi A, Welser-Alves JV, Tigges U, Milner R. Chronic cerebral hypoxia promotes arteriogenic remodeling events that can be identified by reduced endoglin (CD105) expression and a switch in beta1 integrins. J Cereb Blood Flow Metab. 2012;32:1820–1830. doi: 10.1038/jcbfm.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu KG, Wang BW, Chen WJ, Kuan P, Hung CR. Mechanism of the inhibitory effect of atorvastatin on endoglin expression induced by transforming growth factor-beta1 in cultured cardiac fibroblasts. Eur J Heart Fail. 2010;12:219–226. doi: 10.1093/eurjhf/hfq011. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Terushkin V, Wolff MJ, Zhang X, Valacca C, Poggio P, et al. TGF-beta1 induces endothelial cell apoptosis by shifting VEGF activation of p38(MAPK) from the prosurvival p38beta to proapoptotic p38alpha. Mol Cancer Res. 2012;10:605–614. doi: 10.1158/1541-7786.MCR-11-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yan H. Simvastatin increases circulating endothelial progenitor cells and reduces the formation and progression of diabetic retinopathy in rats. Exp Eye Res. 2012;105:1–8. doi: 10.1016/j.exer.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Rabkin SW. Arterial stiffness: detection and consequences in cognitive impairment and dementia of the elderly. J Alzheimers Dis. 2012;32:541–549. doi: 10.3233/JAD-2012-120757. [DOI] [PubMed] [Google Scholar]
- Panizo S, Cardus A, Encinas M, Parisi E, Valcheva P, Lopez-Ongil S, et al. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ Res. 2009;104:1041–1048. doi: 10.1161/CIRCRESAHA.108.189001. [DOI] [PubMed] [Google Scholar]
- Ishida A, Fujita N, Kitazawa R, Tsuruo T. Transforming growth factor-beta induces expression of receptor activator of NF-kappa B ligand in vascular endothelial cells derived from bone. J Biol Chem. 2002;277:26217–26224. doi: 10.1074/jbc.M111093200. [DOI] [PubMed] [Google Scholar]
- Ueberham U, Zobiak B, Ueberham E, Bruckner MK, Boriss H, Arendt T. Differentially expressed cortical genes contribute to perivascular deposition in transgenic mice with inducible neuron-specific expression of TGF-beta1. Int J Dev Neurosci. 2006;24:177–186. doi: 10.1016/j.ijdevneu.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Mun JH, Kim YM, Kim BS, Kim JH, Kim MB, Ko HC. Simvastatin inhibits transforming growth factor-beta1-induced expression of type I collagen, CTGF, and alpha-SMA in keloid fibroblasts. Wound Repair Regen. 2014;22:125–133. doi: 10.1111/wrr.12136. [DOI] [PubMed] [Google Scholar]
- Tsubaki M, Satou T, Itoh T, Imano M, Yanae M, Kato C, et al. Bisphosphonate- and statin-induced enhancement of OPG expression and inhibition of CD9, M-CSF, and RANKL expressions via inhibition of the Ras/MEK/ERK pathway and activation of p38MAPK in mouse bone marrow stromal cell line ST2. Mol Cell Endocrinol. 2012;361:219–231. doi: 10.1016/j.mce.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Ueberham U, Ueberham E, Bruckner MK, Seeger G, Gartner U, Gruschka H, et al. Inducible neuronal expression of transgenic TGF-beta1 in vivo: dissection of short-term and long-term effects. Eur J Neurosci. 2005;22:50–64. doi: 10.1111/j.1460-9568.2005.04189.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Canabal A, Wheeler AL, Sarkis D, Lerch JP, Lu WY, Buckwalter MS, et al. Chronic over-expression of TGFbeta1 alters hippocampal structure and causes learning deficits. Hippocampus. 2013;23:1198–1211. doi: 10.1002/hipo.22159. [DOI] [PubMed] [Google Scholar]
- Morales I, Guzman-Martinez L, Cerda-Troncoso C, Farias GA, Maccioni RB. Neuroinflammation in the pathogenesis of Alzheimer's disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci. 2014;8:112. doi: 10.3389/fncel.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.